?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Production of precipitated calcium carbonate (PCC) by indirect carbonation of BOF (Basic Oxygen Furnace) slag usually requires a calcium extraction stage to promote the dissolution of calcium. Previous works have shown that the grinding of BOF slag to smaller particle size accelerates calcium leaching at the expense of a high energy input and further environmental constraints such as dust management. In order to minimize the excessive consumption of energy due to grinding, this study focused on the optimization of calcium extraction from unmilled BOF slag using ammonium chloride (NH4Cl) and ammonium nitrate (NH4NO3). The study was conducted with a solvent-to-slag mass ratio of 10/1, to avoid the dissolution of impurities such as iron, silicon, magnesium and manganese and guarantee minimum equipment costs. The results obtained using umilled BOF slag show that the efficiency of calcium extraction reached ~75% for NH4NO3 and ~69% for NH4Cl in two extraction stages. A calcium carbonate precipitation yields of 80% was reached at 25°C and 6.5bars. Scanning Electron Microscopy (SEM) analysis of the dry PCC obtained showed that it is mostly dependent on the flow rate of CO2 and the carbonation pressure. Finally, the PCC was evaluated for the treatment of acid mine drainage.
1. Introduction
Recent trends in highly alkaline industrial residues (e.g. steel slag, bauxite processing residue (red mud) and ash from coal combustion) management include
i) newer or reengineered processes resulting in decreasing quantities of carbon dioxide (CO2) emissions as the aqueous dissolution of these materials creates high pH solutions that dissolves CO2 to store carbon in the form of solid carbonate minerals or dissolved bicarbonate ions. (LaGrega, Buckingham, and Evans Citation2010; Krausmann et al. Citation2017) and
ii) strategies to maximize current levels of these waste materials utilization and/or development of high value products.
During steel production in a Basic Oxygen Furnace (BOF), for every ton of crude steel produced, 300–400 kg of slag is produced in an highly energy intensive process which requires significant amount of electrical energy produced through coal-based thermal power plants as in the case of South Africa, leading to additional solid waste generation in the form of fly ash (Bezuidenhout and Cock Citation2009). In South Africa, ArcelorMittal Steel generates about 4 million tons of by-products each year, including 7 million tons of waste BOF slag (0.6 ton of waste per ton of steel produced) (Strunz et al. Citation2018). Although on a global stage, the recycling of BOF slag has made relatively significant progress in recent years with much of the material being utilised as construction aggregate and in road applications (Takeshi, Hiroyuki, and Keiji Citation2018), the recycling of BOF slag still faces many technical, economic, and environmental challenges (Setién, Hernández, and González Citation2009; Arribas et al. Citation2015) mainly due to:
i) The high levels of free lime in the BOF slag affect its performance and limit its widespread applications for road and concrete production.
ii) In-plant recycling of the by-products has achieved limited success because of its phosphorus content.
In South Africa, the BOF slag generated is only partially reprocessed in the steel-making process and a significant proportion is stockpiled at various sites across the country, for instance, at the Vanderbijlpark and New Castle. As a result, large amounts of waste BOF slag have been stockpiled at various sites across South Africa (about 300,000 tons/year BOF slag at the Gauteng plant and ~250,000 tons BOF slag /year at the New Castle plant) (Mulopo, Mashego, and Zvimba Citation2012). Moreover; the National Environmental Management Waste Act 59 of 2008 in South Africa places a strong emphasis on solid waste management (Strydom and King Citation2009). Cleaner production is the most preferred waste management route and is a guiding principle for many local policies, especially in the development of new processes and new operations (Strydom and King Citation2009; Mulopo, Mashego, and Zvimba Citation2012). Previous studies have shown that mineral carbonation could be one of the most effective methods of avoiding or reducing carbon dioxide emissions into the atmosphere from industrial activities (Gerdemann et al., Citation2007; Pan et al. Citation2017). BOF slags are alkaline and appear to be potential raw materials for CO2 sequestration by accelerated carbonation because they are generally rich in metal oxides including calcium, magnesium, aluminium, iron, and manganese oxide (Pan, Chang, and Chiang Citation2012). Among the mineral carbonation processes, indirect carbonation is usually considered as the most efficient and least energy demanding (Bobicki et al. Citation2012). Indirect carbonation of highly alkaline industrial residues requires two stages that are; the extraction of divalent metal using a selective mineral solution and the carbonation with gaseous carbon dioxide (Kakizawa et al., Citation2001). Indirect carbonation of highly alkaline industrial residues offers the potential of separate optimisation of the two successive steps and the possibility to increase the efficiency of carbonation compared to the other methods by acting selectively on carbonation parameters (Eloneva et al., Citation2008; Bobicki et al. Citation2012). Accelerated carbonation has been investigated through the indirect carbonation method, where the reacting alkaline element (Ca, Mg or both) is first extracted from the feedstock, and in a separate stage will react with CO2 to form carbonates following the reactions:
RX is the solution and M the metal for carbonation.
Recent studies have shown that ammonium salts dissolve calcium selectively and effectively, and among the most effective ammonium salts for calcium extraction are ammonium nitrate and ammonium chloride (Eloneva et al. Citation2012; Lee et al. Citation2016). During calcium extraction from highly alkaline industrial residues, the main extraction parameters are usually the concentration of the solvent, the liquid/solid ratio and the slag particles size. However, data from several studies suggest that the particle size of waste residues has the single most effect on the energy requirement of extraction process (Hall et al. Citation2014; Rahmani, Tyrer, and Junin Citation2014; Vanderzee and Zeman Citation2018). Moreover, the finer the particle size, the higher the calcium extraction efficiency but with the inherent penalty of high energy costs. On the other hand, to improve the extraction efficiency, the ratio liquid-to-slag is generally increased which may lead to increased capital and operation costs (Eloneva, Teir, and Revitzer et al. Citation2009; Said et al. Citation2013).
