Abstract
The novel “Palette Modular Device” (PMD) technology, addressing the recovery of wastes from Physical Vapour Deposition (PVD) maintenance, is evaluated according to a Life Cycle Assessent (LCA) approach. The PMD recovery technology was recently developed with the aim of an easier and more sustainable separation of the film layers used in PVD, with a particular emphasis on multi-material film productions, frequently adopted in the electronic industry. The PMD is briefly presented in the paper, along with three implementations for industrial purposes. Each implementation adopts a different light solvent for the metal recovery like acetone and formic acid. The usage of light solvents is a peculiar feature enabled by the system considered, as an alternative to traditional approaches. The LCA starts with the objectives and prosecutes with inventories of all material and energy flows for each different scenario considered. Additionally, a global impact assessment is provided in a specific section, in order to enable a quantitative comparison of the potential effects on the environment in every scenario. The results of this study allow the industrial designer to perform an overall environmental evaluation of the three strategies proposed and to compare the performances of the novel technology. The research, furthermore, highlights some critical points in the adoption of the PMD and suggests how to improve the implementation of this recovery process.
1. Introduction
In recent decades, the continued growth of technology and product volumes has been in contrast with sustainable business models for a variety of reasons, including a lack of environmental responsibility and overall commercial strategies adopted by manufacturers. This growth is evident especially in the field of microelectronics and has triggered a huge supply of raw materials, encouraging researchers to develop advanced solutions to support the recovery from disposed devices, a reduction in material usage or the decrease in impacts during extraction activities.
It must be underlined that mining is one of the main causes of pollution on the planet. The extraction of precious metals has a significant impact on the environment (Horowitz Citation2006) in terms of energy consumption, pollutant emissions, waste processing and impact on landscapes (Coelho, Teixeira, and Goncalves Citation2011). The processing and the refinement of extracted materials, moreover, require a large amount of oil, natural gas, coal and hydropower, due to the low concentration of metals in rocks and complex production processes. As a consequence, the energy consumption brings along important pollutant emissions. Therefore, improving the reuse and recycling of resources (from production wastes, as well as disposed products) reduces ex-ante resources’ consumption and identification of novel mines, thus saving energy and preserving natural heritage.
Among different cases available for metals’ life cycles, the problem gold recovery is here addressed for two reasons: (i) gold is a “critical” raw material, e.g. used in electronics for its properties; (ii) its extraction is a good example of a polluting mining industry (Kumah Citation2006). In addition, research programmes and a strategic use of this material can be observed in nanotechnology (World Gold Council Citation2010) due to innovative applications in industrial environments (World Gold Council Citation2014). In this context, the present research tries to give a contribution by evaluating within a LCA perspective, the environmental impacts of different implementations of a novel gold recovery process. This process is specifically dedicated to production wastes of the thin-film industry (a sector which has observed an ever-growing number of novel applications involving gold). LCA studies about production wastes deserve to be analyzed, in fact scientific literature focuses often on the materials extraction phase (Coelho, Teixeira, and Goncalves Citation2011) and on the recycling of electronic goods and scrap (Hagelüken and Corti Citation2010).
The paper is structured as follows: in Section 2, a background is presented in order to discuss the context where the novel system for gold recovery is introduced; Section 3 is focused on describing gold recovery process through the new system and the corresponding procedure; Section 4 discusses the environmental aspects of the novel system, considering different hypotheses of specific implementation; Section 5 is dedicated to conclusions and future outlooks.
2. Background
Depositing a film means to superpose a layer of a material on the surface of another one (“substrate”). A thin film has a thickness of a few microns at most (Mattox Citation2010). Different kinds of films can be deposited, each one with certain characteristics that make it suitable for specific applications. The objective of the thin film deposition is to take advantage of the surface properties of a target material (such as electrical conductance/insulation, abrasion resistance, etc.), using it as a coating and, at the same time, maintaining properties of the substrate material (such as robustness, low cost, etc.). Among available techniques for thin film deposition, the “atomistic overlay” is widely used. Here, the target material is deposited by successive small quantities without penetrating deeply in the substrate. The Physical Vapour Deposition, in particular, adopts physical transport (ballistic or diffusive) of the species to be deposited and it is the method taken in consideration for this study. Physical Vapour Deposition (PVD) is particularly appropriate to produce thin films of metallic materials in a rapid and relatively cheap way, but also without the use of complex chemical precursors (Bunshah Citation1994), for this reason, it is widely used for the deposition of metal tracks in microelectronics (Rossnagel Citation1999), only to cite an example of application.
