Abstract
This work investigated the optimisation of biodiesel production from waste cooking oil (WCO) and palm oil using a two-step transesterification process for WCO and base catalysed transesterification for palm oil. Transesterification reactions were carried out to investigate the effects of prepared catalyst CaO, methanol/WCO and methanol/palm oil ratio and temperature on the yield of biodiesel. A series of experiments were conducted to determine the best conditions for biodiesel production, using methanol/oil ratio between 4:1 and 11:1 and contact time varying between 2 and 4 h. Biodiesel yield of around 90 and 70% was obtained for palm and waste cooking oil at the methanol/oil ratios of 6:1 and 8:1 at temperature of 60 °C for reaction time of 4 h using prepared CaO as catalyst. The physicochemical properties of palm and WCO biodiesel were carried out using standard methods, while the fatty acid profile was determined using gas chromatography. The investigation concludes that biodiesel obtained from palm and waste cooking oil was within the specified limit.
Introduction
The energy demand of the world is increasing fast mainly because of the rapid growth of human population and industrialisation by fast developing countries (Suresh, Kamath, and Banapurmath Citation2014). Fortunately, people all over the world are becoming aware of the problem of consuming too much of energy and are making conscious efforts to conserve it and avoid pressure on the mother earth. Conservation of energy helps to lower the amount of pollutants released into the air and protect the environment. The interaction between the natural resources and the population needs to be balanced in order to ensure the continuity of the human race. So, energy is essential to life and its conservation has become all more an absolute necessity.
Common sources of energy are petroleum, natural gas and coal from fossil fuels. The growing demand for energy globally has led to the rapid depletion of these non-renewable sources. This growing consumption of energy has rapidly depleted non-renewable sources of energy. Fossil fuels emissions are major contributors of greenhouse gases which may lead to global warming. Combustion from fossil fuels is major source of air pollutants, which consist of CO, NOx, hydrocarbons and particulates. The disadvantages and shortages of fossil fuels have motivated many researchers to find an alternative source of renewable energy. Biodiesel is one of the most promising alternative fuels for diesel engines. Biodiesel is defined as the fuel comprising of monoalkyl esters formed by transesterification of vegetable oil or animal fat with methanol, either pure or blended with fossil fuel. Biodiesel as a diesel engine fuel superior advantages over its fossil diesel counterpart including its renewability, lower emissions of green house gases, improved lubricate, non-toxic, it remarkably reduces carbon dioxide and other pollutants emissions from engines, (Roy, Ching kheihunba, and Pakshirajan Citation2016). Biodiesel alone can help to meet the world energy demands hitherto not yet sufficient. Biodiesel properties tested are superior in terms of cetane number, flash point and volumetric heating value (Awolu and Layokun Citation2013). It is evidenced that biodiesel is sulphur free and non-aromatic while petro-diesel can contain up to 500 ppm SO2 and 20–40 wt.% aromatic compounds (Phan and Phan Citation2008). Many researchers have reported work on biodiesel. The effect of operating variables and types of catalyst on yield of biodiesel is studied. The efficiency of producing biodiesel depends on the properties of oil (Phan and Phan Citation2008). Phan and Phan (Citation2008) have reported in his work that yield of biodiesel is around 90% with WCO as feedstock for methanol/oil ratio of 8:1 (Dawodu, Ayodele, and Bolanle-Ojo Citation2014). The economics of biodiesel production greatly depends on the cost of feed-stock. The use of vegetable oils such as sunflower, peanut, olive oil and palm oil in the production of biodiesel as being promoted in many countries due to rapid decline in crude oil reserves. Depending upon climate and soil conditions, various countries are searching for appropriate vegetable oils for biodiesel production (Barnwal and Sharma Citation2005).
Presently, use of partially or fully refined and edible – grade vegetable oils, such as soybean, rapeseed and sunflower are the predominant feedstock for biodiesel production. Use of this feedstock may result in high price of biodiesel (Dawodu, Ayodele, and Bolanle-Ojo Citation2014). The use of waste and palm oil has received immense interest. However, use of such low – cost materials which contains free fatty acids (FFAs) requires two-step catalysis process. Extensive literature shows that biodiesel can run in a conventional diesel engine for an extended time.
