?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A systematic study of the diffusion mechanism and effect of mass transfer limitation during the adsorption of CO2 onto polyaspartamide is presented using a differential adsorption bed method, carried out in a 100 × 60 × 40 mm packed-bed adsorption unit. The rate-limiting step where mass transfer limitation is dominant was studied using diffusion models. It was observed that intraparticle diffusion mechanism is the major contributor to the resistance offered to the transport of gas molecule through polyaspartamide. The behaviour of polyaspartamide, based on the intraparticle diffusion rate parameter derived from the plots of CO2 adsorbed versus the square root of time, signified that the adsorption mechanism involved both film and intraparticle diffusion. The intraparticle diffusion parameter (kid) obtained was dependent on temperature as well as intraparticle convection effects and ranged from 1.24 × 10−4 to 2.13 × 10−4 ms−1. The Biot number (Bi) values were all greater than 10 (ranged from 17.80 – 30.74), confirming that the intraparticle diffusion was the rate-limiting step and heat transfer is more by conduction from the gas film layer than convection within the pores of polyaspartamide. Results from this study provide an important basis for future scale-up and optimisation of CO2 capture process using polyaspartamide.
1. Introduction
Carbon capture, storage and utilisation (CCSU) is widely accepted as a promising option that aims at reducing CO2 emissions from industrial point sources (e.g. coal-fired power plants) and it also allows for the continual use of fossil fuels (e.g. coal) for sustainable energy generation with a significant reduction of CO2 emission. One of the technologies employed in CCSU is adsorption using CO2-selective adsorbents. However, for adsorption of CO2 to be economically feasible for CCSU, adsorbents with high adsorption capacities for CO2 should be developed and the mechanism involved in the adsorption process well understood. The adsorption of CO2 by most materials is usually affected by pore diffusion, external film diffusion, or by the combination of the two which constitute the rate-limiting step during adsorption processes.
So far, different adsorbents with high CO2 adsorption capacity and selectivity have been developed and suggested for CO2 capture (Plaza et al. Citation2016; Singo et al. Citation2017; Titinchi et al. Citation2014; Wang et al. Citation2011; Sekoai and Yoro Citation2016; Yoro and Sekoai Citation2016); but details on their diffusion mechanism and mass transfer limitation effect have not attracted sufficient attention. Most studies in literature on CO2 capture have focused on the synthesis and characterisation of new adsorbents for CO2 capture (Chitsiga et al. Citation2016; Loganathan and Ghoshal Citation2017; Ngoy et al. Citation2017; Osler et al. Citation2017a, Citation2017b) and novel strategies to minimise material wastage during CO2 capture (Yoro, Isafiade, and Daramola Citation2018). Generally, it has been identified that there is a dearth of report on diffusional behaviour of adsorbents especially in gas-solid systems. The few reports available on diffusion mechanism of adsorbents dwelt majorly on liquid-solid adsorption systems with respect to the adsorption of dyes by different materials (Allen, McKay, and Khader Citation1989; Costa and Rodrigues Citation1985a, Citation1985b, Citation1985c, Citation1985d; Cheung, Szeto, and McKay Citation2007; Dotto, Buriol, and Pinto Citation2014; Ho and Mckay Citation1998b; Piccin et al. Citation2013). As far as could be ascertained, not much report has been published on the diffusion mechanisms involving gas-solid adsorption systems with a keen interest in polymer-based materials. Furthermore, the effect of mass transfer limitation on most adsorbents for CO2 capture has not been adequately studied in the past; hence they are considered in the current study. Before now, some researchers speculated that adsorption kinetics can be presented in the same manner as a rate of reaction to represent diffusion mechanism (Ocampo-Pérez et al. Citation2012; Qiu et al. Citation2009; Sadare et al. Citation2018). As such, most performance evaluation studies for CO2 capture materials have focused mainly on investigating just the simple kinetic behaviour of adsorbents (Álvarez-Gutiérrez et al. Citation2017; Dantas et al. Citation2011; Grande, Ribeiro, and Rodrigues Citation2010; Liu et al. Citation2011; Teng et al. Citation2016; Wang et al. Citation2011; Yoro Citation2017; Yoro et al. Citation2017, Citation2018) without considering their diffusion mechanism and effect of mass transfer limitation. Since there are insufficient reports in literature on the application of diffusional mass transfer models to investigate the effect of mass transfer limitation during CO2 capture, detailed studies like this are necessary in this field before logical conclusions on the behaviour of adsorbents during CO2 capture can be made.
Polyaspartamide is an amine-grafted polymer-based material recently developed for CO2 capture. It is obtained when polysuccinimide reacts with Ethylenediamine (EDA). This material has been studied previously, and it has proven to be a potential adsorbent for CO2 capture because of its rich amine content, high thermal stability (473.15 K), high adsorption capacity (365 mg/g) and porosity (Chitsiga et al. Citation2016; Ngoy et al. Citation2014, Citation2017); but detailed investigation on its diffusional behaviour and effect of mass transfer limitation during CO2 capture has not been given adequate consideration. Synthesis and characterisation of polyaspartamide have been carried out and reported in our previous works (Chitsiga et al. Citation2016; Ngoy et al. Citation2017). Nuclear Magnetic Resonance analysis was carried out on the adsorbent (polyaspartamide) by Chitsiga et al. (Citation2016) to confirm if the synthesis of the adsorbent was qualitatively successful. Among other analyses carried out on this adsorbent were Fourier Transform Infrared Spectroscopy (FTIR) to identify all the functional groups present on the adsorbent’s surface, Thermogravimetric analysis (TGA) to determine the thermal stability of polyaspartamide, Scanning Electron Microscopy (SEM) was carried out to obtain clear images showing the opened pores and cavities in the adsorbent, while the BET analysis was carried out on polyaspartamide to determine its pore size, pore volume and the surface area (Chitsiga et al. Citation2016). Here, we mainly report the diffusion mechanism and effect of mass transfer limitation on polyaspartamide during the adsorption of CO2 in a packed bed column.
Polyaspartamide can be re-used after the trapped CO2 is desorbed in regeneration studies. Regeneration and re-use of this adsorbent can be carried out by applying higher temperatures in a desorption process. However, it does not fall within the scope of this study. The main focus of the current study is to apply diffusional mass transfer models to determine the mechanism of diffusion and effect of mass transfer limitation during the adsorption of CO2 onto polyaspartamide. The diffusion model developed by Boyd, Myers, and Adamson (Citation1947) was adapted in this study to investigate if the adsorption of CO2 onto polyaspartamide was controlled by film diffusion (diffusion through the gas film layer) or pore diffusion (diffusion within the pores of polyaspartamide) while inter and intra-particle diffusion models were considered to investigate the actual rate-limiting step. The diffusional mass transfer models took into account external and internal mass transfer diffusion steps which were used to study the experimental curves under different conditions of gas flow rates.
