Abstract
Thermally polymerised soybean oil (SBO) is compared with several other vegetable oils, including ordinary SBO and high-oleic SBO (HO SBO). Acid values (AVs) and kinematic viscosities of the oils were measured over 28 days on oils stored at 85°C. As expected, the AVs and viscosities increased with time and the HO SBO demonstrated similar but smaller effects. The thermally modified oil was not better than ordinary SBO necessitating the need for an optimised blending strategy. Lubricant blends were prepared by mixing thermally modified SBO with a series of compatible ester-based synthetic fluids. These displayed oxidative stabilities, by pressurised differential scanning calorimetry, similar to the bio-based oil. Furthermore, the kinematic viscosity and pour point of the lubricant blend could be accurately controlled by careful tuning of the blend ratio.
Introduction
Lubricants allow the world we live on to turn smoothly. From the manufacture of almost all metal products to the gears of the vehicles used to transport them, lubricants function to improve process efficiency and economy. There are many types of lubricants including neat fluids, blended solutions (Mang et al. Citation2007) and lubricating emulsions (Szeri Citation1996, Hollinger et al. Citation2001, Doll and Sharma Citation2011).
Approximately 50% of all lubricants end up lost to the environment (Schneider Citation2006) through improper disposal, leaks or normal use. This amplifies the importance of environmental issues and has made the use of bio-based lubricants an attractive area of research in recent years (Gawrilow Citation2004, Kurth et al. Citation2005, Adhvaryu et al. Citation2006, Hill Citation2006, Erhan et al. Citation2008). Soybean oil (SBO) has many properties which make it ideal for use as a lubricant including a very good viscosity index of ∼225 and friction-reducing ability superior to many petroleum-based materials (Adhvaryu et al. Citation2006). Strategies including genetic modification of soybean plants (Abidi et al. Citation1999, McKeon Citation2007) and chemical modification of the oil (Erhan et al. Citation2005, Ming et al. Citation2005, Schneider Citation2006, Sharma et al. Citation2006, Citation2007a, Zhao et al. Citation2006, Doll et al. Citation2007, Citation2008) have been undertaken in an effort to develop a variety of industrial SBO-based products. The simplest of these is a thermal polymerisation which will increase viscosity from its normal value of ∼55 mm2 s− 1 at 25°C. At high temperatures, the unsaturated bonds in the oil will polymerise creating a higher viscosity fluid (Radlove and Falkenburg Citation1948, Cowan Citation1949, Cowan et al. Citation1949, Gast et al. Citation1963). Materials with viscosities >600 mm2 s− 1 can be obtained through this reaction, which was discovered some time ago. However, because industrial applications call for a variety of applications, a blending strategy must be employed where targeted viscosities can be achieved using a strategy of where the bio-based fluid base stock is mixed with lower viscosity synthetic fluids in order to achieve targeted properties. This strategy also addresses deficiencies in the bio-based material including oxidative degradation (Cosgrove et al. Citation1987, Gunstone Citation1994, Dunn Citation2005, Citation2008, Frankel Citation2005, Colakoglu Citation2007, Kockritz and Martin Citation2008) and high pour points (Li et al. Citation2004, Ming et al. Citation2005, Moser et al. Citation2007). The effect of this blending strategy on these properties, and on the viscosity of the blended lubricant, is studied in this report.
Results and discussion
Initial oil study
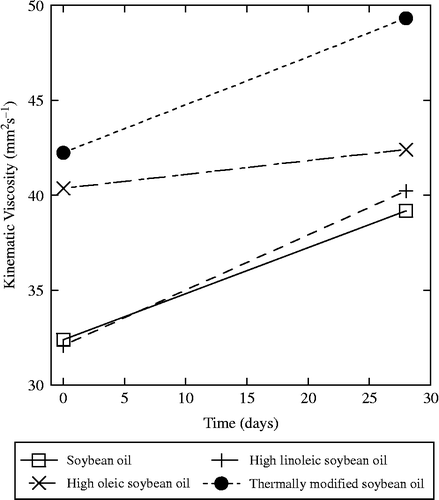
Initially, several types of SBO, such as normal SBO, high-oleic SBO (HO SBO) and high-linoleic SBO (H LIN SBO), were compared to thermally modified SBO (Erhan et al. Citation2003). Samples were placed in oven at 85°C for 28 days. The results show an increase in kinematic viscosities, measured at 40°C, which was observed in all oils (Figure ). The SBO and H LIN SBO became ∼6 mm2 s− 1 more viscous, whereas the viscosity of HO SBO only changed by ∼2 mm2 s− 1. The thermally modified sample was not better than the ordinary SBO, indicating thermal modification of oil does not significantly stabilise it.