A number of studies have been reported on the use of various catalysts for synthesis of biodiesel from waste cooking oil (WCO) and Palm oil (PO). Margaretha et al. (Citation2012) found that the biodiesel yield reached 90% at the methanol oil ratio of 7:1. While Phan and Phan (Citation2008) revealed the best result obtained at the molar ratio of methanol/WCO ratio of 8:1 for WCO using KOH as catalyst. Zhao and Li (Citation2015) in his work on biodiesel production form palm oil using supported solid base catalyst CaO/MgO/γ-Al2O3 reported optimum condition was obtained at methanol to oil of 9:1, a catalyst dosage of 3.5%, a reaction temperature of 65 °C, and a reaction time of 3 h. Malpani, Varma, and Mondal (Citation2016) found optimum condition at methanol to (Algal) oil ratio of 15.68, catalyst concentration of 5.12 wt.% and reaction time of 8.5 h. Biodiesel yield under optimum conditions was reported to be 86.4% for CaO as catalyst.
Homogeneous catalyst has been used extensively for biodiesel production. However, use of homogenous catalyst has several draw backs: it cannot be recovered or regenerated after the reaction and also it produces toxic wastewater (Margaretha et al. Citation2012). These homogenous catalysts pose difficulty in separation of end products. These further increase the cost of biodiesel and recovered glycerine (Malpani, Varma, and Mondal Citation2016). To replace the homogenous catalyst, heterogeneous catalysts can be developed and studied. Heterogeneous base catalysis is the most viable process for the transesterification of triglyceride into biodiesel. The heterogeneous catalysis features lower corrosiveness, environmental friendliness, easy catalyst recovery and high process integrity, lower investment costs, easy separation by filtration (Malpani, Varma, and Mondal Citation2016) all at levels superior to those of homogeneous catalysis.
Several studies have been reported on the use of homogenous and heterogeneous catalyst using different types of edible and non-edible oil, but no extensive work has been reported on the use of CaO (heterogeneous catalyst) in production of biodiesel from WCO. Therefore, present study was conducted on biodiesel production using CaO as a solid base catalyst. CaO catalyst has many advantages such as higher activity, mild reaction conditions, long catalyst lifetimes, low catalyst cost (Liu et al. Citation2008).
In the study reported herein, a WCO and palm oil was used for the transesterification with a heterogeneous catalyst instead of homogenous catalyst. This paper proposes to optimise the operating conditions for production of biodiesel. The study was also carried to compare between heterogeneous catalyst and homogenous catalyst.
Experimental
Materials
WCO was obtained from local restaurant. The collected oil was filtered to remove inorganic residues. Fresh palm oil was procured form local super market. All chemicals and reagents used for the research work were of analytical reagent grade and 99% pure. The composition of WCO and palm oil was analysed by gas chromatographic method and the results are shown in . Oil of both types was heated at 100 °C for 10 min to remove all the moisture (Verma, Sharma, and Dwivedi Citation2016).
Table 1. Fatty acid composition of palm and waste cooking oil (WCO).
Catalyst preparation
Calcium oxide of 24 grams was dipped in 200 ml of ammonium carbonate solution (12% by wt). The corresponding mixture was stirred for 30 min at room temperature. The resulting mixture was filtered and dried to get precipitate at 110 °C for about 6 h. Dried solid was milled and calcined at 900 °C for 1.5 h in a muffle furnace. The prepared catalyst was removed from muffle furnace and kept in a desiccator to prevent contact with air (Margaretha et al. Citation2012).
Transesterification
Biodiesel production is carried out using a two step transesterification process. WCO and palm oil was filtered to remove all insoluble impurities (Verma, Sharma, and Dwivedi Citation2016). show FFA profile of palm oil and WCO. Fatty acid profile of both the oil was determined using gas-chromatography. Owing to low acid number (<1%) of palm oil, here base catalysed transesterification processes was adopted for palm oil. The saponification value of WCO obtained was 268 mg KOH/g oil and that of palm oil was 264 mg KOH/g oil (Vermani and Narula Citation2008). However, FFA content of WCO was high, due to which two-step transesterification was followed.
Acid transesterification for WCO
Acid catalysed transesterification process, a pre-treatment step was carried to reduce the FFA in waste oil. Forty millileter methanol and 4 ml of tetraoxosulphate (iv) acid (98%) was mixed and placed in a heated water bath. The mixture was letter poured into 750 ml three-neck reound bottom glass flask with a reflux condenser containing 200 ml ‘Waste Cooking oil’. Three-neck round bottom glass flask was equipped with thermometer and magnetic heating mantle (Awolu and Layokun Citation2013). The experiment was carried out at a temperature of 60 °C with agitation speed around 600 rpm for 1.5 h. (Margaretha et al. Citation2012).