The economic and technical efficiencies of the indirect carbonation process using BOF slag are therefore directly related to the particle size of the feed slag material and to the liquid-to-slag ratio used during the extraction stage which is usually the first step towards the recovery of valuable products or components from waste slag. Several studies have focussed on the extraction of calcium from steel slags with the view of developing long-term carbon dioxide sequestration and storage to fix carbon dioxide as calcium carbonate as a valuable by-product. In the case where the treatment of BOF slag has calcium carbonate as the end product the quantity of available calcium for carbonation in the extract is usually the limiting reagent and it is therefore crucial to optimise the extractions conditions. It is against this background that we believe there exists a tremendous need for low energy cost strategy for calcium extraction from the slag matrix with the view to achieve environmentally and economically sound overall indirect carbonation processes. In particular, this paper considers the use of simple graphical mass balance targeting strategy for low energy calcium extraction and the implications thereof on the calcium carbonate produced as final valuable product for carbon sequestration.
2. Experiments and methods
2.1. Experimental protocol
A sample of the BOF slag was collected from ArcelorMittal Vanderbijlpark, South Africa. Sampling was done with a Rotary Splitter Eriez of ten pots rotating with variable speeds regulator. Four samples were weighed on a Mettler Toledo Scale and dried in a labotec oven at 130°C for 3 h to determine the moisture. Using the splitter, 4 aliquots were taken from each sample and subsequently pulverised and prepared for the chemical analysis. The dried slags were subjected to a particles size analysis using a Macsalab Electronic Sieve Shaker. The mesh sizes were in the range from 4000 to 75 µm arranged in descending order from top to bottom during 3 h each. The holding proportions of each sieve were weighed and pulverised before chemical analysis. Moisture was determined according to the formula:
Where; ρh is the wet weight of the sample and ρs the dry weight of the sample and H is the moisture in %. Subsequently, a particle size analysis was conducted to determine a particle size distribution of the samples and calculate the P80 which is the size of the sieve which passes more than 80% of the load using the following formula:
The grinding energy required was estimated using the Bond’s formula as follows:
Where: W represents the energy required to reduce a particle of dimension d0 to dimension d1 and W1 the experimental work index of the steel slag in Kwh/t. In our case, the work index was 12.2 (Doering international, Citation2015). By adding to EquationEquation (3)(3)
(3) the over grinding due to the grinding of the particles that have already reached the optimal dimension for calcium extraction which was experimentally found to be 125 µm, then the EquationEquation (3)
(3)
(3) becomes:
Where Ws represents the over grinding energy of fine and ultrafine particles.
2.2. Chemical analyses
The determination of the chemical composition of the solid samples (untreated slag, different particle size fractions and extraction residues) was carried out using a PANalytical PW 2400 Wavelength-Dispersive spectrometer to determine the contents of each constitutive chemical element of the samples analysed. The chemical elements contained in the extraction and precipitation filtrates were determined using the Agilent Technologies 200 Series AA atomic absorption spectrophotometer (AAS) with an air/acetylene flame. The morphology of solid precipitate was determined by using a scan electron microscopy (SEM) analyser.
2.3. Extraction
The extraction experiments were carried out using two solvents namely ammonium chloride and ammonium nitrate. Different solvent-to-slag ratios were tested to determine the optimum parameters for efficient extraction using the 2 different solutions. The ammonium salt solutions were prepared in different concentrations (2, 2.5 and 3 mol/L) by diluting the corresponding mass with distilled water in volumetric flasks of 500 ml each. BOF slag /solution mixture of different ratios (1.5, 3, 5, 7, 10, 20, 50) were prepared in a 500 ml beaker. 25 g of dried BOF sample is mixed with NH4NO3 or NH4Cl 2, 2.5 or 3 mol/L at different liquid/solid ratios ranging from 1.5 to 50. The mixture is put in a 500 ml beaker and then placed in a Precisterm Selecta water bath to maintain the temperature at 25°C. The mixture is stirred using a mechanical stirrer Labotec (HEIDOLPH) rotating at 400 RPM. The pH during reaction was measured with a Starter 3100 pH metre (OHAUS). After a determined time, a sample of the reaction mixture was collected and filtered off using a 47 mm filter paper (pore size: 0.45 μm) well placed in a funnel, and the filtrate was collected in a 250 ml flask and later distributed in test tubes for chemical analysis.
2.4. Carbonation
The carbonation was carried out in a 100 ml autoclave reactor fed with carbon dioxide stored in a pressurised gas cylinder equipped with a pressure regulator and fed to the carbonation reactor using a metal pipe. Precipitates obtained were filtered on a 47 mm filter paper (pore size: 0.45 μm) placed in a funnel subtended by a 250 ml volumetric flask. The precipitate obtained were dried in an oven at 150 °C for 2 h and 30 min. The calcium extraction yield was calculated using EquationEquation (5) by relating the calcium contained in the filtrate to the total calcium contained in the primary steel slag sample.
The following formula (6) was used to calculate the conversion yield of Ca to CaCO3, and it was assumed that only the calcium contained in the post-extraction solvent was carbonated and that there was no loss:
The carbonation yield was calculated using EquationEquation(7) below:
The amount of carbon dioxide captured was determined by EquationEquation (8) as follows:
In this equation PCO2 in and PCO2 out, denotes the mean value of PCO2 (atm) at the input and the output, respectively, Q and Δt are the flow rate (min−1) and the time interval (min), respectively, R gives the gas constant (0.082057 L.atm.mol−1K−1), M and T are, respectively, the mass (g) and the temperature (K) of the gas.
3. Results and discussions
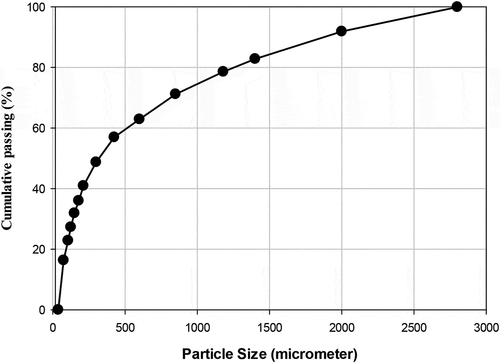
XRF analysis of the raw BOF slag shows that it contains 26.91% of calcium as shown in . The particle size distribution of the raw BOF slag sample shown in gives a P80 of 1247.22 µm using EquationEquation (2)(2)
(2) above. Because 80% of the particles are less than 1250 µm, which is a relatively fine size, it is expected that the grinding of these particles is likely to lead to a considerably higher energy penalty.