Among the most common practices for PVD the evaporation is considered. In this technique, the emission from the target material occurs by thermal runaway. The need to minimize unwanted contaminations and to have a sufficiently long mean free path of the emitted particles requires the use of chambers in ultra high vacuum (UHV). Within the same deposition chamber, several depositions of different materials in succession may occur, activating a single target material for each step, or sometimes even simultaneous targets (co-evaporation).
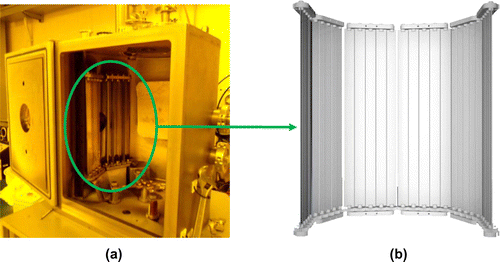
State-of-art maintenance of PVD facilities involves the adoption of static screens: shields made of vacuum-compatible materials that are mounted inside the deposition chamber. These collect most of the deposited material, which does not condense on the substrate, thus protecting chamber walls. If static screens are adopted (or even worse no screen is used, so walls are exposed to the flux of particles), standard maintenance involves abrasive removal techniquesFootnote1 for screen cleaning. Whenever the chamber is used for multi-material depositions, these techniques produce a powder, which is a solid mixture of those same metals composing the overlapped film layers on the substrate. The difficulty to recover this “wasted” fraction is mainly due to the separation of these metals, which requires complex chemical procedures. The Palette Modular Device (PMD) (Gentile and Modeo Citation2012) is a patented system,Footnote2 which tries to overcome the main drawbacks of current maintenance techniques for PVD equipment. In particular, its main characteristics are:
| • | Removable rotating palettes – exposed to the deposition materials – that screen and protect the surface of the vacuum chamber. | ||||
| • | Non-abrasive removal techniques to clean screen surfaces: this reduces or even avoids the need to recondition the chamber after maintenance procedures. | ||||
| • | Good adhesion between screening surfaces and deposited films, in order to reduce spontaneous unrolling and flaking of deposited layers due to mechanical stress. | ||||
| • | Preservation of appropriate standards for use in UHV. | ||||
Focusing just on the recovery process from a deposited film, a standard technique is the dissolution with aqua regia (Sheng and Etsell Citation2007). This substance is a mixture composed of one part of nitric acid and three parts of concentrated hydrochloric acid that oxidizes gold and dissolves it as a chloride. The practice suggests to dissolve scraps of gold alloy by means of a series of little additions of aqua regia in order to use only a small excess of acid and without leaving residues of undissolved gold. When all the gold is dissolved, the solution has a greenish-yellow colour (chloroauric acid), while the other metals remain on the bottom together with the non-metallic materials such as abrasive grains. At this point, the process of recovery proceeds with the filtering of the solution and the cementation of the metals deposited. The gold in solution is subjected to precipitation with sodium bisulphite (Brug and Heidelberg Citation1974) or other selective salts/resins. The precipitation of gold must be done with mechanical ventilation under a fume hood, so as to allow the evacuation of sulphur dioxide generated during the process. Then, the process continues with the filtration of the solution under vacuum and the subsequent siphonage. The gold remaining on the filter is subjected to several washings with hot and cold water, before being sent to the final fusion in ingots. The use of aqua regia can be replaced by the employment of other substances (e.g. cyanides) in order to obtain quite similar results in terms of recovered quantity of material.
