ABSTRACT
Material generated during industrial activities that do not have further use in the production process are often categorised as refused industrial byproducts. This paper briefly discusses the utilisation of such refused byproducts in the cement manufacturing process and evaluates their use at different stages of the cement manufacturing process. The evaluation was based on the byproducts’ physicochemical properties without affecting the quality of the final cement product. Dealing with hazards caused by byproduct mismanagement and storage is a tremendous task; however, such production continues to rise. The worldwide diminishment of raw materials offers ample opportunities to replace these materials with refused byproducts. Many refused byproducts remain unexplored even though they might be used in cement production and to develop newer types of cement. Using refused byproducts as alternatives to more traditionally used raw materials will help maintain the world’s ecological balance while contributing to society’s development.
Introduction
Portland cement underpins modern global development. As such, it is the most abundantly produced material in the world (Deolalkar Citation2016). The world has an abundance of the primary raw materials for cement manufacturing and, compared to other construction materials, cement’s cost is low and it has good durability (Hewlett Citation2003).
In 2017, China and India, the world’s biggest producers, together produced 64% of the world’s cement, or 2.61 million tons of cement out of 4.05 million tons. In 2018, these countries together estimated production of 2.66 million tons of the total 4.10 million tons, or 65% of the world’s total. In the Middle East, Saudi Arabia, the region’s major cement producer, manufactured 0.47 and 0.45 million tons for 2017 and 2018, respectively. In comparison, in the same years, the United States produced 0.86 and 0.88 million tons of cement.
Demand for cement in the North Africa and Middle East (MENA) region is expected to grow at a compounded annual rate of 5.5% (Uwasu, Hara, and Yabar Citation2014). Increasing urbanisation has led to the steady growth of cities; hence, cement demand is inevitable. Infrastructure development in the MENA region primarily has been driven by considerable investment in the construction sector. Further construction of road and rail networks across major cities and infrastructure development are likely to continue to supplement the growth of the regional market (Abbass, Kumar, and El-Gendy Citation2018). The healthy increase in construction activity throughout the MENA region suggests that demand for cement will continue to rise.
According to the World Bank, carbon dioxide (CO2) emissions are generated from industrial processes such as cement manufacturing and burning fossil fuels. Cement manufacturing accounts for 5–6% of all anthropogenic CO2 emissions (Farzanegan and Markwardt Citation2018). Around 50% of the total emissions are from limestone decarbonisation, and about 40% result from the fuel used in the kiln process. The MENA countries are the world’s top per capita emitters for CO2 and SO2 and have had high pollution records since the 1960s, exceeding the world average from 1995 onwards. This region has around 57% of the world’s proven oil reserves and 41% of proven natural gas reserves, and about 85% of all greenhouse gas (GHG) emissions in this region come from energy production and consumption.
The global industrial market is facing environmental and regulatory issues regarding water disposal and usage, CO2 and sulphur oxide (SOx) emissions, depletion of reserves, and other industrial activities. To overcome these challenges, many industries are working on alternative sources of fuel and raw materials. Considering the predicted increase in demand for cement, there is pressure on the cement industry to maintain or reduce CO2 emissions. Alternative fuels and raw materials are increasingly used to minimise cement-related CO2 emissions, and increased plant efficiency is desirable from both economic and environmental perspectives. Also, there has been significant research into new, lower carbon cement types, including blended cements, which may contain industrial waste and byproducts. If these alternative cements can be made economically viable, they may contribute to a considerable reduction in CO2 output from the cement industry.
Cement production
The process of cement making
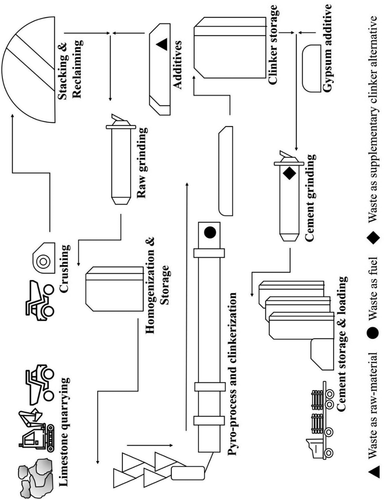
Cement is produced from suitable raw materials in six stages (). Mining limestone/raw materials; grinding, homogenisation, and blending various raw mix components; the preheater phase; processing and pyro processing in the kiln; clinkerizing; and grinding, packing, and transporting materials (Hewlett Citation2003; Peray and Waddell Citation1986).
In stage one, the essential raw materials-limestone, laterite, bauxite, kaolinite, clay, iron ore, sandstone, etc-are mined. Limestone is a source of calcium, while bauxite and kaolinite meet the requirement for aluminium. Laterite, red ochre, and iron ore fulfill the need for iron and, to some extent, silica. Quartz-phyllite and sandstone provide a source for silica. Because the requirement for limestone greatly outweighs that of other components, cement kilns are usually located close to plentiful limestone supplies, with other raw materials being transported to the plant. Erecting cement kilns near limestone mines allows easy handling of the primary raw material, thereby saving energy and making cement production more economical. In recent years, computer-aided mining plans have been widely adopted to assist with quality limestone production. The extracted limestone boulders are crushed in crushers for size reduction, with the advanced technology of modern crushers resulting in optimal crushing. Piles of various raw materials stacked in stockpiles also receive further processing as part of the mining process.
Stage two involves the characterisation of various raw materials. This quality control process establishes the composition of the raw materials. Then, with the help of various quality control tests, suitable raw mix designs are prepared. The raw materials are then ground in suitable ball mills/vertical roller mills (VRMs), both of which are common grinding mills, in order to achieve the targeted fine powder, which is stored in pre-homogenisation silos (Aïtcin and Flatt Citation2015; Hewlett Citation2003).
In stage three, the raw mix is fed into a preheating chamber. This chamber consists of 5–6 vertical cyclones, through which the raw material passes and undergoes decarbonation. The preheating chamber utilises the hot gases emitted from the kiln, thus saving energy depending on the number of stages. This approach makes the cement plant more environmentally friendly by reducing fuel consumption by 30–50% (Wasilewski and Duda Citation2012).
In stage four, the pre-heated decarbonised raw mix is fed into the rotary kiln for clinkerization. The retention time of the kiln feed also varies from plant to plant, taking into consideration the reactivity of the raw mix and defining the production capacity, which is generally calculated as tons per day. The kiln inlet temperature is usually maintained at around 900 °C, and the final temperature is around 1450 °C (Deolalkar Citation2016; Hewlett Citation2003; Peray and Waddell Citation1986). During heating, a series of chemical reactions occurs between calcium, silicon, aluminium, iron, etc. resulting in phase formation. Kilns are typically heated from the exit side, with fuel type depending on availability and suitability.
In stage five, the clinker exiting the kiln is rapidly cooled by forced air. The resulting hot air is circulated back into the kiln or the pre-heater, thus reducing energy demand. The cooled clinker is then ground in a suitable grinding mill with about 5% gypsum or another calcium sulphate source and other trace components such as limestone and stannous or ferrous sulphate.
In the final stage, the ground cement is conveyed to storage silos using a suitable transport system. The silos need to prevent contamination and hydration, for example. Stored cement is then either packaged in waterproof bags of 20–50 kg or transported in bulk.
Raw materials chemistry
Cement manufacture requires a source of calcium, such as CaCO3 or CaMg (CO3)2, which comprises almost 80–90% of the total and the rest is iron oxide (Fe2O3, Fe3O4), quartz (SiO2), and clay/bauxite (Al-silicates) (Aïtcin and Flatt Citation2015; Hewlett Citation2003; Peray and Waddell Citation1986). Traces of Na2O, K2O, TiO2, and MnO2 are also present in the various raw materials used for cement making (Gineys, Aouad, and Damidot Citation2010; Vollpracht and Brameshuber Citation2016). Some of these trace elements can be beneficial to the clinkering process while others are not.
Besides chemical composition, the dissociation of CaCO3 in carbonate rocks depends on the rock’s textural and microstructural features. Holocrystalline calcite (CaCO3) dissociates at higher temperatures than fine-grained calcite (Treiman and Essene Citation1984).
It is known that MgO in limestone can be present in various forms, including magnesium silicates, dolomite, and magnesite; each has different dissociation characteristics because of thermal stability and the break-down temperature of the lattice structure of magnesium compounds, such as for dolomite (Ca Mg (CO3)2), is 700–750 °C, and ankerite (Ca (Mg, Fe) (CO3)2) is 580 °C. The presence of Al2O3, SiO2, Fe2O3, Fe3O4, etc., further influences these dissociation characteristics (Kääntee et al. Citation2004; Madlool et al. Citation2011).
The mineralogical nature and dispersion characteristics of silica have a strong bearing on the reactivity of the cement raw mix. Experiments have shown that a slight increase in silica content in the raw mix may profoundly reduce phase formation in the clinker. Silicates react more readily than free silica or uncombined silica such as quartz (Herfort et al. Citation2010; Ludwig and Zhang Citation2015).
Clay minerals used in cement making are essentially hydrous aluminosilicates and provide a combined source of silica and alumina (Hewlett Citation2003; Ludwig and Zhang Citation2015). Clays vary incredibly in composition and hence show hugely variable thermal behaviours. However, all clays show a similar pattern of change: dehydration then dihydroxylation followed by the breakdown of crystal structures. Through this process, the clay releases Al2O3 and SiO2 in a reactive form. The presence of this reactive state is then exploited in cement production.
The presence of iron oxide in the ferrous state favours raw material reactivity. The appearance of FeO depends upon the breakdown temperature of the parent phase, and the temperature ranges of the occurrence of FeO and its oxidation are goethite at 300 °C, glauconitic at 450–500 °C, ferriferous chlorite at 500 °C, siderite/biotite/hornblende at 500–900 °C, and phlogopite at 1050–1250 °C (Herfort et al. Citation2010; Peray and Waddell Citation1986). Fe3O4 may contain more iron than FeO, but it only becomes reactive at temperatures above 900 °C. Limonite (FeO, OH.H2O) often presents in laterite, and it generally contains amorphous silica and is more reactive (Snellings, Salze, and Scrivener Citation2014).
From a chemical viewpoint, the reactivity of a raw mix is strongly dependent on the availability of reactive components. As such, it is favourable that raw mix components become reactive at similar temperatures to the carbonate decomposition temperature. It is evident that highly reactive fine carbonates combine poorly with low reactive clays containing silica, iron, magnesium, potassium, and so on.
Environmental impact
The environmental impact of the cement industry
Every production industry impacts the environment in both minor and major ways. This phenomenon is true for the cement industry also (Hendriks et al. Citation1998; Kajaste and Hurme Citation2016). Wet process cement plants impact their surroundings by slurry disposal while dry and semi-dry plants also create solid waste, which is an environmental concern (Deolalkar Citation2016; Uwasu, Hara, and Yabar Citation2014). Today, however, very few wet process plants remain. Appropriate environmental protection laws, and norms and emission limits are formulated by every country, and the adoption of a proper environmental management plan (EMP) can minimise ecological degradation. Most cement plants worldwide take adequate steps to protect the environment, and assessments of environmental norms are improved and modified annually.
Mediators of the environmental impact of cement production
Several environmental impacts riddle the cement production process and, as a result, personal protective measures, such as helmets, goggles, masks earplugs, and protective clothing are employed by operatives to address health and safety issues. Drilling limestone beds during mining produces large quantities of dust, for example, but wet drilling, where possible, minimises dust generation (Donoghue Citation2004; Fthenakis Citation2003).
Other steps are also taken to minimise environmental impact. Rock blasting, which is most commonly conducted in limestone mines, may lead to ground vibration, flying rock, dust generation, and high noise levels. Sequential and controlled blasting helps minimise ground vibration, while blast holes can be optimised to avoid excessive generation of flying rock. Reducing the powder factor (i.e., the quantity of explosive used per ton of rock broken) helps to minimise dust generation. Similarly, detonators and explosives can be managed so as to avoid high noise levels and control the peak particle velocity of the entire blasting operation. During surface mining, watering limestone can reduce dust generation (Donoghue Citation2004).
