Abstract
A series of alkene cross-metathesis reactions were performed using a homogeneous ruthenium-based catalyst. Using this technology, a variety of functional groups can be incorporated into the bio-based starting material, methyl oleate. Trans-stilbene, styrene, methyl cinnamate and hexen-3-ol have all been shown to give desirable products. Using this technology, aromatics, alcohols or additional esters can be incorporated into the products. For example, the cross-metathesis reaction of methyl oleate with methyl cinnamate by the second-generation Grubbs catalyst showed 70% conversion of methyl oleate into products where half of the observed products contain an aromatic group and over one-third of the products contain an α,β-unsaturated methyl ester. This promising green route is versatile, and with an appropriate selection of starting materials, it is applicable to the synthesis of polymer precursors, industrial fluids or any other application where the upgrade of natural oils is necessary.
Keywords::
Introduction
The interchange of groups around a double bond, or alkene metathesis, has recently been utilized as a valuable tool in the cabinets of organic (Nicolaou, Bulger, and Sarlah Citation2005) and industrial chemistries (Mol Citation2004). The first report of this useful reaction was a 1964 publication focusing on a molybdenum–alumina catalyst which could be used to convert propene into n-butene and ethene. Initially, the more accurate term ‘olefin disproportionation’ (Banks and Bailey Citation1964) was used before the less accurate metathesis terminology became dominant. The possibility of using this reaction in the industrial synthesis of specialty chemicals was envisioned by the 1980s (Banks et al. Citation1982).
The use of alkene metathesis in natural oil-based materials was communicated in the 1970s (van Dam, Mittelmeijer, and Boelhouwer Citation1972, Citation1974; Verkuijlen et al. Citation1977), where a brew of tungsten hexachloride mixed with tetramethyl tin in chlorobenzene was shown to convert methyl oleate into metathesis products. This system was shown to be inactive for methyl linoleate at that time, but, later, it was adapted for use on mixtures such as the methyl esters of sunflower oil (Nicolaides et al. Citation1990) or crude palm oil (Ahmad et al. Citation1995; Yarmo et al. Citation1992). A few applications based on materials of this type, such as soya-containing inks and coatings (Erhan, Bagby, and Nelsen Citation1997), were developed in this era, but overall catalyst sensitivity and intolerance of functional groups limited the area.
Ruthenium-based homogeneous metathesis catalysts (Schwab et al. Citation1995; Wu et al. Citation1995) have found many uses in various organic syntheses (Despagnet-Ayoub and Grubbs Citation2005; Dias, Nguyen, and Grubbs Citation1997; Hong, Day, and Grubbs Citation2004; Hong et al. Citation2005; Rölle and Grubbs Citation2002; Sandford, Love, and Grubbs Citation2001), and even made it into non-traditional arenas such as in supercritical carbon dioxide-based reactions (Fürstner et al. Citation2001). This catalyst family's increased functional group tolerance has opened up the arena of natural oil modification, where initial work could make polymeric oils (Refvik, Larock, and Tian Citation1999). In addition, because unsaturation was retained in the oil, hydrogenated or epoxidized versions of metathesis polymers are also possibilities (Refvik and Larock Citation1999). The polymer area has also been studied in a report using alkene metathesis and meadowfoam oil (Warwel et al. Citation2004).
The use of this catalyst in the biodiesel area was pioneered in 2006, where the catalyst was shown to be active (Holser, Doll, and Erhan Citation2006), and cross-metatheses have recently been shown to add functionality to methyl oleate (Behr and Gomes Citation2011) and to modify the distillation profile of biodiesel into something closer to that of petroleum diesel (Montenegro and Meier Citation2012).
Industrially, metathesis work on naturally based materials has been patented where 9-decenoic acid has been used as a coating preservative (Zullo et al. Citation2011), or in industrially useful alkene and diene formulations (Millis et al. Citation2011). This technology spawned a joint venture between a company specializing in this catalyst and a leading vegetable oil producer (Tullo Citation2008). Other partnerships in surfactant, alcohol and specialty lubricants have been formed. Industrial support has also been given to research work where the reactions of trioleyl glycerol have been reported (Li, Hojabri, and Narine Citation2012) and also to the use of a different family of catalyst. Most recently, a patent has been sought on a method of production of synthetic hydrocarbon lubricant base stocks and plasticizers from natural oils in a process that utilizes alkene metathesis (Wu et al. Citation2010).