The next sections describe how the use of PMD can provide an alternative to remove gold or other precious metals from the deposited film, avoiding the overlapping of different materials and involving the employment of less aggressive solvents (“light solvents”). This aim is supported by a recent research, envisaging better results of this new technique when compared with standard procedures, in terms of different environmental impact categories and also time efficiency (Gentile et al. Citation2014).Footnote3
3. Description of gold recovery process
The workflow related to the recovery process described below is enabled by the installation of a PMD in the deposition facility. The PMD is able to collect different materials used during the PVD process selectively. In fact, different materials are collected on different sides of palettesFootnote4 uniformly. Moreover, each side is covered by a layer of “sacrificial polymer”, which can be easily removed by the action of light solvents, when palettes are removed and cleaned, avoiding any abrasive procedure. This facilitates operations for the recovery of valuable materials. Further features of this system can be observed in Figures (a) and (b) (Gentile and Modeo Citation2012). In this study, the test case analyzed for the maintenance process presents the following characteristics:
| • | The chamber is protected by a screening surface 1 m2 sized, which is a typical dimension for PVD shields (or shield tiles, according to the facility dimensions). | ||||
| • | Screen maintenance is required after 30 production cycles. | ||||
| • | The screen has a lifetime of 1000 maintenance cycles before its replacement (i.e. Iron in traces has been considered in the maintenance outputs, due to the erosion of the screen). | ||||
| • | The chamber is employed for multi-layer depositions, involving Si and GaAs/GaN substrates (Oktyabrsky and Ye Citation2010). | ||||
| • | Therefore, the materials mainly used in deposition processes are: gold and aluminium as contact metals (respectively, 35 and 8% of “wasted material” on the screen); titanium and chromium as adhesion layers (respectively, 30 and 25%) (Baenard et al. Citation1996, Brillson Citation1994), plus negligible traces of other metals. | ||||
It should be emphasized how this is a typical set-up for evaporation chambers used in R&D and pre-production facilities of microelectronics industries.
At the end of the PVD process, gold is deposited on one side only of the palettes (side “A”), while the rest of the metals used are settled on the back (side “B”) due to the rotation of palettes during the deposition. We suppose to adopt a sandblasting process for side B of each palette. Avoiding to deposit gold on this side, the final thickness of the film to be removed will be smaller on this side, which allows an estimated reduction of 35% of the time necessary for sandblasting. Metals produced by this step are considered wastes as most of them are non-valuable, and they should be separated via state-of-art techniques from the mixed silicon and metallic powders resulting from sandblasting.
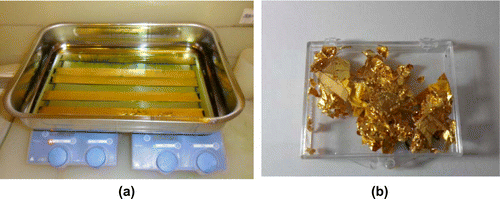
The gold recovery is performed, instead, by etching the sacrificial polymer (previously applied on the side A of the palettes) with an acetone bath, using a sonicatorFootnote5 (Figure (a)) to make its dissolution more rapid and complete. After the gold recovery procedure has been performed (Figure (b)), palettes have to be “regenerated”, that is, a new layer of sacrificial polymer must be applied.
4. Environmental analysis
4.1. Definitions of boundaries
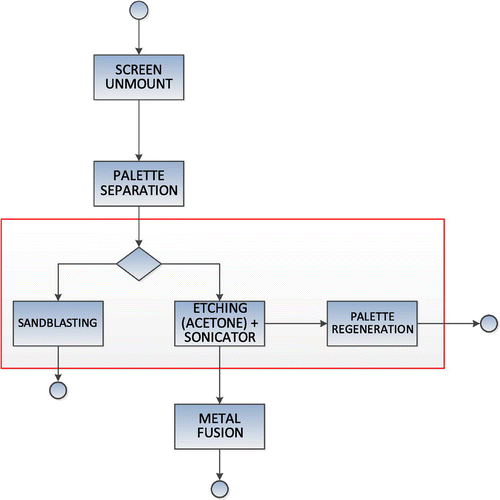
As emphasized in the introduction, the goal of this study is to analyse a novel approach for gold recovery from PVD shields maintenance. In particular, all the scenarios described are enabled by the adoption of the PMD. We will consider three cases: the first two adopt acetone (in different quantities) as the solvent for removing the “sacrificial layer”; a third hypothesis is characterized, instead, by the use of formic acid. The final purpose is to investigate which, among these different scenarios, has a lower overall impact on the environment, assessing it as the most favourable.
