Abstract
Hydrogen produced by microorganisms is a topic of growing interest because of its potential for derivation from several agro-industrial by-products. In this study, we evaluated the hydrogen production of strains of genus Clostridium (Clostridium acetobutylicum and Clostridium butyricum) using glycerol as a carbon source. Fermentation studies were conducted using three initial concentrations of glycerol: 10, 30 and 50 g/L. The micro-organism growth kinetics and the amounts of solvents and gases were recorded over 48 h. The strain C. acetobutylicum exhibited the best results in terms of hydrogen production, the highest production yield (Y p/s) of 0.37 mol H2/mol glycerol and the highest level of productivity (0.75 mg H2/(L·h)). Based on these results, it is reasonable to conclude that glycerol could be effectively exploited as a carbon source for hydrogen production, which adds value to this primary by-product of standard biodiesel processes.
Introduction
Modern and contemporary society has experienced several energetic crises throughout history, especially in the last three decades. However, this has not limited its energy consumption, which has continued to grow in response mainly to global economic and population growth (Tasri and Susilawati Citation2014). In fact, 2014–2015 studies manifest that the total global energy demand was doubled since 1980 (OECD Citation2015), and is expected that the worldwide demand for energy will increase at an average rate of 1.8%/year between 2000 and 2030 (Tasri and Susilawati Citation2014) where almost 90% of the total energy supply in 2030 will continue dominated by fossil fuels (European Commission Citation2003). In this scenario, fossil fuels such as carbon, natural gas and petroleum will continue being the world’s primary energy sources and will supply near 81% of the total energy demanded (EIA Citation2014). Also, projections show that at 2025, the demand for energy will increase by more than 50%, which creates a foreseeable problem because the proven reserves of both oil and petroleum will decrease by between 40 and 60% by 2030 (OECD Citation2015). In contrast, the use of these fuels is linked to environmental challenges that are reflected in the depletion of the ozone layer, climate change and global warming in addition to the environmental damage caused by CO2 emissions. The amount of CO2 released into the atmosphere as a result of the use of fossil fuels is expected to more than double, from 21 in 1900s up to 45 Gt projected in 2030 (European Commission Citation2003; Tasri and Susilawati Citation2014).
For these reasons, an increasing number of researchers around the world have been promoting the use of alternative energies and energy carriers suitable to replace or at least (in the worst case) reduce the use of fossil fuels (Tanneru, Parapati, and Steele Citation2014). In fact, this promotion and research in renewable, environmentally friendly or environmentally neutral energy resources, is conceived with the aim to mitigate the environmental damage incurred up to date by the use of conventional energy sources.
Among several options, hydrogen, as energy carrier, has several advantages such as: (1) A clean energy release without producing carbon dioxide emissions, (2) Ability to be used in systems such as fuel cells for electricity generation, which are more efficient than traditional thermal devices, (3) The standard use in combustion engines that produce water vapour as a by-product and (4) The high energetic yield about 122 kJ/g, which is approximately 2.75 times as high as that of conventional hydrocarbons (Kapdan and Kargi Citation2006).
Also, the possibility to be produced (or obtained) not only from non-renewable energy sources like oil, natural gas, but also (and most important) from renewable sources such as wind, solar and biomass (Bartels, Pate, and Olson Citation2010; Won et al. Citation2017), becomes the hydrogen technology a relevant field for research and technological development at all phases of its life cycle: production, storage and utilisation.
However, and spite of the fact of the advantage of numerous production alternatives, the main concern of using hydrogen is related to its production cost. In the case of biomass, pyrolysis and gasification are not economically feasible due to the production price is approximately the same to the gasoline price per gallon (EIA Citation2014). Therefore, and based on biomass as raw material, it is important to explore different alternatives different to classical thermochemical processes for hydrogen production. In fact, biological processes for hydrogen production seems to be an interesting alternative to gasification and pyrolysis in which high environment conditions, mainly in pressure and temperature makes the process inherently expensive (Kapdan and Kargi Citation2006; Antonopoulou et al. Citation2011).