During crushing, dust generation and high noise levels are normal. The effects can be mitigated by watering adjacent land. Dense shrubs, such as as Quercus ilex, Psidium guajava, Psidium cattleianum and Mangifera indica, can be planted to help trap dust (Rai Citation2016).
In the preheater section of the cement plants, harmful gases are commonly generated. Proper methodologies should be adopted by installing modern techniques to minimise the excess generation of hazardous gases, excess heat, and dust (Mikulčić et al. Citation2016).
In the kiln section, excess heat generation should be controlled by adopting the waste heat recovery technique (Khurana, Banerjee, and Gaitonde Citation2002; Zhang et al. Citation2013). The excess heat recovered should be appropriately used in the required sections to save energy and protect the environment. Waste heat recovery is one of the most critical parameters to be controlled in cement plants because doing so helps to minimise energy conservation and safeguard the environment.
Noxious gases like NOx, SO2, CO2, and CO should be controlled properly and must be monitored on a regular basis to avoid environmental damage to the surroundings. Gas emissions may impact global warming (Barcelo et al. Citation2014; Benhelal et al. Citation2013; Van Oss and Padovani Citation2002). Dust generation in the cooler and packing plant must be monitored on a day-to-day basis. Proper mitigation measures should be adopted to avoid generation more dust than is permissible. Transport systems in every section of the plant should be adequately maintained to protect the cement plant’s surroundings.
The cement plant’s chimneys should be appropriately monitored to avoid excess dust emission to the atmosphere. Installing electro static precipitators (ESP), or baghouses with filter bags, is a must for arresting the dust generated during the cement manufacturing process (Hewlett Citation2003; Uwasu, Hara, and Yabar Citation2014). Proper, regular maintenance of ESP and baghouses is necessary to achieve environmental norms.
CO2 emissions and global warming
The cement production process consumes a lot of energy. According to some estimates, the cement industry contributes as much as 5% of total global CO2 emissions (Gartner Citation2004). The cement industry has several sources of CO2 emissions, including fuel combustion and calcination. The cement industry’s primary energy consumption is through carbon-based fuels such as coal, pet coke, natural gas, and petroleum products (Ali, Saidur, and Hossain Citation2011; Barcelo et al. Citation2014). During combustion, a high percentage of CO2 emission takes place, and the cement industry’s CO2 emissions in 2008 accounted for 1.8 Gt/Y worldwide (Barcelo et al. Citation2014). The cement industry generates CO2 by a calcination process and the consumption of natural carbon-based fuels. During the calcination process, CO2 is emitted mainly from limestone, which is the primary raw material consumed during production. It is estimated that about 0.5 kg CO2/kg of clinker is generated during the calcination process (Van Oss and Padovani Citation2002).
The primary use of fossil fuels in cement production occurs during pyro-processing. CO2 emissions during this process depends on the type of production process and its efficiency and also on the type of fuel used in the pyro process. The role of the percentage of additives used and the clinker-to-cement ratio also play significant roles. It has been observed that as the clinker-to-cement ratio increases, CO2 emissions also increase. However, the carbon intensity of cement manufacturing is not reported uniformly by different agencies. This reality is the subject of some variation in the literature. Total CO2 emissions per ton of cement from energy and calcination also ranges widely. Along with CO2, other harmful substances like NOx, SOx, CO, dust, and some heavy metals in trace amounts also are emitted.
The cement industry’s CO2 emissions can be reduced by improving the energy efficiency of the process, using low carbon fuels or alternative fuels (e.g., waste-derived fuels, maintaining a lower clinker-to-cement ratio), and removing CO2 from flue gases (Valderrama et al. Citation2012). Many of these techniques have already been adopted by cement plants worldwide. However, more stringent norms should be followed to control CO2 emission levels.
Waste generation
Industrial waste generation
Every process industry in the world generates some waste, and this waste generation may be major or minor. No matter the level of waste generated, it is generally dumped in the plant’s surroundings. Hundreds of years ago, waste generation was not a major concern, but with increasing industrialisation and a growing population, society has become more aware of the need for waste management. The cement industry has been no exception to this growing awareness. During the wet process era, a vast quantity of waste in the form of slurry was generated and disposed of in plants’ surrounding open lands, rivers, ponds or lakes. These dump yards created a nuisance in cement plants’ surroundings. Most of these cement plants are closed now, but those dump yards of slurry remain untouched. Similar situations have arisen with the paper, sugar, copper, iron, pharmaceutical, textile, leather, ceramic, and marble industries, to name a few. Nowadays, industries like mining, petroleum refineries, thermal and nuclear plants, and construction also generate a massive quantity of waste. However, the dump yards created by the electronics industry are currently the biggest in the world (Kiddee, Naidu, and Wong Citation2013; Nnorom and Osibanjo Citation2008). The situation is alarming.
Waste management and an increase in waste generation
With increasing quantities of waste being generated across a range of industries, appropriate waste management strategies are a global concern. Hence, sustainable waste management has been incorporated as a core principle of industries worldwide. Many environmentally friendly waste management options have been prescribed by various international environmental agencies. Restrictions have been put on sea-based and farmland waste disposal for various environmental reasons.
Advanced waste management techniques are formulated for suitable reuse of the waste generated by different industries. Methodologies have been developed from the viewpoint of waste disposal, improvements required in the ratio of recycled-to-non-recycled resources and how to recycle waste. Hence, developing a waste management plan (WMP) as part of an industry’s startup is a fundamental step which is crucial to a venture’s success (Aranda Usón et al. Citation2013; Schneider et al. Citation2011). The volume of waste generated, along with its quality and physical and chemical properties must be determined in advance alongside estimations of production capacity. Accordingly, the industry’s WMP and EMP should encompass a minimum of two-thirds of the life of the industry. Simultaneously, the WMP should aim to overcome adverse environmental degradation. This advance planning will not only safeguard the environment but also help generate more eco-friendly products and reduce environmental degradation. Developments in science and technology have opened many new fields that can utilise various industries’ waste products, and few steps are required to adopt and implement these approaches. If the gap between research and implementation is narrowed, then ecosystem improvements will be noted immediately.
In the past few decades, proper WMPs have not been developed within the cement industry. This finding is the main cause of today’s severe environmental issues. However, if appropriate steps are taken in relation to waste dumps, materials can be used depending on their physical and chemical properties. For example, in many instances, the quality of refuse from limestone mines will support the manufacture of gypsum and slag and, in many cases, rejected limestone can be used as composite cement components (ASTM, Citation2013; EN 197-1. Citation2011 ; Hawkins, Tennis, and Detwiler Citation2003; Voglis et al. Citation2005). Even fine materials present in these dump yards may have utility in brick and tile manufacturing (Scott et al. Citation2005). Use of waste as both coarse and fine aggregates can fulfill all the requisite characteristics of strength and durability in concrete. Certain discarded materials may also be used in manufacturing of retrofitted concrete (Gesoǧlu et al. Citation2012; Khankhaje et al. Citation2016; Nagaratnam et al. Citation2016; Neupane Citation2016).
As stated earlier, global cement production is growing yearly. While an increase in production means more waste generated and more gas emitted, an increase in the number of cement plants also means an increase the CO2 emission and other noxious gases in the atmosphere, which is a matter of significant environmental concern. Proper planning for the gaseous waste generated must be borne in mind to avoid any further environmental degradation and adequately utilise all types of waste generated.
Planning for cement plants’ waste generation should be mandatory. Such planning will not only save the environment but also reduce the amount of refuse generated. Auxiliary industries which can use the generated waste must be planned to avoid waste overload.
Waste types
The list of waste that humans generate is endless despite environmental norms, rules, and regulations, and no perfect solution has been devised to deal with waste products. By the time waste of one type is controlled, another type of waste is generated. Improper industrial planning, coordination, and cooperation are mainly responsible for this situation. It is the need of the hour to think on this issue globally; otherwise the day is not far off when everything will perish. Waste can be classified broadly into the following three categories (Nemerow Citation2007):
Non-hazardous waste,
Hazardous waste, and
Industrial waste.
Non-hazardous waste is mostly generated as non-metal mining byproducts and can be categorised as rejects, demolished construction materials, old metal machinery, and biodegradable household waste. However, the volume of non-hazardous waste occupies an increasing amount of the world’s available land annually. In many locations, this waste covers useful land, which is of great concern. Hence, proper planning should focus on this waste’s potential uses. Research in this area has been carried out globally, and many results have been achieved to direct the handling of non-hazardous waste.
A few examples of hazardous waste generating industries are the pharmaceutical, automobile, paper, leather, textile, and rubber industries; nuclear and thermal power plants; nuclear explosion tests; and petroleum refineries (Fthenakis Citation2003; Gidarakos and Aivalioti Citation2012). This type of waste has the potential to damage the global ecosystem (Gidarakos and Aivalioti Citation2012) as it disturbs the natural environment, human and animal health, and flora and fauna. Essentially, hazardous waste causes severe damage to the microbiological environment. Hazardous waste is generally dumped in barren lands, forest covered areas, lakes, and the sea. These dumping sites have been used by various industrial agencies over the years and have caused severe disturbances in the world’s overall ecosystem. Numerous steps have already been taken to solve this problem. In many cases, considerable success has been achieved but, to improve results, continuous efforts are required.
Different cement plants have identified non-hazardous and hazardous waste products, and research has suggested their proper utilisation. Such waste is being used in clinker production and even in some cases as post-production material. However, while considerable success has been achieved in a few areas to safeguard the environment, human health, and ecology from hazardous waste, the cement industry has yet to achieve widespread success in this area. Different international healthcare and environmental agencies are putting great effort to minimise the impact of hazardous waste.
Industrial waste is mainly the byproduct of manufacturing units. One of the biggest producers of industrial waste is the world’s building material producing industries (Huntzinger and Eatmon Citation2009; Van Oss and Padovani Citation2002), but other sectors contribute to the waste issue as well. These sectors include those involved in thermal power production; metal, metalliferous, non-metal ore, and coal mining; and paper, sugar, and textile production. During building material production, numerous toxic minerals in the form of fine powder are generated. These fine airborne products spread in the atmosphere carried by ambient air. These toxic fine-grained minerals affect the vegetation cover in production units’ surroundings, impacting human and animal health, soil, water bodies, and crops. Thermal power plants worldwide also contribute to this issue by producing a massive quantity of fly ash. This underutilised fly ash causes several diseases in humans and in the animal kingdom (Singh et al. Citation2016). The different metal manufacturing industries also generate waste which endangers the health of humans and animals and contaminates both surface and sub-surface water.
Waste used as raw materials in clinker production
The world’s manufacturing development process started with the production of new materials beneficial to human beings. From the beginning of the metal age, necessary materials have been used and unwanted materials have been rejected. However, at the beginning of the metal age, no one could have visualised the sheer volume of waste occurring both before and after the manufacturing process, especially in cement production. Perhaps this short-sightedness is the reason that people involved in industrial development never thought of optimising natural resources. This pattern continued for centuries, and natural resources started to diminish. At the beginning of the nano-technological era, experts started thinking of the use of both pre- and post-production materials. Whereas waste was previously defined as materials that do not have further use in the industrial production, the current definition has evolved. Nothing on earth should be wasted, whether natural or human made, because today’s waste is tomorrow’s raw materials. The industrial application of waste generated by process/manufacturing industries has become the main thrust of technological developments worldwide.
Cement technologists have also come to this realisation and put effort into using waste materials differently (). Definitions of what constitutes raw materials also have been modified, natural resource use is now maximised, and new items have been added to cement manufacturers’ lists of potential materials for productions.