The best recent academic metathesis work quantified diesters and diacids (Ngo and Foglia Citation2007) formed in this reaction, and an interesting report on the incorporation of a phosphorous group onto an unsaturated methyl ester (Sacristan et al. Citation2011). Other reports include a diacetate-methyl oleate cross-metathesis (Behr and Gomes Citation2011) and a study where the ω terminus of a fatty chain has been functionalized with an isopropylidine (Zerkowski and Solaiman Citation2012).
Despite this work, surprisingly little has been done in the incorporation of either alcohol or aromatic groups into a bio-based oleochemical using alkene metathesis. These moieties have been shown to improve the oxidative stability of vegetable oil-based lubricants, and also have important film formation and viscosity-modifying properties. This work reports the production of these materials in a low-temperature and solvent-free process using a variety of catalysts (Table ).
Table 1 Homogeneous catalysts used in this study.
Materials and methods
Reagents
Methyl oleate (>99%; Nu-Check Prep, Elysian, MN, USA), methyl linoleate (>99%; Nu-Check Prep), methyl palmitate (>99%; Nu-Check Prep), trans-stilbene (96%; Sigma-Aldrich, St Louis, MO, USA), hexen-3-ol (98%; Sigma-Aldrich), styrene (99%, inhibited with 10–15 ppm 4-tert-butylcatechol; Sigma-Aldrich), cinnamic acid (>99.0%; Sigma-Aldrich) and n-heptane (EMD, Gibbstown, NJ, USA, Omnisolve) were used as received. Partially epoxidized methyl linoleate was synthesized by the epoxidation method used in the literature using reagents specified therein (Doll and Erhan Citation2005b), except that only the peroxide amount was reduced and the reaction stopped before conversion to diepoxide was complete. The catalysts (G-1: Strem Chemical, Newburyport, MA, USA; all others: Sigma-Aldrich) were used as received. They were stored in an inert atmosphere glovebox to avoid possible degradation.
Instruments
The gas chromatograph–mass spectrometer (GC–MS) used was an Agilent (Santa Clara, CA, USA) 7890A GC equipped with a 7683B series injector and a 5975 C quadrapole mass detector. The column used was an Agilent/J&W DB35-MS with Agilent MSD Enhanced Chemstation software, version E01.00.237, controlling the instrument. Samples of ∼10 mg were diluted in ∼1 ml of heptane for analysis. Products were identified by comparative retention time and using mass spectra. The molecular ion was present in all of the samples except the α,β-diester (M, from Scheme ), and library matches were satisfactory in all cases where the library compound was present in the database.
Metathesis reactions
A stock solution containing 99% methyl oleate and 1% methyl palmitate, as an internal standard, was made. Reactions were typically run on an ∼5 g scale. In a dried vial containing a stirbar, a carefully weighed amount of a catalyst was added, the vial purged with nitrogen and then capped with a septa. The liquid co-metathesis substrates and the methyl oleate stock solution were injected into the vial. In the cases where the co-metathesis substrate was a solid, it was dissolved in the methyl oleate stock solution and that mixture was added to the catalyst-containing vial. The sealed reaction vials were stirred on a Reacti-vap® heating block that was set at the reaction temperature. In some cases, reaction aliquots were removed from the vials using a needle and syringe either initially or after 1 h.
