Abstract
Intense electric fields up to 107 V/m generate very interesting and useful applications for the environment. Several processes using high electrical voltage for environment preservation were developed in the laboratory. The purpose of this study was to review these applications, highlighting their economic and environmental benefits: (1) electrostatic separators of particles used in industrial wastes recycling; (a) role-type electrostatic separator for granular mixtures of plastic–metal particles; (b) free-fall and rotating belt triboelectric separators for mixtures of plastic–plastic particles; (c) plate-type electrostatic separator for mixtures of metal–metal particles, (2) electrostatic precipitators for gas cleaning and (3) ozone generators for air and water treatment.
1 Introduction
High voltage levels were at first primarily used in power systems for transportation of energy. Later, many applications have been developed during the past century (Aguet and Lanoz Citation2004). Besides the equipment being used in power systems such as high voltage circuit breakers and lightning arresters, there are many applications using high voltage levels such as electrostatic processes. There are also several applications in physics including electron microscopy and particle accelerators, and in electronics such as cathode ray tube, piezoelectric generator, electric ignition, electronic flash and gas discharge lamp and so on. High voltage levels are also being introduced in medicine, in which there are applications such as biological effects of electric fields, X-ray diagnostics, radiation therapy, ozone therapy and so on. We report in this study some of these applications commonly used for waste management, air cleaning and water treatment.
2 Electrostatic separators of particles
Electrostatic techniques have been widely used for dry separation of small particles with a large difference in conductivity. Electrostatic separation is a generic term given to an important class of materials processing technology, widely used for sorting granular mixtures through electrical forces acting on particles. Electrostatic separation of copper, aluminium and insulating material is economically viable for the preservation of the environment because it provides high-quality recycled plastic and is more efficient for the recovery of metals than conventional methods of waste treatment (Howard Citation1974, Inculet et al. Citation1998, Knoll et al. Citation1988).
In this section, we present four different electrical methods for separating granular mixtures from industrial wastes such as electrical cables wastes.
2.1 Role-type electrostatic separator
The role-type electrostatic separator makes possible the separation of insulator–metal granular mixtures, such as PVC–copper particles, with purities that can reach 99.9%. They are mainly used for recovery of metal from crushed printed circuit boards and electric cables wastes (Hongzhou et al. Citation2008, Jiang et al. Citation2008, Li et al. Citation2008a, Citation2008b, Wu et al. Citation2008, Citation2009).
2.1.1 Description of the process
Figure shows the various elements of this type of separator (Dascalescu Citation1993). Electric charges are created by air ionisation due to the intense electric field generated by an electrode with ionising tips (1) called ‘corona electrode’, connected to a high voltage direct-current supply. Metal–plastic particles are deposited on the rotating cylinder (2) by the vibratory feeder (3) and acquire an electric charge with the same polarity as the high voltage potential. The particles behave differently according to whether they are electrically conducting or insulating.
Figure 1 Descriptive schematic of the role-type electrostatic separator. 1, Corona electrode connected to a DC high voltage supply; 2, grounded cylindrical rotating electrode; 3, electromagnetic vibratory feeder; 4, collecting hoppers; 5, brush and 6, static electrode connected to the same high voltage supply.
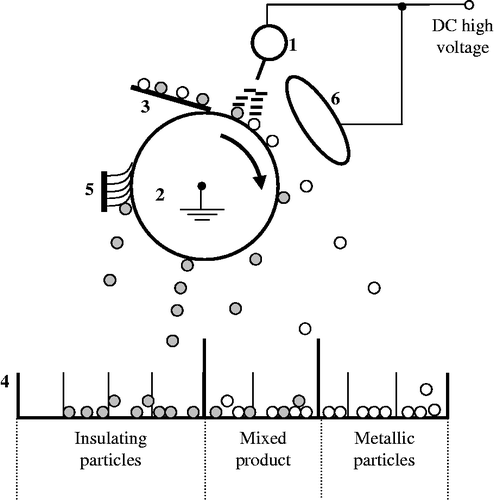
Due to the attractive electric force, insulating granules remain attached to the rotating roll and adhere to its surface. They fall down into the collector (4) when the gravitational force becomes greater than the attractive electric force. To make sure that these particles can be removed from the cylinder surface, some separators are equipped by another electrode with emitting tips, called a ‘neutralisation electrode’ to eliminate the electric charge acquired by ion bombardment. In addition, a brush (5) is used to detach mechanically the remaining granules from the surface of the cylinder.
