Abstract
The pharmaceutical industry traditionally uses complex batch-type processes in the manufacture of medicines, although the production of specific medicines by continuous processes is currently envisaged. Due to the diversity of these processes, it is difficult to define a general set of waste prevention guidelines that would apply to all drug manufacturing. The most applicable methods of prevention can, however, be delineated for each of the five steps in the pharmaceutical manufacturing, i.e. (i) research and development, (ii) chemical synthesis, (iii) natural product extraction, (iv) fermentation and (v) product formulation. Waste streams generally arise from cleaning and sterilising equipment, chemical spills, rejected by-products and the processes themselves. Prevention mainly involves waste reduction by materials substitution, process modification/optimisation, waste stream segregation and solvent waste recycling. These measures are assessed and lead to guidelines for waste minimisation methods according to the waste streams under scrutiny.
The pharmaceutical industry and its environmental impact
Pharmaceuticals are emerging as a rapidly growing global industry. Worldwide pharmaceutical sales were $693 billion in 2010, and are expected to grow to over $900 billion by 2015. This growth is driven by higher demands due to concerns for better health in advanced economies of North America and Western Europe, along with improving living standards in Latin America, the Asia-Pacific Rim and Central and Eastern Europe: 51.8% of the global market value is currently generated in the USA, 28.2% in Europe and 20% in the Asia-Pacific region. The market performance is expected to accelerate in the years to come with the fastest growth taking place in the Asia-Pacific market (+9.2%), thanks to low costs and a favourable regulatory environment. With a market share of 52.4%, Japan is the leading country in this region. China, India and South Korea together represent another 37.2% market share. North America will remain the largest market (42% market share) because of new product launches, an ageing population and an increased reimbursement for prescription drugs by government-sponsored health programmes and private health insurance plans as discussed in several references (Grassman et al.Citation2008, Datamonitor Citation2010, Pharmaceutical Industry Citation2012, Wikipedia Citation2012).
The four major pharmaceutical multinationals (Pfizer, Merck and Co., Sanovi-Aventis and Novartis) are headquartered in the USA and Europe. These companies generate about 25% of the total worldwide revenues and employ about 400,000 people (Pharmaceutical Industry Citation2012, Wikipedia Citation2012). There are however numerous additional actors in the pharmaceutical field such as Johnson & Johnson, GlaxoSmithKline, Bayer, Abbott Laboratories, Bristol-Myers Squibb and Eli Lilly. The pharmaceutical market is hence very fragmented and characterised by strong market rivalry. Pharmaceutical companies are primarily involved in manufacturing, formulating and processing medicinal chemicals and pharmaceutical products and which grind, grade and process botanical products. Finished products are sold in various dosage delivery forms such as tablets, capsules, ointments, solutions, suspensions and powders. Two segments of the industry focus on living organisms. The in vivo and in vitro diagnostic sectors produce chemical, biochemical and radioactive substances used in diagnosing and monitoring health. The biological products sector makes bacterial and virus vaccines, toxoids, serums, plasmas and other blood derivatives for human and veterinary use.
Although the pharmaceutical industry is not considered a ‘dirty’ industry compared to many other industries, it faces new challenges in controlling and preventing environmental pollution as it expands. In 1995, the US Environmental Protection Agency reported that pharmaceutical facilities in the USA alone were annually releasing more than 50,000 tons of air, water and soil pollutants composed of 104 different chemicals (Von Zahren Citation1996, EPA Citation1997). In 1997, the US pharmaceutical industry released more than 6500 tons of carbon monoxide (CO), 19,000 tons of nitrogen oxide (NOx), 1500 tons of particulate matter of < 10 μm (PM10), 4500 tons of total particulates, 21,000 tons of sulphur dioxide (SO2) and 37,000 tons of volatile organic compounds (VOCs). Due to a strict environmental management programme, the 2010 releases were reduced to < 10% of the 1995 data, but still considered as very important due to the hazardous nature of the remaining pollutants, as only the common pollutants (CO, NOx, SO2 and particulates) have been tackled as priorities.
The sources of pollution are diverse. Material inputs to the pharmaceutical manufacturing processes generate air, water and soil pollution. Chemical synthesis processes involve reactions, separation, purification and drying, and use solvents, catalysts, reactants and intermediates that generate air emissions, wastewater and reaction residues (Everaert and Baeyens Citation2008).