Table 1. Wastes used as raw material in cement production
Numerous problems are faced in the process of clinker manufacturing when materials derived from fuels are used. These problems have led to intensive focus from the cement industry to understand the cause of these problems. The most frequent problems faced are related to volatile compounds such as SO3, alkalis (Na2O and K2O), chlorine (Cl), and moisture content. These oxides create a cycle in the preheater tower in which they build until the formation that blocks the production path, leading to lower production. In many cases, this stoppage in the rotary kiln requires cleaning to remove the formation. This issue usually occurs at temperatures between 950–1250 °C. Because alternative fuels and materials introduce water vapour into the preheater, when sulphur is present in the alkalis cycle, the reaction becomes more complicated. The presence of SO2 tends to decrease the impact of the alkalis’ cycle while the presence of Cl and water vapour tends to increase it. If sulphur is present in the form of CaSO4, then the oxygen released from the decomposition of CaSO4 at very high temperatures (from 1200–1400 °C) leads to the formation of more undesirable complex compounds.
Additionally, the presence of alkalis can lead to the formation of free lime (i.e., uncombined lime) in the clinker (, chemical equations 6 and 7), affecting the quality of the clinker and producing cement that has low compressive strength and higher expansion (Chatterjee and Sui Citation2019). Using alternative fuels such as polyvinyl chloride (PVC), chlorinated hydrocarbons or sewage sludge, biomass raises the amount of chlorine in the preheater and kiln system. Furthermore, the occurrence of volatile chlorine in the kiln gases’ stream introduces environmental concerns and can cause volatilisation of metals such as mercury, lead, and cadmium.
Figure 2. The chemical reactions happen in the preheater and kiln during the process of clinker formation at present of alkalis (Na2O and K2O), Sulphur (SO3), chlorine (Cl) and moisture content (Chatterjee and Sui Citation2019)
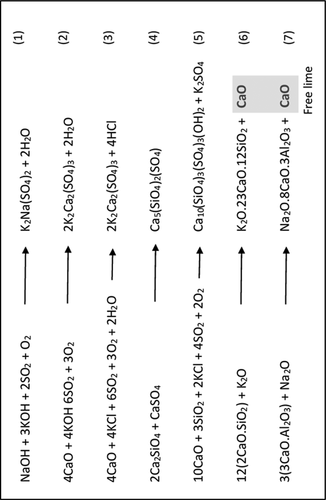
Some remedial measures have been successfully implemented in the cement industry to counteract the problems caused by alkalis, including preparing the kiln feed with a molar ratio of SO3 to a total alkali oxide of 1:2.
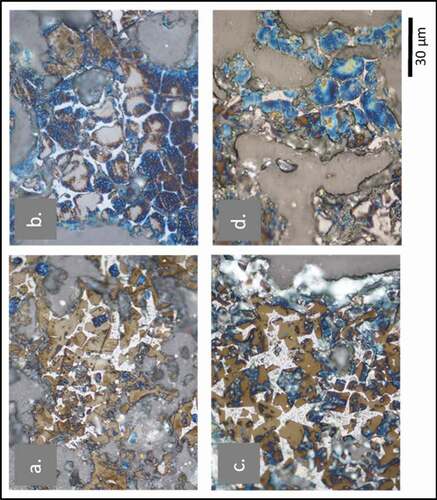
Another effect of using waste as alternative fuel or material in clinker preparation is that it causes changes in the clinker phase microstructures. These changes, which can be conceptualised as microstructure modifications, happen when trace elements, including heavy metals, alkalis, chlorine, and others, appear in the crystal structure of clinker phases. shows clinker analysis by optical microscopy for two types of clinker. and B show clinker obtained from a cement plant that does not use any waste in their process. The clinker phases formed in these processes are alite and belite. However, and D show the microstructure for clinker prepared using 55% hazardous waste obtained from the oil drilling process. The alite and belite crystals are relatively more substantial when compared with clinker prepared with non-waste clinker.
Sludge from water treatment
Raw water from various sources is treated in water treatment plants. Impurities are removed by coagulation-flocculation, sedimentation, and the filtration process, and potable water is achieved. During this process of purification, and especially in conventional water treatment plants, massive quantities of sludge are generated, but discharging the sludge into rivers, lakes, sea, drains, landfills, etc. is not a disposal solution because it damages the environment.
While the chemical composition of sludge may vary regionally, usually SiO2, Al2O3, Fe2O3, CaO, Na2O, K2O, and MgO are the chemical constituents present in sludge and TiO2, P2O5, MnO, ZnO, and PbO are present in smaller percentages. Trace elements like Ba, Zr, Rb, Ce, Sr, Cu, Ni, Cr, Ga, As, Nd, Nb, and Co are also present in sludge. As stated previously, discharging sludge is not sustainable as it will damage local and regional environments; however, the chemical and mineralogical composition of sludge suggests that it can be used in cement production (Lin and Lin Citation2005; Taruya, Okuno, and Kanaya Citation2002). More specifically, research worldwide has suggested that water treatment sludge could be used in lower percentages as a raw mix component of cement manufacturing because its elemental composition is fine sand, silt, and clay, which is sometimes rich in lead, chromium, arsenic, barium, and other metals. In fact, this sludge has been used as cementitious material, as a fine aggregate component in the construction industry, in the ceramics industry, and in lightweight brick and aggregate manufacturing (Weng, Lin, and Chiang Citation2003). Research indicates that recycling sludge for use in the building and construction industries is safe, and its strength and durability have shown encouraging results.
Sewage sludge waste
Sewage sludge waste is produced from municipal wastewater coming from hospitals, nursing homes, and households. The fresh sludge is extracted mechanically and passed to a separate sludge digestion tank which operates at a high temperature. The digested sludge is generally disposed of in landfills, which causes numerous health hazards and disturbs an area’s flora and fauna. Contaminants and hazardous substances are present in sewage sludge, including plasticisers, synthetic fibres, biosolids, and various Cd-, Cu-, Pb-, Hg-, Mo-, Ni-, Se-, and Zn-rich complex compounds. In addition, sewage sludge contains pathogens, micropollutants, undetected components, and heavy metals (Donatello and Cheeseman Citation2013; Fytili and Zabaniotou Citation2008).
In many countries, it has been discovered that huge swaths of farmland, forests, and seawater are contaminated due to the continuous disposal of this type of waste. As a result of such research, many places have banned the disposal of sewage sludge. However, if this sludge is adequately treated, it can be used in the construction industry and, in many countries, sewage sludge waste is used in small proportions by the construction industry.
Industrial waste
Several industries produce industrial waste. Like other types of waste, industrial waste is not without its problems and, if it is not disposed of properly, can cause land and water pollution. High moisture content, the nature of the waste, the extent of impurities present, its availability, its negligible effect on production costs and profitability, its physical properties, etc. make the waste critical for use in cement manufacturing. Many byproducts of chemical industries are rich in lime and enhance phase formation so are used as a minor percentage of the raw material needed for cement production (Herfort et al. Citation2010; Schneider et al. Citation2011; Van Oss and Padovani Citation2002). Numerous characteristic features of industrial waste make it suitable for the manufacture of blended and special cements:
The source of industrial waste and its byproducts is well defined;
Such waste does not require any mining so only handling costs are involved;
Industrial waste is often fine grained; hence, extensive grinding and crushing may not be required, which saves energy and, consequently, cost; and
Most industrial waste does not need much processing, (e.g., drying, calcining) which reduces energy consumption and cost (Aïtcin and Flatt Citation2015; EN 197-1. Citation2011 ; Hewlett Citation2003).
Waste used in the cement industry includes blast furnace slag, fly ash, fertiliser slag, red mud from the aluminium industry, Pb-Zn slag, Cu-slag, agricultural waste, paper sludge, etc. (Ishak and Hashim Citation2015; Stefanović et al. Citation2010). Other than this petroleum refinery sludge, sugar industry baggase, rice husks, refuse from the aluminium scrap handling industry, marble slurry, jarosite, etc. are also used to a minor extent by several cement industries.
Numerous mineral and chemical constituents present in the different waste categories mentioned above enhance the better phases in the clinker and improve the quality of cement. These minerals include dehydrated gypsum, melilite, K-aluminosilicates, Ca-rich minerals, active quartz, etc. However, if the proportion of these minerals constitutes more than 15% of the mixture, then the chances of poor phase formation in the clinker increases. Even large size layered alite grains and free lime are formed. An optimum proportion of minerals in the waste helps create good silicate, aluminate and ferrite phases in the clinker. In such conditions, grain size distribution is uniform in the clinker.
Fly ash, which is a fine-grained material consisting of quartz, mullite, haematite, and clay minerals as major minerals and carbonate minerals, gypsum, and pyrite as secondary phases, is a byproduct of coal-based thermal power plants. The quality of fly ash is ascertained by the presence of a glass phase. Numerous types of glass grains (mostly spherical) are present in fly ash. These glass grains may be clear, solid, spongy, partly crystalline, or porous. Grain sizes in fly ash can vary drastically. Technical assessment of fly ash is determined by physical and chemical characteristics which meet certain requirements. In a few cement plants, fly ash is used as a raw mix component but, in most cases, fly ash is added to cement to produce Portland Pozzolana cement (PPC). Standards have been developed by quality assurance organisations worldwide, which is a prerequisite for manufacturing PPC. These standards mainly have been developed because of variation in the quality of the fly ash produced by different thermal power plants.
Fly ash utilisation in the cement and construction industries can lower GHG emissions because such use offsets the emissions that result from mining activities and CO2 generation during cement production. Fly ash can decrease a higher percentage of the consumption of cement during construction. Using fly ash as a substitute for cement in roller compacted concrete results in reductions when 15–30% of the Portland cement is replaced with fly ash (ASTM C595/C595M-16. Citation2003. ASTM C595-03 ; EN 197-1. Citation2011). This phenomenon results in energy savings, and reductions in GHGs and the emission of dust and noxious gases. Fly ash has also been used successfully to produce bricks and in roads to save soil (Dabhade, Chaudari, and Gajbhaye Citation2014). However, fly ash use is restricted in countries where coal-based thermal power plants do not exist.
Blast furnace slags are formed when iron ore, coke, and limestone or dolomite are heated at high temperatures. During this process, the limestone/dolomite acts as a flux and is chemically combined with the silicates and aluminates present in ore. Coke ash and the above products are mixed and produce blast furnace slag. This molten product can be cooled in several ways to form various types of slag, including ground granulated blast furnace slag (GGBS), which is rapidly cooled with large quantities of water to produce granules (Bijen Citation1996).
GGBS is mixed with Portland cement clinker to make a blended cement known as Portland slag cement (PSC) (ASTM Committee C01.10, . ASTM C150/C150M-16 ; Bijen Citation1996; EN 197-1. Citation2011). It is preferable that MgO, SO3, and sulphide Sulphur (S) loss on ignition and insoluble residue should not exceed 8.0%, 3.0%, 1.5%, 4.0%, and 2.5%, respectively. GGBS with the following compositions can produce suitable PSC: SiO2 27–32%, Al2O3 17–31%, Fe2O3 0.0–1.0% CaO 30–40%, MgO 0.0–17% and sulphide 0.2% (Zhang, Hama, and Na Citation2015). Further, the GGBS should show hydraulicity, which is a function of glass content. As per practice, the glass content should not be less than 85–90%. A lesser percentage of glass implies the presence of slag minerals such as melilite, and wollastonite in greater amounts (Collins and Sanjayan Citation2000).
The more basic the slag, the higher the hydraulic activity in the presence of alkaline activators. Ordinary air-cooled slag has no or very few cementing properties. Granulated slag alone has a negligible cementing action due to activators like lime and Portland cement, and alkalies such as soda, sodium carbonate, or sulphate of the alkalies. An average or moderately high alumina content of slag enhances the quality of cement.
Various phases are present in the slag including glass (supercooled liquid silicates), semi-glass, quartz, Ca-rich silicates, aluminosilicates, the presence of modified C3S and C2S phases, and in melilite, gehlenite, akermanite, merwinite, rankinite, pseudo wollastonite, monticellite, oldhamite, anorthite, forsterite, perovskite, spinel, etc. in minor amounts (Yildirim and Prezzi Citation2011).