By this way, biological hydrogen production by microorganisms of genus Clostridium spp. becomes an interesting alternative for research and technological development (Bernal et al. Citation2013; Lo et al. Citation2013). The following paragraphs describe the evaluation of two strains of genus Clostridium, C. acetobutylicum and C. butyricum, with special focus of glycerol as a carbon source (Bernal et al. Citation2013). Such micro-organisms generate hydrogen and solvents such as butyric and lactic acids, ethanol and 1,3 propanediol as its primary metabolites during dark fermentation (Bernal et al. Citation2013).
The idea behind use of glycerol as carbon source is because glycerol is a coproduct of transterification process in biodiesel production (Seifert et al. Citation2009; Dincer Citation2012; Sarma et al. Citation2012; Singh et al. Citation2015; Trchounian and Trchounian Citation2015). Finally, this study presents a potential alternative to use the glycerol in a complementary process of hydrogen production in a biodiesel facility. The process shown here is evaluated by its main output variables, i.e. the concentrations of the biomass, glycerol, solvents and hydrogen obtained, and their evolution over time.
Materials and methods
Micro-organisms
This study uses the strains Clostridium acetobutylicum ATCC 824 and Clostridium butyricum DSM 2578. Strains were donated by the bank of strains and genes of the Biotechnology Institute of the National University of Colombia (Instituto de Biotecnología, Universidad Nacional de Colombia, IBUN) at Bogotá (Marin and Vargas Citation2012). The micro-organisms were kept in a liquid medium in spore suspensions in Oxoid® RCM (reinforced clostridium medium).
Culture medium
The strains were actived by transferring 10% (v/v) of stock culture to 25 ml of RCM and incubated at 37 °C for 24 h in anaerobic conditions. When the strains growth, they were transferred to a culture medium described by Papanikolau et al. (Citation2000). This medium used in the fermentation process has the following composition, in g/L unless otherwise noted (Papanikolaou et al. Citation2000): K2HPO4 1, KH2PO4 0.5, yeast extract 1, MgSO4.7H2O 0.2, (NH4)2SO4 2, CaCl2 15 mg/L, FeSO4.7H2O 5 mg/L and micro-mineral solution 2 mL/L. The composition of the micro-mineral solution was as follows: ZnCl2 70 mg/L, MnCl2.4H2O 100 mg/L, H3BO3 60 mg/L, CoCl2.2H2O 200 mg/L, CuCl2.2H2O 20 mg/L, NiCl2.6H2O 25 mg/L, Na2MoO4.2H2O 35 mg/L and HCl 37% 0.9 mL/L. HCl was added to adjust the initial pH to 7. Also, Resazurin with a concentration of 10 μL/100 mL was added as an indicator for anaerobiosis.
Fermentation process
The experimental conditions used for the fermentation process were as follows: an effective working volume of 30 mL, an inoculum of 10% (v/v), an initial pH of 7 and a temperature of 37 °C. Each strain was kept under anaerobic conditions in a Petri dish into an anaerobic jar. When fermentation process started, strain was inoculated in a vial with 3 ml of culture medium. After 24 h, the vial was transferred to another vial with 27 ml of culture medium. Samples of 500 μl of the liquid phase and 1000 μl of the gaseous phase were taken every 6 h. The samples were centrifuged at 5000 × G for 15 min, the pellet was collected for biomass determination and the supernatant was employed for HPLC analysis. Biomass was determined by dry-weighting and the solvent composition analysed using high-pressure liquid chromatography (HPLC). The composition of the gas samples were determined using gas chromatography (GC).