Table 1. XRF analysis of the raw BOF slag
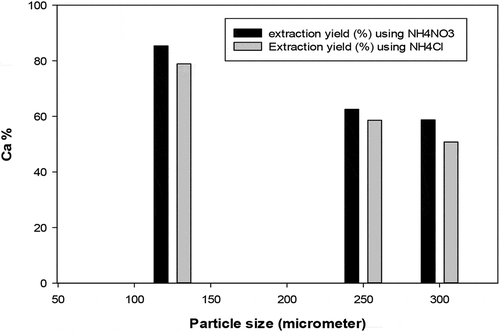
Results of calcium extraction experiments show that the calcium extraction efficiency from the unmilled slag reaches more than 70% for solvent–to-slag ratio exceeding 30 for a solution concentration of 3 mol/l, as shown in . The implication of such high solvent–to-slag ratio is that one may have to use large amount of solvent and possibly large equipment for extraction which will result in high capital and operating costs for industrial production. There was therefore a need to assess which additional extraction parameter may be considered i.e. extraction stage number in order to achieve an efficient extraction while maintaining the liquid-to-slag at an ‘economically’ acceptable value. The smaller particle size (<125 um) leached out more than 80% calcium from the BOF slag during 90 min of leaching for both solvent NH4NO3 and NH4Cl, while the coarser particle size (>125 um) leached out significantly less calcium within the same reaction time period with the solvent NH4NO3 performing slightly better than NH4Cl. In the composition changes during leaching for different particle sizes and solvent-to-slag ratio were examined using XRF analysis. The XRF results show that the leaching of Ca from BOF slag particle is more impacted by the feed particle size than it is by the solvent-to-slag ratio probably due to the selective leaching of the different fractions of the feed slag that take place leaving behind an undissolved layer of mixed non-reactive oxides in different phases. It is therefore clear that tough the extent of the leaching reaction is controlled in the first place by the solvent-to-slag ratio, when this ratio is less than the stoichiometric requirement for the different components of the slag matrix, the calcium leaching rate is significantly reduced as the available solvent has been completely consumed.
Figure 2. Calcium extraction yield (%) versus solvent-to-slag ratio and NH4NO3 (solvent) concentration at 90 min reaction time
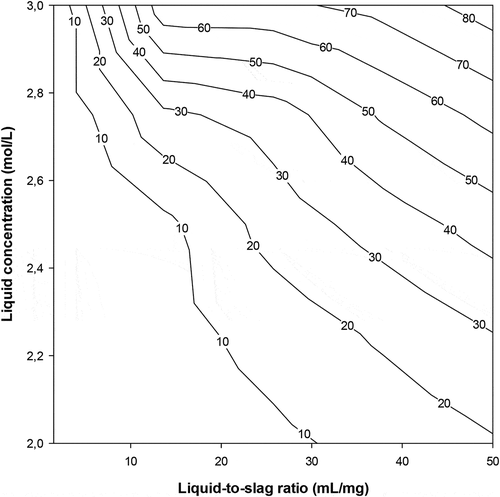
Figure 3. Calcium extraction yield versus liquid-to-slag ratio and NH4Cl (solvent) concentration at 90 min reaction time
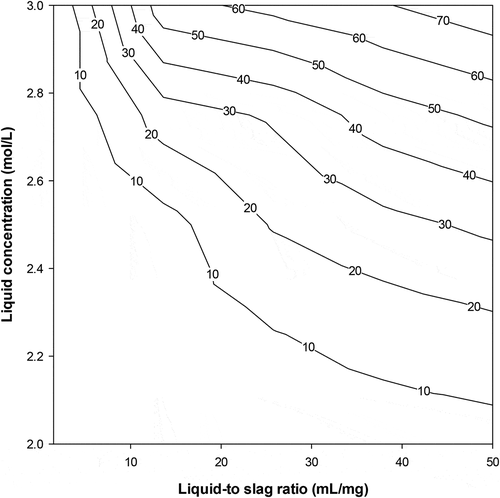
Table 2. Calcium distribution in different particle size ranges of the steel slag where X: particle size
Table 3. XRF composition of BOF steel slag residues from extraction for different liquid-to slag ratio and particle size at 90 min reaction time
Table 4. XRF and LECO analysis of produced and commercial calcium carbonate
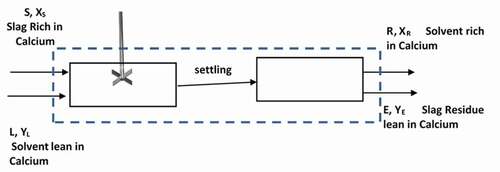
In order to propose a low energy cost calcium extraction scheme taking into account all above interactions of extraction parameters, let’s revisit a simple mass balance around a simple batch extraction stage p shown in , which is a basic combination of mixing and filtration steps:
Figure 4. Calcium extraction efficiency versus particle size at 90 minutes reaction time for liquid-to slag ratio=10
S and L are the mass (or volume) of slag and solvent, respectively
R and E are the mass (or volume) of spent solid (residues) and the rich calcium extract (filtrate) as shown in .
The calcium compositions in different streams are expressed by the mass fractions XS (in feed slag sample), XR (in the residue), YL (in the feed solvent) and YE (in the filtrate or spent solid). If we assume that all streams are equilibrium with each other i.e. steady state is reached after a long mixing time and if the solid extract is clear meaning does not contains solid residue then we may assume that S = R and L = E
The calcium balance gives:
EquationEquation 12 shows that at steady state XR and YE follows an equilibrium line passing through the point (XS, YL) after p extraction stage.