The various types of slag cements produced are Portland blast furnace slag, Eisen Portland cement, Hochofen cement, Cimetanlaiter. et aux Cenacles, super sulphated cement, and Cimet de laiter a La Chaise.
Cu-Zn and Pb slags are also used in cement manufacturing (Bijen Citation1996; Zhang, Hama, and Na Citation2015). In these types of slags, the morphometric complexity in the glass is typical, and semi-glass grains may behave as mineralisers. There is a direct effect of this slag on the formation of belite grains. However, these slags reduce the size of grains of both C3S and C2S if the pyro-processing system is disturbed.
Red mud is a byproduct of the aluminium industry. It contains numerous in situ mineralisers, which help to enhance quick phase formation in the clinker. However, the phases’ forms are different in shape and size. Red mud also affects the morphology of phases, which is fragmentation of alite and belite, thereby increasing the granulometry of phases. Caution should be taken to avoid free lime formation in the clinker. The primary aims when using different waste is
To reduce energy consumption and CO2 release,
To enhance reactivity,
To limit the time content,
To use alternative source materials,
To create cement based on sulpho alumina tests,
To characterise waste materials using different instrumental methods, and
To emphasise the importance of grain morphology and size percent distribution for motion, strength, etc.
Building waste
Building waste is generally generated by the demolition of old buildings. These are also sometimes considered construction and demolition (C&D) waste. Many definitions of C&D wastes have been adopted (Medina et al. Citation2014; Wagih et al. Citation2013). C&D waste is very complex in composition and contains masonry materials like dirt, rock, concrete, and brick mixed with wood, roofing, drywall, and a small amount of metal. These materials are usually broken and smashed, complicating recovery from debris loads. Additionally, certain materials have further complicating factors. Wood, for example, is typically weathered, painted and in many cases attached to some other material. Roofing materials and masonry materials such as brick, concrete, rock, and dirt within a demolition site are generally mixed with other demolition materials.
Despite their special considerations, C&D waste can have value in cement production. Masonry materials can be used both as coarse and fine aggregates of concrete depending on their physical properties. Different grades of concrete were first developed in laboratories, and various tests, including durability, compressive strength, bleeding, physical properties, leaching, etc. were conducted. In most cases, these materials yielded promising results, with pavement concrete developed for C&D waste in particular showing very promising results. In many instances, C&D materials are used for road construction, and some masonry materials are even used to develop lightweight concrete. However, more periodic research is required for more use of these waste products.
Waste used as alternative addition in cement grinding
Various types of cement have been introduced in the recent past by cement technologists the world over (). The majority of these cements were developed by the addition of alternative waste (also known as SCM, supplementary cementitious materials) produced by other industries. Fly ash and various slags produced by metal industries are the two most significant components (ASTM C595/C595M-16. Citation2003. ASTM C595-03 ; EN 197-1. Citation2011). Additionally, limestone has been used as a component of cement. These additives were added independently as well as in combination in permissible percentages in the cement mixture along with clinker. Fly ash and GGBS slag were added in cement grinding to produce PPC and PSC cement. However, the combination of clinker, fly ash, and slag along with gypsum is used in cement grinding.
Table 2. Wastes used as clinker replacement
Similarly, clinker, fly ash, and limestone have been used to produce cement. These permutations and combinations have been decided based on the physical and chemical characteristics of the waste materials. This scientific approach to cement manufacturing was introduced to reduce CO2 emission and energy conservation within the cement industry. More research is being done worldwide to find ways to conserve limestone deposits and use waste materials to produce new types of cement. During the development of new cement, quality aspects are strictly monitored and, to this end, during grinding, every possible physical and chemical parameter is maintained to produce high-quality cement.
shows a list of SCMs which are used as fillers, are chemically active in hydrating cement, and replace clinker. Adding SCMs results in improved strength and durability in the cement composite, and they are widely used in concrete admixtures. One advantage of cement concrete prepared with fly ash is the binder’s low hydration temperature. The fly ash maintains a low temperature in the concrete, thus decreasing the concrete’s risk of thermal impact. Likewise, fly ash increases the chemical resistance of concrete and decreases exposure to an alkali-aggregate reaction (Czarnecki and Justnes Citation2012; Pacewska and Wilińska Citation2013).
Table 3. Chemical analysis of some wastes used as additives in cement grinding
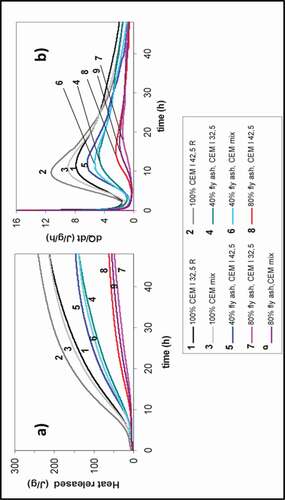
Pacewska and Wilińska (Citation2013) studied the effect of using fly ash as a replacement for cement using the isothermal calorimetry analytical method which measures the total heat released and the rate of heat evolution after adding water to the mixture. The total heat released is measured as shown in and B. The five stages of heat released (i.e., initial dissolution, induction period, acceleration period, deceleration, slow period) were observed for the eight samples with different ratios of fly ash. The reference sample without fly ash shows typical shapes where the total heat released was observed to decrease (). This influence of the delaying process is due to the developing of the pozzolanic activities of fly ash cement and low amount of original cement in the mix (20% cement and 80% fly ash).
Figure 4. Rate of heat release for cement paste made of different percentages of fly ash by isothermal calorimetry method (Pacewska and Wilińska Citation2013)
Cement standards and specifications
Cement is a hydraulic binder, or a fine-grained inorganic material, which forms a paste when water is mixed with its coarse and fine aggregates. Cement hardens into concrete or mortar over time through hydration reactions. The process of hardening allows cement to retain specified strength and long-term stability with ageing. Common types of cement include Portland slag cement, PPC, Portland fly ash cement, Portland limestone cement, Portland-burnt shale cement, composite cement and others (ASTM C595/C595M-16. Citation2003. ASTM C595-03 ; EN 197-1. Citation2011). Portland cement clinker is manufactured by defined raw materials containing CaO, SiO2, Al2O3, Fe2O3, MgO, K2O, Na2O, etc. Homogenous mixtures of raw materials are developed for firing in the kiln. The clinker thus produced should contain at least two-thirds by mass of C3S, other phases of C3A, C4AF, and liquid.
All cements are developed by adding different components in prescribed limits, and quality assurances of all these cements are fixed according to standard norms. All these cements should achieve set mechanical, physical, chemical and durability requirements. Compressive strength, initial setting time and soundness in these cements should be maintained according to standards. The requirements for the constituents like Portland cement clinker, GGBS slag, pozzolanic materials fly ash, burnt shale, limestone, and minor constituents are defined in the standards of all the cement producing countries of the world. Targeted limits specified by the standards must be followed to maintain the quality of the cement. These standards, however, have not always existed.
Different countries-initiated cement standards beginning in the late nineties after it was observed that different cements had different qualities, setting times, strengths and soundness. Initially, only a few types of cement were included in the list for standardisation. Before standardisation, many countries developed criteria for cement performance depending on the durability and quality of the product. Therefore, significant variation was found in different countries’ cement compositions. Eventually, standards were based on numerous factors. For example, standards were revised depending on the type of construction for which the cement was being used and whether the material was being used as concrete or mortar. Standards were also developed in consideration of the raw materials available and fuels used in producing the cement, the region’s climatic conditions, and the places the cement would be used along with the types of use that would be employed with the cement (Aïtcin and Flatt Citation2015; ASTM C595/C595M-16. Citation2003. ASTM C595-03 ; ASTM Committee C01.10, . ASTM C150/C150M-16 ; EN 197-1. Citation2011). In recent years, the standards of various new generation cements have also been developed by countries other than the ones that are using the cement. However, standards have been developed only for cements which meet certain mechanical, physical and chemical requirements (ASTM, Citation2007 ; ASTM C595/C595M-16. Citation2003. ASTM C595-03 ; ASTM C807, Citation1999 ; EN 197-1. Citation2011). Even today, cement is generally consumed by local and regional markets; in very few instances, cement is exported to other countries.
The previous discussion highlights the fact that defining fixed specifications for cement is an enormous task because of the different plants’ and different countries’ raw material composition, available fuels, process parameters, and climatic conditions.
Impact on the cement properties
The composition of raw materials, additives, and fuel has a significant bearing on cement’s properties. Limestone used for cement production by different cement plants varies considerably in mineralogy, chemical composition, physical features, and microstructure. Significant components may not vary considerably, but minor constituents may vary largely. Minor variations in raw materials can affect the formation phase, so the composition of cement clinker should be adequately understood, especially because these minor constituents (e.g., magnesia, barium, strontium, titanium, chromium, vanadium, manganese, sulphates, phosphates, fluorine, alkalis) can directly and disproportionately impact clinker properties and the quality of the cement produced (Hewlett Citation2003). These minor constituents can affect the properties of the cement either by changing phase relations and the reaction products formed. Alternatively, they may alter the reactivity of the main cementing compounds towards water by building solid solutions of different properties or by producing water-soluble compounds. These effects may influence setting and hardening processes. Because these effects may manifest singly or in combination, it is desirable to identify the constituents present in the raw materials and the proportion of their content to postulate their possible effect on clinker quality and the cement produced from them. The advantage of their presence or adequate steps to safeguard against the possible harmful effects to control the clinker quality is required. Potentialities for these naturally occurring raw materials or the potential use of industrial waste should be established. Most of these materials have been investigated in detail and are successfully used by cement plants worldwide.
Akin Altun and Sert (Citation2004) used weathered phosphor-gypsum by-product as a set retarder in Portland cement. They compared the setting and mechanical properties of phospho-gypsum cement that were prepared in different ratios with Portland cement containing natural gypsum and found that the highest 28-day compressive strength is associated with 3% phosphor-gypsum cement. Smarzewski and Barnat-Hunek (Citation2016) utilised coal cinder in high-performance concrete and studied mechanical behaviour and durability. Coal cinder, inorganic waste produced by the combustion process in boiler house used as cement additive with 30% showed higher compressive strength, increase in absorptivity and high surface free energy. Simultaneous addition of coal cinder waste cement was found to improve resistance to salt crystallisation of cement concrete. Yan et al. (Citation2020) conducted a feasibility study about using incinerated bottom ash municipal solid waste as a cement-stabilised macadam aggregate. The addition of the ash showed a reduction in properties like density, water-absorption, porosity, hydraulicity, and cementitious activity. The increased substitution rate of the waste in cement (up to 20%) was found to be associated with a reduction in drying shrinkage and an increase in the leaching of heavy metals. Roslan et al. (Citation2020) investigated experimentally the properties of electric arc furnace steel slag waste generated from steel industries as cement replacement (up to 20%). The properties like workability, initial surface absorption, and heavy metals leaching were studied along with the regular physical properties. The results confirmed that the surface and water absorption capacities were enhanced. The leaching tests revealed that the results were within the limits which favoured high potential for application as green concrete.
Nazer et al. (Citation2016) substituted copper slag up to 25% in Portland cement mortars and studied their alkali-activation properties by different analytical methods. The measured slag reactivity was confirmed as the copper slags indicated an interesting binding properties while used in the production of blended cement.