Conducting particles quickly lose the charge, acquired by ion bombardment, through the grounded cylinder. When reaching the zone of the electrostatic field generated by the high voltage electrode of elliptic shape (6), called ‘static electrode’, they acquire by electrostatic induction a charge of opposed polarity. They are then attracted and collected in the right side of the collector.
2.1.2 Application to electric cable wastes
The role-type electrostatic separator can be used for recycling electric cable wastes. Experiments were carried out on two samples:
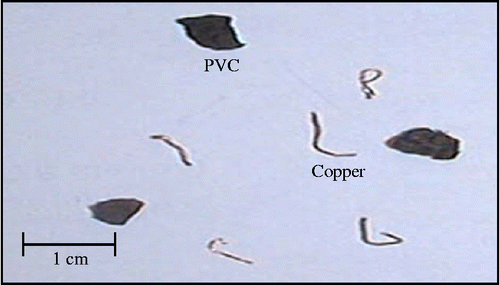
| • | Sample #A (Figure ): a total mass of 200 g containing 50% of PVC and 50% of fine copper.
| ||||
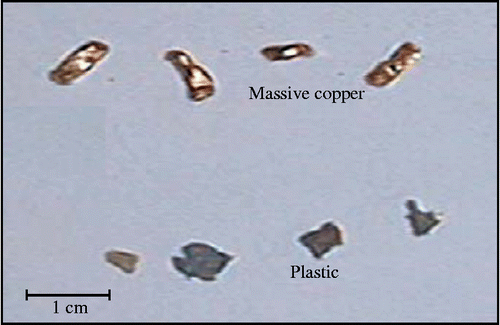
| • | Sample #B (Figure ): a total mass of 200 g containing 5% of PVC and 95% of massive copper.
| ||||
Table 1 Results after processing samples.
| • | Sample #A | ||||
More than 94% of the copper introduced into the separator is recovered with purity higher than 99%. More copper could be recovered by reprocessing the collected product in the mixed compartment, since electrostatic processes consume little electrical energy.
| • | Sample #B | ||||
The electrostatic separation process increases the purity of the copper up to 99.9% (the purity before separation was equal to 190/200 = 95%). This operation makes it possible to obtain metal with higher purity. Knowing its price which varies accordingly, it is beneficial to use the electrostatic process as ‘cleaning operation’ of the metal product.
2.2 Free-fall triboelectric separator
The free-fall electrostatic separator, also called ‘triboelectric separator’, is used for separating granular mixtures generated from plastic wastes containing plastic particles, such as PVC and PE granules (Inculet and Castle Citation1991, Inculet et al. Citation1994, Tilmatine et al. Citation2009, Citation2010a, Citation2010b), and for mineral beneficiation (Kelly and Spottiswood Citation1989a, Citation1989b, Celik and Yasar Citation1995).