Among the most serious air pollutants are VOC emissions from reactor vents, non-contained filtering systems, materials handling and acid gases. Fugitive emissions from pumps, sample collections, valves, tanks and centrifuges and solvent vapours from purification tanks also pollute the air. Fermentation processes emit odiferous gases, particulate matter and extraction solvent vapours. Natural product extraction from plants, roots and animal tissues using solvents such as ammonia, methyl chloride, phenol and toluene can release vapours and VOCs from the extraction chemicals.
Pharmaceuticals production processes also create wastewater that can pollute rivers, streams and groundwater. Chemical synthesis generates wastewater from liquors, catalysts, reactants, pumps, wet scrubbers and equipment cleaning. Wastewater from these sources is generally high in biochemical oxygen demand (BOD), in chemical oxygen demand (COD) and in total suspended solids, with pH values between 1 and about 11. The processes of separation, purification and drying produce wastewater pollutants from equipment cleaning, spills, leaks and spent solvents. Water resources are threatened by ineffective disposal of spent fermentation broth and fermentation liquids.
Pharmaceutical facilities also generate residual wastes such as reaction residuals and reactor bottom wastes, spent raw materials from natural product extraction, waste filter cake, fermentation residues, particulates, waste packaging and rejected tablets or capsules from drug formulation processes.
Key activities of the pharmaceutical industry
The pharmaceutical industry utilises diverse technologies and complex processes mostly in the batch production of pharmaceutical products. Because of this diversity of processes, it is difficult to provide general guidelines that would apply to all drug manufacturing. Its activities are based upon five key activities comprising (i) research and development; (ii) chemical synthesis; (iii) natural product extraction; (iv) fermentation and (v) formulation.
Each of the five key activities is briefly described. Their sources of pollution are dealt with in Section 3.
Research and development
In the pharmaceutical sector, research and development occupy an important position. Research entails various sub-domains, including chemical, microbiological and pharmacological research. The development of a drug is complex, tedious, expensive and financially risky as illustrated before. By taking up this challenge, pharmaceutical companies supply an invaluable contribution in fighting diseases. More than for any other product, research and development of a drug require scientific technologies. The entire production process is characterised by quality, therapeutic effectiveness and safety of the new drug.
Chemical synthesis
Most drugs are produced by means of chemical or biochemical synthesis. In a typical production unit, various types of batch reactors are used to execute a series of reactions, separations and purifications and to consequently form the desired end product. Various reaction types, recovery processes and chemicals are used to produce a multitude of active substances destined for drugs. The process equipment needs thorough cleaning at the end of a campaign. Planning of the different production campaigns is hence essential.
Natural product extraction
Extraction of natural products entails leaching of pharmaceutical products from natural materials such as roots, leaves, animal glands and so on whether or not followed by a transformation. Examples of pharmaceutical products by this production process are allergy relief medicines, insulin, morphine, cocaine and papaverine.
A characteristic of extraction is that the quantity of finished product is small compared to the used quantity of raw materials. Typically, the volume reduction from raw material to finished product is 1000. Because of this volume reduction, conventional batch and continuous processes are usually not suitable. Product recovery and purification processes entail precipitation and solvents extraction (with the usual solvents such as ketones and alcohols).
Fermentation
Steroids, vitamin B12 and antibiotics are typically produced in batches by means of fermentation processes. Overall, fermentation processes consist of two important steps: preparation (seed and inoculum preparation) and fermentation, followed by product recovery and purification.
The sterile inoculums are carefully prepared in the laboratories. Cells hereof end up in a sterile fermenter through different treatment steps. During fermentation, the content of the vessel is agitated and also treated with sterile air. Important parameters that have to be carefully monitored during the fermentation cycle are the quantity of dissolved oxygen, pH and temperature. After cell maturation, the reactor content is usually filtrated. The filtrate is processed further to separate the desired products by means of solvent extraction, precipitation and ion exchange or adsorption chromatography. In solvent extraction, the aqueous filtrate is brought into contact with an organic solvent – typically methylene chloride or butyl acetate – to transfer the desired product to the organic phase. The product is then treated through further extraction processes, precipitation or crystallisation. Ion exchange resins are used for a final purification before achieving the isolation.
Formulation, design and packaging
Pharmaceutical formulation entails mixing the active molecules with inert fillers, sweeteners and other products that should facilitate or regulate absorption. Furthermore, there is the production of various dosage forms such as tablets, capsules or fluids: 90% of oral medicines are in tablet form and are produced in three varieties, namely in compressed, coated and melted form. The tablet form depends on the desired absorption characteristics of the active ingredients. This can be fast, slow or gradual. A possible control of absorption is achieved by mixing the active ingredients with the so-called fillers such as starch or sugar, and binders such as cornstarch. For every dosage form, a unique processing method exists to achieve products with the desired characteristics.