If the process comprises n extraction stages starting from the initial point (Xo and Yn+1) EquationEquation (12) reduces to the following working slurry line;
EquationEquation (7) can be written as:
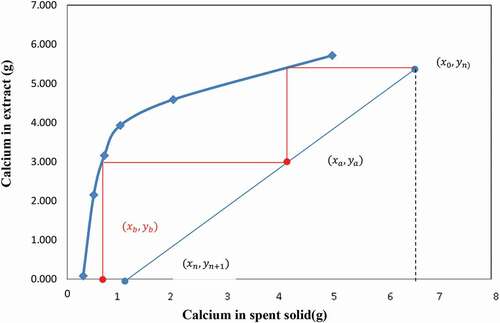
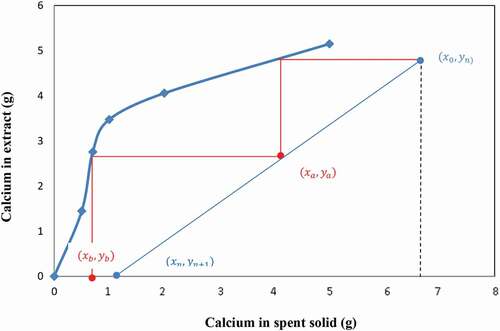
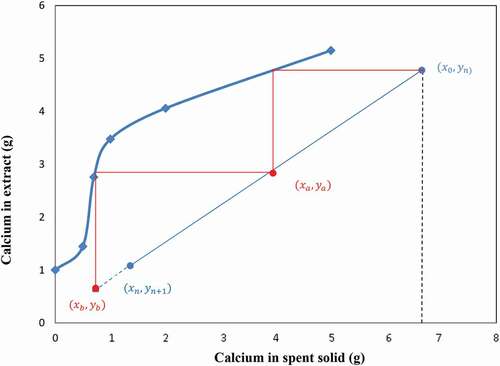
The experimental data for calcium extraction at different liquid/solid ratios may be used to draw both slurry and equilibrium lines (12 & 14) above. One may set the values of xn and yn, as the amount of calcium in the extraction residue (spent solid) and in the solvent, respectively. A graph similar to the classical Ponchot-Savarit diagram (Mathias Citation2009) can be drawn to determine the number of stages necessary to carry out the targeted calcium extraction. From calcium extraction results depicted in , one can deduce that the lowest liquid-to-slag ratio for extracting at least 50% of calcium is 10/1. Two extreme scenarios are the used to build the extraction diagram i) The solvent used is pure (does not contain calcium yn+1 = 0); ii) the solution used is a regenerated solvent that contains at least 4 g/l of calcium (yn+1 ≥ 1) as the set content for calcium in the final residue was 4% (xn = 1). Using the experimentally measured calcium value of the starting sample (x0 = 6.72) the points of the slurry line was then determined. Using calcium extraction data at the different liquid-to-slag ratios one can then plot both the slurry and equilibrium lines. depict the cases using NH4NO3 and NH4Cl as solvent, respectively, and where the points (xa, ya) and (xb, yb) indicate the calcium contents in the 2 phases at each extraction step (first stage = A, second stage = B)
The results in show that the use of at least 2 extraction stages is required in order to produce a residue containing less than 4% of calcium and the spent solid (from first extraction stage) contains more calcium when using NH4NO3 than NH4Cl.
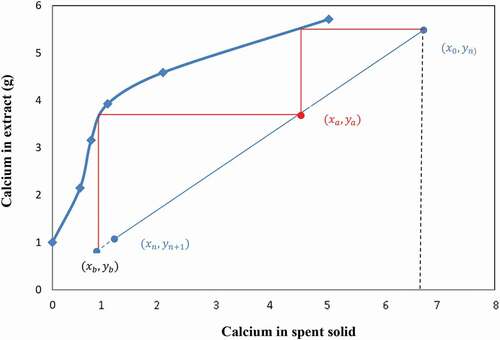
On the other hand, the number of calcium extraction stage using used NH4NO3 or NH4Cl (Ca≥4 g/l in solution) at liquid-to-slag ratios of 10/1 (slurry line slope = 0.1) is determined in the same way as in the first case described above using the mass balance diagrams and is equal to 2 for all solution and liquid-to-slag ratio of 10/1 (). The results in show a decrease in calcium extraction efficiency due to the presence of calcium in the solution at the beginning of the operation, though the end residue from the 2 stages extraction process contains less than 4% of calcium using both NH4NO3 and NH4Cl.
In conclusion, the above results show that 2 extraction stages are required to extract calcium from BOF slag by re-using a regenerated solution from the carbonation stage which may contain at most 15% of calcium and a liquid-to-slag ratio of 10/1 and this offers an interesting potential for an integrated overall process.
XRF analysis of the BOF slag shows that it contains~ 26% of Ca and AA (Atomic Absorption Spectroscopy) analysis shows that the amount of slag calcium leached in solution as Ca2+ ion is ~ 75% for NH4NO3 and ~69% for NH4Cl. The total energy consumed if the BOF slag is ground may be approximated using EquationEquation (3)(3)
(3) and may include the energy of over grinding (Rahmani, Tyrer, and Junin Citation2014). Considering over grinding up to 38 µm the grinding energy is determined as follows:
Therefore, by carrying out the extraction directly without grinding the raw BOF slag, one may save about 0.768 kwh/t which is substantially high than the energy required for the extraction-filtration step (0.25kwh/t) as calculated in previous work Rahmani Citation2018). It is worth mentioning that the process operating cost will depend on the reagents used as the BOF slag is assumed a cheaper waste material and therefore the main cost drivers of the process are primarily considered to be the solvent-to-slag ratio and the concentration of the reagents used. shows that in order to process a tonne of BOF slag, about 10 m3 of solvent (NH4NO3 or NH4Cl) is required and the solvent for the second calcium stage extraction contains at least 4 g/l of Ca while the spent solid contains 4% of calcium. In case of a continuous process, the pure solvent may be used only as supplement for material losses incurred (NH3 evaporation, solvent carried in the residues). It has been widely reported in previous works that the temperature had an effect on the carbonation of calcium by carbon dioxide (Eloneva et al. Citation2012; Hall et al. Citation2014; Lee et al. Citation2017), but in this work we opted for a low energy process and therefore have only consider an ambient temperature process.