Impact on cement strength and hydration
To achieve strength, different grades of concrete (e.g., M-25, M-30, M40, M-50, M-100) have been developed per requirements. These grades mainly define compressive strength and meet all required physical and chemical parameters. Compressive strength tests are conducted at days one, three, seven and 28 to ascertain the development of the concrete’s targeted strength (ASTM, Citation2007 ; Kurdowski Citation2014). Developing a proper concrete formulation and mix design is a must. The water-to-cement ratio must be maintained to achieve the targeted parameters. After attaining the targeted concrete’s basic mechanical properties, compressive and bending strengths of the concrete are measured. Short- and long-term durability tests follow to ensure the concrete’s proper development. Durability tests for the concrete’s chemical resistance in different environments, including resistance against Na2SO4, MgCl2, NH4Cl, HCl, and CO2 in the environment are measured. The water absorption coefficient and apparent moisture diffusivity are measured to ascertain the hygienic and thermal properties of the concrete. These two tests assess the transport of water through the capillary pore to determine the development of pores in the concrete. To achieve good quality concrete, effective fracture toughness, effective toughness, and specific fracture energy are also measured. The role of cement in achieving the features in concrete mentioned above is very important. Positive results of the concrete entirely depend on the quality of cement used in the concrete mix design. However, the coarse aggregate used in the concrete also plays a crucial role. All the physical and chemical properties of the coarse and fine aggregates must be appropriately cross-examined to achieve the targeted quality of the concrete. The alkali-silica reaction (ASR) and alkali-carbonate reaction tests (ACR) are a must to ascertain the durability of the concrete (Kurdowski Citation2014).
Four significant components-alite, belite, calcium, aluminate and calcium alumina ferrite-are present in the anhydrous state of the cement (Herfort et al. Citation2010; Hewlett Citation2003). When water is added, exothermic reactions take place. With the help of conduction calorimetry, the rate of heat evolution can be monitored. Almost immediately on adding water, calcium aluminate reacts to form an aluminate-rich gel. This reaction is strongly exothermic, continues for a few minutes, and is followed by a period of a few hours of dormancy. The first part of the dormant period is when concrete can be poured and the paste is stiff enough to be workable. At the end of the dormant period, both the alite and belite in the cement start to react with each other.
These reactions mainly develop calcium silicate hydrate and calcium hydroxide, which is the main period of hydration during which the time concrete strength increases. The grains react then individually from the surface inwards. Hydration of calcium aluminate stays as fresh crystals become accessible to water. The reaction of Ferrite also begins rapidly as water is added. Then the reaction slows down probably due to the formation of a layer of iron hydroxide, coating the ferrite and standing as a barrier to additional reaction (Marchon and Flatt Citation2016; Scrivener, Juilland, and Monteiro Citation2015). The heat evolution period during concreting after mixing lasts usually between 10 and 20 hours and then gradually starts to fail. In a mix containing only Portland cement, most of the strength gain happens within almost a month. When Portland cement has been partially substituted by other different materials (e.g., fly ash, GGBS slag, composite cement), the strength gain may happen more slowly and persist in for a longer period.
Calcium silicate hydrate, portlandite hydroxide, calcium hydroxide, tricalcium hydrate, and mono sulphate are the four main results of hydration. In some instances, monocarbonate grains also develop. Calcium silicate (C-S-H gel) is the core reaction product which is the main source of the strength of the concrete. No strict SiO2-to-CaO ratio is inferred. The SiO2-to-CaO ratio is rather variable but typically is around 0.45:0.50 in the hydrated Portland cement and up to 0.45:0.6 if slag, fly ash, or micro silica is present, depending on the proportion. Portlandite is often abbreviated as CH and forms mainly by hydration of alite which has a Ca:Si ratio 3:1. C-S-H, on the other hand, has a Ca-to-Si ratio of nearly 2:1; hence, excess lime is available to produce CH. Another common phase developed in the hydrated cement is mono sulphate. Mono sulphate is an ettringite because it contains three molecules of anhydrite.
Similarly, the mono-sulphate phase contains one molecule of anhydrite. Ettringite is existing as rod-like crystals in the first stage of the reaction or from time to time as a huge growth. In mature concrete or mortar, ettringite crystals develop in the available pore spaces. Nomo sulphate have a tendency to arise in the advanced stages of hydration, a day or two days after mixing. Both ettringite and mono sulphate are compounds of C3A, CaSO4, and water in various proportion.
Both of ettringite and mono sulphate have a large amount of water, particularly ettringite in the context of cement. However, the anions of both these hydration products can be changed by differently charged anions. The sulphate of ettringite can be replaced by carbonate or partly substituted by two hydroxyl ions, although practically none of these is often detected.
In concrete that is made from cement containing only cement and gypsum, ettringite forms at early stage after the cement and water are mixed together, but it is slowly substituted by mono sulphate. This phenomenon occurs because the available alumina-to-sulphate ratio is easily available to dissolve, but the calcium aluminate is contained inside cement grains without an initial access to water. Continued hydration progressively liberates alumina, and the proportion of ettringite declines as that of mono sulphate rises. If there is a slightly higher amount of sulphate, the cement paste will have a mixture of mono sulphate and ettringite. With the increasing availability of sulphate, there will be extra ettringite and a lesser amount of mono sulphate, and at even higher levels of sulphate, there will be ettringite and gypsum. If the limestone is present, then carbonation becomes available owing to the reaction of some of the limestone. The carbonate replaces sulphate or hydroxyl in ettringite. Therefore, it decreases as the proportion of mono carbonate increases. The displaced sulphate usually merges with the remaining mono sulphate to form ettringite. The key here is the balance between alumina on the one hand and carbonate and sulphate on the other hand.
The impact of using fly ash and granulated blast furnace slag on compressive strength has been widely investigated. Cement containing large amounts of such industrial waste displays improved compressive strength for an extended period, and mortar and concrete’s physical properties are also improved. The initial compressive strength (within 2 and 7 days’ hydration) of the Ordinary Portland Cement (OPC) without any addition showed the highest strength. However, as hydration progressed, as a function of time, the cement with ground granulated blast furnace slag (GGBFS) improved, but the hydration was still lower than the OPC without any mixing. According to Giergiczny (Citation2019), the mixture of OPC, fly ash, and HCFA showed higher compressive strength than the OPC without any additions ().
Figure 5. Compressive strength of mortars containing cement with fly ash (FA), high-calcium fly ash (HFCA) and granulated blast furnace slag (GBFS) (Giergiczny Citation2019)
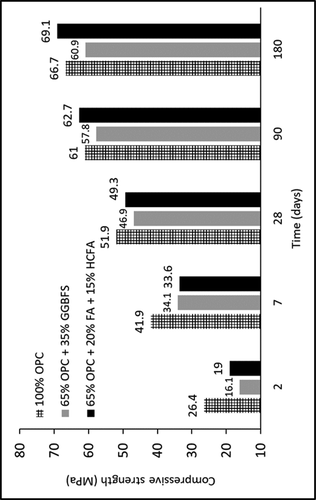
Fly ash is very fine dust with spherical grains that contain pozzolanic properties. As soon as fly ash comes in contact with water, it reacts with calcium hydroxide (Ca(OH)2) to produce calcium silicate hydrates (C-S-H) and calcium aluminate (C-A) products. The calcium hydroxide is released as a result of the cement’s hydration. Calcium hydroxide alone plays no role in cement strength; therefore, the reaction between pozzolanic materials and Ca(OH)2 and the resultant conversion to a calcium silicate product will contribute to the cement’s durability.
Liu, Qin, and Yu (Citation2020) studied the particle size of slag particles that present in the slag cement. The blended cement, containing 50% cement clinker and 50% slag powder, was tested for its compressive strength. The study concluded that the determined strength of slag cement does not only depend on glass content, but also on the particle size distribution of slag present in the cement.
Mehta and Ashish (Citation2019) investigated the effect of silica fume and crushed waste glass as a cement substitute, due to its high content of silicon, in concrete. The addition of silica fume up to 20% was found to increase the compressive, tensile and flexural strengths of concrete due to the increase in the hydration reaction rate. The presence of silica fume in a percentage above 20% was found to improve the durability properties of concrete but reduce the strength due to the decrease of the calcium hydroxide content in the cement concrete. The waste glass addition shows the cement vulnerable to alkali-silica reaction but it was reduced by the addition of silica fume with this cement mix. The lower specific area of glass powder present in cement increased the pump flow even in the lower water-cement ration.
Younes, Abdel-Rahman, and Khattab (Citation2018) prepared ternary blended cement using rice husk ash and waste glass powder with Portland cement with different ratios to study its physical properties. The influence of silica present in them as amorphous showed improvements in compressive strengths of all mortars due to the increase in hydration time. The pozzolanic nature of rice husk ash and glass powder enhanced the mechanical properties of cement mortars.
Impact on CO2 emissions
The production of cement is not only energy intensive but also represents one of the world’s most crucial CO2 emission sources. Based on the technology of the production, CO2 emissions range from 0.73–0.99 tons of CO2 per ton of cement (Ali, Saidur, and Hossain Citation2011; Uwasu, Hara, and Yabar Citation2014). More than half of this amount is produced from calcination, which is an essential part of cement production. Therefore, countless efforts have been made to use supplementary cementitious materials, which can replace at least part of the cement in concrete with more environmentally friendly materials. Even industrial waste with its residual energy and low chloride and heavy metal contents can be appropriated to provide part of the energy required to make Portland cement. Additionally, alternative fuels that do not increase CO2 emission levels can be used. Using alternatives should not increase production costs; instead, they must bring profits. Waste carbons from different industrial processes, or used oil, solvents, tar mud, etc. can be considered as alternative fuels for Portland cement production.
The total carbon emissions estimated from cement production in 1994 were 307 million metric tons of carbon out of which 160 million metric tons were from process carbon emissions and 147 million metric tons were from energy use. Overall, the top 10 cement-producing countries accounted for 63% of global carbon emissions from cement production in 1994. The average intensity of CO2 emissions from total global cement production is 22 kg of cement. Emission mitigation options include improving energy efficiency, introducing new processes, shifting to low carbon fuels, using waste fuels, and increasing the use of additives in cement making. Eventually using alternative cement and removing CO2 from flue gases in kilns might be approaches employed to mitigate carbon emissions.
Improving energy efficiency reduces CO2 emissions from fuel and electricity uses and may reduce the cost of cement production. Improvements may be attained by using more energy efficient equipment, replacing old installations with new ones or shifting to entirely new types of cement production. The most significant gain in reducing energy input may come from improved fuel efficiency. The main opportunities for fuel efficiency in the kiln are the conversion to more energy efficient variants, optimising the clinker cooler, improving preheating efficiency and burners as well as instituting process controls and management systems. Introducing high-efficiency classifiers or motor systems, and process control systems are required. It has been estimated that future technologies will bring energy savings to almost 48%, leading to CO2 emission reductions of 27% (Ali, Saidur, and Hossain Citation2011).
Research direction
The cement considered as the second-highest consumer product next to the water and the consumption reaching around one ton per annum for each person. Considering the level of the carbon footprint of cement concrete from the environmental impact perspective and the life-cycle aspect cement replacements considered vital. The prevailing usage of successful supplementary materials like fly ash, ground-granulated blast furnace slag, and other potential waste by-products play an important role in bringing down the impact. The future trend in concrete focus on self-compacting, geopolymer, demolition and recycling, and supplementary cementitious materials. Further, focusing on the production process such as belite cements, clinkerization factor also of greater concern for the reduction of the carbon footprint.
The supplementary cementing materials (SCM) role in partial replacement of cement due to their pozzolanic and/or cementitious properties beyond their ability in reducing environmental burdens also effectively improve physio-mechanical, mobilisation, and durability properties. Panesar (Citation2019) studied four major SCMs effects on cement hydration, fresh, mechanical, transportation, and durability. The SCMs ability on resisting chloride ingress, alkali-silica reaction, and sulphate attacks were briefly studied by the author in different concretes. Giergiczny (Citation2019) focussed in his study on improving the binder properties like long setting time, low early strengths, etc. due to the addition of SCMs. Addition of high activated additives like silica fumes, metakaolin, etc. minimises the negative futures of high addition of SCMs in the cement concrete which helps in shortening setting times and influencing high early strengths and increasing durability.