Base transesterification for NaOH catalyst
The base transesterification process of WCO (obtained from acid transesterification (pretreated oil) and palm oil was carried out in a 750 ml three-neck round bottom flask equipped with a reflux condenser and magnetic heating mantle. For testing the superiority of catalyst prepared, biodiesel was produced using NaOH as a catalyst (1.5 gm) with methanol to oil ratios varied from 4:1 to 11:1. Use of methanol is most feasible because of its low cost, and physical and chemical advantages (Gürü and Keskïn Citation2016). From extensive literature review, (Meher, Dharmagadda, and Naik Citation2006; Saydut et al. Citation2008; Ferdous and Uddin Citation2012; Yathish, Suresh, and Amruth Citation2013) the range of operating variables are shown in .
Table 2. Range of parameters selected.
Base transesterification for CaO prepared catalyst
The prepared CaO catalyst of 30 g is added to methanol in a beaker. The solution is stirred well to get a solution of methoxide. The WCO and palm oil of 200 g is poured in a 500 ml three-neck round bottom flask equipped with a reflux condenser and magnetic heating mantle. The prepared catalyst was poured into oil at 60 °C and stirring speed of 600 rpm was maintained. Methanol to oil ratios was varied from 4:1 to 11:1. The experiments were carried with similar parameters as explained for NaOH as catalyst.
Separation of biodiesel from glycerine
After the reaction was completed, the catalyst (prepared catalyst) was removed from the solution using filtration. The reaction mixture was poured into separating funnel and allowed to settle for 24 h inside a separating funnel to allow clear separation of biodiesel from glycerine (Ferdous and Uddin Citation2012). The bottom layer obtained consists of glycerine, excess alcohol, catalyst, impurities and traces of unreacted oil. The bottom layer which contains glycerol and impurities was drawn off.
Biodiesel washing and drying
The upper layer was washed with warm distilled water at 50 °C and the mixture was shaken vigorously to remove contaminants like soap, catalyst and other impurities (Muthu et al. Citation2010). From the bottom of separating funnel water was allowed to drain out. This procedure was carried out four times until a clear biodiesel was obtained. After washing, the biodiesel was kept in an oven at 70 °C overnight (Islam et al. Citation2015). Biodiesel was dried until it was crystal clear.
Physicochemical analysis of biodiesel
The analysis of prepared biodiesel using both type of catalyst included flash point determination (Pensky–Martens closed-cup tester), viscosity (Red wood viscometer) (Saydut et al. Citation2008), density, acid value and saponification value.
The higher heating value was evaluated by using a model which relates HHV to iodine value (IV) and saponification value (SV) (Enweremadu and Alamu Citation2010):
Cetane number was calculated using the correlation (Enweremadu and Alamu Citation2010).
Cetane number (CN) does not differ much from cetane index. Cetane number was evaluated using the correlation given below (Enweremadu and Alamu Citation2010):
Fatty acid composition of WCO and palm oil was determined by gas chromatography.
Calculation of biodiesel yield
Biodiesel yield (BY) was determined using the following formula (Betiku and Adepoju Citation2013; Islam et al. Citation2015).