Diffusion effect is very important in porous materials like polyaspartamide and the physical meaning of the diffusion constant ought to be determined in order to know the actual mass transfer mechanism involved during the sorption process (Garcés-Polo et al. Citation2016; Iren and Hristova Citation2011). Diffusional mass transfer models with different level of complexities have been applied in studying the diffusion mechanisms of different materials in the past (Chanie, Díaz, and Pérez Citation2016; Marczewski, Deryło-Marczewska, and Słota Citation2013; Teng et al. Citation2017). For instance, Ocampo-Pérez et al. (Citation2013) used a homogenous surface diffusion model (HSDM) to predict the diffusion of gaseous molecules from the external surface of an adsorbent through its pore surface. The model assumed that internal mass transfer occurs as a result of surface diffusion only with a negligible pore volume diffusion resistance (Ho and McKay Citation1998a). However, this model was limited to the description of only the surface diffusion coefficients in the adsorption process (Russo et al. Citation2016; Sonetaka et al. Citation2017). Viegas et al. (Citation2014) further combined the homogenous surface diffusion model (HSDM) with Boyd’s diffusion model to estimate intra-particle diffusion coefficients in adsorption processes. The model was established based on the assumption of external mass transfer and pore diffusion. Both researchers concluded that HSDM and Boyd’s model of film diffusion can be used to describe the diffusion mechanism and the rates controlling any gas-solid adsorption process. Intra-particle diffusion model is a model proposed by Boyd, Myers, and Adamson (Citation1947) and it is widely used with kinetic data to determine the diffusion mechanism and rate limiting step (mass transfer limitation) controlling adsorption processes. Inter-particle diffusion model has also been used to study adsorption mechanism of separation materials at different operating conditions before now (Song et al. Citation2016; Yao and Chen Citation2017).
2. Materials and methods
2.1. Materials
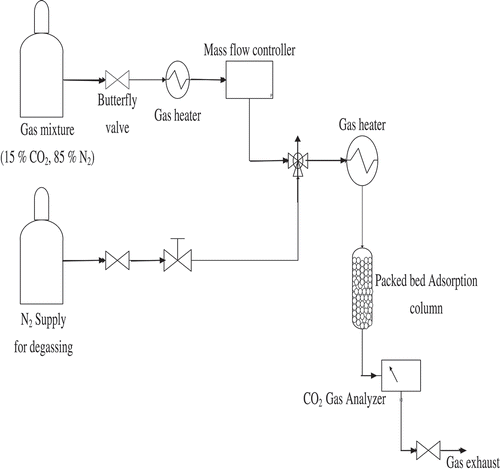
The materials used in this study were polyaspartamide and a binary gas mixture consisting of 15% CO2 and 85% N2. Polyaspartamide was synthesised by reacting polysuccinimide (the root polymer) with Ethylenediamine (EDA) and N,N-Dimethylformamide (with of DMF > 99%) as described by Chitsiga et al. (Citation2016). All gases (CO2 and N2) used in this study were purchased from Afrox (Pty) South Africa and used as delivered without any modification or purification. A schematic diagram of the experimental layout for the investigations in this study is presented in .
Figure 1. Schematic diagram of the experimental set up. (Adapted and modified from Yoro et al. Citation2017).
2.2. Experimental methods
To obtain the experimental values that will fit into the diffusion models explored, continuous gas-solid adsorption experiments were conducted in a laboratory-scale packed bed adsorption column (dimension of the packing is 8.5 mm in diameter and 125 mm in length). The packed bed is characterised by non-uniform flow distribution, radial mass and momentum transfer processes as well as varying diffusion coefficient. A balanced mixture of 15% CO2 and 85% N2 was used to mimic a flue gas source in this study while CO2 concentrations were taken directly from the CO2 gas analyser; Model: ABB-AO2020 (Frankfurt, Germany). The column was packed with polyaspartamide (0.1 g) and the initial temperature of the adsorption bed was increased to 373 K to remove moisture from the adsorbent. After the moisture removal stage, the bed was cooled down to the adsorption temperature of 333, 318 and 303 K, respectively, in a pre-conditioning (degassing) step of 20 min, during which pure N2 was made to flow through the packed bed column at a flow rate of 2.5 mL/s. After this pre-conditioning step, the gas mixture containing 15% CO2 and 85% N2 was fed into the adsorption column while by-passing the reactor in order to measure the initial concentration of CO2 in the gas mixture. The initial concentration of CO2 in the gas mixture was read directly from the CO2 gas analyser; model ABB-AO2020 (Frankfurt, Germany). The gas mixture was then allowed to flow through the reactor bed at specific flow rates after taking note of the initial concentration. Experimental runs were conducted in triplicate (Experiment 1, 2, 3 presented in ) at different gas flow rates but constant operating pressure and temperature. The concentration of CO2 in the adsorption column was recorded from the CO2 gas analyser as it varied with time until equilibrium was attained. The experiment was left to proceed for 1200 s (20 min) at varying gas flow rates of 1.5, 2.0 and 2.5 mL/s. After every experimental run, the used adsorbent was changed and a new polyaspartamide material of same mass (0.1 g) was fed into the reactor for continuation of the adsorption experiment. Equilibrium adsorption capacities of polyaspartamide were determined by applying a mass balance to the bed as well as accounting for the gas accumulated in intraparticle voids and dead space as described by Singo et al. (Citation2017). Relevant data obtained from the experimental study were used with the Boyd’s film diffusion, inter and intra-particle diffusion models to adequately investigate the adsorption mechanism and mass transfer limitation involved in the CO2 adsorption process. Model parameters such as the intraparticle diffusion constants (Kid), Boyd’s constants and Biot numbers were solved for in MATLAB R2014a. The diffusion mechanism that appears to be in a closer agreement with the assertions of the diffusion models stated in Sections 4.2 and 4.3 was considered as the actual diffusion mechanism and rate-limiting step controlling the adsorption process in this study. The flow diagram for the experimental set-up is presented in .
Table 1. Diffusional mass transfer parameters for the adsorption of CO2 on polyaspartamide at specific operating conditions compared with a recent study.
2.3. Theoretical background
Adsorption of CO2 by most materials during CO2 capture is usually affected by pore diffusion, external film diffusion, or by a combination of both and this constitutes the rate-limiting step during CO2 adsorption processes (Choy, Porter, and McKay Citation2004; Kavand et al. Citation2017). The usual gas adsorption process onto solid adsorbents comprises of five steps; bulk diffusion, film diffusion, inter-particle diffusion, surface diffusion and intra-particle diffusion steps. But according to previous studies (Plazinski, Dziuba, and Rudzinski Citation2013; Zhang et al. Citation2009), surface adsorption is usually very fast with rapid adsorption kinetics and if the adsorption is given by a Langmuir model, bulk diffusion is always negligible below a critical radius (Ho Citation2006; Ho and Mckay Citation1998b; Ho et al. Citation2006). In view of this, the current study has considered only the film, inter and intra-particle diffusion models. The fact that adsorption is a multi-step process necessitates the application of diffusion models in this study (Aljeboree, Alshirifi, and Alkaim Citation2017; Javadian et al. Citation2015; Staszak Citation2016). In order to investigate the mechanism of the adsorption of CO2 onto polyaspartamide in the present work, intraparticle diffusion-based mechanism has been studied alongside other mechanisms. This is because, diffusion-controlled mechanisms are considered to be more appropriate in gas-solid adsorption processes (Ho, Ng, and McKay Citation2000). The diffusional mass transfer models selected for this study assume that external mass transfer is responsible for the sluggishness or fastness of the sorption process and describes the actual diffusion mechanism controlling the adsorption of CO2 onto polyaspartamide. One of the aims of this paper is to apply a simple analytical solution to the adsorption of CO2 onto polyaspartamide from a CO2/N2 mixture. presents different outlet compositions of CO2 at different times during the CO2 capture experiment with polyaspartamide while the basic assumptions made for the models used in this study to investigate the diffusion mechanism of CO2 onto polyaspartamide have been summarised in .
Table 2. Captured CO2 at different adsorption times.
Table 3. Models explored and assumptions made.
