Abstract
In this study, methanol and organometallic MnO2 fuels were used to improve the properties of diesel fuel. In addition, the effect of methanol fuel on engine oil and a piston ring was also examined experimentally in a four-cylinder, direct injection diesel engine running at 200 bar. Three different diesel fuels were prepared by adding 5, 10 and 15% methanol to diesel fuel. In order to prevent phase separation, 1% dodecanol was added to the mixture. Organometallic compounds of manganese were synthesised to prepare the solutions. The most effective amount and performance of anti-freeze were determined. From the results, it was observed that carbon monoxide emissions decreased and NOx emissions increased with the increase in the amount of methanol. On the other hand, organometallic MnO2 increased the cetane number and decreased the freezing point, viscosity and flame temperature.
1. Introduction
Today, the world is confronted with an energy crisis due to decreasing resources and increased environmental pollution. The current situation has driven scientists to search for new alternative fuels that are sustainable, renewable and environment friendly. These considerations severely affect internal combustion engines. Therefore, it is necessary to develop low-emission alternative fuels for diesel engines (Bayındır Citation2010; Sayın Citation2011; Cheenkachorn and Fungtammasan Citation2010; Hazar Citation2010, Pang et al.Citation2006).
Legal restrictions have been brought in around the world for diesel engines to reduce emissions at working sites. In order to meet the legal requirements, intensive research is being continued on emission control methods and the development of alternative fuels. In order to use alternative fuels or additives, it is of great importance that renewable resources and existing technology can be used directly without major structural changes. The main alternatives to oil for use as engine fuels include alcohols (ethanol, methanol), natural gas, biogas, hydrogen and vegetable oils. The most widely used alternative fuels are alcohol, alcohol–gasoline mixtures and alcohol diesel. Diesel fuel from alcohols (ethanol, methanol) has a smaller molecular structure and contains oxygen. Alcohol-based fuels do not contain sulphur and heavy metals, and thereby reduce the negative effects of exhaust emissions (Sayın et al.Citation2009; Can, Çelikten, and Usta Citation2005). Gasoline and diesel engines and fuel systems do not require significant changes in order to use methanol (Knowles Citation1984). In this study, the effect of methanol and organometallic MnO2 additives on diesel engine exhaust emissions, engine oil and piston ring was investigated.
2. Materials and methods
Tests were conducted on four-cylinder, four-stroke, direct injection, water-cooled Mitsubishi Canter diesel engines. The technical specifications of the engine (with a maximum torque of 320 Nm at 1500 rpm) are given in Table . Diesel fuel was supplied from commercial petrol offices in Elazig/Turkey. The properties of the fuel have a direct effect on combustion. These are the physical and chemical properties of the fuel. In order to improve the fuel properties, first organometallic Mn was added and MnO2 was obtained. MnO2 was used as a reactive compound. A reactor of 1000 mL was used for the synthesis of the organometallic compound. Spindle oil, salt and abietic acids reacted with MnO2 in the reactor at 180°C for 2 h. The organometallic MnO2 obtained was 11.3% by mass of the mixture. At the end of the reaction, 2% ethanol was added to the fuel mixture to dissolve parts of the sediment. The structures and the amounts used in the synthesis of the organometallic compound are given in Table .
Table 1 Test engine specifications.
Table 2 Structures and the amounts used in the synthesis of organometallic compounds.
Organometallic MnO2 was added to diesel fuel at ratios of 20, 40, 60 and 80 ppm, respectively. Fuel mixtures with additives were prepared 8 h before the experiments. MnO2 was added to 1000 mg diesel fuel in ppm. Fuel compositions are given in Table .
Table 3 Fuel compositions.
In the second phase of the experiments, diesel fuels to which MnO2 was added were analysed and the Mn-related compounds are given in Table . As shown in Table , the freezing point drop in the most ideal fuel was 40 ppm.
Table 4 Fuel analysis results of Mn-related compounds.