This case study can be considered a small step in a rather complex context, and it presents some difficulties in the collection of those data needed for a global study. Therefore, the definition of process boundaries focuses exclusively on the recovery of a single material (gold) from the maintenance of a chamber as a test case, instead of dealing with all possible materials used in PVD facilities (which can be extremely various) (Figure ). Given that the scope of the study is to compare different implementations of the PMD, we are allowed to exclude all those steps and factors that can be identical or rather similar for the recovery practices considered. These include: extraction, refinement and transportation of raw materials; operating impacts; production, disposal of deposition facilitiesFootnote6 and of goods embedding the thin-film structures produced. The focus on the PVD shield maintenance has been further refined, excluding those few steps having intuitively a very low environmental impactFootnote7 or equivalent for the different implementations (such as: the scrap transport or the fusion of gold to be recycled). The boundaries are consistent with the purposes of this study: the environmental comparison of a novel gold recovery process according to different hypotheses. The functional unit is defined as follows: a shield surface of 1 m2 covered by a total thickness of 6.9 μm of deposited material (with the composition outlined in the previous paragraph), but the material is separated into two parts: as introduced in the previous paragraph about 30% on the A side of palettes (that is, almost all of the gold deposited, according to the operating principles of the PMD system) and the remaining 70% on the B side.
4.2. Environmental considerations and inventories
This stage tries to define the flow of material input, energy used and emissions in the environment for each step of the recovery methods that are examined. Please note that only emissions into the atmosphere were taken into account for all scenarios, excluding effects of any exhausted chemical reactants improperly disposed. For the preparation of the sacrificial layer, a commercial photopolymerFootnote8 is considered for this study.Footnote9 The quantity of polymer required is approximately 242 g for the functional unit in order to produce a coating with a thickness in the range 30–50 μm. Volatility considerations (they are based on the vapour pressure characteristics of each component) allow to estimate the portion that will evaporate during the preparation of the sacrificial layer. In particular, at room temperature (20 °C) dimethyl ether, naphtha and acetone have a rather high vapour pressure (respectively: 531 kPa (Hart and Veith Citation2007), 585.2 kPa, 24.46 kPa (OECD SIDS Citation1999)). Therefore, we can consider a full evaporation of these components already in the preparation phase. Relying on the low vapour pressure of the other components, instead, it can be supposed that they will contribute to form a solid layer, which will be etched in acetone when palettes are exhausted and replaced. Palettes, once refurbished with the protective sacrificial layer, are arranged in the vacuum chamber, where they collect deposited metals along a certain number of cycles.
The case study involves the etching operation only on the palette side where gold has been collected. The sandblasting operation on the “b” side, in fact, is considered only for the maintenance of the PVD chamber, but plays no role in the recovery of the deposited material. This step is the most energy consuming, as reported in Table , and is performed via industrial sandblasters requiring roughly 1 kg of silica sand in the cartridge, in order to operate along the process time (20′) needed to accomplish complete removal of the films from the “b side” of the palettes.
Table 1. Inventory of the first recovery scenario.
The etching process, instead, uses a bath in a specific solvent combined with the usage of a sonicator. Starting from original data provided by MRS, and considering the particular functional unit, preliminary tests on prototypes registered the employment of 3 L of acetone ca., which have to reach a temperature range of 70–80 °C, to completely dissolve the sacrificial layer from exhausted palettes. This step requires 0.21 MJ of energy, and can be aided by the usage of a sonicator. In particular, according to the specifications provided by the Scientific Instrument Services, a sonicator characterized by a tank of 6.5 L, a power of 200 W, operating for 2 h, requires 1.44 MJ of energy. Therefore, the etching stage provides indicatively a total consumption of 1.65 MJ of electricity, which corresponds to the production of about 197 g of CO2.