Measurements of glycerol, solvents and hydrogen
The concentration of the soluble metabolites obtained during fermentation was determined using HPLC (Shimadzu – UFPLC). The primary metabolites identified were acetic acid, ethanol, lactic acid, butyric acid and 1,3 propanediol. An Aminex HPX – 87H HPLC column equipped with a Shimadzu® RID 10A refractive index detector and Lab Solutions version 1.25 from Shimadzu® was used during the chromatographic tests. The analyses were performed by injecting 20 μL of the analyte into the column with a 0.5 mL/min flow of 0.3 mM sulphuric acid as the mobile phase. The column was kept at a constant temperature of 58 °C and pressure of 450 psi.
The gaseous sample were stored in a syringe and sealed with silicon for chromatographic analysis. The hydrogen content of the samples was quantified using GC (Shimadzu-2014). A Porapak Q (80/100 mesh) chromatography column equipped with a thermal conductivity detector (TDC) was used with a 20 mL/min nitrogen flow rate. The column operated at a temperature of 90 °C with injector and detector temperatures of 100 and 150 °C, respectively. The software employed was Varian Star v.4.5.
Quantification of cellular growth
The morphology and purity of the micro-organisms were determined using Gram staining and microscopy. The pellet obtained after centrifugation of the sample was re-suspended in saline solution (NaCl 0.85 w/v) for absorbance determination in a SMART® Spectro 2000-01 LaMotte spectrophotometer at a wavelength of 600 nm. A curve relating absorbance and dry weight was developed. Also, dry weight was determined employing cellulose nitrate membrane of 0.22 μm of pore size. Laboratory oven (Thermo Scientific) was employed at 90 °C for drying biomass on the membranes.
Statistical analysis
An ANOVA was used (with a significance level of 5%) to evaluate the dependence between the total amount of soluble microbial products (SMPs) generated and the amount of hydrogen produced as a function of the initial glycerol concentration.
Results and discussion
Cellular growth
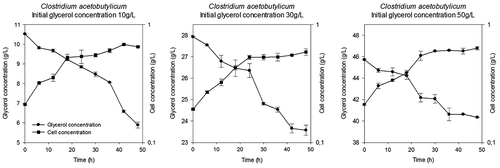
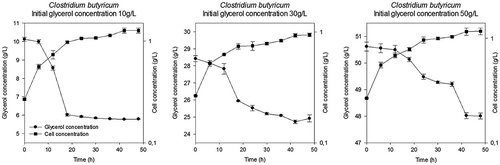
Figures and show increases in the biomass concentration over time for the three (3) concentrations of glycerol tested with both strains evaluated. During 0–18 h, cellular concentration increased due to a decrease in the glycerol concentration; this time lapse coincides with an exponential stage. Inhibition due to high concentration of glycerol was not generated for both strains, because the specific growth rates are similar for any initial glycerol concentration. This behaviour is similar to other reports in which glycerol concentrations are between 30 and 100 g/L (Szymanowska-Powałowska Citation2014), and from 5 to 50 g/L (Gallardo, Alves, and Rodrigues Citation2014). In this study, it is possible that the lag phase would have a duration time less than 6 h (time of sampling). In this sense, we do not have a conclusion about the lag phase.
In contrast, the curves obtained for both strains show that an increase in the initial substrate concentration does not represent a greater consumption. The substrate consumption for each of the three initial glycerol concentrations tested is shown in . The glycerol consumed and the biomass yield are similar for both strains. According to this result, it is possible that, under these conditions, initial glycerol concentration did not affect the behaviour of the strains. Also, at the end of the fermentation process, remained glycerol which was not consumed by Clostridium was observed in the medium. This behaviour is possible due to accumulation of by-products (e.g. hydrogen or acetic acid, see ).
Table 1. The amounts of substrate consumed after 48 hours of fermentation.
The specific growth rate obtained during the exponential growth stage (the first 18 h of fermentation) was fitted, and results presented in . Literature such as Papanikolaou et al. (Citation2000) reports growth rates between 0.10 and 0.15 h−1 for the F2b strain of Clostridium butyricum when the initial glycerol concentration was 30 g/L. These values of specific growth rate are greater than those achieved in this study which ranges between 0.03 and 0.05 h−1. Difference in the results is probably due to the use of a different species even though the genus was the same.