Figure 1 The kinematic viscosity at 40°C of various SBOs, fresh and after heating at 85°C for 28 days.
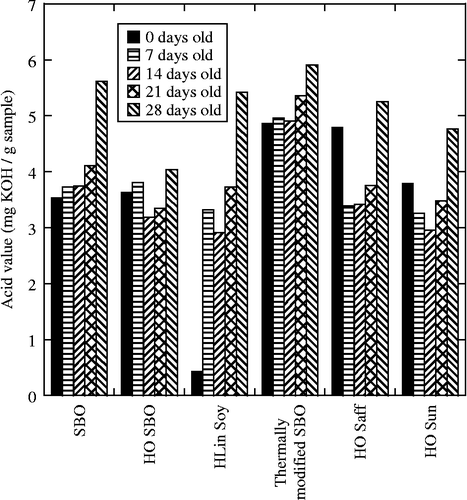
The acid values (AVs) of the same oils followed a similar trend. High-oleic sunflower oil and high-oleic safflower oil were also studied by monitoring AVs with time and were found to degrade as expected (Figure ). That is the amount of titratable acid in most of these oils increased from the starting value of ∼3 mg KOH/g sample to nearly 6 mg KOH/g sample for most of the oils. The HO SBO was significantly better than the others showing an AV increase of only 1 mg KOH/g sample.
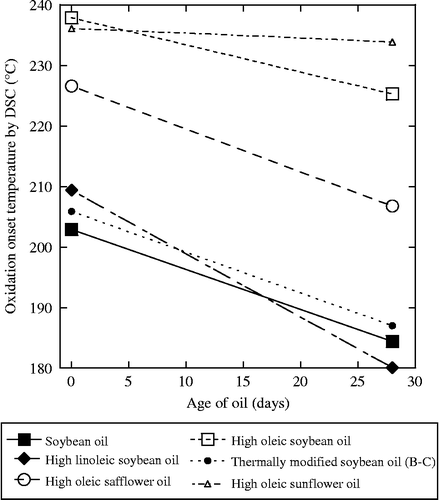
A better measure of the relative stabilities comes from ambient pressurised differential scanning calorimetry (PDSC), which measures the oxidation onset temperatures. Values between 195 and 210°C were observed in normal oils, whereas they were above 225°C for the high-oleic variety oils (Figure ). However, all of the oils became less stable when they were aged under heating. This is especially true for all of the varieties of SBOs. As in the other tests, the thermally modified SBO was not significantly improved over ordinary SBOs.
Blended oil viscosities
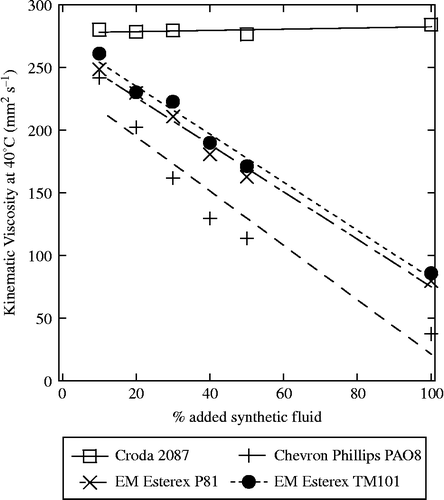
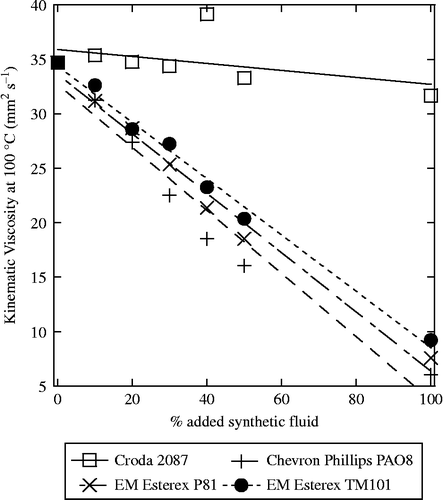
In an effort to prepare a better base stock for a wider range of lubrication applications, we turned to blending. Detailed here are blends of thermally modified SBO of kinematic viscosity 600–630 mm2 s− 1 at 25°C, with several popular lubricant esters: CRODA® 2087, Chevron Phillips PAO® 8, Exxon Mobil Esterex® P81 and Exxon Mobil Esterex® TM 101. All of these were blended in 10% increments up to 50% by volume and showed no visible separation, even after 4 weeks. These samples included a very high-viscosity fluid, CRODA® 2087, and the other lower viscosity fluids. The blends had kinematic viscosities at both 40°C (Figure ) and 100°C (Figure ), which varied with the blend ratio; therefore, a variety of applications could be attainable by the selection of blend ratios. Some other fluids such as Chevron Phillips PAO® 30 and PAO® 40 showed separation and are not suitable for higher blending levels.