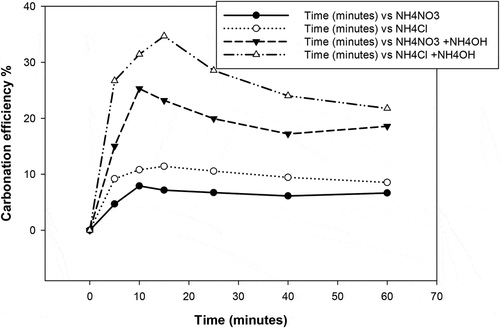
Carbonation results obtained in by varying the pressure of the CO2 injected in the carbonation reactor from 1 to 6.5 bars show that the carbonation efficiency reaches a maximum after 15 min when using NH4NO3 and 10 min when usingNH4Cl. This reduction in carbonation time may be attributed to the strength of aqueous basic solutions of the respective solvents which promotes the formation of the necessary ions from gaseous CO2. Donaldson and Nguyen (Donaldson and Nguyen 3), (Citation1980; Liu et al. Citation2019) have described the reaction as a base-catalysed CO2 hydration with generation of HCO3− resulting in gradual decrease in pH.
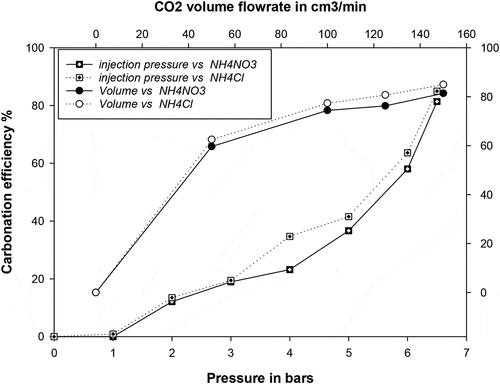
In order to promote carbonation in the carbonation stage, alkaline conditions are usually preferred as CO2 dissolution into carbonate and bicarbonates ions occur under these conditions. Ammonium hydroxide was used in this work to increase the pH of rich calcium filtrate above 10. The results in show that the carbonation reaction proceeds faster when ammonium hydroxide is used (high pH) and even more so for NH4Cl than NH4NO3. The effect of CO2 flow rate and stirring rate induced by the gas injection pressure on the carbonation efficiency is reported in . It can be seen that the carbonation rate proceeds faster at a higher CO2 flow rate (volume injected). In this regard, more than 80% carbonation was achieved at the CO2 injected flow rate of 150 cm3/min and 6.5 bars as shown in .
The effect of CO2 volumetric flow rate on the carbonation efficiency also suggests that the carbonation rate decreases with reaction time even though the overall carbonation reaction is improved. Increasing the injected flow rate from 60 to 150 cm3/min only increase the carbonation efficiency by 10%. The trends in indicate that the Ca carbonation decreases at low CO2 flow rate due probably to a deficiency of dissolved CO2. The carbonation reaction may be divided into 2 parts: (1) diffusion and dissolution of CO2 into solution to generate carbonate ions; (2) reaction of calcium and carbonate ions to produce calcium carbonate. Results in also imply that the declining carbonation reaction rate at high injected CO2 flow rate is an indication that the precipitation of calcium carbonate is likely not the rate-determining step during carbonation and that the moderate influence of the CO2 flow rate on the carbonation at high injected CO2 flow rate may be an indication that the mass transfer of CO2 into the solution is comparatively a more important factor and that diffusion and dissolution of CO2 into the solution is likely the reaction step that determines the overall carbonation rate.
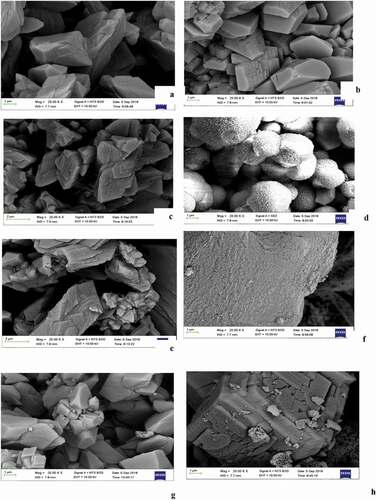
Calcium carbonate exists in one of 3 different polymorphs namely calcite, aragonite and vaterite (De Keyser and Degueldre Citation1950). The vaterite polymorph of calcium carbonate often exhibits nearly perfect spherical shapes. Although these spheres may appear smooth, but can be quite porous. Vaterite is metastable meaning that atoms in the crystals have a natural tendency to rearrange usually to a calcite structure. Calcite is the most common polymorph of calcium carbonate and the most stable and can be found in rhombohedral (cubic), prismatic (barrel-shaped) and scalenohedral (triangular) morphologies. The rhombohedral and prismatic forms are useful in paper coating applications and as strength enhancers in polymer matrixes. Therefore, SEM photomicrograph analysis of the calcium carbonate obtained by carbonation of calcium from unmilled BOF slag was conducted in order to further investigate the effect of CO2 volumetric flow rate, and results in shows the morphologies of the calcium carbonate produced at a CO2 volumetric flowrate of 150 cm3/min and different gas injecting pressures and carbonation reaction time. show that the morphology of the precipitate is closely related to the reaction time and the CO2 injection pressure. Initially, the precipitated calcium carbonate exhibited needle-like shape (associated with aragonite crystals) after 5 min and a pressure of 4 bars ()). When the reaction time was increased to 60 min all things being equal, the initially observed small scalenohedral crystals grew gradually to calcite like crystals shapes as shown in ). It was also observed that the increase of CO2 injection pressure from 4 to 6.5 bars accelerated the formation of prismatic calcite after 15 min for both NH4Cl () and NH4NO3 solvents ()). Vaterite crystals with spherical shape appeared after 25 min at 6.5 bars ()). For higher reaction times bigger than 60 min, the initial orderly crystalline forms previously observed tend to disappear and gives place to a more complex morphology at 6.5 bars as shown on . These evidences probably suggest that CO2 diffusion may control the nucleation process and the morphology of CaCO3 crystals. Additional experiments done by adding EDTA to control pH follow similar trends. All things being equal, the addition of EDTA to the carbonation mixture favoured the formation of a more compact and complex morphology as displayed on . It is hypothesised that the catalytic effect of EDTA is based on the formation of a hydrogen bond between the EDTA and water, weakening the bond between the hydroxyl group (-OH) and hydrogen, resulting in increased water nucleophilic reactivity towards carbon dioxide.