2.4. Diffusional mass transfer models
Different diffusional mass transfer models have been developed in the past to investigate the mechanism of diffusion of different adsorbents (Fuente, Ordóñez, and Pérez Citation2016; Largitte and Pasquier Citation2016; Souza, Dotto, and Salau Citation2017). The level of resistance due to film diffusion is very important in any adsorption process because it accounts for the rapid or sluggish adsorption of gases onto most adsorbents (Yao and Chen Citation2017). More so, diffusion and mass transfer phenomena during the adsorption of CO2 from flue gas are important parameters that could be used to describe the performance of adsorbents for CO2 capture. Boyd, Myers, and Adamson (Citation1947) developed mathematical models to study ion-exchange kinetics of adsorption processes. These models were found to be versatile with a wide range of applications in different adsorption studies to describe adsorption and diffusion mechanisms as well as mass transfer limitations (rate-limiting steps) during adsorption processes (Fulazzaky, Majidnia, and Idris Citation2017; Malash and El-Khaiary Citation2010; Wróbel and Arabczyk Citation2006). The Boyd’s film diffusion model is expressed in Equations (1) and (2).
where Bt is the Boyd number, F (t) is the fractional attainment of equilibrium () and
. The principle of film diffusion during the adsorption of CO2 onto polyaspartamide was investigated in this study using the model developed by Boyd, Myers, and Adamson (Citation1947) while inter and intraparticle diffusion models were used to describe the rate-limiting steps (mass transfer limitation). The molecular diffusion models as proposed by Boyd, Myers, and Adamson (Citation1947) are expressed in Equations (3) and (4), respectively, while the Biot number parameter is expressed in Equation (5).
where qe is the equilibrium concentration of CO2 adsorbed by the adsorbent and qt is the final concentration of CO2 at time (t), B is the Boyd number, is the effective intra-particle diffusion coefficient, Bi is the Biot number and r is the radius of particle. Equation (3) is the interparticle diffusion model, Equation (4) is Boyd number parameter and Equation (5) is the Biot number parameter. Biot number is simply the ratio of the rate of conductive heat transfer resistance within the pores of the particle to the rate of convective heat transfer across the gas-solid surface (Ramakrishna Citation2013; Giner et al. Citation2010). The biot number links the external mass transfer coefficient with diffusion coefficient, it is a dimensionless quantity that could be used to identify controlling mechanisms and it is an appropriate standard used in measuring the thermal-shock resistance of a material. In a case of high Biot numbers (Bi > 1), the temperature remains almost the same at the surface of the adsorbent due to conductive heat transfer from its surface to its surroundings. If the Biot number is high, the heat transfer from the surface becomes analogous to that extended from the irradiated field. This, in turn, subdues the temperature rise at the surface. As the depth below the surface increases, the conductive heat transfer within the pores of polyaspartamide replaces the convective cooling from the surface region to the solid bulk. In this study, Biot number values obtained ranged from 17.80–30.74 (greater than 1). This means that conductive thermal resistance is dominant in this study and polyaspartamide is a thermally thick material. More so, complicated heat transfer equations for transient heat conduction will be required to describe the time-varying and non-spatially-uniform temperature field within the material (polyaspartamide). The high values of the Biot numbers obtained in this study has also provided an explanation for the dominance of external diffusion, and intra-particle diffusion over surface diffusion in polyaspartamide as is the case with most gas-solid adsorption studies.
2.5. Mass transfer effect
Mass transfer occurs when a component from a mixture moves between two points, either in the same or different phase as a result of a difference in concentration commonly referred to as driving force (Lin et al. Citation2017; Wang et al. Citation2012). In adsorption processes, mass transfer occur from a gas phase into a solid phase; this is made possible through the process of physisorption or chemisorption. Mass transfer in porous materials is a complex process. This is due to the presence of multiple mass transfer mechanisms that are dependent on the morphology of the adsorbent. In very small micro-pores (pores with a diameter below 2 nm), inter-molecular interactions and steric effects become the dominant forces for diffusion. In large macro-pores (pores with diameter greater than 50 nm), mass transfer becomes much more like bulk diffusion; that is, atomic diffusion within a crystalline lattice which occurs by either interstitial or substitutional mechanisms. Many amorphous adsorbents have wide pore size distributions that include micro-pores, meso-pores, and macro-pores. Therefore, it is often difficult to determine the relative contribution of diffusion in each region. Given the complexities of mass transfer in porous adsorbents, a myriad of appropriate experimental techniques have been developed to measure the effect of mass transfer limitation. These methods include NMR, chromatography, differential adsorption bed, zero-length column, etc.. The method used in this study considered experimentation in adsorption beds at varying flowrates to study the effect of mass transfer during the adsorption of CO2 by polyaspartamide.
Faster gas flow rates lead to a shorter contact time between the adsorbent and the adsorbate in the packed bed column which leads to restriction in the mass transfer area. This eventually results in a decrease in mass-transfer coefficient during the sorption process which confirms that this variable (gas flow rate) has an important influence on mass-transfer during gas-solid adsorption processes. An increase in the flow rate of the gas fed to the packed bed column in this study led to an increase in the amount of energy supplied to the sorbent in the solid phase. The turbulence on the solid sorbent in the reactor increases as well thereby resulting in an enhanced mass transfer in the gas-solid phase during the sorption process. This further avoids limitations due to gas concentration gradients and diffusion. In this study, a gas mixture with compositions of 15% CO2, 85% N2 was fed to a packed bed adsorption column containing 0.1 g of polyaspartamide evenly distributed within its packing. The outlet composition of CO2 after adsorption was 2%. This means that the material was able to capture about 86.7% of CO2 fed into the adsorption unit which confirms the good performance of polyaspartamide during CO2 capture. In addition, the Biot numbers in this work were much larger (>10), this simply implies that the walls of the material (polyaspartamide) have a great effect on mass transfer, which in turn will have an effect on heat transfer.
3. Results and discussion
3.1. Boyd’s film diffusion model
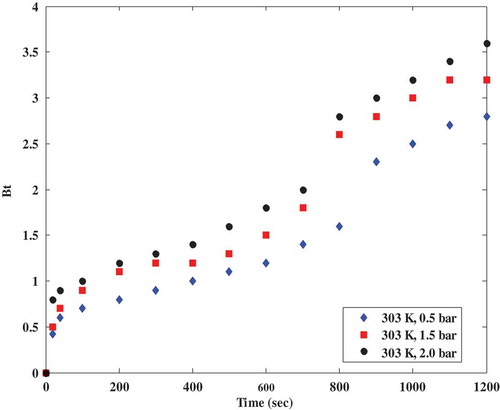
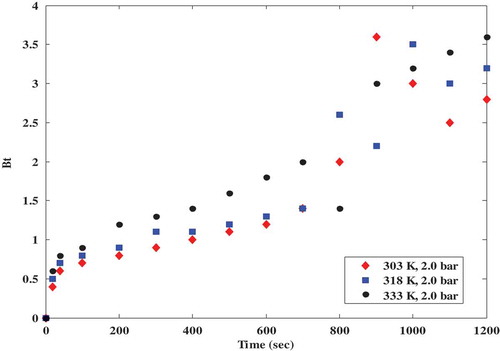
One of the most important factors to consider in any diffusion study is the film diffusion because it is the main factor that governs particle diffusion (Carta Citation2003; Huang, Cheng, and Lin Citation2015; Song et al. Citation2016). Film diffusion is the transport of the adsorbate (CO2) within the pores of the adsorbent (in this case, polyaspartamide). A plot of Bt versus t is called the Boyd plot where a straight line passing through the origin means that the adsorption process is controlled by intraparticle diffusion only (Viegas et al. Citation2014). Otherwise, the adsorption process is controlled by film diffusion or by both film and intraparticle resistance (Chatterjee and Schiewer Citation2014). Other researchers have also confirmed that if a plot of Bt versus time is non-linear, the process is suspected to be controlled by film diffusion but if the plot appears to be linear, pore diffusion is assumed to be the dominant diffusion mechanism controlling the adsorption process (Dotto, Buriol, and Pinto Citation2014; Kapur and Mondal Citation2013). The expressions from the film diffusion models as shown in Equations (1) and (2) were used to investigate whether the adsorption of CO2 onto the amine-grafted polyaspartamide is controlled by film or pore diffusion. The plots of Bt versus time (Boyd plots) at different partial pressures as shown in is non-linear while the Boyd plot at different adsorption temperatures in is also a non-linear scattered plot. The scattered plots in could be attributed to the magnified fluctuation of caused by the logarithmic term in the model. The non-linear descriptions in and signify that the adsorption of CO2 onto polyaspartamide is dominated by film diffusion instead of pore diffusion. In this study, the equilibrium concentration of CO2 (qe) was 0.13 while
was 0.15. This implies that according to Equation (3), the fractional attainment of equilibrium
is 1.15 (which is >0.85). Therefore, Equation (1) was used for the Boyd’s diffusion studies. Important model parameters that can be used to determine the adsorption mechanism have been obtained from this study and compared to that obtained in a similar study (Lin et al. Citation2017; Piccin et al. Citation2013) as shown in . Slight discrepancies observed in the constants obtained from this study and that reported in literature as presented in could be attributed to the different adsorbents considered in both studies. The calculated Boyd’s numbers presented in establish that intra-particle diffusion is the adsorption rate-controlling step in this gas-solid adsorption study.