Although the utilisation of SCMs supporting the cement replacement, in some regions like the Sultanate of Oman, the availability is limited, or uncertain. For example, the main fuel utilised for power generation in the Gulf region, natural gas, phasing out coal-based power units which limit the fly ash availability. The availability of another major SCM, slag, highly depends on the economy and therefore uncertain. In such regions degraded concrete infrastructures generate as a result of ageing and infrastructure development can be used as SCMs. Another conventional substitute to some degree is Belite cements. Schneider (Citation2019) in his study briefs that belite clinkers around 4090% belite. It can be produced in a conventional cement kiln with low process CO2 emission by lowering the lime saturation factor. The calcium sulphoaluminate cements produced using bauxite as one of the major raw materials which contain ye’elimite, belite, and gypsum show around 20 to 30% less CO2 emissions during the production process. The measures to be taken in optimising the energy demand for cement manufacturing are also of major concern with regard to clinkerization process and finer grinding processes. The current electrical energy requirement of 104 to 110 kWh/t could be bringing down to 90 to 95 kWh/t with efficient equipment.
The carbon capture technology, a breakthrough innovation but still requires industrial level study, shows promising influence in supporting to achieve commitment under the Paris Agreement which calculates the reduction of annual CO2 emission by the energy sector by 60% from current levels in 2050. By capturing post-combusted carbon dioxide by suitable absorbers like certain amines, special capturing membranes, or by calcium looping using calcium oxide and utilisation as carbon feedstock or for fuel production seen as the best option for a significant reduction in CO2. Schneider (Citation2019) presented in his paper about the usage of such technology in the cement manufacturing more feasible due to the availability of sufficient heat which requires capturing the CO2. Further, the separation of nitrogen from the combustion air which enters the cement kiln using oxyfuel technology results in the lesser thermal requirement, and a high concentration of CO2 and water vapour enable effective carbon capturing. Such process implementation requires industrial level trails to assess the complexities and further adjustments required in an existing cement manufacturing plant.
Conclusion
Because most of the world’s raw materials are quickly diminishing, the most significant challenge that cement technologists will face in the coming years is an investigation of cement’s raw materials and their optimum use. A second challenge is to address environmental issues related to raw material production. Cement plants worldwide must put more effort into improving the different types of machinery used in various production units. Breakthroughs can be achieved by continuous monitoring of existing units of the plant. Meticulous use of suitable fuels is necessary to limit energy conservation. Optimal use of diverse kinds of fuel used during production must be defined to avoid any underuse of fuels.
Quality control in clinker production must be dealt with strictly to avoid problems faced by construction industries. Developing new types of cement has become the need of the hour.
Waste generation and utilisation must be managed effectively. More research-based efforts should be undertaken to achieve targeted results. More scientific work is required for using the refuse generated by various industries as waste that could be used in clinker production or to develop newer types of cement go untouched.
Using waste generated by various production units in the concrete industry is another area warranting examination. Ample scope remains for investigations of the proper utilisation of waste. One further step has to be taken up by the construction industries on the technology transfer and the adaptation of the research work done by the scientists and engineers. More stress should be placed on testing to help in the development of quality concrete. Proper construction fields should be identified that might be able to use concrete formulated with waste. Undertaking such ventures will help maintain the ecological balance as well as societal development.
In conclusion, cement is a critical part of concrete. As stated earlier, to initiate the hydration process, Portland cement is mixed with fine and coarse aggregates and water, and the hydration process becomes simple or complex depending on the concrete mix design. Dry powder cement is anhydrous in nature when produced by grinding of clinker and gypsum. During the process of concrete mixing, water is added to develop concrete slurry. With the advancement of the reactions, the products of the hydration process gradually bond together the individual components of the concrete mix. The mix of concrete components forms a solid mass.
Acknowledgments
The research leading to these outcomes has received Research Project Funding from The Research Council of the Sultanate of Oman (TRC), Research Agreement No. ORG/SQU/EI/15/009.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors

Sabah Ahmed Abdul-Wahab
Sabah Ahmed Abdul-Wahab received her doctorate from Bath University, UK. She is currently a Professor in the Department of Mechanical and Industrial Engineering, Sultan Qaboos University in the Sultanate of Oman. She is a Chemical Engineer experienced in energy and environmental engineering, with an emphasis on environmental protection. She has published more than 200 referred international journal articles and around 10 chapters in books. The publications covered chemical and environmental engineering topics as well as various academic and educational issues.

Hilal Al-Dhamri
Hilal AL-Dhamri received his MSc (2010) in Science Chemistry from SQU, Oman, and PhD (2019) in Civil Engineering from the University of Leeds, United Kingdom. His PhD thesis research has been chosen to be among the best six scientific researches in the field of Cement and Concrete in the UK for the year 2019. Hilal is a professional in the field cement manufacturing technology and industrial operation management with a 20 years of experience. He is currently the General Manager Manufacturing at Oman Cement Company, Oman. He has contributed towards achieving several research projects in the cement industry.

Ganesh Ram
Ganesh Ram, Research and Development Chemist, with Oman Cement Company, has MSc (2000) in Science from Bharathidasan University, India. He has experience in cement manufacturing and exploring environment-friendly alternatives and practices in the process. Ram is an expert in chemical analysis such as XRF, XRD, Optical microscopy, Atomic Absorption Spectroscopy and TGA/DSC Analysis.

Vishnu P. Chatterjee
Vishnu Chatterjee has received his PhD (1995) from Allahabad University, Earth and Planetary Science, India. Dr. Chatterjee had worked as Joint Director and Unit Head, at National Council for Cement and Building Materials, India. The main research interests are focused on raw materials for cement manufacturing, petrographic studies of various rocks, concrete, clinker, fly ash, slag, powder samples of cement, fire-damaged building rocks, Blasting techniques and preventive measures, igneous petrology, experimental petrology of natural and synthetic systems, mineralogy, and refractory materials.
References
- Abbass, R. A., P. Kumar, and A. El-Gendy. 2018. “An Overview of Monitoring and Reduction Strategies for Health and Climate Change Related Emissions in the Middle East and North Africa Region.” Atmospheric Environment 175: 33–43. doi:https://doi.org/10.1016/j.atmosenv.2017.11.061.
- Abdul-Wahab, S. A., G. A. Al-Rawas, S. Ali, and H. Al-Dhamri. 2016. “Impact of the Addition of Oil-Based Mud on Carbon Dioxide Emissions in a Cement Plant.” Journal of Cleaner Production 112: 4214–4225. doi:https://doi.org/10.1016/j.jclepro.2015.06.062.
- Aïtcin, P. C., and R. J. Flatt. 2015. “Science and Technology of Concrete Admixtures.” Science and Technology of Concrete Admixtures 1–613. doi:https://doi.org/10.1016/C2015-0-00150-2.
- Akin Altun, I., and Y. Sert. 2004. “Utilization of Weathered Phosphogypsum as Set Retarder in Portland Cement.” Cement and Concrete Research 34 (4): 677–680. doi:https://doi.org/10.1016/j.cemconres.2003.10.017.
- Al Dhamri, H. S., S. A. Abdul-Wahab, C. Velis, and L. Black. 2020. “Oil-Based Mud Cutting as an Additional Raw Material in Clinker Production.” Journal of Hazardous Materials 384. doi:https://doi.org/10.1016/j.jhazmat.2019.121022.
- Ali, M. B., R. Saidur, and M. S. Hossain. 2011. “A Review on Emission Analysis in Cement Industries.” Renewable and Sustainable Energy Reviews 15 (5): 2252–2261. doi:https://doi.org/10.1016/j.rser.2011.02.014.
- Allahverdi, A., and S. Ahmadnezhad. 2014. “Mechanical Activation of Silicomanganese Slag and Its Influence on the Properties of Portland Slag Cement.” Powder Technology 251: 41–51. doi:https://doi.org/10.1016/j.powtec.2013.10.023.
- Aranda Usón, A., A. M. López-Sabirón, G. Ferreira, and E. Llera Sastresa. 2013. “Uses of Alternative Fuels and Raw Materials in the Cement Industry as Sustainable Waste Management Options.” Renewable and Sustainable Energy Reviews 23: 242–260. doi:https://doi.org/10.1016/j.rser.2013.02.024.
- ASTM, 2007. C109/C109M-07. “Standard Test Method for Compressive Strength of Hydraulic Cement Mortars.” Chem. Anal. 04, 1–9. 10.1520/C0109_C0109M–05
- ASTM, 2013. C150. “Standard Test Methods for Chemical Analysis of Hydraulic Cement.” Am. Soc Test. Mater. Proc. 10.1520/C0114–13
- ASTM, 2016. C595/C595M-16. ASTM C595-03. “Standard Specification for Blended Hydraulic Cements.” Annu. B. ASTM Stand. https://doi.org/10.1520/C0595.
- ASTM, 1999. C807, “Standard Test Method for Time of Setting of Hydraulic Cement Mortar by Modified.” Current 96, 1–4. https://doi.org/10.1520/C0807-08.2.
- ASTM Committee, 2016. C01.10, ASTM C150/C150M-16. “Standard Specification for Portland Cement.” Annual Book of ASTM Standards 04.1. https://doi.org/10.1520/C0150.
- Baeza-Brotons, F., P. Garcés, J. Payá, and J. M. Saval. 2014. “Portland Cement Systems with Addition of Sewage Sludge Ash. Application in Concretes for the Manufacture of Blocks.” Journal of Cleaner Production 82: 112–124. doi:https://doi.org/10.1016/j.jclepro.2014.06.072.
- Barcelo, L., J. Kline, G. Walenta, and E. Gartner. 2014. “Cement and Carbon Emissions.” Materials and Structures/Materiaux Et Constructions 47 (6): 1055–1065. doi:https://doi.org/10.1617/s11527-013-0114-5.
- Benhelal, E., G. Zahedi, E. Shamsaei, and A. Bahadori. 2013. “Global Strategies and Potentials to Curb CO2 Emissions in Cement Industry.” Journal of Cleaner Production 51: 142–161. doi:https://doi.org/10.1016/j.jclepro.2012.10.049.
- Bijen, J. 1996. “Benefits of Slag and Fly Ash.” Construction and Building Materials 10 (5 SPEC. ISS.): 309–314. doi:https://doi.org/10.1016/0950-0618(95)00014-3.
- Chatterjee, A., and T. Sui. 2019. “Alternative Fuels – Effects on Clinker Process and Properties.” Cement and Concrete Research 123. doi:https://doi.org/10.1016/j.cemconres.2019.105777.
- Chen, H., X. Ma, and H. Dai. 2010. “Reuse of Water Purification Sludge as Raw Material in Cement Production.” Cement and Concrete Composites 32 (6): 436–439. doi:https://doi.org/10.1016/j.cemconcomp.2010.02.009.
- Chen, Z., and C. S. Poon. 2017. “Comparative Studies on the Effects of Sewage Sludge Ash and Fly Ash on Cement Hydration and Properties of Cement Mortars.” Construction and Building Materials 154: 791–803. doi:https://doi.org/10.1016/j.conbuildmat.2017.08.003.
- Collins, F., and J. G. Sanjayan. 2000. “Effect of Pore Size Distribution on Drying Shrinkage of Alkali-Activated Slag Concrete.” Cement and Concrete Research 30 (9): 1401–1406. doi:https://doi.org/10.1016/S0008-8846(00)00327-6.
- Cordeiro, G. C., and C. P. Sales. 2015. “Pozzolanic Activity of Elephant Grass Ash and Its Influence on the Mechanical Properties of Concrete.” Cement and Concrete Composites 55: 331–336. doi:https://doi.org/10.1016/j.cemconcomp.2014.09.019.
- Czarnecki, L., and H. Justnes. 2012. “Sutainable & Durable Concrete.” Cement, Wapno, Beton 6: 341–362.