Blended oil oxidation
For oxidation testing, we turned to the more rigourous PDSC method, which is a rapid method to compare oxidative stabilities and gives accurate oxidation rates for lubricants (Sharma et al. Citation2007b, Cermak et al. Citation2008, Knothe Citation2008). This method is similar to ambient PDSC except that the cell is pressurised to 1379 KPa (200 PSI) using air. This sharpens the observation of the oxidation reaction because the rate of oxygen diffusion is much faster and less limiting under the pressurised conditions. In this test, the thermally modified SBO, used in all of these blends, shows oxidation onset at 158°C. The blended oils showed similar temperature values, except for the one with EM Esterex TM101 which was ∼20°C more stable (Table ).
Table 1 The oxidation onset temperature, measured by DSC, of lubricant fluids and blends with thermally modified SBO.
Pour points
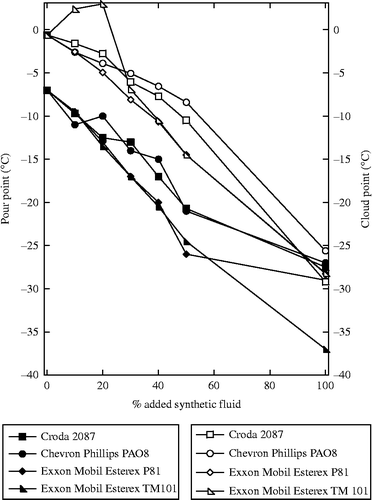
One of the barriers for using bio-based lubricants in cold weather conditions is the tendency of the oil to develop flow problems at low temperatures (Figure ). The pour point, that is the temperature below which the oil will not flow, and cloud point, the temperature at which a fluid has visible regions which scatter light, of the thermally modified SBOs are approximately − 7 and − 1°C, respectively. All of the synthetic fluids have much better values for these temperatures, all below − 25°C. As expected, the blends follow a trend where the oil is improved by adding synthetic fluid. Some slight further improvement can be found by the addition of a pour point depressant, such as Lubrizol LZ7671, which was able to lower the values of a 40% blend of thermally modified SBO in Esterex TM 101 by about 8°C (data not shown).
Experimental
AV was determined using AOCS official method Cd 3d-63. In short, ∼1 g of sample was dissolved in 125 ml of toluene/2-propanol solvent (50/50 volume) along with 2 ml of phenolphthalein indicator solution (1% in 2-propanol). Samples were titrated with 0.1 N KOH in 2-propanol, and compared with a solvent blank titrated with the same solution. Calculations were done to report AVs in mg KOH/g sample.
PDSC experiments were carried out using a PC-controlled DSC 2910 thermal analyser from TA Instruments (New Castle, DE, USA). Typically 2.0 mg of oil was placed in a hermetically sealed aluminium pan with a pinhole lid for interaction of the sample with dry air. The controlled diffusion of the gas through the hole greatly restricts the volatilisation of the oil while still allowing for saturation of the liquid phase with air. This set-up gives a film thickness of < 1 mm, and along with pressure ensures proper oil–air interaction and minimises gas diffusion limitations. The module was first calibrated for baseline, and then for temperature using the melting point of indium metal (156.6°C) at a 10°C min− 1 heating rate. Dry air was pressurised in the module at a constant pressure of 1379 KPa (200 PSI) and a scanning rate of 10°C min− 1 was used throughout the experiment. The onset temperature of oxidation was calculated from the exotherm in each case.