Acetone is a solvent characterized by a high evaporation rate (vapour pressure is 24.46 kPa at 20 °C) and this factor, which is associated with the high temperatures to which it is subjected, leads to the production of harmful vapours, corresponding to approximately 70% of the total quantity of acetone used. For this reason, the high-temperature phase of the etching occurs under a hood using forced aspiration. However, as in the case of the precipitation of gold with bisulphite in the standard scenario, eventual catalytic treatment of emissions will not be considered in this study.
This prototypal implementation has been improved via solubility considerations of the single components of the sacrificial layer in acetone. In fact, theoretical investigations (based on the Abraham descriptors theory), further refined by experimental trials have found that the minimum amount of acetone strictly needed in order to be compliant with the complete dissolution of the photopolymer is roughly 0.25 L. In order to have sufficient acetone to completely cover the blade surface, an initial quantity of 0.949 kg must be used. This quantity will be the reference for calculating the evaporated acetone in the second scenario. This reduction in the initial amount of acetone employed is a first improvement in the etching process. However, a further proposal has been to adopt a closed recipient for the step in the temperature range of 70–80 °C. In fact, most of the acetone vapours are produced during this phase: a sealed recipient avoids these vapours to be dispersed in the environment. Once the sacrificial layer has been removed, the solvent can be cooled down before proceeding with the procedures of filtering and disposal of exhausted solvent. The combination of these two improvements will be in the following referred to as the second scenario. In Table , the inventory of the second scenario is displayed.
Table 2. Inventory of the second recovery scenario.
A third hypothesis can be proposed as an alternative to acetone: the formic acid. It is a caustic liquid with a pungent odour, characterized by a vapour pressure at 20 °C of 2.5 kPa. This latter feature, in particular, indicates a capacity of evaporation rather limited, especially if it is compared with the vapour pressure of acetone which is almost 10 times greater. So, during the etching phase, formic acid will generate an amount of vapour that is assumed to be one-tenth of the amount generated by acetone: that is, about 0.5% of the acid is used to completely cover the palettes (0.13 L). Also for this third scenario, in fact, we hypothesize the adoption of a sealed recipient for the bath during the high-temperature phase, followed by a cool down of the solvent before subsequent steps (Table ).
Table 3. Inventory of the third recovery scenario.
4.3. Environmental impact assessment
The phase of the life cycle inventory analysis allows to identify flows occurring inside the boundaries of the system and provides the starting point for the phase of “Impact Assessment”. Before proceeding to the assessment, categories of impact to be considered must be defined.
On the basis of the purposes of this study, only emissions in the atmosphere are selected. For these impact categories, “CML 2001” method is used, in the following, as main reference. This characterization was developed by the University of Amsterdam–Leiden and focuses on the following categories of environmental impact: consumption of abiotic resources, acidification, eutrophication, climate change, depletion of the stratospheric ozone layer, human toxicity, eco-toxicity and photochemical smog (Hischier et al. Citation2010). From a first qualitative evaluation, the following CML 2001 categories were selected as the most important for the processes considered in this study:
| • | Global Warming Potential (GWP). | ||||
| • | Human Toxicity Potential (HTP). | ||||
| • | Photochemical Ozone Creation Potential (POCP). | ||||
In the following, we will focus on the calculation of relative impact indicators for each of these categories, excluding acidification potential (AP), which has been noticed to have negligible impacts in the considered scenarios: this is clearly an advantage gained avoiding aggressive solvents.
The next step is a quantitative impacts comparison of substances present in the inventory for the categories considered.
As indications for the impacts, the following references have been used:
| • | for Acetone (Hertwich et al. Citation2006; Pachauri and Reisinger Citation2007; Jiménez-González and Constable Citation2011); | ||||
| • | for Formic Acid (Hertwich et al. Citation2006; SNAP Citation2010; Jiménez-González and Constable Citation2011); | ||||
| • | for Dimethyl Ether (Martel Citation2004; Pachauri and Reisinger, Citation2007; Jiménez-González and Constable Citation2011). | ||||
Most of the emissions occur during the stages of removal and replacement of the sacrificial layer, for the innovative process described. An additional contribution to global warming of these processes occurs in the preliminary sandblasting procedure, and is reported in the table as equivalent emissions of carbon dioxide and PM 2.5/10.