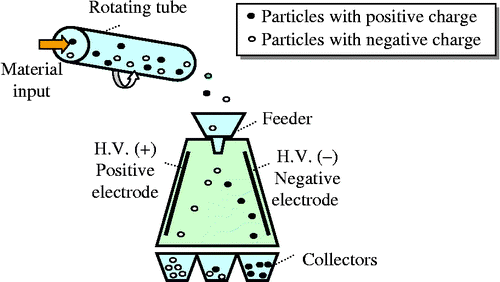
2.2.1 Description of the process
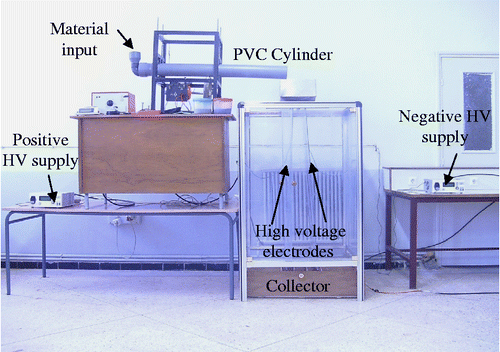
If two different materials are rubbed against each other, or simply brought into contact, they acquire opposite electric charges. This charging mechanism based on electrons transfer is called ‘triboelectricity’. A descriptive schematic is shown in Figure . First, the particles are introduced into a PVC cylinder rotating at an adjustable speed. Then, the particles undergo several collisions inside the tube: particle–particle collisions and particle–wall collisions. These collisions allow the granules to be charged by triboelectric effect. Thus, charged particles exit from the rotating cylinder and fall vertically into an intense horizontal electrical field. This horizontal electrical field is produced by two rectangular metal plates connected to two direct-current high voltage supplies of opposite polarities. The negatively charged particles are attracted towards the positive electrode and those charged positively are attracted towards the negative electrode. The products are recovered in a collector which contains three compartments (material 1, mixed product and material 2).
2.2.2 Application to PVC/HDPE mixtures
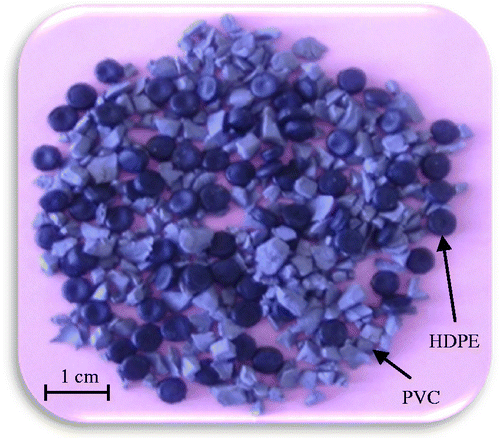
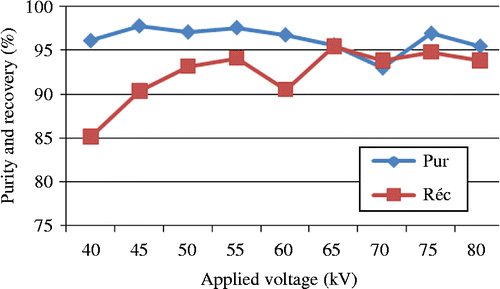
Experiments were carried out using a laboratory experimental bench; both electrodes have dimensions of 100 × 40 cm, with an applied high voltage of 45 kV for the positive electrode and 45 kV for the negative electrode (Figure ). The rotation speed of the cylinder was fixed at 180 rpm. Experiments were carried out on a PVC/PE granular sample of 200 g total mass containing 50% of polyvinyl chloride (PVC) and 50% of high-density polyethylene (HDPE), the particle size lies between 1 and 2 mm (Figure ). Results presented in Figure show and confirm the efficiency of the free-fall electrostatic separator, for the recovery of plastic wastes. Indeed, more than 90% of PVC was recovered with a purity of 98%.
2.3 Plate-type electrostatic separator
The plate-type electrostatic separator is mainly used for sorting of metal–metal particles, such as a granular mixture containing copper and aluminium fine particles (Tilmatine and Dascalescu Citation2010).
2.3.1 Description of the process
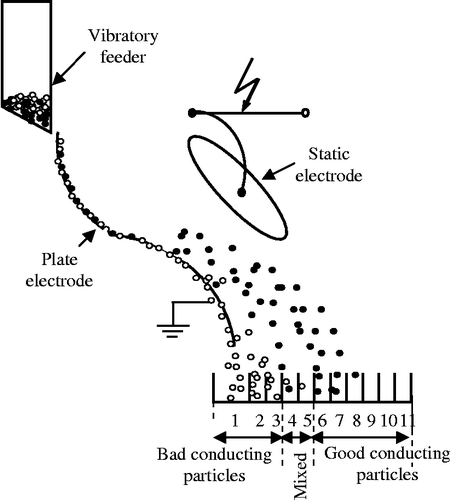
The granules (more or less good conductors) are deposited by a vibratory electromagnetic feeder on the grounded plate electrode (Figure ). The particles then ‘slip’ on the surface of the plate and behave differently according to their electric conductivity.