Results and discussion
Catalyst, concentration, time and temperature optimization
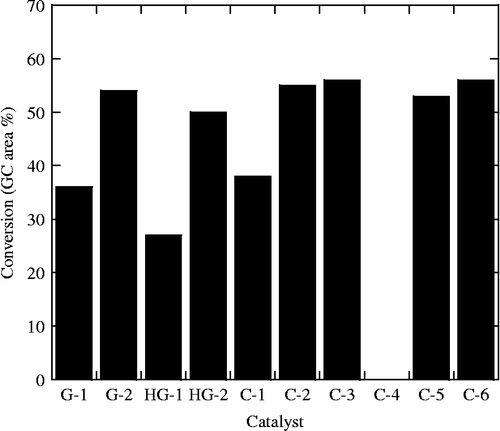
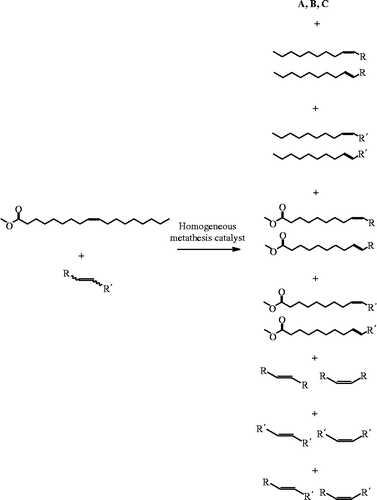
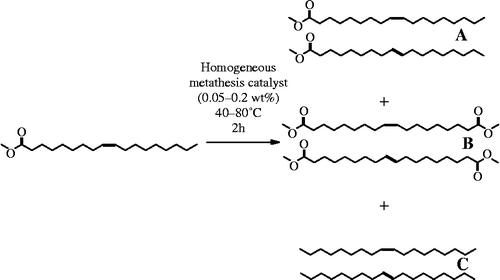
In order to determine reaction conditions for further studies, several variables were optimized using the G-1 catalyst (Table ). In agreement with prior literature reports, the self-metathesis of methyl oleate yields three groups of isomeric products (Scheme ): methyl oleate (methyl 9-cis-octadecenoate) and its trans isomer (A), the cis and trans isomers of dimethyl octadec-9-endioate (B) and cis- and trans-9-octadecene (C). Differences in the isomers were observed by the slightly longer GC retention time of the trans isomer, where its production was favoured by an ∼3:1 ratio in all cases where significant conversion was observed.
The data from these initial optimization experiments show some interesting, though expected, trends (Table ). Allowing sufficient reaction time was shown to be important where increased conversion was observed for times up to 2 h, but no significant further reaction occurred after that point. This is consistent with the expected steady-state conditions where products are also substrates for the catalyst. The temperature dependence data (Table ) show that an increase in temperature actually decreases the observed conversion percentage, whereas the catalyst concentration data indicate that an increased concentration of the catalyst increases the observed conversion. From a combination of these data, a catalyst loading of 0.2 wt%, a reaction time of 2 h and a reaction temperature of 40°C were selected for use in the catalyst selection and cross-metathesis experiments. These parameters were chosen to maximize differences in catalyst performance, yet also being experimentally convenient and cost-efficient.
Table 2 Self-metathesis of methyl oleate under various conditions.
A series of different homogeneous alkene metathesis catalysts were tested in the self-metathesis reaction under the optimized conditions (Table ). The results showed differences in the activity of the catalysts, ranging from over 50% conversion down to completely inactive (Figure ). Generally, catalysts with an imidazolidinylidene were more active than those with a bis-phosphine (G-1, HG-1 and C-1). The pyridinyl catalyst, C-4, was completely ineffective. The lower activity for bis-phosphines is in agreement with the prior literature (Montenegro and Meier Citation2012). This is possibly an effect of low-level impurities in the starting materials, such as peroxides and free acid groups, which are capable of inactivating those catalysts. The literature report did observe activity in the pyridinyl system, in contrast to what was observed here. In this study and the literature, both of the ‘second-generation’ catalysts were strong performers and, therefore, were used in all of the further systems.
Cross-metathesis of methyl oleate
The cross-metathesis of methyl oleate with different alkenes is a more complicated system (Scheme ), but one that enables the introduction of functional groups into the bio-based oleate. All of the products from the self-metathesis of methyl oleate are observed, in addition to the self-metathesis products of the other alkene and the cross-products. This leads to 10 sets of isomeric products including the cis and trans versions of both starting materials. The use of an alkene, where R = R′, reduces the number of different compound sets in the product mixture from 10 down to 6. For example, the six product sets produced in the cross-metathesis reaction of methyl oleate with trans-7-tetradecene by the G-1 catalyst were as follows: (A), both cis and trans-7-tetradecene, (B), (C) and products analogous to (A) and (C), with two fewer carbon atoms. These two shorter chain products are the actual cross-metathesis products containing contents from each monomer. In this study, monomers containing a single alkene group and another functional group were utilized. The aromatic, ester and alcohol groups were chosen (Scheme ) in order to produce products of interest.