The kinematic viscosity of various hydroxy ester derivatives of methyl oleate was measured using Cannon-Fenske calibrated viscometers (Cannon Instrument Co., State College, PA, USA) in a Cannon temperature bath (CT-1000) at 40 and 100°C, as per the ASTM D445-95 method. The viscosities obtained are average values of two to three determinations, and the precision was within the limits of the ASTM specification.
Pour points and cloud points were measured as per the ASTM D-5949 and ASTM D-5773 automated methods, respectively, using a Phase Technology Analyser, Model PSA-70S (Phase Technology, Hammersmith Gate, Richmond, BC, Canada).
Conclusion
The use of blending gives some degree of control to the lubricant formulator. Because thermally modified SBO is available in several different viscosities, and because different compatible synthetic fluids are also available, blended ratios can give lubricants of desired viscosities and pour points in a predictable way. Manufactures can optimise these properties with those such as cost and bio-based content to meet their needs. The only drawback is that the blends produced did not show any enhanced oxidative stability, as compared with the natural oil, a problem which will require further study.
Acknowledgements
We would like to acknowledge Cynthia M. Ruder, Richard H. Henz, JoDean Sarins and Amber L. Durham for experimental work presented herein. This work was a part of the in-house research of the Agricultural Research Service of the United States Department of Agriculture. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.
References
- Abidi , S.L. , List , G.R. and Rennick , K.A. 1999 . Effect of genetic modification on the distribution of minor constituents in canola oil . Journal of the American Oil Chemists' Society , 76 ( 4 ) : 463 – 467 .
- Adhvaryu , A. 2006 . Friction behavior of some seed oils: biobased lubricant applications . Industrial and Engineering Chemistry Research , 45 ( 10 ) : 3735 – 3740 .
- Cermak , S. , Biresaw , G. and Isbell , T. 2008 . Comparison of a new estolide oxidative stability package . Journal of the American Oil Chemists' Society , 85 ( 9 ) : 879 – 885 .
- Colakoglu , A.S. 2007 . Oxidation kinetics of soybean oil in the presence of monoolein, stearic acid and iron . Food Chemistry , 101 ( 2 ) : 724 – 728 .
- Cosgrove , J.P. , Church , D.F. and Pryor , W.A. 1987 . The kinetics of the autoxidation of polyunsaturated fatty acids . Lipids , 22 ( 5 ) : 299 – 304 .
- Cowan , J.C. 1949 . lsomerization reactions of drying oils . Industrial and Engineering Chemistry , 41 ( 2 ) : 294 – 304 .
- Cowan , J.C. 1949 . Polymerization of drying oils . Industrial and Engineering Chemistry , 41 ( 8 ) : 1647 – 1653 .
- Doll , K.M. and Sharma , B.K. 2011 . Emulsification of chemically modified vegetable oils for lubricant use . Journal of Surfactants and Detergents , 14 ( 1 ) : 131 – 138 .
- Doll , K.M. , Sharma , B.K. and Erhan , S.Z. 2007 . Synthesis of branched methyl hydroxy stearates including an ester from bio-based levulinic acid . Industrial and Engineering Chemistry Research , 46 ( 11 ) : 3513 – 3519 .
- Doll , K.M. , Sharma , B.K. and Erhan , S.Z. 2008 . Friction reducing properties and stability of epoxidized oleochemicals . CLEAN – Soil, Air, Water , 36 ( 8 ) : 700 – 705 .
- Dunn , R.O. 2005 . Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel) . Fuel Processing Technology , 86 ( 10 ) : 1071 – 1085 .
- Dunn , R.O. 2008 . Antioxidants for improving storage stability of biodiesel . Biofuels, Bioproducts and Biorefining , 2 ( 4 ) : 304 – 318 .
- Erhan , S.Z. , Sheng , Q. and Hwang , H.-S. 2003 . Volatile by-products during heat polymerization of soybean oil . Journal of the American Oil Chemists' Society , 80 ( 2 ) : 177 – 180 .
- Erhan , S.Z. , Adhvaryu , A. and Sharma , B.K. 2005 . “ Chemically functionalized vegetable oils ” . In Synthetics, mineral oils, and bio-based lubricants chemistry and technology , Edited by: Rudnick , L.R. 14 – 30 . Boca Raton, FL : CRC Press .