The potential CO2 sequestration was further evaluated using EquationEquation (8) as: above.
The classification of Wang et al. (Citation2018) indicates that this value obtained using unmilled steel slag lies within the range of economically feasible process for CO2 sequestration.
4. Application
Acid mine waters contain high concentrations of dissolved heavy metals and sulphate, and can have pH values as low as 2.5. Unless treated, such waters may not be discharged into public water streams. The acid water is formed as a result of bacterial oxidation when pyrites are exposed to oxygen and water after or during the mining process. A wide range of acid mines drainage (AMD) treatment technologies have been developed and proven (Zhuang Citation2009). In South Africa, most of the technologies successfully considered are highly depended on AMD pre-treatment to raise the AMD pH and remove acidity. Neutralisation is generally the first step in treating acid mine water (gold, operational and abandoned coal mines) in South Africa and Limestone has been used for decades to raise pH and precipitate metals in AMD. It has the lowest cost and is the safest and easiest to handle of the AMD chemicals. Unfortunately, its successful application has been limited due to its low solubility and tendency to develop an external coating of ferric hydroxide when added to AMD. The pre-treatment of AMD with limestone has shown that the pH of wastewater during neutralisation only changes from acidic pH to about 6.4 (Vandekerckhove et al. Citation2018). In order to achieve higher alkalinity during AMD pre-treatment and effective acid removal, alternative calcium compounds such as calcium hydroxide are known to be very useful in achieving higher pH values above 11.0 resulting in significant removal of metals including magnesium (Fernando et al. Citation2018). The results of recent applications of Steel slag, BOF slag for active neutralisation of acid mine drainage (AMD) have shown that the free alkalinity imparted by high oxides mass fraction (CaO, MgO) could make BOF slag a good neutralisation agent and AMD ameliorant. However, the direct application of BOF slag has few limitations compared to limestone: i) variations in the chemical composition and mineralogy of BOF slag have a direct influence on the neutralisation reaction and thus, operating conditions may have to be adapted and process controls at full-scale treatments carefully assessed, ii) though the slag can be added directly to the AMD or AMD-affected streams as an alkaline material to remove metals, it has shown a much lower capacity to remove anions such as sulphate, iii) there are not yet pilot plants or industrial-scale plants using BOF slag for direct treatment of acid mine waters and although, the experimental results of batch experiments for the direct treatment of AMD with slags are very promising, AMD treatments in slag leach beds sometimes prove ineffective for prolonged period of reaction time probably due to limitation of dissolvable calcium compounds on the surface of slag particles which lead to low alkalinity loadings. Accumulation of thick layer of precipitation in the effluent piping as well as formation of precipitates within slag result in lower alkalinity values than anticipated (Masindi et al. Citation2020; García-Valero et al. Citation2020; Zheng et al. Citation2020). On the contrary, the integrated limestone/lime process is a mature technology in South Africa and has been implemented at numerous plants. Therefore, the AMD neutralisation potential and acid neutralisation capacity of the steel slag-based precipitated calcium carbonate versus the commercial calcium carbonate has been evaluated. AMD containing about 2000 mg/l SO42-, pH of about 3 and acidity of about 1000 mg/l was collected from Emalahleni Reclamation Plant and used as feed water in this study.
Figure 12. SEM images observed during carbonation using CO2 flowrate of 150 cm3/min: (a) 5 min reaction time and 4 bars (b) 35 min at 6.5 bars -NH4NO3 (c) 25 min at 4 bars (d) 25 min at 6.5 bars; (e) 60 min at 4 bars (f) 60 min at 6.5 bars; (g) 15 min at 6.5 bars -NH4Cl and (h) 15 min at 6.5 bars -NH4NO3+ EDTA
From , both the produced PCC appears to exhibit neutralisation potential quite comparable to the commercial precipitated calcium carbonate (PPC) used in this study as a reference. The achieved pH is in line with the expected theoretical value of about 6.4 due to pH buffering by CO2 during AMD neutralisation.
Figure 13. pH evolution versus time during neutralisation of AMD- AMD treated volume = 200 ml; calcium carbonate used = 0.5 g & 1 g
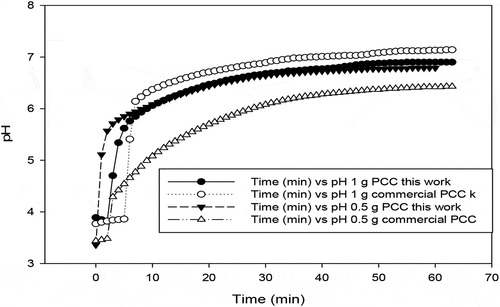
The significantly high Ca about 39.9–41.5% for the calcium carbonate generated from BOF slag and insignificant levels of other major metals components is a clear indication that calcium-based compound of high quality was produced ().
5. Conclusions
Low energy costs conditions for calcium extraction from unmilled BOF slag were assessed using 2 solvents namely ammonium nitrate and ammonium chloride and graphical analysis based on simple mass balance extraction. The effect of solvent concentration, solvent-to-slag ratio, and reaction time were considered. The results obtained show that at a solvent-to-solid ratio of 10/1 and ambient temperature, a calcium extraction of about ~ 75% for NH4NO3 and ~69% for NH4Cl with fewer impurities could be achieved in 2 batch extraction stages. The spent solid obtained after the second extraction stage contained less than 4% of calcium and may find application as aggregates in construction industry due to suitable particle size and low calcium content, which eventually minimise swelling phenomenon due to water absorption. Furthermore, effective CO2 sequestration via carbonation reaction was achieved with a pH > 10, at 6.5 bars pressure and a CO2 flow rate of 150 cm3/min for 15 min reaction. Beyond these operating conditions, it was found that the precipitate quality degraded. The chemical and SEM analyses of the produced calcium carbonate revealed a whitish and fine rhombohedral precipitate with high purity (99.8%). Treatment of the AMD using the obtained calcium carbonate led to treated water with a pH of 6.4 and acidity of less than 200 mg/ml after 90 min.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
G. Kahilu Mwengula
G. Kahilu Mwengula is a PhD candidate at the University of Witwatersrand with a strong academic background and industrial experience in Metallurgical processes and applications mainly in mineral processing and hydrometallurgy of different South Katanga’s minerals in the Congo, Democratic. Kahilu hold a MSc in Chemical Engineering from the University of Witwatersrand.