By-product generation
The main soluble metabolites produced during the fermentation process, as shown in , were ethanol, 1,3-propanediol, butyric, acetate and lactate acid. According to Liquid chromatography (HPLC), butanol and acetone were not synthetised during fermentation process. Similarly, the total amount of SMPs, which corresponds to the sum of the different solvents and acids presented in the medium, is also shown in .
Table 2. The soluble metabolites produced during the fermentation process.
Regarding the synthesis of SMPs, we observed that they evolve during the first 12 h of fermentation, corresponding to the exponential growth phase in both strains for the three glycerol concentrations tested. After this time, the SMPs concentration did not change significantly, suggesting that these products are primary metabolites. The total amounts of SMPs produced did not show a significant difference according to ANOVA with a 5% level of significance (data not shown), so the production of SMPs is essentially independent of the initial concentration of glycerol.
Comparison with literature is also shown in (Lo et al. Citation2013). Although the total SMP production was similar for the CH4 and CH5 strains of Clostridium pasteurianum, noticeable differences in each of their metabolites were observed. Butanol was the most abundant product for the CH4 strain, and 1,3-propanediol was the most abundant product for the CH5 strain. In this work, an absence of butanol and a high percentage of ethanol, which is represented in approximately 40% of the total SMPs, were obtained at the end of the process. This result indicates that strains, according to metabolic pathway reported by Bernal et al. (Citation2013), transform pyruvate in acetyl-coA as intermediate stage and finally are transformed in acetate and ethanol, respectively. This behaviour favours the hydrogen production due to participation of ferredoxin that is able to transfer electrons to an iron-containing hydrogenase.
Some of the SMPs produced during the process are toxic to the micro-organisms. However, and comparing the concentration of SMPs with maximum concentration achieved for strains of genus Clostridium (Zeng et al. Citation1994), only acetic acid exceeded the acceptable amount by a factor of nearly four (0.35 g/L vs. 1.28 g/L). So, high levels of this solvent could inhibit cellular growth and consumption of glycerol.
Hydrogen production
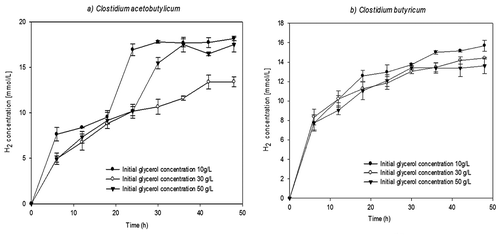
shows the amount of hydrogen produced over time for both strains. During the first 18–20 h of fermentation, there is a timeframe in which the micro-organism exhibits exponential growth. A higher increase in the hydrogen concentration was evident, which indicates that hydrogen is a primary metabolite. Also, it is possible that an increment in the hydrogen concentration would generate an inhibition in the growth of biomass. However, and due to increasing in the concentration of SMP at the same time, it is impossible to conclude if the effect is due to SMP of hydrogen concentration. In the fermentation of genus Clostridium, two stages were identified: acidogenic and solventogenic (Mathews and Wang Citation2009). The former is characterised by the production of acetic acid and butyric acid, fast growth and the production of large amounts of hydrogen. The latter, in contrast, is linked with the production of solvents such as 1,3 propanediol.
In contrast, according to the theory, the fermentation of glycerol produces 1 mol H2/mol glycerol. In this study, C. acetobutylicum had the highest productivity (0.75 mmol H2/L h) and yield (0.37 mol H2/mol glycerol) due to its greater cellular growth, which was related to its greater consumption of the substrate. Lo et al. (Citation2013) reports a yield of 0.74 mol H2/mol glycerol when C. pasteurianum CH4 is grown in a medium containing 16.4 g/L of initial glycerol. The differences of experimental conditions could explain the possible differences with this paper. In the current study, the vials containing the medium were not stirred during the fermentation process. The objective of stirring is to ensure that the medium remains homogeneous and also eliminate concentration and temperature gradients to benefit micro-organism growth.