- Erhan , S.Z. 2008 . Lubricant base stock potential of chemically modified vegetable oils . Journal of Agricultural and Food Chemistry , 56 ( 19 ) : 8919 – 8925 .
- Frankel , E.N. 2005 . Lipid oxidation , 2nd ed. , Bridgewater : The Oil Press .
- Gast , L.E. 1963 . Composition of methyl esters from heat-bodied linseed oils . Journal of the American Oil Chemists' Society , 40 ( 7 ) : 287 – 289 .
- Gawrilow , I. 2004 . Vegetable oil usage in lubricants . Inform , 15 ( 11 ) : 702 – 705 .
- Gunstone , F. 1994 . Fatty acid and lipid chemistry , Glasgow : Blackie Academic and Professional .
- Hill , K. 2006 . “ Industrial development and application of biobased oleochemicals ” . In Biorefineries – industrial processes and products, status quo and future directions , Edited by: Kamm , B. , Gruber , P.R. and Kamm , M. 291 – 314 . Weinheim : Wiley VCH .
- Hollinger , S. 2001 . High-pressure lubrication with lamellar structures in aqueous lubricant . Tribology Letters , 9 ( 3 ) : 143 – 151 .
- Knothe , G. 2008 . Designer biodiesel: optimizing fatty ester composition to improve fuel properties . Energy and Fuels , 22 ( 2 ) : 1358 – 1364 .
- Kockritz , A. and Martin , A. 2008 . Oxidation of unsaturated fatty acid derivatives and vegetable oils . European Journal of Lipid Science and Technology , 110 ( 9 ) : 812 – 824 .
- Kurth , T.L. , Biresaw , G. and Adhvaryu , A. 2005 . Cooperative adsorption behavior of fatty acid methyl esters from hexadecane via coefficient of friction measurements . Journal of the American Oil Chemists' Society , 82 ( 4 ) : 293 – 299 .
- Li , S. 2004 . Linear sulphonate detergents as pour point depressants . Lubrication Science , 16 ( 2 ) : 127 – 137 .
- Mang , T. , Freiler , C. and Horner , D. 2007 . “ Metalworking fluids ” . In Lubricants and lubrication , 2nd ed. , Edited by: Mang , T. and Dresel , W. 384 – 521 . Weinheim : Wiley-VCH .
- McKeon , T.A. 2007 . Castor plant gene for castor oil biosynthesis . Industrial Bioprocessing , 29 ( 5 ) : 7
- Ming , T.C. 2005 . Strategies for decreasing the pour point and cloud point of palm oil products . European Journal of Lipid Science and Technology , 107 ( 7–8 ) : 505 – 512 .
- Moser , B.R. 2007 . Diesters from oleic acid: synthesis, low temperature properties, and oxidation stability . Journal of the American Oil Chemists Society , 84 : 675 – 680 .
- Radlove , S.B. and Falkenburg , L.B. 1948 . Comparative evaluation of polymers of heat-bodied conjugated and non-conjugated linseed and soybean oils . Journal of the American Oil Chemists' Society , 25 ( 1 ) : 1 – 3 .
- Schneider , M.P. 2006 . Plant-oil-based lubricants and hydraulic fluids . Journal of the Science of Food and Agriculture , 86 ( 12 ) : 1769 – 1780 .
- Sharma , B.K. 2006 . Chemical modification of vegetable oils for lubricant applications . Journal of the American Oil Chemists' Society , 83 ( 2 ) : 129 – 136 .
- Sharma , B.K. , Doll , K.M. and Erhan , S.Z. 2007a . Oxidation, friction reducing, and low temperature properties of epoxy fatty acid methyl esters . Green Chemistry , 9 ( 5 ) : 469 – 474 .
- Sharma , B.K. , Perez , J.M. and Erhan , S.Z. 2007b . Soybean oil-based lubricants: a search for synergistic antioxidants . Energy Fuels , 21 ( 4 ) : 2408 – 2414 .
- Szeri , A.Z. 1996 . On the flow of emulsions in tribological contacts . Wear , 200 ( 1–2 ) : 353 – 364 .
- Zhao , F. 2006 . Structural aspects of surfactant selection for the design of vegetable oil semi-synthetic metalworking fluids . Environmental Science and Technology , 40 ( 24 ) : 7930 – 7937 .