The granules with good conductivity, reaching the zone of the electric field generated by the high voltage static electrode of elliptical shape, are subjected to an attractive electric force exerted by this electrode and thus are collected in the appropriate compartment.
The granules with poor conductivity do not acquire a sufficient electric charge, therefore, they are not strongly attracted by the static electrode and thus are recovered in the left compartments of the collector due to the action of gravitational force.
The efficiency of this type of separator varies and largely depends on the granulometric characteristics of the particles.
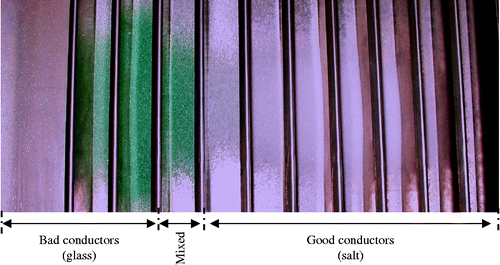
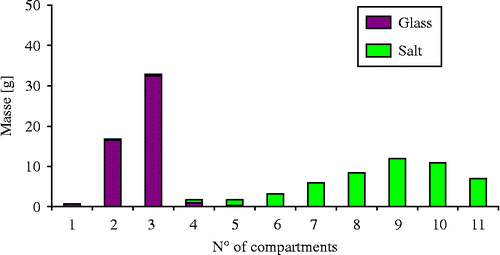
2.3.2 Application to salt–glass powders
The experiments were carried out using a laboratory electrostatic plate-type separator on a sample having an average granulometric size of 0.1 mm: 50% of salt (50 g) and 50% of glass (50 g).
After processing the mixture in the separator, the separated products are recovered in a collector containing 11 compartments. The results of the separation tests shown in Figure are illustrated by histograms in Figure . Obtained results show that it is possible to use the plate-type separator for sorting powder mixtures with high purity products. The efficiency of this process is shown in Figure , in which one can see that the particles are separated, with the resulting products having a higher purity.
The efficiency of this type of electrostatic separator varies and largely depends on the granulometric characteristics of the particles. In addition, let us note that this process can also be used to separate metal particles such as a mixture of copper–Aluminium powders (Medles et al. Citation2006).
3 Electrostatic precipitators
Electrostatic precipitation is used to eliminate solid polluting particles (such as dust and ashes) or liquids (oil mist for example) contained in gases injected into our environment (White Citation1963, Hoenig Citation1981, Takahashi et al. Citation1999, Brocilo et al. Citation2001). Besides the huge electrostatic filters that purify the flue gases of cement plants, foundries or thermal power stations, many smaller size units have been developed for the treatment of ambient air in workshops, offices, hospitals and the like, at very low electric energy consumption and high particle retention efficiency (up to 99.9%).
3.1 Description of the process
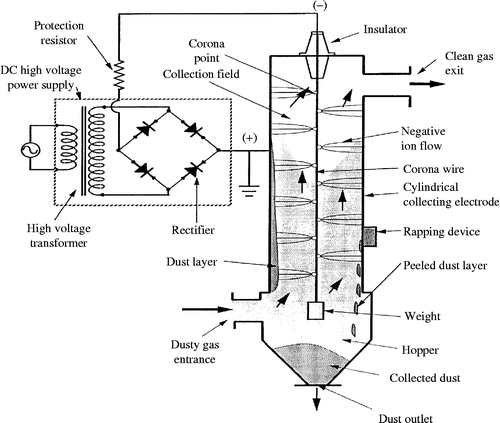
The operation principle of an electrostatic precipitator (ESP) can be easily explained with the cylindrical model (Figure ; Masuda and Hosokawa Citation1995). It consists of a vertical grounded metal cylinder (the collecting electrode) and a wire (the discharge electrode) suspended along its centre by an insulator bushing.