3.2. Inter-particle diffusion model
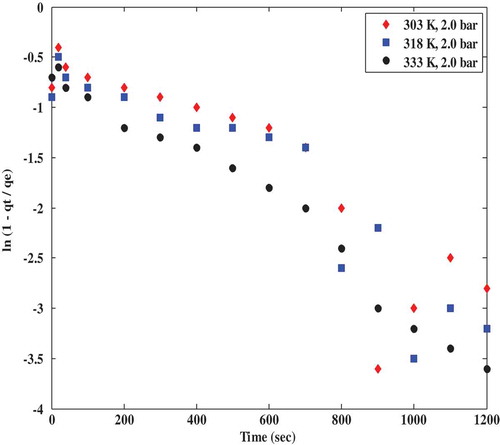
Inter-particle diffusion model was adapted from a similar study by Song et al. (Citation2016) to describe the CO2 adsorption mechanism of polyaspartamide at different adsorption temperatures and CO2 partial pressures in order to ascertain the actual mass transfer limitation controlling the adsorption of CO2 onto polyaspartamide. According to the inter-particle diffusion model reported in literature, a plot of ln (1- qt/qe) against time is expected to be linear having an intercept which is equivalent to the numerical value of ln (6/π2) which when simplified is given as −0.498. From this study, the intercept of a plot of ln (1- qt/qe) versus time as depicted in and were −0.7, −0.8 and −0.9 at different temperatures of 333, 303 and 318 K, respectively, at a constant pressure of 2 bar. At 303 K, the intercepts were −0.4, −0.55 and −0.6 at 0.5 bar, 1.5 bar and 2 bar, respectively
According to the results obtained from and , it is obvious that the intercept values reported in this study were not in agreement with the numerical value of ln (6/π2) as there were some visible deviations. This however indicates a violation of the assertion that inter-particle diffusion model is the rate-limiting step controlling the adsorption mechanism of CO2 onto polyaspartamide. This invariably means that there must be another rate-limiting step governing the adsorption of CO2 onto polyaspartamide.
Figure 5. Interparticle diffusion model plots for CO2 adsorption on polyaspartamide at different CO2 partial pressures.
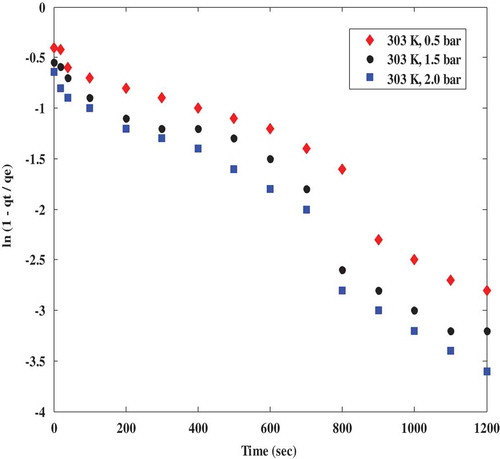
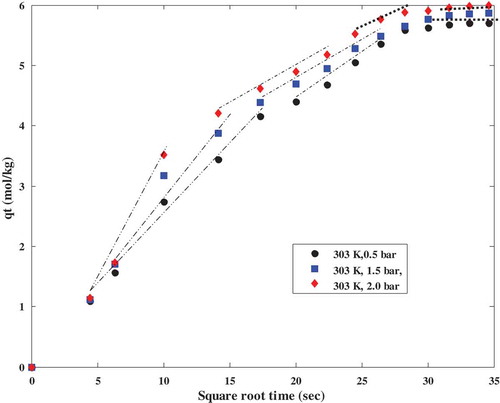
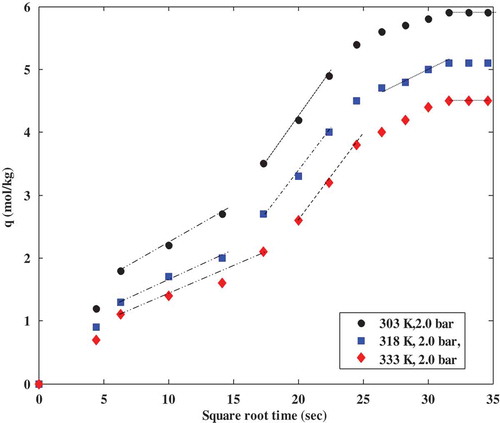
Figure 6. Intraparticle diffusion model plots for CO2 adsorption on polyaspartamide at different adsorption temperatures showing multilinearity.
Since the fractional adsorption capacity in this study is greater than 0.7, Equation (3) can be re-written as:
3.3. Intra-particle diffusion model
Weber and Morris (Citation1963) proposed that if an adsorption process is influenced by intraparticle diffusion, a plot of the adsorption capacity (qt) with the square root of time should vary linearly (Loganathan et al. Citation2014; Yousef, El-Eswed, and Al-Muhtaseb Citation2011). The intraparticle diffusion model considered in this study was derived from Fick’s second law of diffusion with the assumptions that; external resistance of mass transfer is only significant at the beginning of diffusion, diffusion occurs in a radial direction and pore diffusivity is constant. The model is a simple approximation of pore diffusion kinetics; it is capable of identifying the diffusion mechanism governing the sorption process and is expressed in Equation (7).
where qt is the amount of CO2 adsorbed at a particular time (mol kg−1), t is time (s), Kid (mol kg−1s−1/2) is an intraparticle kinetic parameter which determines the dominant diffusion mechanism and C (mol kg−1) refers to the thickness of the boundary layer. The intra-particle diffusion parameter (kid) was estimated from the slope of the plot of qt versus the square root of time and presented in .
According to the results shown in and , the curves obtained at different operating temperatures and pressure display multi-linearity. Multi-linearity nature of intra-particle diffusion model plots confirms that the adsorption mechanism consists of different steps and intra-particle resistance has a role to play during the adsorption of CO2 onto polyaspartamide. The multi- linearity displayed by the curves in and confirm that the mechanism of adsorption of CO2 onto polyaspartamide follows a film diffusion pattern and there are more than one rate-limiting step controlling the adsorption of CO2 onto polyaspartamide of which intra-particle resistance is the dominant one.