Cross-metathesis of methyl oleate with stilbene
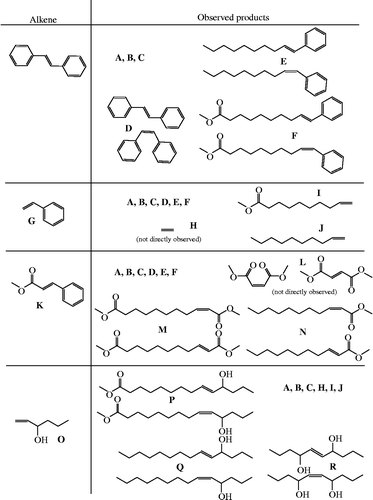
Stilbene (1,2-diphenylethene; D) is a favourable test subject for multiple reasons. First, the introduction of aromatic groups to oleochemicals is of specific interest (Biswas et al. Citation2009) as a method to produce a naturally based industrial oil with improved stability towards oxidation. The expected aromatic group incorporation will give an aromatic group with a long-chain substitution (E) and an aromatic ester (F) (Scheme ). Second, it is symmetric around a lone double bond, R = R′, which limits the possible number of metathesis products and ensures aromatic function introduction into all of the cross-metathesis products. It is also commercially available and a large variety of substituted stilbenes are available.
Reactions were run without an added solvent, 5 wt% trans-stilbene, for 2 h at 40°C. The catalysts employed were G-1, G-2, HG-2, C-1 and C-4, each used at the 0.2 wt% level. The lowest performing catalysts in the metathesis reaction, G-1 or C-1, and C-4 did not display functional group incorporation, consistent with the prior literature using this substrate (Chatterjee et al. Citation2003). However, the two ‘second-generation’ systems, G-2 and HG-2 (Table ), gave conversion of about half of the methyl oleate. The G-2 system gave a small amount of incorporation of aromatic into the products, whereas the HG-2 system produced a significant fraction of that material. Further increases through increased reagent concentrations were postulated, but could not be tested due to the relatively low solubility of stilbene.
Table 3 Conversion and product distributions of cross-metatheses of methyl oleate with various alkenes.
Cross-metathesis of methyl oleate with styrene
The use of styrene (phenylethene; G) presents both some positives and some disadvantages when compared with the stilbene system. These reactions were run under the same temperatures and concentrations as the stilbene reactions with the G-1, G-2 and HG-2 catalysts. As expected, all of the products from the stilbene system, including the aromatic compounds (E and F), were observed in this here as well (Table ). One surprising result was that in the G-1 system, nearly equal amounts of functional incorporation and self-metathesis products were observed. However, this reaction had fairly low conversion, so the actual amounts of the compounds of interest were low. In the second-generation catalyst systems, the aromatic products were produced but in lower amounts than in the stilbene system. Other products, such as 1-decene and 9-decenoic acid, were observed, and, by mass balance, ethene must also be produced in the same molar quantity as stilbene. However, even though these reactions were run in septa-capped vials, the sampling and detection methods employed here were not designed to observe ethene.
Cross-metathesis of methyl oleate with methyl cinnamate
An even more interesting system uses methyl cinnamate (methyl-3-phenyl-prop-2-enoate, K). This substrate will not only produce the same aromatic products as the stilbene system (E and F), but also produce an α,ω-diester that is unsaturated at the α,β position on one end of the chain (M). An α,β-unsaturated mono ester is also produced. Both of these multifunctional products are interesting in the synthesis of bio-based polymeric systems.
Another advantage of this system is solubility. It is convenient to make solutions of methyl cinnamate in methyl oleate of 30 wt%. Using these higher concentrations, in the HG-2 system, it was possible to get aromatic incorporation into more than half of the metathesis products, even at high conversion of methyl oleate (Table ). The G-1 catalyst produced only self-metathesis products in this reaction, indicating inactivity towards the cinnamate monomer.