The gas containing particulate pollutants is introduced into the ESP by a gas inlet at the bottom of the cylinder and flows upward in the cylinder through the inter-electrodes gap. The particles are bombarded by ions from the discharge electrode (corona wire) and are strongly charged; they are driven by coulombic force towards the collecting electrode and are deposited on its inner surface.
The particles deposited on the cylinder are regularly removed by rapping and are collected in hoppers and finally evacuated out of the precipitator. Rapping ensures continuous operation of the precipitator but also presents inconvenience of re-entrainment of particles, thus causing a degradation of filtration outcome.
3.2 Application for precipitation of micronised wood particles
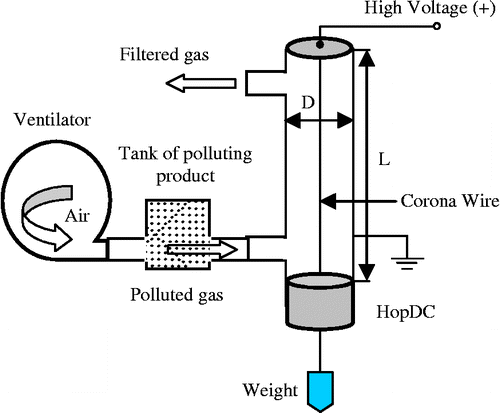
A laboratory model which simulates intermittent operation of electrostatic precipitation was build up when the pollutant is produced in a discontinuous way (Figure ). This operation mode is specific to ESP used in welding or joinery workshops. It consists of a cylinder of aluminium (length L = 50 cm and diameter D = 8 cm) connected to the ground. A central wire of 1 mm diameter is connected to a direct-current high voltage supply (U max = 50 kV, I max = 10 mA). To maintain the wire in a vertical taut position, a weight of mass m = 1.5 kg was fixed at its bottom end. An insulating hopper is used to recover the product (polluting particles) after filtration.
For each experimental run, a well-defined mass m i of polluting product (micronised wood) was blown from a container located upstream of a ventilator (maximum air flow = 2.3 m3/h, at U = 220 V) connected to the inlet of the ESP. In order to simulate a filtration process characterised by discontinuous generation of the pollutant, the electric motor of the ventilator was turned off after less than 1 min of operation. A hopper was used to recover the polluting particles after filtration.
The outcome of the process (i.e. the air filtration efficiency) using η efficiency was calculated the formula
The filtration efficiency was measured according to the applied voltage, the latter being varied from 10 to 30 kV.
The application of a high voltage generates an intense electric field E ch which determines the electric charge acquired by the particle. The saturation electric charge of an insulating particle of spherical form is given by
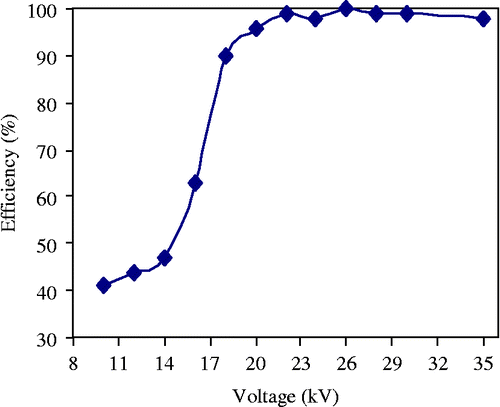
The obtained results (Figure ) show that the increase in voltage U improves the filtration efficiency to a significant degree. It is much better when the applied high voltage approaches the maximum value without sparkover, which is equal to 28 kV for our experimental set-ups. Beyond this value, the frequency of sparks increases and causes reduction in the outcome. Moreover, the high level of the applied voltage (and also a high particle resistivity) can lead to the ‘contra-emission’ phenomena that can affect the efficiency of the filter.