3.4. Effect of flowrates on breakthrough time and mass transfer
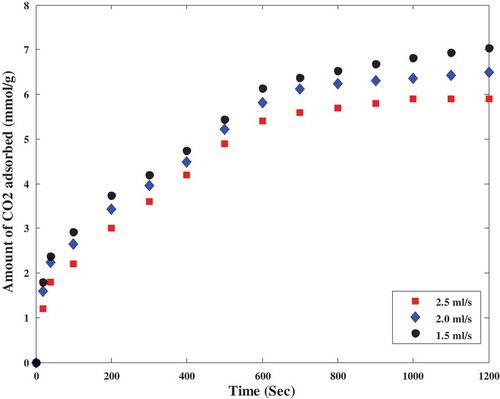
Breakthrough curves provide information for further design of the adsorption column for industrial applications. The breakthrough curve in this study signifies that at higher feed flowrates, the packed bed adsorption column was saturated early and lower flow rates resulted in a shallower adsorption zone and longer contact time between the CO2 and the adsorbent in the packed bed. Early breakthrough time resulted in a higher CO2 uptake by polyaspartamide. The effect of gas flow rates on the breakthrough behaviour during the adsorption of CO2 onto polyaspartamide is depicted in .
Figure 8. Breakthrough curves showing the effect of feed flow rate during the adsorption of CO2 on PAA: (Experimental conditions: feed pressure, 2 bar, and temperature 303 K).
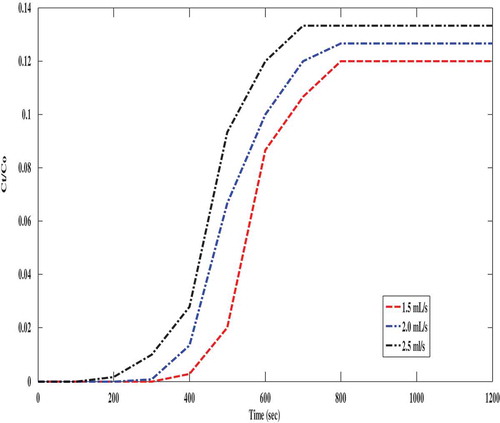
The packed bed column reactor was 8.5 mm in diameter and 125 mm, this helped to maintain an extension of breakthrough point as well as the exhaustion time of the adsorbent. Characteristic parameters describing the breakthrough behaviour have been summarised in . According to the breakthrough curves shown in , breakthrough and saturation time got significantly decreased as the feed flow rate was increased. This could be attributed to the fact that a lower feed flow rates prolong the mass transfer zone thereby leading to a shorter breakthrough time and also a decreased diffusion coefficient. The quantity of CO2 adsorbed by polyaspartamide at breakthrough time decreased from 6.8 mmol CO2/g adsorbent to 5.9 mmol CO2/g adsorbent when the feed flow rate was increased from 1.5 mL/s to 2.5 mL/s as shown in . This decrease is due to the insufficient diffusion time for CO2 molecules within the pores of polyaspartamide before leaving the packed bed. However, the efficiency of the packed bed could also be under threat. Similarly, it was also observed that a higher amount of CO2 was adsorbed by polyaspartamide at a gas flow rate of 1.5 mL/s with improved mass transfer which is attributed to the longer contact time between the sorbent and sorbate. These observations are in good agreement with previous reports (Lin et al. Citation2017; Piccin et al. Citation2013). Furthermore, as the gas flow rates were increased to 2.0 and 2.5 mL/s, the residence time of the gas within the packed bed decreased which resulted to a lower amount of CO2 adsorbed by polyaspartamide due to poor mass transfer between the gas (CO2) and the adsorbent (Polyaspartamide). Steeper slopes of the breakthrough curves were observed as the feed flow rate varied from 1.5 to 2.5 mL/s. Higher flow rates led to shorter contact times. It could also be attributed to the slow intra-particle diffusion within the pores of polyaspartamide. The estimated bed-contact time as the flow rate increases at constant bed depth as well as value for breakthrough times and maximum adsorption capacity of polyaspartamide are presented in .
Table 4. Estimated breakthrough and bed contact times with maximum adsorption capacity of polyaspartamide at different gas flow rates.
Based on the results presented in , operating at a low feed flow rate is recommended for the capture of CO2 from polyaspartamide via adsorption technology because maximum adsorption capacity was observed at the lowest gas flow rate (1.5 mL/s). Lower feed flow rates also led to a better estimated bed contact time (EBCT) of CO2 within the packed bed column as seen in as well as an improved CO2 capture efficiency as seen in . This simply means that at lower gas flow rates, mass transfer between the adsorbate (CO2) and the adsorbent (polyaspartamide) is greatly improved. Hence, the amount of CO2 captured also increases as shown in and because an improved mass transfer between adsorbent and adsorbate positively influences the amount of adsorbate captured an adsorbent.
Table 5. Adsorption efficiencies of polyaspartamide at different gas flow rates.
4. Conclusions
For the first time, diffusional mass transfer models have been used to study the actual adsorption mechanism and effect of mass transfer during the adsorption of CO2 onto an amine-grafted polymer-based material. The following conclusions have been drawn:
The adsorption of CO2 onto polyaspartamide involves more than one diffusion mechanism; film diffusion has been observed to be the dominant diffusion mechanism in his study, and it was responsible for the rapid adsorption of CO2 onto polyaspartamide before it gradually declined as equilibrium was approached.
The values of the Biot numbers obtained throughout this study were greater than 10; this confirms that the thermal resistance on the interface of polyaspartamide exceeds the thermal resistance in its interior and there is non-uniformity of temperature distribution within polyaspartamide during the adsorption process.
Intra-particle resistance is the actual rate-limiting step controlling the sorption process while inter-particle surface diffusion parameters obtained throughout this study have confirmed that inter-particle diffusion has a negligible role to play during the adsorption of CO2 onto polyaspartamide.
Considering the results obtained in this study, intra-particle and Boyd diffusion models can be used to facilitate the design of the packed-bed column while saving experimental expense and improving work efficiency.
Mass transfer was greatly influenced by the abundant narrow pores on the polyaspartamide material when the feed flow rates were varied; this accounted for the latter sluggish steps during the sorption process considered in this study. In addition, it is worthy to note that if CO2 capture efficiency and cost are to be taken into consideration, operating at a lower feed flow rate is most ideal because lower feed flow rates increase the sorbate-sorbent residence/contact time in the reactor thereby improving mass transfer and efficiency of the adsorbent during the sorption process.
Based on the results obtained in this study as compared with previous works, improvements on the process synthesis of polyaspartamide may be considered in future research since it is obvious that there are deviations between the estimated values of Kid and F (t). The heat transfer mode of diffusion studies can also be looked into in future research.
Acknowledgments
The authors are grateful to the National Research Foundation, South Africa (NRF grant numbers 107867 and 95061, respectively) and the University of the Witwatersrand, Johannesburg South Africa for the postgraduate merit awards (WITS-PMA 2017).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Kelvin O. Yoro
Kelvin O. Yoro obtained his MSc. Eng. degree in chemical engineering from University of the Witwatersrand, South Africa in 2017. He is currently a doctoral student in the school of chemical and metallurgical engineering at the same university. His main research interests are in CO2 capture storage and utilisation as well as process modelling and optimisation, heat and mass integration as well as environmental sustainability.
Mutiu K. Amosa
Mutiu K. Amosa is an international research expert with the department for management of science and technology development and the faculty of environment and labour safety at Ton Duc Thang University in Vietnam. His main research interests are in environmental process engineering, process systems engineering, separation processes, and materials synthesis & development.