Sources of pollution
Research and development
Because of the multiple research domains of interest in the pharmaceutical industry, a multitude of waste materials is produced, such as halogenated and non-halogenated solvents, acids, bases and oxidising agents. Moreover, large quantities of specific waste are produced such as biological and medical waste. An important waste stream originates from the use of laboratory animals and the testing of active ingredients such as carrions, clinical tissue, excrements and so on.
Methods of chemical synthesis
The waste streams generated by chemical synthesis vary significantly. Nevertheless, most waste streams contain organic as well as inorganic substances from the excess of some reagent, from by-products separated during purification, etc.
Almost every step in an organic synthesis generates a stream of non-reacted regents, reaction by-products and residues. Also acids, bases, cyanides and heavy metals can be present. Typically, waste streams are treated onsite by means of distillation or extraction. Yet, this solvent recovery results into new waste streams such as tar soil. When gaseous solvents are used, there is a chance of emissions in the sky. The latter can be controlled by using scrubbers, activated carbon fillers or by means of cryogenic condensation.
An aqueous waste stream arises from soluble solvents, filtrates, concentrates, cleaning of process equipment, scrubbers and spills. Sometimes pretreatments are required before discharging the waste stream because of the concentration or toxicity. Waste streams of synthetic processes are characterised by a high BOD, COD (>103–104 mg/l) and total quantity of dissolved substances. Moreover, waste streams can vary between pH 1 and 11.
Natural product extraction
Waste streams coming from natural product extraction consist, on the one hand, of unprocessed raw materials such as leaves, seeds or roots, but, on the other hand, also of water soluble organics, vapours of solvents and wastewater streams. Wastewater of extraction processes typically contains a low BOD, COD and total quantity of dissolved substances, whereas pH values range from 6 to 8.
Methods of fermentation
Waste streams of fermentation processes are characterised by large volumes, especially the spent fermentation medium and solid cell debris. The streams can contain many impurities such as unused raw materials or fermentation liquid. Separation processes result into large quantities of solid waste in the form of filter cakes. The liquid waste stream consists of the filtrate stream after product recovery, cleaning of process equipment and streams coming from the scrubbers.
Wastewater from fermentation processes typically contains high BOD, COD (>104 mg/l) and total quantity of dissolved substances, with pH values from 4 to 8. Moreover, atmospheric emissions may occur when using volatile solvents.
Methods of formulation
Waste streams generated during formulation processes result from the cleaning and sterilisation of process components, chemical spills, rejected products and from the formulation processes itself. During the mix or tablet forming processes, dust can be generated, which is then recycled to the formulation processes. Yet, small quantities of dust can be released. The primary wastewater stream is caused by the cleaning of process equipment. This stream contains inorganic salts, sugars and syrups. The wastewater is characterised by a low BOD, COD and total quantity of dissolved matter, while the pH is almost neutral.
Table illustrates the various process of waste streams in the different steps of the pharmaceutical industry.
Table 1 Waste streams of pharmaceutical processes.
Prevention and reduction
Waste reduction can be achieved by means of product changes, a better choice of raw materials and process technologies and procedural or organisational measures. In this section, these substitutions, process modifications and good manufacturing principles are discussed in more depth.
The pharmaceutical industry is a versatile, very competitive and very confidential industry. It is hence difficult to present a general discussion of substitution and process modification. At the same time, one has to take into account the governmental regulations that are imposed on the pharmaceutical companies. Consequently, it is not possible to simply change processes, as they all have to follow carefully prepared and well-defined methods (ISO standards; Good Manufacturing Practices).
Materials substitution
Substitution envisages the modification of a raw material used in the production process to achieve a reduction in the volume or toxicity of waste streams. This substitution is difficult in the pharmaceutical industry since much testing is required to ensure that after the substitution the process has the same therapeutic effects, stability and purity as the original product. Furthermore, it requires considerable costs and a long time span before the product is accepted. Another aspect to consider is the fact that customers may reject the product due to changes in colour, form, taste or dosage.