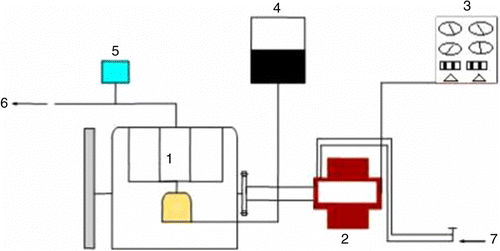
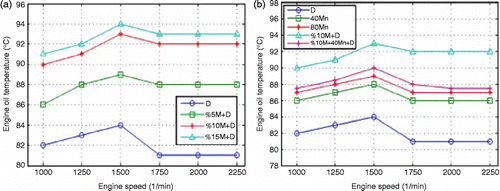
The cetane number and the cetane index of the test fuels were measured using a Zeltex ZX440 device. Samples were calibrated to ASTM standards. Several measurements were taken. The average of these values was included. The cetane number and the cetane index device have 3% error. Saybolt Universal Viscosity was measured according to ASTM D88 standards. These measurements were recorded in mm2/s. Measurements were taken at 40° according to the EN 14214 standards. The flash point was determined using an APM-7 model closed cup flash-point tester. The range of measurement was between 24 and 370°C. Flash point measurements were taken according to standard ASTM D93 (ISO 2719, IP 34) A+B, special (Fast Search), simulated TAG modes. The range of measurement for the freezing point was between +51°C and − 40°C. Measurements were taken according to standard ASTM D6749/D97, ISO 3016 (PP). The engine was run for 15 min using diesel fuel to achieve a normal working temperature. The values were recorded on a computer from the engine. After the first experiment using standard diesel fuel, tests were conducted using diesel fuel+methanol+dodecanol. In these tests, 1% dodecanol was added to 5, 10 and 15% methanol+diesel fuel. Alcohol is heavier than methanol in molecular mass. This ensured the stability of the mixture added to dodecanol. Thus, the phase separation of the mixture is prevented. An organometallic additive was added to MnO2. The tests were repeated under full load conditions of the same engine. The exhaust emissions were measured using an A Test 354 device. The exhaust device was calibrated for 30 min before measurements. Data from the engine were recorded on a computer, and 20/50 diesel engine oil was used in the experiments to examine the effect of methanol on engine oil and the components. The experiments were carried out with a Lombardini 6LD 400 single cylinder diesel engine, unloaded, at room temperature for 100 h using diesel fuel. After the experiments, the engine oil was transferred into a container to determine the changes that had occurred. The same engine was operated for 100 h under the same experimental conditions using 10% M+D fuel. Crucibles at 1200°C held in the oven according to an ASTM standard sensitive scale were weighed. After 2 h in the drying oven to achieve a stable dried weight, the crucibles were filled with specific quantities of two different motor oils. Figure shows the schematic of test set-up.
3. Results and discussion
3.1 The effect of methanol over combustion
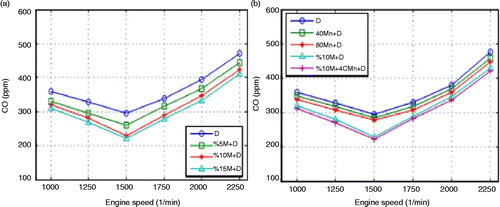
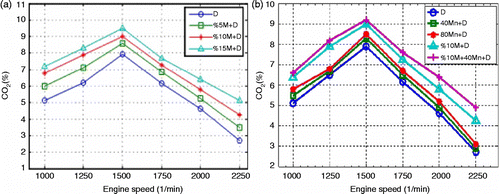
From Figure (a), which shows the relation between carbon monoxide (CO) emissions and engine speed, it is observed that CO emissions decrease at medium engine speeds for all fuels. This can be attributed to the increase in the post combustion temperature as a result of more hydrocarbons (HC) burning and the decrease in the heat losses during cooling. In various studies, it was reported that the more homogeneous the air mixture in the cylinder, the less the CO emission (Heywood Citation1988; Hazar and Temizer Citation2012). Regarding Figure (a), the increase in methanol rate in the mixture causes CO emissions to decrease. The carbon to hydrogen (C/H) ratio of the methanol fuel is less than that of the diesel fuel. This means that there is more air within the cylinder. Excess oxygen results in a better combustion and less CO emission. Moreover, the viscosity of methanol is 0.59 mPas. And that of the diesel fuel was measured as 2.8 mPas. Good atomisation of the fuel spray probably gave a positive contribution to the combustion. The decreases in average CO emissions for all speeds are 7.6, 12.6 and 15.3% for 5, 10 and 15% M+D fuels, respectively. As can be seen in Figure , the most significant decrease was recorded for the 15% M+D fuel mixture. Excess oxygen within the rich part of methanol increases the oxygen/fuel ratio and thus combustion efficiency. CO emission declines at the maximum engine torque as seen in Figure (b). Minimum CO emission was obtained at 1500 rpm. The time which is long enough increases the quality of combustion during this period. It is thought that extension in combustion time due to the air admission during the intake period affects the combustion positively, thus decreasing CO emission (Cheung, Zhu, and Huang Citation2009).