Patrick T. Sekoai
Patrick T. Sekoai completed his doctoral study in 2017 at the school of chemical Engineering, University of the Witwatersrand South Africa. His research interests are mainly in the area of sustainable and renewable energy.
Jean Mulopo
Jean Mulopo is an associate professor at the school of chemical and metallurgical engineering, University of the Witwatersrand, South Africa with interest in environmental sustainability, modelling and simulation.
Michael O. Daramola
Michael O. Daramola is an associate professor of chemical engineering at the University of the Witwatersrand, South Africa. His core research interests are in the area of CO2 capture and storage, kinetics and catalysis, Process modelling and simulation.
References
- Aljeboree, A. M., A. N. Alshirifi, and A. F. Alkaim. 2017. “Kinetics and Equilibrium Study for the Adsorption of Textile Dyes on Coconut Shell Activated Carbon.” Arabian Journal of Chemistry 10: S3381–93. doi:10.1016/j.arabjc.2014.01.020.
- Allen, S. J., G. McKay, and K. Y. H. Khader. 1989. “Equilibrium Adsorption Isotherms for Basic Dyes onto Lignite.” Journal of Chemical Technology and Biotechnology 45 (4): 291–302. doi:10.1002/jctb.280450406.
- Álvarez-Gutiérrez, N., M. V. Gil, F. Rubiera, and C. Pevida. 2017. “Kinetics of CO2 Adsorption on Cherry Stone-Based Carbons in CO2/CH4 Separations.” Chemical Engineering Journal 307: 249–257. doi:10.1016/j.cej.2016.08.077.
- Boyd, G. E., L. S. Myers, and A. W. Adamson. 1947. “The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. III. Performance of Deep Adsorbent Beds under Non-Equilibrium Conditions1.” Journal of the American Chemical Society 69 (11): 2849–2859. doi:10.1021/ja01203a067.
- Carta, G. 2003. “Adsorption Calculations Using the Film Model Approximation for Intraparticle Mass Transfer.” Adsorption 9 (1): 55–65. doi:10.1023/A:1023815330732.
- Chanie, Y., I. Díaz, and E. Pérez. 2016. “Kinetics and Mechanisms of Adsorption/Desorption of the Ionic Liquid 1-Buthyl-3-Methylimidazolium Bromide into Mordenite.” Journal of Chemical Technology and Biotechnology 91 (3): 705–710. doi:10.1002/jctb.4632.
- Chatterjee, A., and S. Schiewer. 2014. “Multi-Resistance Kinetic Models for Biosorption of Cd by Raw and Immobilized Citrus Peels in Batch and Packed-Bed Columns.” Chemical Engineering Journal 244: 105–116. doi:10.1016/j.cej.2013.12.017.
- Cheung, W. H., Y. S. Szeto, and G. McKay. 2007. “Intraparticle Diffusion Processes during Acid Dye Adsorption onto Chitosan.” Bioresource Technology 98 (15): 2897–2904. doi:10.1016/j.biortech.2006.09.045.
- Chitsiga, T., M. O. Daramola, N. Wagner, and J. Ngoy. 2016. “Effect of the Presence of Water-Soluble Amines on the Carbon Dioxide (CO2) Adsorption Capacity of Amine-Grafted Poly-Succinimide (PSI) Adsorbent during CO2 Capture.” Energy Procedia 86: 90–105. doi:10.1016/j.egypro.2016.01.010.
- Choy, K. K. H., J. F. Porter, and G. McKay. 2004. “Film-Pore Diffusion Models - Analytical and Numerical Solutions.” Chemical Engineering Science 59 (3): 501–512. doi:10.1016/j.ces.2003.10.012.
- Costa, C., and A. Rodrigues. 1985a. “Design of Cyclic Fixed‐Bed Adsorption Processes. Part I: Phenol Adsorption on Polymeric Adsorbents.” AlChE Journal 31 (10): 1645–1654. doi:10.1002/aic.690311008.
- Costa, C., and A. Rodrigues. 1985b. “Intraparticle Diffusion of Phenol in Macroreticular Adsorbents: Modelling and Experimental Study of Batch and CSTR Adsorbers.” Chemical Engineering Science 40 (6): 983–993. doi:10.1016/0009-2509(85)85012-0.
- Costa, C., and A. Rodrigues. 1985c. “Regeneration of Polymeric Adsorbents in a CSTR.” Chemical Engineering Science 40 (5): 707–713. doi:10.1016/0009-2509(85)85023-5.
- Costa, C., and A. Rodrigues. 1985d. “Design of Cyclic Fixed‐Bed Adsorption Processes. Part II: Regeneration and Cyclic Operation.” AIChE Journal 31 (10): 1655–1665. doi:10.1002/aic.690311009.
- Dantas, T. L. P., F. M. T. Luna, I. J. Silva Jr, D. C. S. de Azevedo, C. A. Grande, A. E. Rodrigues, and R. F. P. M. Moreira. 2011. “Carbon Dioxide-Nitrogen Separation through Adsorption on Activated Carbon in a Fixed Bed.” Chemical Engineering Journal 169: 11–19. doi:10.1016/j.cej.2010.08.026.
- Dotto, G. L., C. Buriol, and L. A. A. Pinto. 2014. “Diffusional Mass Transfer Model for the Adsorption of Food Dyes on Chitosan Films.” Chemical Engineering Research and Design 92 (11): 2324–2332. doi:10.1016/j.cherd.2014.03.013.
- Fuente, A., C. Ordóñez, and R. Pérez. 2016. “Diffusional Mass Transfer Coefficient at the Water–Sediment Interface for Wind-Induced Flow in Very Shallow Lagoons.” Environmental Fluid Mechanics 16 (3): 539–558. doi:10.1007/s10652-015-9437-9.
- Fulazzaky, M. A., Z. Majidnia, and A. Idris. 2017. “Mass Transfer Kinetics of Cd(II) Ions Adsorption by Titania Polyvinylalcohol-Alginate Beads from Aqueous Solution.” Chemical Engineering Journal 308: 700–709. doi:10.1016/j.cej.2016.09.106.
- Garcés-Polo, S. I., J. Villarroel-Rocha, K. Sapag, S. A. Korili, and A. Gil. 2016. “A Comparative Study of CO2 Diffusion from Adsorption Kinetic Measurements on Microporous Materials at Low Pressures and Temperatures.” Chemical Engineering Journal 302: 278–286. doi:10.1016/j.cej.2016.05.057.
- Giner, S. A., R. M. Torrez-Irigoyen, S. Cicuttín, and C. Fiorentini. 2010. “The Variable Nature of Biot Numbers in Food Drying.” Journal of Food Engineering 101 (2): 214–222. doi:10.1016/j.jfoodeng.2010.07.005.
- Grande, C. A., R. P. P. L. Ribeiro, and A. E. Rodrigues. 2010. “Challenges of Electric Swing Adsorption for CO2 Capture.” ChemSusChem 3: 892–898. doi:10.1002/cssc.201000059.
- Ho, Q. T., B. E. Verlinden, P. Verboven, S. Vandewalle, and B. M. Nicolaï. 2006. “A Permeation–Diffusion–Reaction Model of Gas Transport in Cellular Tissue of Plant Materials.” Journal of Experimental Botany 57 (15): 4215–4224. doi:10.1093/jxb/erl198.
- Ho, Y. 2006. “Review of Second-Order Models for Adsorption Systems.” Journal of Hazardous Materials 136 (3): 681–689. doi:10.1016/j.jhazmat.2005.12.043.