Heavy emissions contributing to the HTP and POCP are detected (specifically, they are related to acetoneFootnote10 and dimethyl ether outputs from the etching and regeneration of palettes). Table shows the overall values for emissions matched with impact categories.
Table 4. Characterization of environmental impact related to the first scenario.
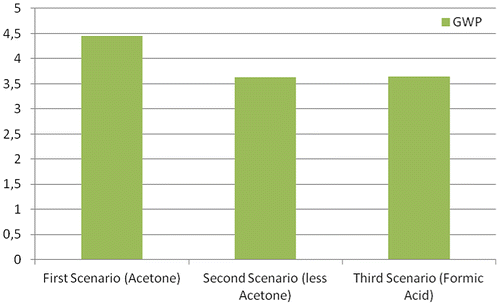
For the second scenario, a hypothesis of a different dissolution process for the sacrificial layer is made, as it is described in detail in the previous paragraph. Here, however, acetone is used again as the solvent. This improved approach greatly reduces the acetone emitted as vapours during the etching phase (one-sixth of the amount corresponding to the first case), thus contributing to a considerable cut-off in the POCP and HTP impacts. This alternative also benefits of a reduced initial quantity of solvent, therefore also the GWP is slightly reduced compared to the first scenario (Table ).
Table 5. Characterization of environmental impact related to the second scenario.
The third alternative introduced consists of replacing acetone with another solvent, which has a lower POCP potential: specifically, formic acid. For the case study summarized in Table , it supposed the usage of formic acid with the process described as the third scenario. With this approach, we observe a further reduction in the POCP impact: roughly 18% of the corresponding case with acetone, with a small increase in the GWP impacts, due to the bigger amount of formic acid needed to completely cover the palettes. Notice how a recirculation of the solvent in an engineered recipient may eventually solve this issue. We also observe that further important reductions in the POCP factor would require the adoption of a different sacrificial layer. In fact, the contribution from acetone vapours during the regeneration of the sacrificial layer is now dominating the POCP impact of the process.
Table 6. Characterization of environmental impact related to the third scenario.
4.4. Results interpretation
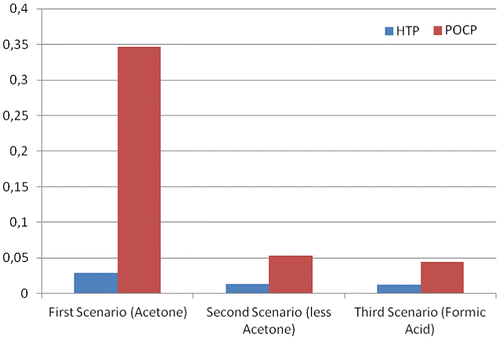
The graphs in Figures and provide the impact indicators for selected impact categories. As suggested by Tables and , the second and the third scenarios present almost the same environmental impacts for the GWP category. The worst performance attained by the first scenario is related to the ozone formation, due to the intense usage of acetone to dissolve the sacrificial layer. The second and in particular third scenarios, instead, display improvements in this aspect, reducing the amount of acetone used and avoiding the evaporation of raw solvents. We also emphasize how the POCP contributions of these solvents should be taken carefully. For instance, the corresponding indicator used for acetone (0.182 kg C2H4 eq./kg) has been under debate in the scientific communityFootnote11.
We recall once again, how the eventual introduction of emission reduction systems (e.g. catalytic converters) has been explicitly excluded from the analysis. This has avoided a detailed discussion about available systems for emission treatment and their impact on final results. That is, a comparison of the three scenarios from the same perspective of “primary emissions of pollutants”.