Results and discussion
Effect of reaction time on biodiesel production
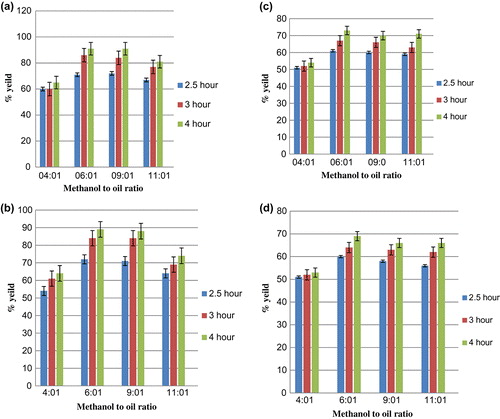
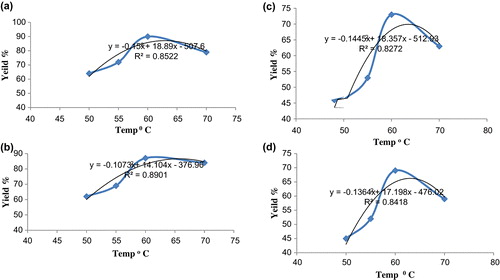
Reaction temperature is important parameter for esterification reaction as prolonged heating can reverse the reaction. The transesterification process can occur at different temperature depending on the type of oil used. In some cases, it would be at ambient temperature or at temperature close to boiling temperature of methanol (Phan and Phan Citation2008). Studies were carried out at divergent temperature from 50 to 70 °C with both catalysts. In this study, contact time of 4 h and ratio was fixed to 6:1. The result indicates maximum yield was obtained at 60 °C at 4 h of reaction time and lowest yield was observed at 50 °C for both the catalyst. Increase in temperature increases solubility which may cause increase in yield of biodiesel. With rise in temperature yield also increased. Maximum yield was found to be 91 and 87% for NaOH and prepared CaO catalyst (palm oil) as shown in at a temperature of 60 °C. For WCO maximum yield was observed to be 69% for prepared CaO catalyst (). However, previous studies (Phan and Phan Citation2008) showed that yield of 88% was obtained for WCO as feed at temperature of 50 °C with KOH as catalyst. At 70 °C yield tends to decrease due to the enhancement of transesterification and Saponification reaction (Meher, Dharmagadda, and Naik Citation2006; Yathish, Suresh, and Amruth Citation2013). Moreover, at high temperature vaporisation of methanol may take place.
The regression of the data obtained from the polynomial equation gives R2 () in the range 0.82–0.86, which verifies the quality of the results obtained and satisfactory nature of this analysis. Statistical software Minitab was used to develop these equations.
Comparison between heterogeneous catalyst and NaOH catalysed transesterification
It has been reported in most of the previous works that the catalyst has a profound role in enhancement of the products yield. The yield obtained from the process with heterogeneous catalyst was slightly less compared to NaOH as catalyst for Palm oil. However, for WCO the maximum yield obtained was around 72% using NaOH as catalyst and 69% using CaO as catalyst. Use of the heterogeneous catalyst offers more advantages compared with homogeneous one, such as environmentally friendly, the catalyst is re-useable. During 2 h of contact, the yield was low. Initially the reaction is slow due to the dispersion of the reactants in the mixture (Colombo, Ender, and Barros Citation2016). The trend observed for both the catalyst was similar. However, the yield for WCO was lower in comparison with fresh palm oil. The maximum yield obtained with heterogeneous catalyst was comparable with NaOH as a catalyst. In our present study, solid heterogeneous catalyst was used as it can be easily removed and reused. We have tested the potential of prepared CaO catalyst for transesterification of WCO and palm oil and compared with NaOH catalyst for 4:1 to 11:1 methanol/oil ratio. The reactions were run for 2–4 h. Prepared catalyst showed higher yield of around 90% for ratio of 6:1 for methanol/oil for contact time of 4 h. However, low conversion was obtained for contact time of 2 h. It can be seen from Figures and that at various operating conditions yield obtained by using CaO as catalyst had similar results compared to homogenous catalyst. The heterogeneous solid catalyst has longer lifetime (Sun, Qiu, and Yang Citation2016) Similar results were obtained for (Margaretha et al. Citation2012) while using CaO catalyst.
Biodiesel characterisation
The suitability of biodiesel produced using both the catalyst was verified using certain physical tests. The physical properties of liquid product are illustrated in . Comparison of the test results is done with ASTM standard values as shown in . Physical properties of liquid product obtained closely match with the ASTM values. Density is an important characteristic of biodiesel (Saydut et al. Citation2008; Margaretha et al. Citation2012). The density of biodiesel obtained from palm oil was 900 kg/m3 and with WCO as feed 860 kg/m3 which is comparable with that of diesel. Viscosity is also an important parameter of biodiesel. It affects the atomisation of fuel upon injection into the combustion chamber. Fuel with low viscosity will not provide sufficient lubrication in the combustion system while fuel with high viscosity tends to form deposit in the engine. The viscosity of biodiesel obtained from both the catalyst is within the range of ASTM standard as shown in .The other properties such as flash point, viscosity, cetane number, HHV and iodine values confirm to the ASTM standards. The maximum value of acid number as per ASTM standard for pure biodiesel is 0.50 mgKOH/g. The acid numbers for biodiesel produced from WCO and palm oil is 0.3 and 0.2 which is below as per ASTM standard. The GC analysis revealed that biodiesel produced (palm oil) using prepared catalyst shows high content of oleic acid (41.4%) and Pamitic acid (39.3%). Similar results were obtained by (Baskar and Soumiya Citation2016; Verma, Sharma, and Dwidedi Citation2016).