Jean Mulopo
Jean Mulopo is currently an associate professor in the school of Chemical Engineering at the University of Witwatersrand. He is the Coordinator and founder of the Sustainable Energy and Environment Research Unit (SEERU) within the School of Chemical and Metallurgical Engineering. One of the key focus areas of his research is on understanding the opportunities and constraints provided by the general and hazardous waste generation towards growing a green economy particularly in the context of water and energy challenges. Prior to joining Wits University, Jean was a Senior Engineer/Scientist in the Council of Science and Industrial Research (CSIR) Natural Resources and the Environment competency area (Waste Research Group). Jean holds a PhD in Chemical Engineering from the University of Witwatersrand, a postgraduate diploma in higher education from the Wits School of education and a certificate in Advanced Management and Business Leadership from the Wits Business School.
References
- Arribas, I., A. Santamaría, E. Ruiz, V. Ortega-López, and J. M. Manso. 2015. “Electric Arc Furnace Slag and Its Use in Hydraulic Concrete.” Construction and Building Materials 90: 68–79. doi:https://doi.org/10.1016/j.conbuildmat.2015.05.003.
- Bezuidenhout, A., and J. Cock. 2009. “Corporate Power, Society and the Environment: A Case Study of ArcelorMittal South Africa.” Transformation: Critical Perspectives on Southern Africa 69 (1): 81–105. doi:https://doi.org/10.1353/trn.0.0033.
- Bobicki, E. R., Q. Liu, Z. Xu, and H. Zeng. 2012. “Carbon Capture and Storage Using Alkaline Industrial Wastes.” Progress in Energy and Combustion Science 38 (2): 302–320. doi:https://doi.org/10.1016/j.pecs.2011.11.002.
- De Keyser, W. L., and L. Degueldre. 1950. “Contribution à l’étude de la formation de la calcite, aragonite et vatérite.” Bulletin des Sociétés chimiques belges 59 (1–2): 40–71. doi:https://doi.org/10.1002/bscb.19500590105.
- Doering International GmbH. (2015, January 13). Grinding media size formula. Retrieved from http://www.doeringsinn.de/fileadmin/download/mahlkoerper/Mahlkoerperformel_nach_Bond_en.pdf
- Donaldson, T. L., and Y. N. Nguyen, 1980. “Carbon Dioxide Reaction Kinetics and Transport in Aqueous Amine Membranes.” Industrial & Engineering Chemistry Fundamentals 19: 260–266. doi:https://doi.org/10.1021/i160075a005.
- Eloneva, S., S. Teir, J. Salminen, C. J. Fogelholm, and R. Zevenhoven. 2008. “Steel Converter Slag As A Raw Material For Precipitation Of Pure Calcium Carbonate”. Industrial & Engineering Chemistry Research 47 (18): 7104–7111. doi:https://doi.org/10.1021/ie8004034
- Eloneva, S., A. Said, C. J. Fogelholm, and R. Zevenhoven. 2012. “Preliminary Assessment of a Method Utilizing Carbon Dioxide and BOF Slags to Produce Precipitated Calcium Carbonate.” Applied Energy 90 (1): 329–334. doi:https://doi.org/10.1016/j.apenergy.2011.05.045.
- Eloneva, S., S. Teir, H. Revitzer, J. Salminen, A. Said, C.J. Fogelholm, and R. Zevenhoven. 2009. “Reduction of CO2 Emissions from Steel Plants by Using BOF Slags for Production of Marketable Calcium Carbonate.” Steel Research International 80 (6): 415–421.
- Fernando, W. A. M., I. M. S. K. Ilankoon, T. H. Syed, and M. Yellishetty. 2018. “Challenges and Opportunities in the Removal of Sulphate Ions in Contaminated Mine Water: A Review.” Minerals Engineering 117: 74–90. doi:https://doi.org/10.1016/j.mineng.2017.12.004.
- García-Valero, A., S. Martínez-Martínez, A. Faz, J. Rivera, and J. A. Acosta. 2020. “Environmentally Sustainable Acid Mine Drainage Remediation: Use of Natural Alkaline Material.” Journal of Water Process Engineering 33: 101064. doi:https://doi.org/10.1016/j.jwpe.2019.101064.
- Gerdemann, S., O'Connor, W., Dahlin, D., Penner, L. and Rush, H., 2007. Ex Situ Aqueous Mineral Carbonation. Environmental Science & Technology, 41(7), pp.2587–2593
- Hall, C., D. J. Large, B. Adderley, and H. M. West. 2014. “Calcium Leaching from Waste BOF Slag: Significance of Leachate Chemistry and Effects on Slag Grain Mineralogy.” Minerals Engineering 65: 156–162. doi:https://doi.org/10.1016/j.mineng.2014.06.002.
- Kakizawa, M., A. Yamasaki, and Y. Yanagisawa. 2001. “A New CO2 Disposal Process Via Artificial Weathering Of Calcium Silicate Accelerated By Acetic Acid”. Energy 26 (4): 341–354. doi:https://doi.org/10.1016/s0360-5442(01)00005-6
- Krausmann, F., D. Wiedenhofer, C. Lauk, W. Haas, H. Tanikawa, T. Fishman, and H. Haberl (2017). “Global Socioeconomic Material Stocks Rise 23-fold over the 20th Century and Require Half of Annual Resource Use.” Proceedings of the National Academy of Sciences, USA, 201613773.