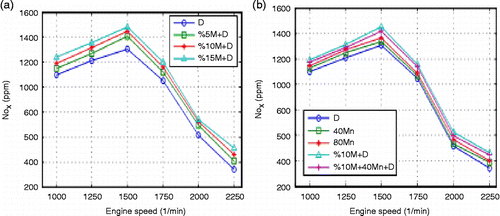
Figure (b) shows that CO emission is high at low engine speeds, decreases at medium engine speeds and increases at high speeds for all fuels. High Mn content in fuel helps the completion of the combustion. The flash points are measured as 69.3, 70, 73°C for 80 manganese+diesel (Mn+D), 40 Mn+D and pure diesel fuels, respectively. Fragmentation of the fuel helps combustion. In addition, adding MnO2 to the fuel increased the cetane number, thus decreasing ignition delay and providing better combustion. The average decrease in CO emission was measured to be 3.4% for 40 Mn+D and 5.9% for 80 Mn+D fuels in comparison with the diesel fuel. Figure (b) shows that the maximum decrease in CO emission is associated with 10% M+40Mn+D fuel. This can be attributed to the excess oxygen in this fuel mixture, which helps to increase the cetane number in the fuel. The average decrease in CO emission for 10% M+40Mn+D is 2.6% in comparison with the 10% M+D and 15% in comparison with the diesel fuel. The results show that NOx emissions increase with the increase in the proportion of methanol within the mixture. Excess oxygen in methanol decreases the cetane number of methanol from 47 to 40, and this is the main reason for the increase in ignition delay. Figure (a) shows the NOx emissions for the engine speed and methanol content of the fuel.
This can be described with the decrease in cylinder temperature, and thus the decrease in NOx content. NOx emissions increased with the increase in engine speed for all fuels. In methanol fuels, a low C/H ratio causes the temperature in the cylinder to increase. The highest NOx emission was recorded at an engine speed of 1500 rpm. The average decrease in NOx emission was determined as 16.1% for 15% M+D, 12.1% for 10% M+D and 7.7% for 5% M+D fuel in comparison to the diesel fuel. The addition of MnO2 increases NOx emissions. Catalyst addition, on the other hand, raises combustion temperature. The decrease in the viscosity and ignition point of the fuel is thought to affect the combustion positively (Karakaya Citation2000).
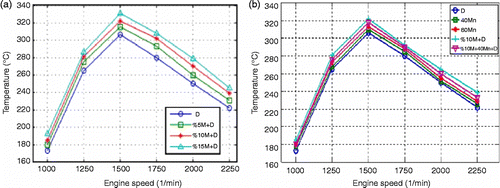
The higher ignition delay recorded for methanol is due to its high ignition temperature, high latent vaporisation heat and low cetane number. When Mn, which increases the cetane number, is added to the methanol fuel NOx emissions decreased slightly compared to 10% M+D fuel. Considering all test fuels used in the engine, increase of 5 and 3.5% in NOx emissions was detected for Mn additions of 80 and 40 ppm, respectively. While methanol increased NOx emissions, MnO2 addition decreased them. Figure (b) shows the effect of engine speed and methanol on NOx emissions. Due to the increase in the amount of air within the cylinder, combustion improves, and consequently, the inner temperature increases. Figure (a) shows the effect of methanol on the exhaust temperature for various engine speeds.
Exhaust gas temperature increases with methanol addition. This increase is 6.8% for 10% M+D, 9.6% for 15% M+D and 4% for 5% M+D fuels when compared to diesel fuel. The improvement in combustion in the ranges of engine speeds where maximum torque is achieved causes the temperature to rise. However, the shortening in ignition time as a result of the increase in engine speed affects the combustion reaction negatively. This results in some increases in exhaust emissions and decreases in NOx emissions. Mn addition caused an increase in exhaust gas temperature at all speeds when compared with diesel fuel. An increase in the cetane number of fuel, on the other hand, causes a decrease in the combustion temperature and ignition delay (Cheung, Zhu, and Huang Citation2009; Uyaroglu, Yucesu, and Cıtak Citation2010).
Mn addition raised the combustion temperatures of all fuels slightly regarding diesel fuel.
CO2 (carbon dioxide) emissions are directly related to combustion and are low for all the fuels. Methanol has lower viscosity than diesel fuel, and therefore can easily be injected and atomised. CO2 emissions versus motor speed are shown in Figure . Maximum torque was obtained at 1500 rpm, and the CO2 emissions increased with the improvement in combustion. Maximum exhaust gas temperature was recorded at medium engine speeds. From the experimental results, it is seen that oxygen in methanol combines with CO to form CO2, thus increasing the amount of CO2. This increase is 11.8% for 5% M+D, 33% for 15% M+D and 23% for 10% M+D fuel. Organometallic MnO2 additive had a positive effect on combustion, and therefore, the amount of CO2 in both the diesel and diesel–methanol mixture increased. For all engine speeds, CO2 emissions of the diesel, methanol and MnO2 mixture increased up to 5.5% when compared to ethanol–diesel fuel.