Table 3. Physicochemical analysis of prepared biodiesel compared with standards.
Conclusion
A heterogeneous catalyst (CaO) was prepared for the production of biodiesel. Result obtained reveals that operating temperature of 60 °C, transesterification time of 4 h and alcohol to oil ratio of 6:1 are the optimum conditions for biodiesel production using both the catalyst. The physical and chemical properties of biodiesel produced from WCO and palm oil using both the catalyst confirm to the available standards. The result obtained using prepared CaO as catalyst had similar results compared to homogenous catalyst. The work concludes that WCO and palm oil serves as a potential feed stock for producing biodiesel. It also suggests that the prepared CaO heterogeneous catalyst have the potential to be developed as catalysts for the biodiesel production.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Santosh A. Kadapure is working as an associate professor in the Department of chemical engineering at KLE Dr MSSCET, Belgaum. He has widely published research papers on bacterial concrete and biodiesel production in various national and international journals.
Prasanna Kirti is a research student in the Department of chemical engineering at KLE Dr MSSCET, Belgaum.
Sabhaya Singh is a research student in the Department of chemical engineering at KLE Dr MSSCET, Belgaum.
Sagar Kokatnur is a research student in the Department of chemical engineering at KLE Dr MSSCET, Belgaum.
Neeraj Hiremath is a research student in the Department of chemical engineering at KLE Dr MSSCET, Belgaum.
Akhil Variar is a research student in the Department of chemical engineering at KLE Dr MSSCET, Belgaum.
Shagufta Shaikh is a research student in the Department of chemical engineering at KLE Dr MSSCET, Belgaum.
Raju Chittaragi is a research student in the Department of chemical engineering at KLE Dr MSSCET, Belgaum.
Acknowledgement
The authors would like to thank the management authorities of KLE DR. M.S. Sheshgiri College of Engineering and Technology, Belgaum for their kind support. The authors gratefully acknowledge support of Dr. Basavaraj G. Katageri for giving all the encouragement needed which kept the enthusiasm alive.
References
- Awolu, O. O., and S. K. Layokun. 2013. “Optimization of Two-step Transesterification Production of Biodiesel from Neem (Azadirachta Indica) Oil.” International Journal of Energy and Environmental Engineering 4 (1): 1–9.
- Barnwal, B. K., and M. P. Sharma. 2005. “Prospects of Biodiesel Production from Vegetable Oils in India.” Renewable and Sustainable Energy Reviews 9 (4): 363–378.10.1016/j.rser.2004.05.007
- Baskar, G., and S. Soumiya. 2016. “Production of Biodiesel from Castor Oil Using Iron (II) Doped Zinc Oxide Nanocatalyst.” Renewable Energy 98 (2016): 101–107.10.1016/j.renene.2016.02.068
- Betiku, E., and T. F. Adepoju. 2013. “Methanolysis Optimization of Sesame (Sesamum Indicum) Oil to Biodiesel and Fuel Quality Characterization.” International Journal of Energy and Environmental Engineering 4 (1): 1–8.
- Colombo, K., L. Ender, and A. C. Barros. 2016. “The Study of Biodiesel Production Using CaO as a Heterogeneous Catalytic Reaction.” Egyptian Journal of Petroleum 26 (2): 341–349.
- Dawodu, F. A., O. O. Ayodele, and T. Bolanle-Ojo. 2014. “Biodiesel Production from Sesamum Indicum L. Seed Oil: An Optimization Study.” Egyptian Journal of Petroleum 23 (2): 191–199.10.1016/j.ejpe.2014.05.006
- Enweremadu, C. C., and O. J. Alamu. 2010. “Development and Characterization of Biodiesel from Shea Nut Butter.” International Agrophysics 24: 29–34.
- Ferdous, K., and M. R. Uddin. 2012. “Biodiesel from Sesame Oil: Base Catalysed Transesterification.” International Journal of Engineering & Technology 1 (4): 420–431.