There are however numerous examples of materials substitution, as discussed by several authors (Shearouse et al.Citation2009, Raymond et al.Citation2010, Li et al.Citation2011, Nicponski and Ramachandran Citation2011). These examples include e.g. using water-based coating material or cleaners to replace their solvent-based alternatives, using citrus-based solvent to strip labels off bottles instead of using trichloroethane, and using toluene to replace dochloromethane.
Process modification
Waste reduction can also be achieved by applying improvements to existing processes. By controlling the reaction parameters, the reactor efficiency can be improved and the formation of by-products can be reduced. An increasing automation can substantially reduce operating errors. For example, an automation system for treating and transferring products can reduce the loss of the latter.
Deposits inside process equipment – among others formed through crystallisation, sedimentation, polymerisation and even corrosion – cause a reduction in the process efficiency and an increase in waste generation. This can be avoided by making use of an optimal mixing system and by maintaining optimal process temperatures.
Although process modifications can result in significant waste reduction, this type of waste minimisation also implies various problems. For example, the cost of comprehensive procedures and the required downtime for installing new process equipment can be important obstacles. Moreover, new processes require extensive testing to ensure that the resulting product is acceptable, and usually permission of the competent authorities is required to apply process changes once a drug has been accepted.
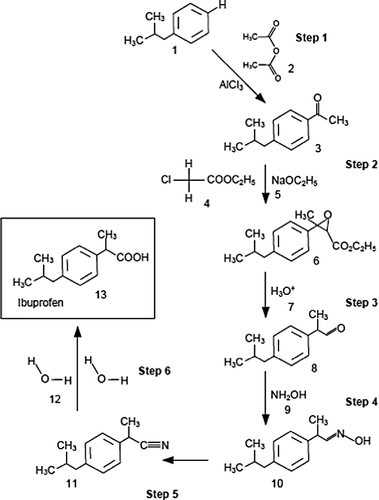
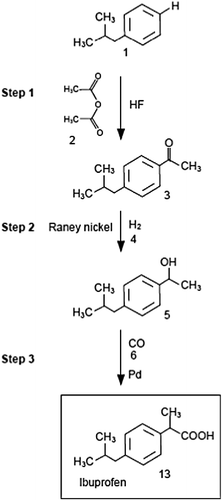
Over the past decade, principles of ‘green’ chemistry have also been introduced in the pharmaceutical industry as discussed by several authors (Anastas and Kirchhoff Citation2002, Welch Citation2007, Thissen Citation2010). A typical and widely quoted example is the production of ibuprofen, where the ‘brown’ and ‘green’ synthesis routes can be compared on the basis of the synthesis process, as illustrated in Figure (a), with the respective atom economies of Table .
Table 2 Atom economies of the ibuprofen synthesis by a ‘brown’ and ‘green’ manufacturing process.
Waste stream segregation
Segregation of waste streams contains separation of hazardous and harmless materials per contaminant, sorting of hazardous waste per contaminant and segregation of fluid and solid substances. This segregation results in a reduction in waste volumes and easy removal, recovery and recycling (ICF Technology, Inc. Citation1991, Everaert and Baeyens Citation2008).
Recovery and recycling
Recovery and recycling include direct reuse of waste material, recovering used materials for a separate use and removing impurities from waste to obtain relatively pure substances. The final purpose of recovery and recycling is the recovery of resources and products for reuse in the same or a different process. Yet, reuse is usually difficult due to the strict quality control in the pharmaceutical industry. Therefore, a high degree of cleaning is required before reuse can be considered. Recycling can be done either onsite or offsite. Onsite recycling can be integral either to an operation or in a separate operating area.
Advantages of onsite recycling include (i) a reduced amount of waste discharged from the plant; (ii) a reduced cost and liability of waste transported offsite; (iii) reduced reporting requirements in fulfilment of the legislation and (iv) a reduced unit cost for raw materials use. Disadvantages are related to the required recycling equipment (capital expenditure, additional operating and maintenance costs, required operator training, etc.) and to the necessity in obtaining additional permitting requirements. These disadvantages do not apply when recycling is included in the initial design of a process.
Offsite recycling, performed at commercial recycling facilities, is well suited for small quantity generators and firms, which cannot accept the technical, economical and managerial requirements of onsite recycling. The recycler may charge the generator a straight fee or may base fees on waste volumes and, in some instances, may credit the generator for the value of saleable wastes. The value of a waste depends on the type, market, purity, quantity and frequency of generation and distance between the generator and the recycling operation.