- LaGrega, M. D., P. L. Buckingham, and J. C. Evans. 2010. Hazardous Waste Management. USA: Waveland Press..
- Lee, M. G., D. Kang, Y. Yoo, H. Jo, H. J. Song, and J. Park. 2016. “Continuous and Simultaneous CO2 Absorption, Calcium Extraction, and Production of Calcium Carbonate Using Ammonium Nitrate.” Industrial & Engineering Chemistry Research 55 (45): 11795–11800. doi:https://doi.org/10.1021/acs.iecr.6b02880.
- Lee, S. M., S. H. Lee, S. K. Jeong, M. H. Youn, D. D. Nguyen, S. W. Chang, and S. S. Kim. 2017. “Calcium Extraction from steelmaking Slag and Production of Precipitated Calcium Carbonate from Calcium Oxide for Carbon Dioxide Fixation.” Journal of Industrial and Engineering Chemistry 53: 233–240. doi:https://doi.org/10.1016/j.jiec.2017.04.030.
- Liu, S., H. Gao, X. Luo, and Z. Liang. 2019. “Kinetics and New Mechanism Study of CO2 Absorption into Water and Tertiary Amine Solutions by Stopped Flow Technique.” AIChE Journal 65 (2): 652–661. doi:https://doi.org/10.1002/aic.16469.
- Masindi, V., M. M. Ramakokovhu, M. S. Osman, and M. Tekere (2020). “Advanced Application of BOF and SAF Slags for the Treatment of Acid Mine Drainage (AMD): A Comparative Study.” Materials Today: Proceedings, India.
- Mathias, P. M. 2009. “Visualizing the McCabe-Thiele Diagram.” Chemical Engineering Progress 105 (12): 36–44.
- Mulopo, J., M. Mashego, and J. N. Zvimba. 2012. “Recovery of Calcium Carbonate from Steelmaking Slag and Utilization for Acid Mine Drainage Pre-treatment.” Water Science and Technology 65 (12): 2236–2241. doi:https://doi.org/10.2166/wst.2012.143.
- Pan, S. Y., E. E. Chang, and P. C. Chiang. 2012. “CO2 Capture by Accelerated Carbonation of Alkaline Wastes: A Review on Its Principles and Applications.” Aerosol Air Qual Res 12 (5): 770–791. doi:https://doi.org/10.4209/aaqr.2012.06.0149.
- Pan, S. Y., K. J. Shah, Y. H. Chen, M. H. Wang, and P. C. Chiang. 2017. “Deployment of Accelerated Carbonation Using Alkaline Solid Wastes for Carbon Mineralization and Utilization toward a Circular Economy.” ACS Sustainable Chemistry & Engineering 5 (8): 6429–6437.
- Rahmani, O. 2018. “CO2 Sequestration by Indirect Mineral Carbonation of Industrial Waste Red Gypsum.” Journal of CO2 Utilization 27: 374–380. doi:https://doi.org/10.1016/j.jcou.2018.08.017.
- Rahmani, O., M. Tyrer, and R. Junin. 2014. “Calcite Precipitation from By-product Red Gypsum in Aqueous Carbonation Process.” RSC Advances 4 (85): 45548–45557. doi:https://doi.org/10.1039/C4RA05910G.
- Said, A., H. P. Mattila, M. Järvinen, and R. Zevenhoven. 2013. “Production of Precipitated Calcium Carbonate (PCC) from BOF Slag for Fixation of CO2.” Applied Energy 112: 765–771. doi:https://doi.org/10.1016/j.apenergy.2012.12.042.
- Setién, J., D. Hernández, and J. J. González. 2009. “Characterization of Ladle Furnace Basic Slag for Use as a Construction Material.” Construction and Building Materials 23 (5): 1788–1794. doi:https://doi.org/10.1016/j.conbuildmat.2008.10.003.
- Strunz, T., F. Grassmann, J. Gayán, S. Nahkuri, D. Souza-Costa, C. Maugeais, and B. H. Weber. 2018. “A Mega-analysis of Expression Quantitative Trait Loci (Eqtl) Provides Insight into the Regulatory Architecture of Gene Expression Variation in Liver.” Scientific Reports 8 (1): 5865.
- Strydom, H. A., and N. D. King, eds. 2009. Environmental Management in South Africa. South Africa: Juta and Company .
- Takeshi, M., T. Hiroyuki, and W. Keiji (2018). “Iron and Steel Slag Products and New Effective Utilization Technologies.” JFE Technical Report, 23.
- Vandekerckhove, T. G., K. Kobayashi, J. Janda, S. Van Nevel, and S. E. Vlaeminck. 2018. “Sulfur-based Denitrification Treating Regeneration Water from Ion Exchange at High Performance and Low Cost.” Bioresource Technology 257: 266–273. doi:https://doi.org/10.1016/j.biortech.2018.02.047.
- Vanderzee, S., and F. Zeman. 2018. “Recovery and Carbonation of 100% of Calcium in Waste Concrete Fines: Experimental Results.” Journal of Cleaner Production 174: 718–727. doi:https://doi.org/10.1016/j.jclepro.2017.10.257.
- Wang, F., D. B. Dreisinger, M. Jarvis, and T. Hitchins. 2018. “The Technology of CO2 Sequestration by Mineral Carbonation: Current Status and Future Prospects.” Canadian Metallurgical Quarterly 57 (1): 46–58. doi:https://doi.org/10.1080/00084433.2017.1375221.
- Zheng, Q., Y. Zhang, Z. Zhang, H. Li, A. Wu, and H. Shi. 2020. “Experimental Research on Various Slags as a Potential Adsorbent for the Removal of Sulfate from Acid Mine Drainage.” Journal of Environmental Management 270: 110880. doi:https://doi.org/10.1016/j.jenvman.2020.110880.
- Zhuang, J. M. 2009. “Acidic Rock Drainage Treatment: A Review.” Recent Patents on Chemical Engineering 2 (3): 238–252. doi:https://doi.org/10.2174/2211334710902030238.