4 Ozone generators
Sterilisation and purification of water by ozone has been used for many years in drinking water and waste water treatment plants throughout the world. Ozone is a very reactive form of oxygen that can destroy many varieties of liquid waste materials and toxins. It is a simple, highly effective agent capable of killing a wide variety of micro-organisms. Viruses, bacteria, spores and some chemical impurities are attacked and destroyed by ozone. In addition, toxic materials treated with ozone are nearly always converted into less toxic compounds.
Ozone is almost exclusively produced by dielectric barrier discharge (DBD) which is effective for generation of ozone with high concentration and high efficiency than other methods (Rice and Netzer Citation1982, Langlais et al. Citation1991, Rice Citation1996, Kogelschatz Citation2000, Gottschalk et al. Citation2000). Ozone is formed by recombination of ionised oxygen atoms and unionised molecular oxygen. In this process, only 4–12% of the energy is used for the formation of ozone and the rest is transformed into heat.
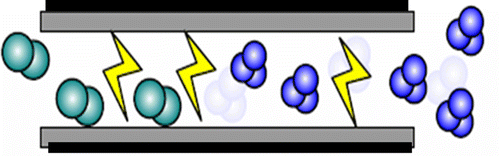
DBD is usually generated between two metallic electrodes, of which at least one is covered by a dielectric barrier, with an AC high voltage applied to these electrodes. The dielectric barrier limits the discharge current, preventing the transition of the DBD discharge to an arc discharge to ensure that stable non-equilibrium plasma can be generated even under atmospheric pressure, which is suitable for ozone generation (Figures and ).
When a high voltage is applied to the discharge gap, electron avalanches soon reach a critical stage by which the local electric field, caused by space charge accumulation at the avalanche heads, leads to a situation in which extremely fast streamer propagation becomes possible. As a result, thin conductive channels are formed. The properties of these micro-discharges have been investigated experimentally as well as theoretically. Typical parameters for air discharges in a 1-mm gap are summarised in Table .
Table 2 Microdischarge characteristic properties.
4.1 Ozone water treatment
Technical ozone generators use cylindrical discharge tubes of about 20–50 mm diameter. Borosilicate glass tubes have for a long time been the favourite dielectric material. They are mounted inside stainless steel tubes to form annular discharge gaps of about 1 mm radial width. Metal coatings, e.g. thin aluminium films, inside the glass tubes serve as high voltage electrodes, which are contacted by metal brushes.
Traditionally, low-frequency high-turns-ratio transformers are used to supply ozonisers. These systems require a very-high-voltage output, since they must operate close to the sparking potential (about 20 kV for a 1-mm gap) in order to reach the required discharge power density. This can limit the ozone production due to the restrictions imposed by the dielectric strength of the dielectric material. Low-frequency systems also present high volume, low efficiency and difficulty in controlling the ozone production.
The use of supply frequencies above 50–60 Hz allows an increase in the power density applied to the ozoniser electrode surface and an increase in ozone production for a given surface area, while decreasing the necessary peak voltage. The higher the frequency, the higher the power density and the lower the applied voltage. With the introduction of switching converters based on fast power electronic devices such as MOSFETs or insulated gate bipolar transistors, it is possible to increase the frequencies up to several kilohertz, thus allowing for an increase in the efficiency of the ozoniser. In addition, the equipment volume is decreased and ozone production can easily be controlled.
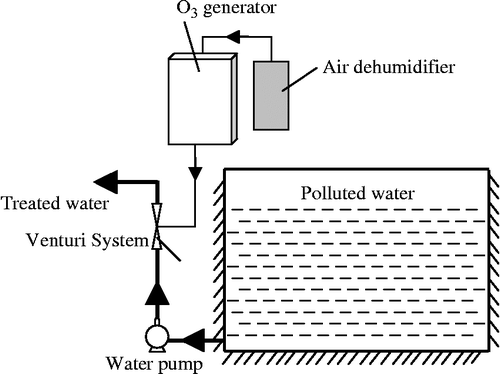
The ozone water treatment installation used is shown in Figure . The water to be treated is sucked into a water pump; ozone is then mixed with polluted water through a venturi injection system. After a few minutes of reaction, according to the ozone generator power and the water flow, water is disinfected. The dehumidifier is used to dry the air in order to increase the process efficiency.