The choice between onsite and offsite recycling depends on the capital investment, operating costs and expertise needed. If waste volumes are small or in-house expertise is unavailable, offsite recycling is more likely to be the alternative chosen. Because generators can be held liable for future clean-up cost of wastes leaving their plants, it is important to select a recycler that is reliable.
Solvent waste recycling
Solvents are used for equipment cleaning, reaction media, extraction media and coating media. Processes for solvent recovery from concentrated waste streams include distillation, evaporation, liquid–liquid extraction, sedimentation, decantation, centrifugation and filtration. Many standard references provide a good description of these unit operations.
Solvent waste recyclability improves when solvent wastes are segregated, e.g. chlorinated from non-chlorinated solvent wastes; aliphatic from aromatic solvent wastes and water wastes from flammables. It is also important to minimise solids concentration in solvent wastes.
Overall picture of minimisation techniques used in the pharmaceutical industry
The overall picture is given in Table , providing suggestions of waste minimisation in terms of the waste stream under scrutiny.
Table 3 Waste reduction in the pharmaceutical industry.
Conclusions
Pharmaceuticals are emerging as a rapidly growing global industry. The pharmaceutical industry uses diverse processes, although mostly of batch nature. Activities cover different areas, i.e. research and development, chemical synthesis, natural product extraction, fermentation and product formulation.
Due to the diverse processes, waste streams are also diverse and arise from cleaning and sterilising equipment, chemical spills, rejected by-products and the processes themselves. The paper assessed the different production processes, each with its waste production. Prevention involves waste reduction by materials substitution, process modification/optimisation, waste stream segregation, recovery and recycling and solvent waste recycling. The assessment of these prevention measures leads to guidelines for waste minimisation methods according to the waste streams under scrutiny.
REFERENCES
- Anastas, P. and Kirchhoff, M.M., 2002. Origins, current status, and future challenges of green chemistry. Accounts of Chemical Research, 35 (9), 686–694.
- Datamonitor, 2010. Industry profile global pharmaceuticals. www.datamonitor.com.
- Environmental Protection Agency (EPA), 1997. Profile of the pharmaceutical manufacturing industry. Washington, DC: EPA 310-R-97-005.
- Everaert, K. and Baeyens, J., 2008. The pharmaceutical industry, Preventieve Pollutiebeperking van Processen. Belgium: Wolters Kluwer, 123–129, ISBN 978-90-4651-959-2 (in Dutch), Chapter 4.
- Grassman, O., Reepmeyer, G., and Von Zedtwitz, M., 2008. Leading pharmaceutical innovation; trends and drivers for growth in the pharmaceutical industry. Leipzig: Springer.
- ICF Technology, Inc., 1991. Guides to pollution prevention: the pharmaceutical industry. EPA/625/7-91/017;10/91.
- Li, Z.D., Zhang, S.S., Zhang, Y., Zhang, Y., and Wei, L., 2011. Evaluation of clearner production audit in pharmaceutical production industry: case study of the pharmaceutical plant in Dalian, P.R. China. Clean Technologies and Environmental Policy, 13 (1), 195–206.
- Nicponski, D.R. and Ramachandran, P.V., 2011. The role of solvent selection at exploratory and production stages in the pharmaceutical industry. Future Medicinal Chemistry, 3 (12), 1469–1473.
- Pharmaceutical Industry, 2012. Available from: www.pharmaceutical-industry.info (consulted on 27 July 2012).
- PhRMA, 2012. Available from: http://www.phrma.org/illustrative-pharmaceutical-lifecycle (consulted on 27 July 2012).
- Raymond, M.J., Slater, C.S., and Savelski, M.J., 2010. LCA approach to the analysis of solvent waste issues in the pharmaceutical industry. Green Chemistry, 12 (10), 1826–1834.
- Shearouse, W.C., Wassell, D.C., and Mack, J., 2009. Alternative solvent-free methodologies in the synthesis of pharmaceutical drugs. Current Opinion in Drug Discovery and Development, 12 (6), 772–783.
- Thissen, N., 2010. Mass and energy flow analysis supports process improvement. Chemical Engineering and Technology, 33 (4), 573–581.
- Von Zahren, W.M., 1996. ISO 14000: Understanding the environmental standards. Rockville, MD: Government Institutes, Inc.
- Welch, C.J., 2007. Challenges and opportunities for the greening of separation science in the pharmaceutical industry. Drug Discovery World, 8 (4), 71–77.
- Wikipedia, 2012. Available from: en.wikipedia.org/pharmaceutical_industry (consulted on 27 July 2012).