Cross-metathesis of methyl oleate with 1-hexene-3-ol
Ricinoleic acid (Yao et al. Citation2010) and its hydrogenated version, 12-hydroxy stearic acid, are commonly used in the lubricant and personal care industries, where the hydroxyl-containing fatty acid chain is of value. However, the production is problematic due to agronomic reasons, and use of a metathesis catalyst to introduce an alcohol functionality to more common fatty materials, such as methyl oleate, is of interest. The use of 1-hexene-3-ol in a cross-metathesis reaction with methyl oleate produces 11-hydroxy fatty acid methyl esters (P) as well as long-chain alcohols (Q). This product is similar to methyl ricinoleate, without the drawbacks. Additionally, this reaction demonstrates the versatility of the method, where any number of other unsaturated alcohols could be made to work in a similar manner. Using the G-2 catalyst, essentially all of the alcohol was consumed, yielding a significant quantity of the ricinoleic-like product.
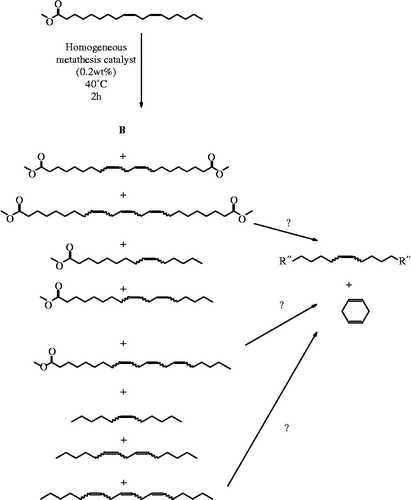
Self-metathesis of methyl linoleate
Despite all of the work that has been done using homogeneous metathesis of oleates and natural oils (Biermann et al. Citation2011; Holser, Doll, and Erhan Citation2006; Ngo and Foglia Citation2007; Refvik and Larock Citation1999; Rybak and Meier Citation2007), a specific description of the product distribution from self-metathesis of the doubly unsaturated methyl linoleate, the most common fatty chain of many natural lipids, such as soyabean, cottonseed and corn oils, has not been previously reported. When the G-2 catalyst is used in that self-metathesis reaction at 40°C, what is produced are the expected nine products (Scheme ). These arise from the expected combinations of groups where they can be placed into two different ways into categories of three, i.e. three alkenes, three monoesters and three diesters are produced, and also three monounsaturates, three diunsaturates and three triunsaturates. By mass balance, the relative amounts of monounsaturated diester should equal the amount of triunsaturated alkatriene and, conversely, the amount of monounsaturated alkene should equal the amount of triunsaturated diester. What was actually observed was an excess of the monounsaturated materials. A possible hypothesis for the missing trienes is an intramolecular metathesis reaction that converts them to 1,4-cyclohexadiene and an additional monounsaturated material. This side reaction could not be verified by the GC–MS analysis used here, due to the low retention time of the side product.
Self-metathesis of a mixture of partially epoxidized methyl linoleate
The use of epoxidized methyl oleate (Biswas et al. Citation2009; Bunker and Wool Citation2002; Doll and Erhan Citation2008; Doll, Sharma, and Erhan Citation2008) as a reaction substrate as well as that of multiple epoxide systems (Doll and Erhan Citation2005a; Doll, Sharma, and Erhan Citation2008) have been reported. By limiting the epoxidation reaction to incomplete conversion, it is possible to synthesize partially epoxidized methyl linoleate. This material contains an unsaturation as well as an epoxy group, and makes a good test of the functionality of the metathesis catalyst in the presence of an oxirane-containing substrate. A synthetic mixture containing 23% methyl linoleate, 61% singly epoxidized methyl linoleate, 8% doubly epoxidized methyl linoleate and 6% polyhydroxy species was used as a substrate for metathesis. The reaction was run at 40°C for 2 h, with either the G-1 or G-2 catalyst at 0.2 wt%. The G-1 catalyst showed less than 5% conversion of either the methyl linoleate or the singly unsaturated material. However, the G-2 catalyst was able to convert 62% of the available methyl linoleate into the expected products and 48% of the partially epoxidized substrate was converted.