4.2 Application to urban and industrial wastewater
This process was applied for the treatment of urban and industrial wastewater. For urban wastewater, satisfactory results were obtained after physicochemical analysis:
| • | suspended solids before treatment: 2636 mg/l | ||||
| • | suspended solids after treatment: 20 mg/l. | ||||
Furthermore, Figure shows water colour transformation obtained after treatment.
In addition, results reported in Table show the efficiency of this process for eliminating heavy metals, of industrial wastewater rejected by a national company of zinc production in Algeria. The physicochemical analysis was done after 3 and 6 min of treatment. Heavy metals would be totally disappearing if several ozone generators were employed instead of one as used in this experiment.
Table 3 Obtained results after 3 and 6 min of industrial wastewater treatment using ozone.
5 Conclusion
High intensity electric fields can be helpful and suitable for the environmental protection, through the applications it generates. The intense electric fields produce forces that are significant when applied to small particles; electrostatic separators and precipitators are a typical example. Industries are a major source of waste and modern methods of recovery are being developed with more and more new applications. Recovery of copper and aluminium from industrial wastes has real advantages because it requires less than 40% of energy than that consumed to obtain the metal from ore. ESPs have been modernized over the last few decades. In recent years, many new methods have been proposed with the goal of increasing cleaning efficiency, particularly for particles in the submicrometer size range. Furthermore, high voltage generates plasma energy for converting oxygen into ozone. Ozone is nowadays increasingly used instead of chemicals to disinfect water.
References
- Aguet , M. and Lanoz , M. 2004 . “ Traité d'électricité ” . In Haute tension , Vol. 12 , Lausanne, Switzerland : Presses Polytechniques et Universitaires .
- Brocilo , D. , Chang , J.S. and Findlay , R.D. 2001 . “ Modelling of electrode geometry effects on dust collection efficiency of-plate electrostatic precipitator ” . In Proceedings of the 8th International Conference on Electrostatic Precipitation Vol. 1 , 1 – 18 .
- Celik , M.S. and Yasar , E. 1995 . Effect of temperature and impurities on electrostatic separation of boron minerals . Mineral Engineering , 8 ( 7 ) : 829 – 833 .
- Dascalescu , L. 1993 . “ Progresses in corona-electrostatic separation technologies and equipment ” . In Proceedings of the 1st meeting of the institute of electrostatics of Japan 31 – 54 . Tokyo
- Gottschalk , C. , Libra , J.A. and Saupe , A. 2000 . Ozonation of water and waste water: a practical guide to understanding ozone and its applications , New York : John Wiley & Sons .
- Hoenig , S.A. 1981 . New application of electrostatic technology to control of dust, fumes, smokes and aerosols . IEEE Transactions on Industry Applications , IA-17 : 386 – 391 .
- Hongzhou , L. 2008 . Movement behavior in electrostatic separation: recycling of metal materials from waste printed circuit board . Journal of Materials Processing Technology , 197 : 101 – 108 .
- Howard , J.B. 1974 . Electrostatics and its applications . AlChE Journal , 20 ( 2 ) : 413
- Inculet , I.I. and Castle , G.S.P. 1991 . “ Tribo-electrification of commercial plastics in air ” . In Proceedings of the 8th international conference on electrostatics ELECTROSTATICS'91 , Institute of Physics Conference Series No 118 217 – 222 . Oxford
- Inculet , I.I. , Castle , G.S.P. and Brown , J.D. 1994 . “ Tribo-electrification system for electrostatic separation of plastics ” . In Proceedings of industry applications society annual meeting 1397 – 1399 . Denver, CO
- Inculet , I.I. , Castle , G.S.P. and Brown , J.D. 1998 . Electrostatic separation of plastics for recycling . Particulate Science and Technology , Vol. 16 : 91 – 100 . International Journal of Sustainable Engineering, Vol. 2, No. 3, pp: 184–191
- Jiang , W. , Jia , L. and Zhen-Ming , X. 2008 . Optimization of key factors of the electrostatic separation for crushed PCB wastes using roll-type separator . Journal of Hazardous Materials , 154 ( 1–3 ) : 161 – 167 .