- Gürü, M., and A. Keskïn. 2016. “Evaluation of Biodiesel Production, Engine Performance, and Emissions.” Journal of Electronic Materials 45 (8): 3882–3888.
- Islam, A. K. M., Z. Yaakob, N. Anuar, M. Osman, and S. R. P. Primandari. 2015. “Preparation of Biodiesel from Jatropha Hybrid Seed Oil through Two-step Transesterification.” Energy Sources, Part a: Recovery, Utilization, and Environmental Effects 37 (14): 1550–1559.
- Liu, X., H. He, Y. Wang, S. Zhu, and X. Piao. 2008. “Transesterification of Soybean Oil to Biodiesel Using CaO as a Solid Base Catalyst.” Fuel 87 (2): 216–221.
- Malpani, M., A. K. Varma, and P. Mondal. 2016. “Production of Bio-oil from Algal Biomass and Its Upgradation to Biodiesel Using CaO-based Heterogeneous Catalysts.” International Journal of Green Energy 13 (10): 969–976.10.1080/15435075.2015.1088445
- Margaretha Y. Y., Prastyo H. S., Ayucitra A. and Ismadji S. 2012. “Calcium oxide from Pomacea Sp. Shell as a Catalyst for Biodiesel Production”. International Journal of Energy and Environmental Engineering 3 (1): 33.
- Meher, L. C., V. S. Dharmagadda, and S. N. Naik. 2006. “Optimization of Alkali-catalyzed Transesterification of Pongamia Pinnata Oil for Production of Biodiesel.” Bioresource Technology 97 (12): 1392–1397.10.1016/j.biortech.2005.07.003
- Muthu, H., V. SathyaSelvabala, T. K. Varathachary, D. Kirupha Selvaraj, J. Nandagopal, and S. Subramanian. 2010. “Synthesis of Biodiesel from Neem Oil Using Sulfated Zirconia via Tranesterification.” Brazilian Journal of Chemical Engineering 27 (4): 601–608.10.1590/S0104-66322010000400012
- Phan, A. N., and T. M. Phan. 2008. “Biodiesel Production from Waste Cooking Oils.” Fuel 87 (17–18): 3490–3496.10.1016/j.fuel.2008.07.008
- Roy, Arindam Sinha, Akoijam Ching kheihunba, and Kannan Pakshirajan. 2016. “An Overview of Production, Properties, and Uses of Biodiesel from Vegetable Oil.” Green Energy and Technology 83–105.10.1007/978-3-319-30205-8
- Saydut, A., M. Z. Duz, C. Kaya, A. B. Kafadar, and C. Hamamci. 2008. “Transesterified Sesame (Sesamum Indicum L.) Seed Oil as a Biodiesel Fuel.” Bioresource Technology 99 (14): 6656–6660.10.1016/j.biortech.2007.11.063
- Sun, C., F. Qiu, and D. Yang. 2016. “Preparation, Characterization of Graphite Oxide Loaded with K2CO3 as Heterogeneous Catalyst and Its Transesterification Application.” Arabian Journal for Science and Engineering 41 (1): 89–96.10.1007/s13369-015-1575-3
- Suresh, G., H. C. Kamath, and N. R. Banapurmath. 2014. “Studies on the Use of Low-volatile Non-edible Oils in a Thermal Barrier-coated Diesel Engine.” International Journal of Sustainable Engineering 7 (4): 341–351.10.1080/19397038.2013.862581
- Verma, P., M. P. Sharma, and G. Dwivedi. 2016. “Evaluation and Enhancement of Cold Flow Properties of Palm Oil and Its Biodiesel.” Energy Reports 2: 8–13.10.1016/j.egyr.2015.12.001
- Vermani, O. P., and A. K. Narula. 2008. Applied Chemistry. Delhi: New Age International.
- Yathish, K. V., R. Suresh, and E. Amruth. 2013. “Optimization of Biodiesel Production from Mixed Oil (Karanja & Dairy Waste Scum Oil) Using Homogeneous Catalyst.” IOSR Journal of Applied Chemistry 3 (6): 9–15.10.9790/5736
- Zhao, H., and H. P. Li. 2015. “Application of Supported Solid Base Catalyst in the Preparation of Biodiesel from Palm Oil.” Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 37 (8): 825–831.10.1080/15567036.2011.598904