?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Power generation without environmental impairment is a challenging task for humankind. Presently pyrolysis process using various catalysts is among the finest technologies for generating alternate energy. This research aims to explore the pyrolysis of discarded bus/truck tires with zeolite ZSM-5. A fixed-bed reactor was employed to pyrolyse scrap tires at a temperature range of 300–580°C. Furthermore, it investigates the temperature and catalyst-to-tire ratio impact on the evolved oils’ generation and composition. Density, kinematic viscosity, and gross calorific value of the obtained oil, are analysed. CT ratio has increased the gas generation but decreases oil production. Elemental analysis of pyrolytic liquid oil revealed carbon percentage increment and decrement of sulphur percentage when CT ratio was increased. FT-IR showed data revealed the strong presence of aromatic compounds and other valuable hydrocarbons in the upgraded pyrolytic oils. The pyrolytic oil showed provided a high calorific value of 40.38 MJ/kg, comparable to conventional diesel oil of 42 MJ/kg. GC-MS analysis identified a higher amount of o-xylene, toluene, naphthalene, and d-limonene in derived oil when the CT ratio of the process was increased from 0.1 to 0.25, denoting the capability of the evolved oil as an alternative energy source and value-added chemical substances.
1. Introduction
Major concerning issues of the current world are energy shortage and destruction of the environment. Explosive population growth, increasing industrial development, and unorganised solid waste management are exceedingly responsible for these issues. Energy recovery from diverse waste materials using adequate technology can minimise energy shortage and environmental damage. Therefore, scientists have carried out extensive studies to find appropriate methods for energy recovery from non-biodegradable solid waste materials like rubbers and plastics (Ahamed et al. Citation2020). Tire waste contains around 24% of synthetic rubber, which is not biodegradable, and the presence of this type of rubber causes substantial ecological problems. This type of waste takes a considerably much longer time if compared to the photodegradation of biomass materials. There is a dominant rise of waste tires in Bangladesh because vehicles’ quantity is increasing day by day. The total yearly generation of scrap tires in this country is around 90,000 tonnes (BBS Citation2008).
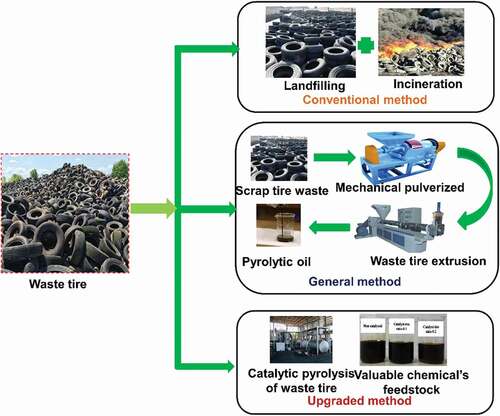
Approximately 20.5 million rickshaw/bicycle tires turn into waste each year (M. Rofiqul Islam, Tushar, and Haniu Citation2008). In terms of weight, 84,247 kg/day of rickshaw/bicycle tires, 14,137 kg/day of motorbike tires, and 79,178 kg/day of bus/truck tires are becoming useless and discarded annually in Bangladesh (M. R. Islam et al. Citation2011). Khulna, the third-largest city of Bangladesh, produces 2,200 kg/day of recyclable tire waste (Moniruzzaman, Bari, and Fukuhara Citation2011). Unorganised management of these wastes can cause severe harm to the ecology and the living beings. Another recent study shows that around 14,400 numbers of waste tires have been recycled per year in Khulna city (Han, Haon, and Oniruzzaman Citation2014).Usually, scrap tires are non-biodegradable and could not be dumped into landfills (M. Rofiqul Islam, Tushar, and Haniu Citation2008). Landfilling for the disposal of used tires has some drawbacks. It demands a huge area as the volume of tires cannot be compacted. Furthermore, landfilling poses a risk due to the likelihood of unintentional fires resulting in high emissions of dangerous gases. (Tang et al. Citation2016) reported while incineration decreases the volume and mass of waste plastic. It also produces a significant amount of toxic and harmful undesirable gases (e.g. CO2, HCl, and other toxic gases), which harms the atmosphere causes secondary pollution. The various methods for disposal of waste tires are shown in .
Waste tires can be a valuable chemical commodity and a source of energy, and their thermolysis allows the retrieval of beneficial compounds. The author of this study suggested a revolutionary waste tire disposal technique that is environmentally friendly and low in pollution and emissions.While there are numerous recycling processes for energy recovery, such as retreading, reclaiming, incineration, grinding, and so on, each has its own set of disadvantages (De Marco Rodriguez et al. Citation2001). Pyrolysis is an appealing alternative solution for the disposal of used tire waste. The pyrolytic process produces liquid oil capable of energy production, and the char potentially as industrial activated carbon. However, because of the increased presence of arenes (Benallal et al. Citation1995; De Marco Rodriguez et al. Citation2001; M. F. Laresgoiti et al. Citation2004; Williams and Bottrill Citation1995), the pyrolysis-derived oil from tires was not suitable for use as a fuel. (Benallal et al. Citation1995) investigated pyrolytic oil from tire pyrolysis and compared it to petroleum naphtha. They discovered that naphtha had a higher octane number than petroleum naphtha, but it had to be hydrofined and reformed before it could be used.