- Kelly , E.G. and Spottiswood , D.J. 1989a . The theory of electrostatic separations: a review part I . Fundamentals Minerals Engineering , 2 ( 1 ) : 33 – 46 .
- Kelly , E.G. and Spottiswood , D.J. 1989b . The theory of electrostatic separations: a review part III. The separation of particles . Minerals Engineering , 2 ( 3 ) : 337 – 349 .
- Knoll , F.S. , Lawver , J.E. and Taylor , J.B. 1988 . “ Electrostatic separation ” . In Ullmann's encyclopedia of industrial chemistry , 5th ed , Vol. B2 , 20-1 – 20-11 . New York : John Wiley & Sons .
- Kogelschatz , U. 2000 . Fundamentals and applications of dielectric-barrier discharges , Baden, Switzerland : ABB Corporate Research Ltd .
- Langlais , B. , Reckhow , D.A. and Brink , D.R. 1991 . Ozone in water treatment , Paris : Lewis Publishers .
- Li , J. 2008a . Optimizing the operating parameters of corona electrostatic separation for recycling waste scraped printed circuit boards by computer simulation of electric field . Journal of Hazardous Materials , 153 ( 1–2 ) : 269 – 275 .
- Li , J. 2008b . Critical rotational speed model of the rotating roll electrode in corona electrostatic separation for recycling waste printed circuit boards . Journal of Hazardous Materials. , 154 : 331 – 336 .
- Masuda , S. and Hosokawa , S. 1995 . “ Electrostatic precipitation ” . In handbook of electrostatic processes , 441 – 480 . New York : Marcel Dekker .
- Medles , K. 2006 . “ Optimisation d'un processus de séparation électrostatique à électrode plaque ” . In 5ème Conférence de la Société Française d'Electrostatique SFE' 2006, 30–31 August, Grenoble, France
- Rice , R.C. 1996 . Ozone treatment for industrial wastewaters treatment: a review . Ozone Science & Engineering , 18 ( 6 ) : 477 – 515 .
- Rice , R.C. and Netzer , A. 1982 . Handbook of ozone technology and applications , Ann Arbor, MI : Ann Arbor Science .
- Takahashi , T. 1999 . Influence of re-entrainment phenomena on particle deposit in electrostatic precipitator . T. IEE Japan , 119-A ( 3 ) : 254 – 260 .
- Tilmatine , A. 2009 . Experimental analysis and optimisation of a free-fall triboelectric separator of granular plastic particles . International Journal of Sustainable Engineering , 2 ( 3 ) : 184 – 191 .
- Tilmatine , A. 2010b . Electrostatic separators of particles: Application to the recovery of industrial wastes . European Journal of Electrical Engineering , 13 ( 3 ) : 283 – 296 .
- Tilmatine , A. and Dascalescu , L. 2010 . Set-point identification of a free-fall triboelectrostatic separation process for plastic particles . International Journal of Environmental Studies , 67 ( 1 ) : 27 – 40 .
- Tilmatine , A. 2004 . Séparation électrostatique: complément des procédés mécaniques de recyclage des déchets industriels . Journal of Electrostatics , 61 : 21 – 30 .
- Tilmatine , A. 2010a . Roll-type versus free-fall electrostatic separation of tribocharged plastic particles . IEEE/IAS Industry Application Society , 46 ( 4 ) : 1564 – 1569 .
- White , H.J. 1963 . Industrial electrostatic precipitation , Reading, MA : Wesley .
- Wu , J. , Li , J. and Xu , Z. 2008 . Electrostatic separation for multi-size granule of crushed printed circuit board waste using two-roll separator . Journal of Hazardous Materials , 159 ( 2–3 ) : 230 – 234 .
- Wu , J. 2009 . Impact of nonconductive powder on electrostatic separation for recycling crushed waste printed circuit board . Journal of Hazardous Materials , 164 ( 2–3 ) : 1352 – 1358 .