The hydrogen production curves obtained for C. butyricum, and presented in , show very similar yields and productivities. When the glycerol concentration was 30 g/L, the yield obtained was higher than reported for Heyndrickx et al. (Citation1991) who obtained a value of 0.38 mol H2/mol glycerol for an initial concentration of 10 g/l of glycerol. The differences are possible due to the initial concentration of glycerol employed in the culture medium. These findings confirm that hydrogen production is independent of the initial concentration of glycerol. Similar results are reported by Seifert et al. (Citation2009). A final concentration of 14 mmol H2/L was obtained for each of the three glycerol concentrations analysed as shown in .
Table 3. The yields and productivities of hydrogen generation.
The low yield obtained for 1,3 propanediol (PD) is consistent with the amount of hydrogen produced, and as previously described in the literature, high hydrogen yields are always obtained under conditions that result in lower levels of 1,3 PD production (Selembo et al. Citation2009). The hydrogen produced is a product of the re-oxidation of ferredoxin, which acts as an electron transporter in the transformation of pyruvate to acetyl-CoA (Selembo et al. Citation2009). Alternatively, re-oxidation can be achieved by transferring protons to NAP (P) and through the activity of ferredoxin-NAP(P) reductase. In the latter case, more NADH2 is generated but must be regenerated by means of a reductive pathway in which 1,3-PD and NAD are the main products of the reaction (Kubiak et al. Citation2012). Previous studies claim that to produce 1 mol of PD, 1 mol of H2 is necessary (Sarma et al. Citation2012), which implies that the resulting hydrogen yield is lower because part of it is reduced to propanediol, which, in turn, depletes the substrate that could be used to generate additional hydrogen.
Conclusions
The production of biofuels from renewable sources is currently one of the most important technological challenges because of the high demand for energy, the depletion of fossil fuels and the severe environmental problems associated with their use. Therefore, this study describes an approach to the production of hydrogen, an alternative and renewable energy source. Our main contribution is to determine the feasibility of producing hydrogen using glycerol as a substrate for the dark fermentation performed by bacteria of the genus Clostridium spp. Two strains of the genus Clostridium were evaluated, and hydrogen yields between 0.3 mol H2/mol glycerol and 0.37 mol H2/mol glycerol were obtained with initial glycerol concentrations of 10, 30 and 50 g/L. These results suggest that hydrogen generation is independent of the initial concentration of glycerol. The present study shows that producing hydrogen from the glycerol generated during biodiesel production is technically feasible.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the FODEIN of Universidad Santo Tomas under [grant number 17304078].
Notes on contributors
Manuel Alejandro Jáuregui is a chemical engineer who works at Ecopetrol as engineer and consultant in projects and research of renewable energies.
Dionisio Malagón-Romero , PhD, works as a senior lecturer and researcher at GEAMEC research group in the Faculty of Mechanical Engineering at Universidad Santo Tomás, Colombia. His main research interests are in renewable energies focused in production and characterization of Hydrogen from biomass, Optimization of biodiesel production based on oil mixtures. Previous publications have focused in biohydrogen separation, transterification of biodiesel mixtures.
Alexander Ladino , MSc, is an assistant professor and researcher of Fluid Dynamics and Renewable Energies division of GIFS research group in the School of Mechanical Engineering at Universidad del Valle, Colombia. His main interests are focused in renewable energies particularly in separation process of hydrogen obtained from biomass. Combustion modeling via CFD and OpenFOAM, modeling of hydrodynamic cavitation reactors and design and optimization of turbomachinery using CFD. Previous publications are mainly in the field of CFD applied to optimization in turbomachinery, cavitation modeling and physics of hydrogen separation.