5. Conclusions
This research has analyzed actual and potential environmental impacts of different implementations for a novel technique, currently available for gold recovery process from the maintenance of PVD equipment. The study was developed according to the methodological guidance provided by ISO standards 14040-43 and it was articulated according to the four steps defined in the LCA methodology in order to maximize standardization and readability of results. In the paragraph dedicated to “goal and scope definition”, boundary conditions of the research and the functional unit were declared explicitly. A full description of the inventory for all processes included in the system boundaries and methods for data processing were defined, calculating inputs and outputs respectively. The research continued with the assessment of impacts using the “CML 2001” method.
We finally provided a first quantitative interpretation of the results, highlighting some critical points in the adoption of the “PMD”. Among unexpected findings, we emphasized how the original PMD scenario revealed a strong contribution to the photochemical ozone formation during the etching phase. This is due to the use of acetone as a solvent in a step of the process at high temperature and in an open container. Starting from this preliminary result, two proposals for refinements in the original process have been proposed, so as to reduce impacts in the POCP. In fact, given that the PMD system is at a proposal stage for industrial use, the underlying process and implementation can be easily subject to major revisions and improvements.
In the first instance, the focus has been on the amount of acetone used: compared to the use in overabundance (typical of prototypal implementations), according to the analyses outlined, it has been found how a smaller initial amount is indeed enough, to ensure the complete dissolution of the sacrificial polymer components (deposited on the palette during the refurbishing phase).
Moreover, replacing the open container with a sealed one, during the high temperature step, also contributes to avoid unnecessary dispersion of acetone vapour in the atmosphere. In fact, the operation of filtering the recovered metal foils can be safely postponed after a cool down of the solvent bath. A quantitative evaluation of this approach has established how this simple modification can dramatically improve the performance of the original implementation of the PMD technique.
Finally, a study of the components of the sacrificial layer has outlined how formic acid can also be a valid replacement of acetone as a solvent, with even lower POCP impacts.
In conclusion, we expect a few spaces for further refinements on the etching phase, without the adoption of a novel formulation for the sacrificial layer. Therefore, we envisage how studying different composition for the sacrificial layer (eventually a biodegradable polymer) could be an interesting direction for further investigations in the environmental improvement of the PMD technique impacts.
Acknowledgments
The authors thank Mr Luca Scala for his expert technical help in accomplishing some of the experimental activities. Part of these activities was funded via the Regional Program “Principi Attivi”, along with awards granted by the Agenzia Regionale Tecnologia ed Innovazione (ARTI) in Apulia (ITA).
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
1. For example, sandblasting, bead-blasting, etc.
2. Patented with ID US 20130008375 A1 by MRS™.
3. Authors address readers to this publication, for an in-depth comparison between PMD based and standard gold recovery practices in the field.
4. For example, in the following of this paper, we will make the hypothesis that gold forms a uniform layer on one of the palette sides.
5. The sonicator is a device that generates ultrasonic waves in a liquid medium, creating microscopic vacuum bubbles, which rapidly expand and compress themselves. This phenomenon is called cavitation, and acts as high speed miniature brushes.
6. With or without the PMD system embedded.
7. For example, consider the unmounting of the static shields/PMD, which can be done by hand.
8. The photopolymer is supplied by RS Components Ltd.
9. Given the trade secret of MRS about its sacrificial layer proprietary formulations, we have here made use of a commercial equivalent, exhibiting similar behaviour in the etching process, as a reference.
10. Notice that other references (SimaPro) – compared to the ones used here – predict a POCP potential as low as 0.1655 kg C2H4 eq., which is roughly half of the contribution in Table .
11. Starting from the work of Carter (Carter et al. Citation1993), several results against the influence of low concentrations of acetone vapors on the air quality have addressed the recent decision by US EPA to declassify acetone as “volatile organic compound” (VOC) of negligible reactivity.
References
- Baenard, W. O., G. Myburg, F. D. Auret, S. A. Goodman, and W. E. Meyer. 1996. “Metal Contacts to Gallium Arsenide.” Journal of Electronic Materials 25 (11): 1695–1702.10.1007/s11664-996-0024-1
- Brillson, L. J. 1994. “Metal-semiconductor Interfaces.” Surface Science 299–300: 909–927.10.1016/0039-6028(94)90706-4
- Brug, J., and E. Heidelberg. 1974. “Recovery of gold from solution in aqua regia” US Patent 3856507.