- Ho, Y. S., and G. Mckay. 1998b. “The Kinetics of Sorption of Basic Dyes from Aqueous Solution by Sphagnum Moss Peat.” The Canadian Journal of Chemical Engineering 76 (4): 822–827. doi:10.1002/cjce.5450760419.
- Ho, Y. S., and G. McKay. 1998a. “Sorption of Dye from Aqueous Solution by Peat.” Chemical Engineering Journal 70 (2): 115–124. doi:10.1016/S0923-0467(98)00076-1.
- Ho, Y. S., J. C. Y. Ng, and G. McKay. 2000. “Kinetics of Pollutant Sorption by Biosorbents: Review.” Separation and Purification Methods 29 (2): 189–232. doi:10.1081/SPM-100100009.
- Huang, P., H. Cheng, and S. Lin. 2015. “Adsorption of Carbon Dioxide onto Activated Carbon Prepared from Coconut Shells. Research Article.” Journal of Chemistry. doi:10.1155/2015/106590.
- Iren, T., and E. Hristova. 2011. “Comparison of Different Kinetic Models for Adsorption of Heavy Metals onto Activated Carbon from Apricot Stones.” Bulgarian Chemical Communications 43 (3): 370–377.
- Javadian, H., F. Ghorbani, H. Tayebi, and S. M. Hosseini. 2015. “Study of the Adsorption of Cd (II) from Aqueous Solution Using Zeolite-Based Geopolymer, Synthesized from Coal Fly Ash; Kinetic, Isotherm and Thermodynamic Studies.” Arabian Journal of Chemistry 8 (6): 837–849. doi:10.1016/j.arabjc.2013.02.018.
- Kapur, M., and M. K. Mondal. 2013. “Mass Transfer and Related Phenomena for Cr(VI) Adsorption from Aqueous Solutions onto Mangifera Indica Sawdust.” Chemical Engineering Journal 218: 138–146. doi:10.1016/j.cej.2012.12.054.
- Kavand, M., N. Asasian, M. Soleimani, T. Kaghazchi, and R. Bardestani. 2017. “Film-Pore-[Concentration-Dependent] Surface Diffusion Model for Heavy Metal Ions Adsorption: Single and Multi-Component Systems.” Process Safety and Environmental Protection 107: 486–497. doi:10.1016/j.psep.2017.03.017.
- Largitte, L., and R. Pasquier. 2016. “A Review of the Kinetics Adsorption Models and Their Application to the Adsorption of Lead by an Activated Carbon.” Chemical Engineering Research and Design 109: 495–504. doi:10.1016/j.cherd.2016.02.006.
- Lin, X., Q. Huang, G. Qi, S. Shi, L. Xiong, C. Huang, X. Chen, H. Li, and X. Chen. 2017. “Estimation of Fixed-Bed Column Parameters and Mathematical Modeling of Breakthrough Behaviours for Adsorption of Levulinic Acid from Aqueous Solution Using SY-01 Resin.” Separation and Purification Technology 174: 222–231. doi:10.1016/j.seppur.2016.10.016.
- Liu, Z., C. A. Grande, P. Li, J. Yu, and A. E. Rodrigues. 2011. “Multi-Bed Vacuum Pressure Swing Adsorption for CO2 Capture from Flue Gas.” Separation and Purification Technology 81: 307–317. doi:10.1016/j.seppur.2011.07.037.
- Loganathan, S., and A. K. Ghoshal. 2017. “Amine Tethered Pore-Expanded MCM-41: A Promising Adsorbent for CO2 Capture.” Chemical Engineering Journal 308: 827–839. doi:10.1016/j.cej.2016.09.103.
- Loganathan, S., M. Tikmani, S. Edubilli, A. Mishra, and A. K. Ghoshal. 2014. “CO2 Adsorption Kinetics on Mesoporous Silica under Wide Range of Pressure and Temperature.” Chemical Engineering Journal 256: 1–8. doi:10.1016/j.cej.2014.06.091.
- Malash, G. F., and M. I. El-Khaiary. 2010. “Piecewise Linear Regression: A Statistical Method for the Analysis of Experimental Adsorption Data by the Intraparticle-Diffusion Models.” Chemical Engineering Journal 163 (3): 256–263. doi:10.1016/j.cej.2010.07.059.
- Marczewski, A. W., A. Deryło-Marczewska, and A. Słota. 2013. “Adsorption and Desorption Kinetics of Benzene Derivatives on Mesoporous Carbons.” Adsorption 19 (2–4): 391–406. doi:10.1007/s10450-012-9462-7.
- Ngoy, J. M., M. O. Daramola, T. L. Chitsiga, R. Falcon, and N. Wagner. 2017. “CO2 Adsorption Using Water-Soluble Polyaspartamide.” South African Journal of Chemical Engineering 23: 139–144. doi:10.1016/j.sajce.2017.04.004.
- Ngoy, J. M., N. Wagner, L. Riboldi, and O. Bolland. 2014. “A CO2 Capture Technology Using Multi-Walled Carbon Nanotubes with Polyaspartamide Surfactant.” Energy Procedia 2230–2248. doi:10.1016/j.egypro.2014.11.242.
- Ocampo-Pérez, R., R. Leyva-Ramos, M. Sanchez-Polo, and J. Rivera-Utrilla. 2013. “Role of Pore Volume and Surface Diffusion in the Adsorption of Aromatic Compounds on Activated Carbon.” Adsorption 19 (5): 945–957. doi:10.1007/s10450-013-9502-y.
- Ocampo-Pérez, R., J. Rivera-Utrilla, C. Gómez-Pacheco, M. Sánchez-Polo, and J. J. López-Peñalver. 2012. “Kinetic Study of Tetracycline Adsorption on Sludge-Derived Adsorbents in Aqueous Phase.” Chemical Engineering Journal 213: 88–96. doi:10.1016/j.cej.2012.09.072.
- Osler, K., D. Dheda, J. Ngoy, N. Wagner, and M. O. Daramola. 2017a. “Synthesis and Evaluation of Carbon Nanotubes Composite Adsorbent for CO2 Capture: A Comparative Study of CO2 Adsorption Capacity of Single-Walled and Multi-Walled Carbon Nanotubes.” International Journal of Coal Science and Technology 4 (1): 41–49. doi:10.1007/s40789-017-0157-2.
- Osler, K., N. Twala, O. O. Oluwasina, and M. O. Daramola. 2017b. “Synthesis and Performance Evaluation of Chitosan/Carbon Nanotube (Chitosan/Mwcnt) Composite Adsorbent for Post-Combustion Carbon Dioxide Capture.” Energy Procedia 114: 2330–2335. doi:10.1016/j.egypro.2017.03.1368.
- Piccin, J. S., L. A. Feris, M. Cooper, and M. Gutterres. 2013. “Dye Adsorption by Leather Waste: Mechanism Diffusion, Nature Studies, and Thermodynamic Data.” Journal of Chemical and Engineering Data 58 (4): 873–882. doi:10.1021/je301076n.
- Plaza, M. G., I. Durán, N. Querejeta, F. Rubiera, and C. Pevida. 2016. “Experimental and Simulation Study of Adsorption in Post-Combustion Conditions Using a Microporous Biochar. 1. CO2 and N2 Adsorption.” Industrial and Engineering Chemistry Research 55 (11): 3097–3112. doi:10.1021/acs.iecr.5b04856.
- Plazinski, W., J. Dziuba, and W. Rudzinski. 2013. “Modeling of Sorption Kinetics: The Pseudo-Second Order Equation and the Sorbate Intraparticle Diffusivity.” Adsorption 19 (5): 1055–1064. doi:10.1007/s10450-013-9529-0.