References
- Antonopoulou, Georgia, Hariklia N. Gavala, Ioannis V. Skiadas, and Gerasimos Lyberatos. 2011. “Effect of Substrate Concentration on Fermentative Hydrogen Production from Sweet Sorghum Extract.” International Journal of Hydrogen Energy 36 (8): 4843–4851. Elsevier Elsevier . doi:10.1016/j.ijhydene.2011.01.077.
- Bartels, Jeffrey R., Michael B. Pate, and Norman K. Olson. 2010. “An Economic Survey of Hydrogen Production from Conventional and Alternative Energy Sources.” International Journal of Hydrogen Energy 35 (16): 8371–8384. doi:10.1016/j.ijhydene.2010.04.035.
- Bernal, Mauricio, Lizeth K. Tinoco, Luz Torres, Dionisio Malagón-Romero, and Dolly Montoya. 2013. “Evaluating Colombian Clostridium spp. Strains’ Hydrogen Production Using Glycerol as Substrate.” Electronic Journal of Biotechnology 16 (2): 6.
- Dincer, Ibrahim. 2012. “Green Methods for Hydrogen Production.” International Journal of Hydrogen Energy 37 (2): 1954–1971. Elsevier Elsevier. doi:10.1016/j.ijhydene.2011.03.173.
- EIA. 2014. “Weekly Retail Gasoline and Diesel Prices.” Energy Information Administration. https://www.eia.gov/dnav/pet/pet_pri_gnd_dcus_nus_a.htm.
- European Commission. 2003. World Energy, Technology and Climate Policy Outlook. Energy. http://ec.europa.eu/research/energy/pdf/weto_final_report.pdf.
- Gallardo, R., M. Alves, and L. R. Rodrigues. 2014. “Modulation of Crude Glycerol Fermentation by Clostridium pasteurianum DSM 525 towards the Production of Butanol.” Biomass and Bioenergy 71: 134–143. doi:10.1016/j.biombioe.2014.10.015.
- Heyndrickx, M., P. De Vos, M. Vancanneyt, and J. De Ley. 1991. “The Fermentation of Glycerol by Clostridium butyricum LMG 1212t2 and 1213t1 and C. pasteurianum LMG 3285.” Applied Microbiology and Biotechnology 34: 637–642. doi:10.1007/BF00167914.
- Kapdan, Ilgi Karapinar, and Fikret Kargi. 2006. “Bio-Hydrogen Production from Waste Materials.” Enzyme and Microbial Technology 38 (5): 569–582. doi:10.1016/j.enzmictec.2005.09.015.
- Kubiak, P., K. Leja, K. Myszka, E. Celińska, M. Spychała, D. Szymanowska-Powałowska, K. Czaczyk, and W. Grajek. 2012. “Physiological Predisposition of Various Clostridium Species to Synthetize 1,3-Propanediol from Glycerol.” Process Biochemistry 47: 1308–1319. doi:10.1016/j.procbio.2012.05.012.
- Lo, Yung-Chung, Xue-Jiao Chen, Chi-Yu Huang, Ying-Jin Yuan, and Jo-Shu Chang. 2013. “Dark Fermentative Hydrogen Production with Crude Glycerol from Biodiesel Industry Using Indigenous Hydrogen-Producing Bacteria.” International Journal of Hydrogen Energy 38 (35): 15815–15822. doi:10.1016/j.ijhydene.2013.05.083.
- Marin, Juan Sebastian, and Lorena Vargas. 2012. “Identificación De La Cepa Nativa Del Género Clostridium Más Eficiente En La Producción De Hidrógeno Según Su Hábitat De Procedencia.” [Identification of the higher Hydrogen yield of Native Clostridium Strains According to the Provenance Habitat.] Universidad Nacional de Colombia.
- Mathews, Juanita, and Guangyi Wang. 2009. “Metabolic Pathway Engineering for Enhanced Biohydrogen Production.” International Journal of Hydrogen Energy 34 (17): 7404–7416. doi:10.1016/j.ijhydene.2009.05.078.