- Bunshah, R. F. 1994. Handbook of Deposition Technologies for Films and Coatings: Science, Technology and Applications. Noyes, NJ: William Andrew.
- Carter, W. P., D. Luo, I. L. Malkina, and J. A. Pierce. 1993. An Experimental and Modelling Study of the Photochemical Ozone Reactivity of Acetone. Final Report to Chemical Manufacturers Association. California (US): Riverside.
- Coelho, P., J. Teixeira, and O. Gonçalves. 2011. “Mining Activities: Health Impacts.” Encyclopedia of Environmental Health 3: 788–802.10.1016/B978-0-444-52272-6.00488-8
- Gentile, A. A., and S. Modeo. 2012. Palette Modular Device for Collection and Recovery of Metals in Thin Film Deposition Equipment. US Patent 20130008375 A1
- Gentile, A. A., C. Rocco, S. Modeo, and T. Romano. 2014. “Gold Recovery from Thin Film Deposition Facilities: Environmental Aspects of a Novel Method.” Journal of Cleaner Production 83: 473–482.10.1016/j.jclepro.2014.07.077
- Hagelüken, C., and C. W. Corti. 2010. “Recycling of Gold from Electronics: Cost-effective Use through Design for Recycling.” Gold Bulletin 43 (3): 209–220.10.1007/BF03214988
- Hart, J., and G.D. Veith. 2007. Applying Chemical Categories to Classification & Labelling: A Case Study of Volatile Aliphatic Ethers. A report to the Danish Environmental Protection Agency. Available at www.qsari.org/documents/aliphaticethers.pdf
- Hertwich E. G., S. F. Mateles, W. S. Pease, and T. E. McKone. 2006. An Update of the Human Toxicity Potential with Special Consideration of Conventional Air Pollutants. Trondheim, Norway: University of Science and Technology and Industrial Ecology Programme.
- Hischier, R., B. Weidema, H. J. Althaus, C. Bauer, G. Doka, R. Dones, and T. Nemecek. 2010. “Implementation of Life Cycle Impact Assessment Methods”. Ecoinvent Report. Report n.3.
- Horowitz, L. 2006. “Mining and Sustainable Development.” Journal of Cleaner Production 14 (3–4): 307–308.10.1016/j.jclepro.2005.03.005
- Jiménez-González, C., and D. J. Constable. 2011. Green Chemistry and Engineering: A Practical Design Approach. New Jersy (NJ): John Wiley & Sons.
- Kumah, A. 2006. “Sustainability and Gold Mining in the Developing World.” Journal of Cleaner Production 14: 315–323.10.1016/j.jclepro.2004.08.007
- Martel, B. 2004. Chemical Risk Analysis: A Practical Handbook. London, UK: Butterworth-Heinemann.
- Mattox, D. M. 2010. Handbook of Physical Vapor Deposition (PVD) Processing. Oxford, UK: William Andrew.
- OECD SIDS. 1999. SIDS Initial Assessment Report for the 9th SIAM. France: UNEP Publications.
- Oktyabrsky, S., and P. Ye, eds. 2010. Fundamentals of III–V Semiconductor MOSFETs. London: Springer. ISBN: 978-1-4419-1546-7 (Print) 978-1-4419-1547-4 (Online).
- Pachauri, R. K., and A. Reisinger. 2007. Climate Changes 2007: Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Switzerland: Geneva.
- Rossnagel, S. M. 1999. “Sputter Deposition for Semiconductor Manufacturing.” IBM Journal of Research and Development 43 (1–2): 163–179.10.1147/rd.431.0163
- Sheng, P. P., and T. H. Etsell. 2007. “Recovery of Gold from Computer Circuit Board Scrap Using Aqua Regia.” Waste Management & Research 25 (4): 380–383.
- SNAP Regulations. 2010. Notice 25. www.epa.gov
- World Gold Council. 2014. Gold Demand Trends Full Year 2013. www.gold.org.
- World Gold Council. 2010. White Paper: “Gold for Good – Gold and Nanotechnology in the Age of Innovation. www.gold.org