- Dabhade, A. N., S. R. Chaudari, and A. R. Gajbhaye. 2014. “Effect of Flyash on Recycle Coarse Aggregate Concrete.” International Journal of Civil Engineering Research 5 (35–42). http://www.ripublication.com/ijcer.htm
- Deolalkar, S. P. 2016. “Designing Green Cement Plants, Designing Green Cement Plants.” https://doi.org/10.1016/B978-0-12-803420-0.00023-8
- Donatello, S., and C. R. Cheeseman. 2013. “Recycling and Recovery Routes for Incinerated Sewage Sludge Ash (ISSA): A Review.” Waste Management 33 (11): 2328–2340. doi:https://doi.org/10.1016/j.wasman.2013.05.024.
- Donoghue, A. M. 2004. “Occupational Health Hazards in Mining: An Overview.” Occupational Medicine 54 (5): 283–289. doi:https://doi.org/10.1093/occmed/kqh072.
- EN 197-1. 2011. “Cement - Part 1: Composition, Specifications and Conformity Criteria for Common Cements.” European Committee for Standardization. NSAI Standards. https://infostore.saiglobal.com/preview/is/en/2011/i.s.en197-1-2011.pdf?sku=1492629
- Farzanegan, M. R., and G. Markwardt. 2018. “Development and Pollution in the Middle East and North Africa: Democracy Matters.” Journal of Policy Modeling 40 (2): 350–374. doi:https://doi.org/10.1016/j.jpolmod.2018.01.010.
- Fthenakis, V. M. 2003. “Overview of Potential Hazards.” Practical Handbook of Photovoltaics: Fundamentals and Applications 1–984. doi:https://doi.org/10.1016/B978-1-85617-390-2.X5000-4.
- Fytili, D., and A. Zabaniotou. 2008. “Utilization of Sewage Sludge in EU Application of Old and New Methods-A Review.” Renewable and Sustainable Energy Reviews 12 (1): 116–140. doi:https://doi.org/10.1016/j.rser.2006.05.014.
- Gartner, E. 2004. “Industrially Interesting Approaches to “Low-co2” Cements.” Cement and Concrete Research 34 (9): 1489–1498. doi:https://doi.org/10.1016/j.cemconres.2004.01.021.
- Gesoǧlu, M., E. Güneyisi, M. E. Kocabaǧ, V. Bayram, and K. Mermerdaş. 2012. “Fresh and Hardened Characteristics of Self Compacting Concretes Made with Combined Use of Marble Powder, Limestone Filler, and Fly Ash.” Construction and Building Materials 37: 160–170. doi:https://doi.org/10.1016/j.conbuildmat.2012.07.092.
- Gidarakos, E., and M. Aivalioti. 2012. “Industrial and Hazardous Waste Management.” Journal of Hazardous Materials 207-208: 1–2. doi:https://doi.org/10.1016/j.jhazmat.2011.10.083.
- Giergiczny, Z. 2019. “Fly Ash and Slag.” Cement and Concrete Research 124. doi:https://doi.org/10.1016/j.cemconres.2019.105826.
- Gineys, N., G. Aouad, and D. Damidot. 2010. “Managing Trace Elements in Portland Cement - Part I: Interactions between Cement Paste and Heavy Metals Added during Mixing as Soluble Salts.” Cement and Concrete Composites 32 (8): 563–570. doi:https://doi.org/10.1016/j.cemconcomp.2010.06.002.
- Hawkins, P., P. Tennis, and R. Detwiler. 2003. “The Use of Limestone in Portland Cement: A State-of-the-Art Review.”, Portland Cement Association. ISBN: 0-89312-229-7.Skokie, Ill. : Portland Cement Association, ©2003. https://www.worldcat.org/title/use-of-limestone-in-portland-cement-a-state-of-the-art-review/oclc/52539405
- Hendriks, C. A., E. Worrell, D. de Jager, K. Blok, and P. Riemer. 1998. “Emission Reduction of Greenhouse Gases from the Cement Industry” in Fourth International Conference on Greenhouse Gas Control Technologies, Interlaken, Switzerland. https://doi.org/10.1101/lm.722908
- Herfort, D., G. K. Moir, V. Johansen, F. Sorrentino, and H. Bolio Arceo. 2010. “The Chemistry of Portland Cement Clinker.” Advances in Cement Research 22 (4): 187–194. doi:https://doi.org/10.1680/adcr.2010.22.4.187.
- Hewlett, P. C. 2003. “Lea’s Chemistry of Cement and Concrete.” Lea’s Chemistry of Cement and Concrete 1–1057). doi:https://doi.org/10.1016/B978-0-7506-6256-7.X5007-3.
- Hsu, S., M. Chi, and R. Huang. 2018. “Effect of Fineness and Replacement Ratio of Ground Fly Ash on Properties of Blended Cement Mortar.” Construction and Building Materials 176: 250–258. doi:https://doi.org/10.1016/j.conbuildmat.2018.05.060.
- Huntzinger, D. N., and T. D. Eatmon. 2009. “A Life-Cycle Assessment of Portland Cement Manufacturing: Comparing the Traditional Process with Alternative Technologies.” Journal of Cleaner Production 17 (7): 668–675. doi:https://doi.org/10.1016/j.jclepro.2008.04.007.
- Husillos Rodríguez, N., S. Martínez-Ramírez, M. T. Blanco-Varela, M. Guillem, J. Puig, E. Larrotcha, and J. Flores. 2011. “Evaluation of Spray-Dried Sludge from Drinking Water Treatment Plants as a Prime Material for Clinker Manufacture.” Cement and Concrete Composites 33 (2): 267–275. doi:https://doi.org/10.1016/j.cemconcomp.2010.10.020.
- Ishak, S. A., and H. Hashim. 2015. “Low Carbon Measures for Cement Plant - A Review.” Journal of Cleaner Production 103: 260–274. doi:https://doi.org/10.1016/j.jclepro.2014.11.003.
- Ishak, S. A., H. Hashim, and T. S. Ting. 2016. “Eco Innovation Strategies for Promoting Cleaner Cement Manufacturing.” Journal of Cleaner Production 136: 133–149. doi:https://doi.org/10.1016/j.jclepro.2016.06.022.
- Kääntee, U., R. Zevenhoven, R. Backman, and M. Hupa. 2004. “Cement Manufacturing Using Alternative Fuels and the Advantages of Process Modelling.” Fuel Processing Technology 85 (4): 293–301. doi:https://doi.org/10.1016/S0378-3820(03)00203-0.
- Kajaste, R., and M. Hurme. 2016. “Cement Industry Greenhouse Gas Emissions - Management Options and Abatement Cost.” Journal of Cleaner Production 112: 4041–4052. doi:https://doi.org/10.1016/j.jclepro.2015.07.055.
- Kenai, S., W. Soboyejo, and A. Soboyejo. 2004. “Some Engineering Properties of Limestone Concrete.” Materials and Manufacturing Processes 19 (5): 949–961. doi:https://doi.org/10.1081/AMP-200030668.
- Khankhaje, E., M. W. Hussin, J. Mirza, M. Rafieizonooz, M. R. Salim, H. C. Siong, and M. N. M. Warid. 2016. “On Blended Cement and Geopolymer Concretes Containing Palm Oil Fuel Ash.” Materials & Design 89: 385–398. doi:https://doi.org/10.1016/j.matdes.2015.09.140.
- Khurana, S., R. Banerjee, and U. Gaitonde. 2002. “Energy Balance and Cogeneration for a Cement Plant.” Applied Thermal Engineering 22 (5): 485–494. doi:https://doi.org/10.1016/S1359-4311(01)00128-4.
- Kiddee, P., R. Naidu, and M. H. Wong. 2013. “Electronic Waste Management Approaches: An Overview.” Waste Management 33 (5): 1237–1250. doi:https://doi.org/10.1016/j.wasman.2013.01.006.
- Kurdowski, W. 2014. “Cement and Concrete Chemistry.” Cement and Concrete Chemistry 9789400779457: 1–700. doi:https://doi.org/10.1007/978-94-007-7945-7.
- Lee, N. K., and H. K. Lee. 2013. “Setting and Mechanical Properties of Alkali-Activated Fly Ash/slag Concrete Manufactured at Room Temperature.” Construction and Building Materials 47: 1201–1209. doi:https://doi.org/10.1016/j.conbuildmat.2013.05.107.
- Li, H., W. Xu, X. Yang, and J. Wu. 2014. “Preparation of Portland Cement with Sugar Filter Mud as Lime-based Raw Material.” Journal of Cleaner Production 66: 107–112. doi:https://doi.org/10.1016/j.jclepro.2013.11.003.
- Lin, K.-L., and C.-Y. Lin. 2005. “Hydration Characteristics of Waste Sludge Ash Utilized as Raw Cement Material.” Cement and Concrete Research 35 (10): 1999–2007. doi:https://doi.org/10.1016/j.cemconres.2005.06.008.
- Liu, J., Q. Qin, and Q. Yu. 2020. “The Effect of Size Distribution of Slag Particles Obtained in Dry Granulation on Blast Furnace Slag Cement Strength.” Powder Technology 362: 32–36. doi:https://doi.org/10.1016/j.powtec.2019.11.115.
- Liu, X., and N. Zhang. 2011. “Utilization of Red Mud in Cement Production: A Review.” Waste Management and Research 29 (10): 1053–1063. doi:https://doi.org/10.1177/0734242X11407653.
- Ludwig, H.-M., and W. Zhang. 2015. “Research Review of Cement Clinker Chemistry.” Cement and Concrete Research 78: 24–37. doi:https://doi.org/10.1016/j.cemconres.2015.05.018.
- Madlool, N. A., R. Saidur, M. S. Hossain, and N. A. Rahim. 2011. “A Critical Review on Energy Use and Savings in the Cement Industries.” Renewable and Sustainable Energy Reviews 15 (4): 2042–2060. doi:https://doi.org/10.1016/j.rser.2011.01.005.
- Marchon, D., and R. J. Flatt. 2016. “Mechanisms of Cement Hydration.” Science and Technology of Concrete Admixtures 129–145. doi:https://doi.org/10.1016/B978-0-08-100693-1.00008-4.
- Medina, C., W. Zhu, T. Howind, M. I. Sánchez De Rojas, and M. Frías. 2014. “Influence of Mixed Recycled Aggregate on the Physical-Mechanical Properties of Recycled Concrete.” Journal of Cleaner Production 68: 216–225. doi:https://doi.org/10.1016/j.jclepro.2014.01.002.
- Mehta, A., and D. K. Ashish. 2019. “Silica Fume and Waste Glass in Cement Concrete Production: A Review.” Journal of Building Engineering. doi:https://doi.org/10.1016/j.jobe.2019.100888.
- Mikulčić, H., H. Cabezas, M. Vujanović, and N. Duić. 2016. “Environmental Assessment of Different Cement Manufacturing Processes Based on Emergy and Ecological Footprint Analysis.” Journal of Cleaner Production 130: 213–221. doi:https://doi.org/10.1016/j.jclepro.2016.01.087.
- Nagaratnam, B. H., M. E. Rahman, A. K. Mirasa, M. A. Mannan, and S. O. Lame. 2016. “Workability and Heat of Hydration of Self-Compacting Concrete Incorporating Agro-Industrial Waste.” Journal of Cleaner Production 112: 882–894. doi:https://doi.org/10.1016/j.jclepro.2015.05.112.
- Nazer, A., J. Payá, M. V. Borrachero, and J. Monzó. 2016. “Use of Ancient Copper Slags in Portland Cement and Alkali Activated Cement Matrices.” Journal of Environmental Management 167: 115–123. doi:https://doi.org/10.1016/j.jenvman.2015.11.024.
- Nemerow, N. L. 2007. “Industrial Waste Treatment.” Industrial Waste Treatment. doi:https://doi.org/10.1016/B978-0-12-372493-9.X5031-5.
- Neupane, K. 2016. “Fly Ash and GGBFS Based Powder-Activated Geopolymer Binders: A Viable Sustainable Alternative of Portland Cement in Concrete Industry”. Mechanics of Materials 103: 110–122. doi:https://doi.org/10.1016/j.mechmat.2016.09.012.