3.2 The effect of methanol over engine oil and piston rings
The results showed that after 100-h engine tests, the lubricating oil from the engine using 10% M+D fuel contained 2.08% ash, whereas the ash content of lubricating oil from the engine using diesel fuel was 1.3%. Looking at the amount of ash of the test oils at the end of the study, the ash content of lubricating oil in the engine was reduced by 60% for 10% M+D compared with the lubricating oil of the diesel fuel. Based on this rate of increase in the methanol-additive engine, the lubricating oil properties break, and it can be expected to increase the amount of sediment (Uyaroglu, Yucesu, and Cıtak Citation2010). Methanol fuel dilutes the engine oil lubrication and leads to a decrease in viscosity. Methanol fuel also caused the decrease in the oil viscosity, as shown in Table , at the end of the 100-h experimental period.
Table 5 Engine oil viscosity after 100 h of operation using diesel and methanol–diesel fuel.
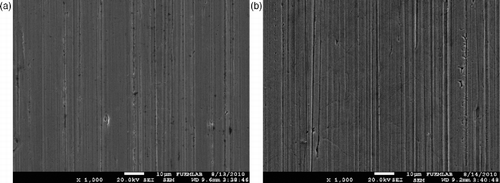
In order to show the effect over the first compression ring, the engine lubricating oil was taken out and prepared for scanning electron microscope (SEM) analysis. The 10% M+D fuel was studied for 100 h in another engine that had the same specifications and testing requirements. The piston ring was first cleared and then made ready for SEM analysis. The most corrosion is observed on the ring, pistons and cylinders. However, the compression and oil rings continuously scratched the lubricating oil over the surface of the cylinder wall. In the wear mechanism, this case causing a partial tear of the oil film starts an abrasive corrosion. In general, when we look at the SEM photographs of the engine rings, in which the diesel and methanol fuels are used, it is seen that there are deeper wear lines on the surface of the rings when the engine was run with the methanol fuel. This can be explained by the fact that the solvents in the chemical structure of methanol decrease the viscosity of the lubricating oil and wear increases (Figures and ).
4. Conclusions
Regarding diesel fuel, methanol with a lower carbon content, smaller molecular structure and oxygen content had a positive effect on combustion. Excess oxygen from methanol increased the combustion temperature. The highest NOx emission was recorded with 15% M+D fuel depending on the combustion temperature. Additives in diesel fuel decreased the viscosity, ignition and freezing points. Organometallic MnO2 addition had a positive effect on the cetane number.
Acknowledgements
The authors would like to thank Firat University (FUBAP – Project No. 1944) for their support.
REFERENCES
- Bayındır, H.2010. “The effects of cotton seed oil-kerosene blends on a diesel engine performance and exhaust emissions.” Energy Sources32 (10): 901–908.
- Can, Ö., I.Çelikten, and N.Usta. 2005. “Effects on exhaustemissions of mixed ethanol diesel fuel a diesel engine.” Journal of Engineering Sciences2: 219–224.
- Cheenkachorn, K., and B.Fungtammasan. 2010. “An investigation of diesel-ethanol-biodiesel blends for diesel engine: part 2—emission and engine performance of a light-duty truck.” Energy Sources32 (10): 894–900.
- Cheung, C. S., L.Zhu, and Z.Huang. 2009. “Regulated and unregulated emissions from a diesel engine fueled with biodiesel and biodiesel blended with methanol.” Atmospheric Environment43 (32): 4865–4872.
- Hazar, H.2010. “Cotton methyl ester usage in a diesel engine equipped with insulated combustion chamber.” Applied Energy87 (1): 134–140.
- Hazar, H., and I. Temizer. 2012. “Effects of metanol-organometal MnO2 – diesel fuel fuels in a diesel engine.”, Paper presented at the 5th Sustainable Energy & Environmental Protection, (SEEP 2012), Dublin, Ireland, July 4–8.
- Heywood, J. B.1988. Internal Combustion Engine Fundamentals. New York: McGraw-Hill.
- Karakaya, U.2000. “Development with additives of diesel fuel properties.”, Master Thesis, Gazi University.
- Knowles, D.1984. Alternative Automotive Fuels. Reston, VA: Reston Publishing Company.
- Pang, X., X.Shi, Y.Mu, H.He, S.Shuai, H.Chen, and R.Li. 2006. “Characteristics of carbonyl compound semission from a diesel-engine using biodiesel–ethanol–diesel as fuel.” Atmospheric Environment40: 7057–7065.
- Sayın, C.2011. “An experimental investigation on the effect of injection pressure on the exhaust emissions of a diesel engine fueled with methanol-diesel blends.” Energy Sources33 (23): 2206–2217.
- Sayın, C., M.Ilhan, M.Canakcı, and M.Gumus. 2009. “Effect of injection timing on the exhaust emissions of a diesel engine using diesel–methanol blends.” Renewable Energy34 (5): 1261–1269.
- Uyaroglu, A., H. S.Yucesu, and R.Cıtak. 2010. “Analysis of deformations of piston.” Journal of Technical-Online9 (2): 110–130.