Conclusion
The search for tools to modify natural materials, such as soyabean oil, is an ongoing effort where homogeneous metathesis catalysts are now one of those tools. This report shows that the catalysts can not only modify the boiling point of biodiesel, but actually add functional groups to the material as well. The potential applications for this technology span the entire spectrum of bio-based products.
Examples include the possibility of the addition of multiple unsaturations onto an oleic chain forming a linseed oil-type structure, or the addition of hydroxyl functional groups to the oil, as was done here. Judging this technology using a value-added approach can be done by the comparison of commodity oil prices. Soyabean oil is currently $0.54 USD # − 1 whereas linseed oil is $0.69 USD # − 1 (http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID = 1290). Although directly comparable prices are not available, castor oil is typically traded at a $0.10 USD #− 1 premium over linseed oil. In addition, the supply of castor oil and its derivative, ricinoleic acid, is not available from a domestic source in the USA. These factors show at least the potential for soyabean oil-based ricinoleic acid or linseed oil to be favourable from a market perspective as long as the catalyst and processing costs can be kept below $0.15–0.25 USD #− 1. It shows that an area that will need to be improved for economic viability is the maximum total turnover number of the catalysts, where 1466 was the highest observed in this study. In any case, a variety of commercially available materials that are active for cross-metathesis with methyl oleate enable the possibility for any functional group inclusion into a bio-based material, an enticing proposition.
Acknowledgements
The author acknowledges Cynthia M. Ruder, Walter G. Rivera Guzman and Erin L. Walter for the synthetic and analytical work reported here. This work was a part of the in-house research of the Agricultural Research Service of the US Department of Agriculture.
References
- Ahmad, F. B. H. , S. Hamdan , M. A. Yarmo , and A. Alimunir . 1995. “Co-metathesis Reaction of Crude Palm Oil and Ethene.” Journal of the American Oil Chemists' Society 72 (6): 757–758.
- Banks, R. L. , and G. C. Bailey . 1964. “Olefin Disproportionation.” Industrial and Engineering Chemistry Product Research 3 (3): 170–173.
- Banks, R. L. , D. S. Banasiak , P. S. Hudson , and J. R. Norell . 1982. “Specialty Chemicals via Olefin Metathesis.” Journal of Molecular Catalysis 15: 21–33.
- Behr, A. , and J. P. Gomes . 2011. “The Cross Metathesis of Methyl Oleate with Cis-2-Butene-1,4-Diyl Diacetate and the Influence of Protecting Groups.” Beilstein Journal of Organic Chemistry 7: 1–8.
- Biermann, U. , U. Bornscheuer , M. A. R. Meier , J. O. Metzger , and H. J. Schafer . 2011. “Oils and Fats as Renewable Raw Materials in Chemistry.” Angewandte Chemie International Edition in English 50 (17): 3854–3871.
- Biswas, A. , B. K. Sharma , K. M. Doll , S. Z. Erhan , J. L. Willett , and H. N. Cheng . 2009. “Synthesis of an Amine Oleate Derivative Using an Ionic Liquid Catalyst.” Journal of Agricultural and Food Chemistry 57 (18): 8136–8141.
- Bunker, S. P. , and R. P. Wool . 2002. “Synthesis and Characterization of Monomers and Polymers for Adhesives from Methyl Oleate.” Journal of Polymer Science A 40: 451–458.
- Chatterjee, A. K. , T.-L. Choi , D. P. Sanders , and R. H. Grubbs . 2003. “A General Model for Selectivity in Olefin Cross Metathesis.” Journal of the American Chemical Society 125 (37): 11360–11370.
- Despagnet-Ayoub, E. , and R. H. Grubbs . 2005. “A Ruthenium Olefin Metathesis Catalyst with a Four-Membered N-Heterocyclic Carbene Ligand.” Organometallics 24 (3): 338–340.
- Dias, E. L. , S. T. Nguyen , and H. Grubbs . 1997. “Well-Defined Ruthenium Olefin Metathesis Catalysts: Mechanism and Activity.” Journal of the American Chemical Society 119 (17): 3887–3897.
- Doll, K. M. , and S. Z. Erhan . 2005a. “The Improved Synthesis of Carbonated Soybean Oil Using Supercritical Carbon Dioxide at a Reduced Reaction Time.” Green Chemistry 7 (12): 849–854.