- OECD. 2015. Energy Technology Perspectives 2015: Mobilising Innovation to Accelerate Climate Action. International Energy Agency.
- Papanikolaou, Seraphim, Patricia Ruiz-Sanchez, Bernard Pariset, Fabrice Blanchard, and Michel Fick. 2000. “High Production of 1,3-Propanediol from Industrial Glycerol by a Newly Isolated Clostridium butyricum Strain.” Journal of Biotechnology 77 (2–3): 191–208. doi:10.1016/S0168-1656(99)00217-5.
- Sarma, Saurabh Jyoti, Satinder Kaur Brar, Eduardo Bittencourt Sydney, Yann Le Bihan, Gerardo Buelna, and Carlos Ricardo Soccol. 2012. “Microbial Hydrogen Production by Bioconversion of Crude Glycerol: A Review.” International Journal of Hydrogen Energy 37 (8): 6473–6490. doi:10.1016/j.ijhydene.2012.01.050.
- Seifert, K., M. Waligorska, M. Wojtowski, and M. Laniecki. 2009. “Hydrogen Generation from Glycerol in Batch Fermentation Process.” International Journal of Hydrogen Energy 34 (9): 3671–3678. doi:10.1016/j.ijhydene.2009.02.045.
- Selembo, Priscilla A., Joe M. Perez, Wallis A. Lloyd, and Bruce E. Logan. 2009. “Enhanced Hydrogen and 1,3-Propanediol Production from Glycerol by Fermentation Using Mixed Cultures.” Biotechnology and Bioengineering 104 (6): 1098–1106. doi:10.1002/bit.22487.
- Singh, Shuchi, Mayank Agarwal, Shyamali Sarma, Arun Goyal, and Vijayanand S. Moholkar. 2015. “Mechanistic Insight into Ultrasound Induced Enhancement of Simultaneous Saccharification and Fermentation of Parthenium Hysterophorus for Ethanol Production.” Ultrasonics Sonochemistry 26: 249–256. Elsevier B.V. doi:10.1016/j.ultsonch.2015.02.011.
- Szymanowska-Powałowska, Daria. 2014. “1,3-Propanediol Production from Crude Glycerol by Clostridium butyricum DSP1 in Repeated Batch.” Electronic Journal of Biotechnology 17 (6): 322–328. doi:10.1016/j.ejbt.2014.10.001.
- Tanneru, Sathish K., Divya R. Parapati, and Philip H. Steele. 2014. “Pretreatment of Bio-Oil Followed by Upgrading via Esterification to Boiler Fuel.” Energy 73: 214–220. doi:10.1016/j.energy.2014.06.039.
- Tasri, Adek, and Anita Susilawati. 2014. “Selection among Renewable Energy Alternatives Based on a Fuzzy Analytic Hierarchy Process in Indonesia.” Sustainable Energy Technologies and Assessments 7 (September): 34–44. doi:10.1016/j.seta.2014.02.008.
- Trchounian, Karen, and Armen Trchounian. 2015. “Hydrogen Production from Glycerol by Escherichia coli and Other Bacteria: An Overview and Perspectives.” Applied Energy 156: 174–184. Elsevier Elsevier. doi:10.1016/j.apenergy.2015.07.009.
- Won, Wangyun, Hweeung Kwon, Jee-Hoon Han, and Jiyong Kim. 2017. “Design and Operation of Renewable Energy Sources Based Hydrogen Supply System: Technology Integration and Optimization.” Renewable Energy 103: 226–238. doi:10.1016/j.renene.2016.11.038.
- Zeng, A. P., A. Ross, H. Biebl, C. Tag, B. Günzel, and W. D. Deckwer. 1994. “Multiple Product Inhibition and Growth Modeling of Clostridium butyricum and Klebsiella pneumoniae in Glycerol Fermentation.” Biotechnology and Bioengineering 44 (8): 902–911.10.1002/bit.v44:8