- Qiu, H., L. Lv, B. Pan, Q. Zhang, W. Zhang, and Q. Zhang. 2009. “Critical Review in Adsorption Kinetic Models.” Journal of Zhejiang University-Science A 10 (5): 716–724. doi:10.1631/jzus.A0820524.
- Ramakrishna, V. 2013. “Effect of Biot Number in Batch Studies of Adsorption.” International Journal of Engineering Research and Technology 2 (10): 587–595.
- Russo, V., R. Tesser, D. Masiello, M. Trifuoggi, and M. Di Serio. 2016. “Further Verification of Adsorption Dynamic Intraparticle Model (ADIM) for Fluid–Solid Adsorption Kinetics in Batch Reactors.” Chemical Engineering Journal 283: 1197–1202. doi:10.1016/j.cej.2015.08.066.
- Sadare, O. O., M. Masitha, K. O. Yoro, and M. O. Daramola. 2018. “Removal of Sulfur (E.G DBT) from Petroleum Distillates Using Activated Carbon in a Continuous Packed-Bed Adsorption Column”. Lecture Notes in Engineering and Computer Science: Proceedings of The World Congress on Engineering and Computer Science, San Francisco, CA, October 23–25, 509–513.
- Sekoai, P. T., and K. O. Yoro. 2016. “Biofuel Development Initiatives in Sub-Saharan Africa: Opportunities and Challenges.” Climate 4 (2): 33. doi:10.3390/cli4020033.
- Singo, M. C., C. X. Molepo, O. O. Oluwasina, and M. O. Daramola. 2017. “Chitosan-Impregnated Sod-Metal Organic Frameworks (Sod-Zmof) for CO2 Capture: Synthesis and Performance Evaluation.” Energy Procedia 114: 2429–2440. doi:10.1016/j.egypro.2017.03.1390.
- Sonetaka, N., Y. Seida, T. Nakano, and E. Furuya. 2017. “Determination of Intraparticle Diffusivity and Fluid-to-Solid Mass Transfer Coefficient from Single Concentration History Curve in Circulated-Type Fixed-Bed Reactor.” Adsorption Science and Technology 36 (1–2): 571–585. doi:10.1177/0263617417707866.
- Song, G., X. Zhu, R. Chen, Q. Liao, Y. Ding, and L. Chen. 2016. “An Investigation of CO2 Adsorption Kinetics on Porous Magnesium Oxide.” Chemical Engineering Journal 283: 175–183. doi:10.1016/j.cej.2015.07.055.
- Souza, P. R., G. L. Dotto, and N. P. G. Salau. 2017. “Detailed Numerical Solution of Pore Volume and Surface Diffusion Model in Adsorption Systems.” Chemical Engineering Research and Design 122: 298–307. doi:10.1016/j.cherd.2017.04.021.
- Staszak, M. 2016. “A Linear Diffusion Model of Adsorption Kinetics at Fluid/Fluid Interfaces.” Journal of Surfactants and Detergents 19 (2): 297–314. doi:10.1007/s11743-016-1789-8.
- Teng, Y., L. Li, G. Xu, K. Zhang, and K. Li. 2016. “Promoting Effect of Inorganic Alkali on Carbon Dioxide Adsorption in Amine-Modified MCM-41.” Energies 9 (9): 667. doi:10.3390/en9090667.
- Teng, Y., Z. Liu, G. Xu, and K. Zhang. 2017. “Desorption Kinetics and Mechanisms of CO2 on Amine-Based Mesoporous Silica Materials.” Energies 10 (1): 115. doi:10.3390/en10010115.
- Titinchi, S. J. J., M. Piet, H. S. Abbo, O. Bolland, and W. Schwieger. 2014. “Chemically Modified Solid Adsorbents for CO2 Capture.” Energy Procedia 63: 8153–8160. doi:10.1016/j.egypro.2015.12.337.
- Viegas, R. M. C., M. Campinas, H. Costa, and M. J. Rosa. 2014. “How Do the HSDM and Boyd’s Model Compare for Estimating Intraparticle Diffusion Coefficients in Adsorption Processes.” Adsorption 20 (5–6): 737–746. doi:10.1007/s10450-014-9617-9.
- Wang, C., M. Perry, G. T. Rochelle, and A. F. Seibert. 2012. “Packing Characterization: Mass Transfer Properties.” Energy Procedia 23: 23–32. doi:10.1016/j.egypro.2012.06.037.
- Wang, Q., J. Luo, Z. Zhong, and A. Borgna. 2011. “CO2 Capture by Solid Adsorbents and Their Applications: Current Status and New Trends.” Energy and Environmental Science 4 (1): 42–55. doi:10.1039/C0EE00064G.
- Weber, W. J., and J. C. Morris. 1963. “Kinetics of Adsorption on Carbon from Solution.” Journal of the Sanitary Engineering Division 89 (2): 31–60.
- Wróbel, R., and W. Arabczyk. 2006. “Solid−Gas Reaction with Adsorption as the Rate Limiting Step.” The Journal of Physical Chemistry A 110 (29): 9219–9224. doi:10.1021/jp061947b.
- Yao, C., and T. Chen. 2017. “A Film-Diffusion-Based Adsorption Kinetic Equation and Its Application.” Chemical Engineering Research and Design 119: 87–92. doi:10.1016/j.cherd.2017.01.004.
- Yoro, K. O. 2017. “Numerical Simulation of CO2 Adsorption Behaviour of Polyaspartamide Adsorbent for Post-Combustion CO2 Capture”. M. Sc. thesis, University of the Witwatersrand, South Africa.
- Yoro, K. O., M. K. Amosa, P. T. Sekoai, and M. O. Daramola. 2018. “Modelling and Experimental Investigation of Effects of Moisture and Operating Parameters during the Adsorption of CO2 onto Polyaspartamide.” International Journal of Coal Science and Technology. doi:10.1007/s40789-018-0224-3.
- Yoro, K. O., A. J. Isafiade, and M. O. Daramola. 2018 October 23–25. “Sequential Synthesis of Mass Exchanger Networks for CO2 Capture.” In Lecture Notes in Engineering and Computer Science: Proceedings of the World Congress on Engineering and Computer Science, Vol. 2 vols, 503–508. San Francisco, USA.
- Yoro, K. O., and P. T. Sekoai. 2016. “The Potential of CO2 Capture and Storage Technology in South Africa’s Coal-Fired Thermal Power Plants.” Environments 3 (3): 24. doi:10.3390/environments3030024.
- Yoro, K. O., M. Singo, J. L. Mulopo, and M. O. Daramola. 2017. “Modelling and Experimental Study of the CO2 Adsorption Behaviour of Polyaspartamide as an Adsorbent during Post-Combustion CO2 Capture.” Energy Procedia 114: 1643–1664. doi:10.1016/j.egypro.2017.03.1294.
- Yousef, R. I., B. El-Eswed, and A. H. Al-Muhtaseb. 2011. “Adsorption Characteristics of Natural Zeolites as Solid Adsorbents for Phenol Removal from Aqueous Solutions: Kinetics, Mechanism, and Thermodynamics Studies.” Chemical Engineering Journal 171 (3): 1143–1149. doi:10.1016/j.cej.2011.05.012.
- Zhang, Q., J. Crittenden, K. Hristovski, D. Hand, and P. Westerhoff. 2009. “User-Oriented Batch Reactor Solutions to the Homogeneous Surface Diffusion Model for Different Activated Carbon Dosages.” Water Research 43 (7): 1859–1866. doi:10.1016/j.watres.2009.01.028.