- Doll, K. M. , and S. Z. Erhan . 2005b. “Synthesis of Carbonated Fatty Methyl Esters Using Supercritical Carbon Dioxide.” Journal of Agricultural and Food Chemistry 53 (24): 9608–9614.
- Doll, K. M. , and S. Z. Erhan . 2008. “Synthesis of Cyclic Acetals (Ketals) from Oleochemicals Using a Solvent Free Method.” Green Chemistry 10 (6): 712–717.
- Doll, K. M. , B. K. Sharma , and S. Z. Erhan . 2008. “Friction Reducing Properties and Stability of Epoxidized Oleochemicals.” CLEAN – Soil, Air, Water 36 (8): 700–705.
- Erhan, S. Z. , M. O. Bagby , and T. C. Nelsen . 1997. “Drying Properties of Metathesized Soybean Oil.” Journal of the American Oil Chemists' Society 74 (6): 703–706.
- Fürstner, A. , L. Ackermann , K. Beck , H. Hori , D. Koch , K. Langemann , M. Liebl , C. Six , and W. Leitner . 2001. “Olefin Metathesis in Supercritical Carbon Dioxide.” Journal of the American Chemical Society 123 (37): 9000–9006.
- Holser, R. A. , K. M. Doll , and S. Z. Erhan . 2006. “Metathesis of Methyl Soyate with Ruthenium Catalysts.” Fuel 85 (3): 393–395.
- Hong, S. H. , M. W. Day , and R. H. Grubbs . 2004. “Decomposition of a Key Intermediate in Ruthenium-Catalyzed Olefin Metathesis Reactions.” Journal of the American Chemical Society 126 (24): 7414–7415.
- Hong, S. H. , D. P. Sanders , R. H. Grubbs , and C. W. Lee . 2005. “Prevention of Undesirable Isomerization During Olefin Metathesis.” Journal of the American Chemical Society 127 (49): 17160–17161.
- Li, S. , L. Hojabri , and S. S. Narine . 2012. “Controlling Product Composition of Metathesized Triolein by Reaction Concentrations.” Journal of the American Oil Chemists' Society 89 (11): 2077–2089.
- Millis, J. R., M. J. Tupy, T. W. Abraham, and M. L. de Souza . 2011. Method for making industrial chemicals. US Patent 7960599, filed June 14.
- Mol, J. C. 2004. “Industrial Applications of Olefin Metathesis.” Journal of Molecular Catalysis A 213 (1): 39–45.
- Montenegro, R. E. , and M. A. R. Meier . 2012. “Lowering the Boiling Point Curve of Biodiesel by Cross-Metathesis.” European Journal of Lipid Science and Technology 114 (1): 55–62.
- Ngo, H. L. , and T. A. Foglia . 2007. “Synthesis of Long Chain Unsaturated-Alpha-Omega-Dicarboxylic Acids from Renewable Materials via Olefin Metathesis.” Journal of the American Oil Chemists' Society 84 (8): 777–784.
- Nicolaides, C. P. , J. H. Opperman , M. S. Scurrell , and W. W. Focke . 1990. “Metathesis of Fatty Esters Derived from South African Sunflower Oil.” Journal of the American Oil Chemists' Society 67 (6): 362–363.
- Nicolaou, K. C. , P. G. Bulger , and D. Sarlah . 2005. “Metathesis Reactions in Total Synthesis.” Angewandte Chemie International Edition in English 44 (29): 4490–4527.
- Refvik, M. D. , and R. C. Larock . 1999. “The Chemistry of Metathesized Soybean Oil.” Journal of the American Oil Chemists' Society 76 (1): 99–102.
- Refvik, M. D. , R. C. Larock , and Q. Tian . 1999. “Ruthenium-Catalyzed Metathesis of Vegetable Oils.” Journal of the American Oil Chemists' Society 76 (1): 93–98.
- Rölle, T. , and R. H. Grubbs . 2002. “Ring Closing Metathesis in Protic Media by Means of a Neutral and Polar Ruthenium Benzylidene Complex.” Chemical Communications 8 (10): 1070–1071.