- Nnorom, I. C., and O. Osibanjo. 2008. “Overview of Electronic Waste (E-waste) Management Practices and Legislations, and Their Poor Applications in the Developing Countries.” Resources, Conservation and Recycling 52 (6): 843–858. doi:https://doi.org/10.1016/j.resconrec.2008.01.004.
- Pacewska, B., and I. Wilińska. 2013. “Hydration of Cement Composites Containing Large Amount of Waste Materials.” Procedia Engineering 57: 53–62. doi:https://doi.org/10.1016/j.proeng.2013.04.009.
- Panesar, D. K. 2019. “Supplementary Cementing Materials.” Developments in the Formulation and Reinforcement of Concrete. doi:https://doi.org/10.1016/B978-0-08-102616-8.00003-4.
- Park, K.-B., S.-J. Kwon, and X.-Y. Wang. 2016. “Analysis of the Effects of Rice Husk Ash on the Hydration of Cementitious Materials.” Construction and Building Materials 105: 196–205. doi:https://doi.org/10.1016/j.conbuildmat.2015.12.086.
- Peray, K. E., and J. J. Waddell. 1986. “The Rotary Cement Kiln.” https://doi.org/10.1007/s13398-014-0173-7.2
- Puertas, F., I. García-Díaz, A. Barba, M. F. Gazulla, M. Palacios, M. P. Gómez, and S. Martínez-Ramírez. 2008. “Ceramic Wastes as Alternative Raw Materials for Portland Cement Clinker Production.” Cement and Concrete Composites 30 (9): 798–805. doi:https://doi.org/10.1016/j.cemconcomp.2008.06.003.
- Rai, P. K. 2016. “Impacts of Particulate Matter Pollution on Plants: Implications for Environmental Biomonitoring.” Ecotoxicology and Environmental Safety 129: 120–136. doi:https://doi.org/10.1016/j.ecoenv.2016.03.012.
- Rashad, A. M. 2013. “Metakaolin as Cementitious Material: History, Scours, Production and Composition-A Comprehensive Overview.” Construction and Building Materials 41: 303–318. doi:https://doi.org/10.1016/j.conbuildmat.2012.12.001.
- Roslan, N. H., M. Ismail, N. H. A. Khalid, and B. Muhammad. 2020. “Properties of Concrete Containing Electric Arc Furnace Steel Slag and Steel Sludge.” Journal of Building Engineering 28. doi:https://doi.org/10.1016/j.jobe.2019.101060.
- Saraya, M. E. S. I. 2014. “Study Physico-Chemical Properties of Blended Cements Containing Fixed Amount of Silica Fume, Blast Furnace Slag, Basalt and Limestone, a Comparative Study.” Construction and Building Materials 72: 104–112. doi:https://doi.org/10.1016/j.conbuildmat.2014.08.071.
- Sathawane, S. H., V. S. Vairagade, and K. S. Kene. 2013. “Combine Effect of Rice Husk Ash and Fly Ash on Concrete by 30% Cement Replacement.” Procedia Engineering 51: 35–44. doi:https://doi.org/10.1016/j.proeng.2013.01.009.
- Schneider, M. 2019. “The Cement Industry on the Way to a Low-carbon Future.” Cement and Concrete Research 124: 105792. doi:https://doi.org/10.1016/j.cemconres.2019.105792.
- Schneider, M., M. Romer, M. Tschudin, and H. Bolio. 2011. “Sustainable Cement Production-Present and Future.” Cement and Concrete Research 41 (7): 642–650. doi:https://doi.org/10.1016/j.cemconres.2011.03.019.
- Scott, P. W., J. M. Eyre, D. J. Harrison, and A. J. Bloodworth. 2005. “Markets for Industrial Mineral Products from Mining Waste.” Geological Society Special Publication 250. doi:https://doi.org/10.1144/GSL.SP.2005.250.01.06.
- Scrivener, K. L., P. Juilland, and P. J. M. Monteiro. 2015. “Advances in Understanding Hydration of Portland Cement.” Cement and Concrete Research 78: 38–56. doi:https://doi.org/10.1016/j.cemconres.2015.05.025.
- Siddique, R., and J. Klaus. 2009. “Influence of Metakaolin on the Properties of Mortar and Concrete: A Review.” Applied Clay Science 43 (3–4): 392–400. doi:https://doi.org/10.1016/j.clay.2008.11.007.
- Singh, G., S. Kumar, M. K. Singh, and S. K. Mohapatra. 2016. “Environmental Impact Assessment of Ash Disposal System of a Thermal Power Plant.” International Journal of Hydrogen Energy 41 (35): 15887–15891. doi:https://doi.org/10.1016/j.ijhydene.2016.03.171.
- Smarzewski, P., and D. Barnat-Hunek. 2016. “Mechanical and Durability Related Properties of High Performance Concrete Made with Coal Cinder and Waste Foundry Sand.” Construction and Building Materials 121: 9–17. doi:https://doi.org/10.1016/j.conbuildmat.2016.05.148.
- Snellings, R., A. Salze, and K. L. Scrivener. 2014. “Use of X-Ray Diffraction to Quantify Amorphous Supplementary Cementitious Materials in Anhydrous and Hydrated Blended Cements.” Cement and Concrete Research 64: 89–98. doi:https://doi.org/10.1016/j.cemconres.2014.06.011.
- Soltanzadeh, F., M. Emam-Jomeh, A. Edalat-Behbahani, and Z. Soltan-Zadeh. 2018. “Development and Characterization of Blended Cements Containing Seashell Powder.” Construction and Building Materials 161: 292–304. doi:https://doi.org/10.1016/j.conbuildmat.2017.11.111.
- Stefanović, G. M., G. D. Vučković, M. M. Stojiljković, and M. B. Trifunović. 2010. “CO2 Reduction Options in Cement Industry - the Novi Popovac Case.” Thermal Science 14 (3): 671–679. doi:https://doi.org/10.2298/TSCI091211014S.
- Taruya, T., N. Okuno, and K. Kanaya. 2002. “Reuse of Sewage Sludge as Raw Material of Portland Cement in Japan.” In Water Science and Technology, 46 (10): 255–258. doi:org/10.2166/wst.2002.0347.
- Treiman, A. H., and E. J. Essene. 1984. “A Periclase-Dolomite-Calcite Carbonatite from the Oka Complex, Quebec, and Its Calculated Volatile Composition.” Contributions to Mineralogy and Petrology 85 (2): 149–157. doi:https://doi.org/10.1007/BF00371705.
- Uwasu, M., K. Hara, and H. Yabar. 2014. “World Cement Production and Environmental Implications.” Environmental Development 10 (1): 36–47. doi:https://doi.org/10.1016/j.envdev.2014.02.005.
- Valderrama, C., R. Granados, J. L. Cortina, C. M. Gasol, M. Guillem, and A. Josa. 2012. “Implementation of Best Available Techniques in Cement Manufacturing: A Life-Cycle Assessment Study.” Journal of Cleaner Production 25: 60–67. doi:https://doi.org/10.1016/j.jclepro.2011.11.055.
- Valdes-Vidal, G., A. Calabi-Floody, and E. Sanchez-Alonso. 2018. “Performance Evaluation of Warm Mix Asphalt Involving Natural Zeolite and Reclaimed Asphalt Pavement (RAP) for Sustainable Pavement Construction.” Construction and Building Materials 174: 576–585. doi:https://doi.org/10.1016/j.conbuildmat.2018.04.149.
- Valipour, M., F. Pargar, M. Shekarchi, and S. Khani. 2013. “Comparing A Natural Pozzolan, Zeolite, to Metakaolin and Silica Fume in Terms of Their Effect on the Durability Characteristics of Concrete: A Laboratory Study.” Construction and Building Materials 41: 879–888. doi:https://doi.org/10.1016/j.conbuildmat.2012.11.054.
- Van Oss, H. G., and A. C. Padovani. 2002. “Cement Manufacture and the Environment - Part I: Chemistry and Technology.” Journal of Industrial Ecology 6 (1): 89–106. doi:https://doi.org/10.1162/108819802320971650.
- Vejmelková, E., D. Koňáková, T. Kulovaná, M. Keppert, J. Žumár, P. Rovnaníková, Z. Keršner, M. Sedlmajer, and R. Černý. 2015. “Engineering Properties of Concrete Containing Natural Zeolite as Supplementary Cementitious Material: Strength, Toughness, Durability, and Hygrothermal Performance.” Cement and Concrete Composites 55: 259–267. doi:https://doi.org/10.1016/j.cemconcomp.2014.09.013.
- Voglis, N., G. Kakali, E. Chaniotakis, and S. Tsivilis. 2005. “Portland-Limestone Cements. Their Properties and Hydration Compared to Those of Other Composite Cements.” Cement and Concrete Composites 27 (2): 191–196. doi:https://doi.org/10.1016/j.cemconcomp.2004.02.006.
- Vollpracht, A., and W. Brameshuber. 2016. “Binding and Leaching of Trace Elements in Portland Cement Pastes.” Cement and Concrete Research 79: 76–92. doi:https://doi.org/10.1016/j.cemconres.2015.08.002.
- Wagih, A. M., H. Z. El-Karmoty, M. Ebid, and S. H. Okba. 2013. “Recycled Construction and Demolition Concrete Waste as Aggregate for Structural Concrete.” HBRC Journal 9 (3): 193–200. doi:https://doi.org/10.1016/j.hbrcj.2013.08.007.
- Wasilewski, M., and J. Duda. 2012. “Influence Construction of Suspension Preheater on Energy Consumption Process during Burning in Rotary Kiln.” XIVth International Symposium on Heat Transfer and Renewable Sources of Energy, Poland .
- Weng, C.-H., D.-F. Lin, and P.-C. Chiang. 2003. “Utilization of Sludge as Brick Materials.” Advances in Environmental Research 7 (3): 679–685. doi:https://doi.org/10.1016/S1093-0191(02)00037-0.
- Xu, W., J. Xu, J. Liu, H. Li, B. Cao, X. Huang, and G. Li. 2014. “The Utilization of Lime-Dried Sludge as Resource for Producing Cement.” Journal of Cleaner Production 83: 286–293. doi:https://doi.org/10.1016/j.jclepro.2014.07.070.
- Yan, K., H. Sun, F. Gao, D. Ge, and L. You. 2020. “Assessment and Mechanism Analysis of Municipal Solid Waste Incineration Bottom Ash as Aggregate in Cement Stabilized Macadam.” Journal of Cleaner Production 244. doi:https://doi.org/10.1016/j.jclepro.2019.118750.
- Yen, C.-L., D.-H. Tseng, and -T.-T. Lin. 2011. “Characterization of Eco-Cement Paste Produced from Waste Sludges.” Chemosphere 84 (2): 220–226. doi:https://doi.org/10.1016/j.chemosphere.2011.04.050.
- Yildirim, I. Z., and M. Prezzi. 2011. “Chemical, Mineralogical, and Morphological Properties of Steel Slag.” Advances in Civil Engineering 463638. doi:https://doi.org/10.1155/2011/463638.
- Younes, M. M., H. A. Abdel-Rahman, and M. M. Khattab. 2018. “Utilization of Rice Husk Ash and Waste Glass in the Production of Ternary Blended Cement Mortar Composites.” Journal of Building Engineering 20: 42–50. doi:https://doi.org/10.1016/j.jobe.2018.07.001.
- Zhang, H., H. Wang, X. Zhu, Y. J. Qiu, K. Li, R. Chen, and Q. Liao. 2013. “A Review of Waste Heat Recovery Technologies Towards Molten Slag in Steel Industry.” Applied Energy 112: 956–966. doi:https://doi.org/10.1016/j.apenergy.2013.02.019.
- Zhang, W., Y. Hama, and S. H. Na. 2015. “Drying Shrinkage and Microstructure Characteristics of Mortar Incorporating Ground Granulated Blast Furnace Slag and Shrinkage Reducing Admixture.” Construction and Building Materials 93: 267–277. doi:https://doi.org/10.1016/j.conbuildmat.2015.05.103.