- Rybak, A. , and M. A. R. Meier . 2007. “Cross-Metathesis of Fatty Acid Derivatives with Methyl Acrylate: Renewable Raw Materials for the Chemical Industry.” Green Chemistry 9 (12): 1356–1361.
- Sacristan, M. , J. C. Ronda , M. Galia , and V. Cadiz . 2011. “Phosphorus-Containing Soybean-Oil Copolymers: Cross-Metathesis of Fatty Acid Derivatives as an Alternative to Phosphorus-Containing Reactive Flame Retardants.” Journal of Applied Polymer Science 122 (3): 1649–1658.
- Sandford, M. S. , J. A. Love , and R. H. Grubbs . 2001. “Mechanism and Activity of Ruthenium Olefin Metathesis Catalysts.” Journal of the American Chemical Society 123 (27): 6543–6554.
- Schwab, P. , M. B. France , J. W. Ziller , and R. H. Grubbs . 1995. “A Series of Well-Defined Metathesis Catalysts – Synthesis of [RuCl2( = Ch′)(Pr3)2] and Its Reactions.” Angewandte Chemie International Edition in English 34 (18): 2039–2041.
- Tullo, A. 2008. “Cargill, Materia Launch New Firm.” Chemical & Engineering News 86 (13): 6.
- van Dam, P. B. , M. C. Mittelmeijer , and C. Boelhouwer . 1972. “Metathesis of Unsaturated Fatty Acid Esters by a Homogeneous Tungsten Hexachloride–Tetramethyltin Catalyst.” Chemical Communications 1972: 1221–1222.
- van Dam, P. B. , M. C. Mittelmeijer , and C. Boelhouwer . 1974. “Homogeneous Catalytic Metathesis of Unsaturated Fatty Esters; New Synthetic Method for Preparation of Unsaturated Mono- and Dicarboxylic Acids.” Journal of the American Oil Chemists' Society 51: 389–392.
- Verkuijlen, E. , F. Kapteijn , J. C. Mol , and C. Boelhouwer . 1977. “Heterogeneous Metathesis of Unsaturated Fatty Acid Esters.” Chemical Communications 1977: 198–199.
- Warwel, S. , F. Bruse , C. Demes , and M. Kunz . 2004. “Polymers and Polymer Building Blocks from Meadowfoam Oil.” Industrial Crops and Products 20 (3): 301–309.
- Wu, M.-S., K. S. Colle, R. Y. Saleh, A. D. Goodwin, and J. E. R. Stanat . 2010. Production of Synthetic Hydrocarbon Fluids, Plasticizers and Synthetic Lubricant Base Stocks from Renewable Feedstocks. US Patent 2010-0160506 A1, filed June 24.
- Wu, Z. , S. T. Nguyen , R. H. Grubbs , and J. W. Ziller . 1995. “Reactions of Ruthenium Carbenes of the Type (PPh3)2(X)2Ru = Ch–Ch = CPh 2 (X = Cl and CF3COO) with Strained Acyclic Olefins and Functionalized Olefins.” Journal of the American Chemical Society 117 (20): 5503–5511.
- Yao, L. , E. G. Hammond , T. Wang , S. Bhuyan , and S. Sundararajan . 2010. “Synthesis and Physical Properties of Potential Biolubricants Based on Ricinoleic Acid.” Journal of the American Oil Chemists' Society 87 (8): 937–945.
- Yarmo, M. A. , A. Alimuniar , R. Abd Ghani , A. R. Sulaiman , M. Ghani , H. Omar , and A. Malek . 1992. “Transesterification Products from the Metathesis Reaction of Palm Oil.” Journal of Molecular Catalysis 76 (1–3): 373–379.
- Zerkowski, J. , and D. Solaiman . 2012. “Omega-Functionalized Fatty Acids, Alcohols, and Ethers Via Olefin Metathesis.” Journal of the American Oil Chemists' Society 89 (7): 1325–1332.
- Zullo, J. L., J. C. Anderson, H. Kaido, R. L. Pederson, Y. Schrodi, W. H. Sperber, M. J. Tupy, and E. H. Wagener . 2011. Surface coating compositions and methods, US Patent 7951232, filed May 31.