As a result, the derived oil’s poor quality and variable properties make it difficult to recycle. (Franck et al. Citation1988) The derived oils contain valuable chemicals like benzene, toluene, xylene, and others that can be recovered and used as raw materials in the chemical industry. Toluene is used to produce pesticides, dyestuffs, surfactants, and solvents, while benzene is used in the production of surfactants, dyestuffs, and pharmaceuticals. Plasticisers, dyes, and pigments are made from o-xylene, derivatives of m-xylene are used in the textile industry, and polyester fibres are made from p-xylene derivatives. However, since their concentrations aren’t high enough to extract them from the pyrolytic oil and use them as a chemical feedstock, catalysts are used to generate more single ring arenes. To figure out this issue, catalytic pyrolysis is a promising and challenging research area. Numerous studies have been conducted to explore the practicability of catalytic pyrolysis of scrap tires for small and large scales. (Laresgoiti et al. Citation2004) examined the maximation of the concentration of certain arenes in derived oil from the pyrolysis of tire wastes with catalyst. (Shah, Jan, and Mabood Citation2009) analysed the consequences of basic, acidic, and mixture of both catalysts on the pyrolysis of scrap tires and generated valuable hydrocarbons (Shah, Jan, and Mabood Citation2009). (Ilkiliç and Aydin Citation2011) generated substitute fuel from the pyrolysis of waste vehicle tires using lime as the catalyst. (Kar Citation2011) reviewed non-catalytic and catalytic pyrolysis of used car tires with expanded perlit. (Hooshmand Ahoor and Zandi-Atashbar Citation2014) conducted pyrolysis of garbage tires using magnesium chloride salt as a catalyst in a batch-type reactor under argon (Ar) environment and maximise the generation of oil in derived products. (Namchot and Jitkarnka Citation2016) investigated waste tire catalytic pyrolysis employing core-shell nanoparticle composite of HY and MCM-41 as catalyst. (Ding et al. Citation2016) discovered significantly higher generation of valuable aromatic compounds in waste tire pyrolysis by using H-Y zeolite.
(Luo and Feng Citation2017) generated combustible gas and fuel oil by catalytic pyrolysis of scrap tire. (Kordoghli et al. Citation2017) identified the influence of various catalysts on gas composition during catalytic pyrolysis of scrap tires. (He et al. Citation2018) produced light olefin products from pyrolysis of scrap tires by using nano-HZSM-5/γ-Al2O3 as catalysts. (Hijazi et al. Citation2019) examined pyrolysis of scrap rubber tires with palladium doped zeolite as the catalyst and revealed considerable increment in hydrocarbon cracking resulting in higher gas yield production. (Khalil et al. Citation2020) found higher production of aromatic compounds using zeolite catalysts. (Wang et al. Citation2020) produced hot char from tire tread pyrolysis and investigated the efficiency of hot char as a cracking catalyst. (Rejeb et al. Citation2020) These studies examine the influences of zeolite catalyst ZSM5 on pyrolytic oil and gas derived from tire waste. The authors of these research works have found that using different types of catalysts enhances the pyrolytic products’ quality. The number of valuable hydrocarbons has increased as well as the yield of gas.
Additionally, the liquid products from the pyrolysis of scrap tires can be transported and stored easily. (Sharma et al. Citation2000) Shows tire wastes have a high proportion of volatiles with small ash content and a heating value higher than biomass and coal, making it a potential energy source. Liquids from pyrolysis of scrap tires have a high GCV of about 41‒44 MJ/kg. It could promote them as a replacement of regular liquid fuels (M. R. Islam et al. Citation2013). The pyrolysis of scrap tires in the non-existence of oxygen produces gas, oil, and char are profitable as its potential for reutilised. Pyrolytic gas has a gross calorific value of 6.4–9.8 MJ/kg, sufficient to supply process energy (Barbooti et al. Citation2004; Felisa et al. Citation2000), and pyrolytic char can be utilised as activated carbon or carbon black. (Sahouli et al. Citation1996; Sainz-Diaz and Griffiths Citation2000) Char from waste tires has a GCV of 17–36 MJ/kg and can supply thermal energy for operating the pyrolysis process. (Czernik and Bridgwater Citation2004) Pyrolytic oil can be used as an alternative fuel (M. S. Hossain and Rahman Citation2015). According to (Md, Shameem, and Iqbal Citation2017) thermal pyrolysis of plastic medical waste was also performed for production of pyrolytic oil like valuable liquid and gaseous fuels.
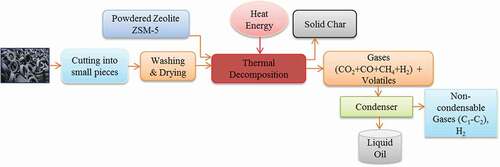
In this work, pyrolysis of scrap tires was conducted in a fixed-bed reactor with powdered zeolite ZSM-5 catalyst. Pyrolytic gases generated in the experiment were passed through the condenser chamber, and the condensable gases ultimately caused pyrolytic oil. Non-condensable gases evolved from scrap tire pyrolysis are mainly low carbon chain compounds (Czajczyńska et al. Citation2017). They can be utilised as a source of energy needed to run the pyrolysis process. The enhanced catalytic activities increased the content of aromatic hydrocarbons in generated pyrolytic liquids, which have been characterised further according to different characteristics. This study also examined the influence of temperature on gas, oil, and char production. FTIR, CHNS, and GC-MS analysis confirmed the potential presence of valuable aromatic hydrocarbons, which can be utilised as a chemical feedstock in different industries.
2. Materials and Methods
2.1 Raw Material
Raw materials used in this experiment were the scrap tires of buses/trucks. Discarded tires were collected from a local garage of Khulna city. To maintain the homogenous size of the samples, scrap tires were shredded into similar-looking pieces. The steel wires, bead, and fabric belts were removed from the samples. The cross-sectional size of tire chips was 0.75 cm3 approximately. The samples were combined to ensure that the components in the sample (such as rubber, dark carbon, and zinc oxide) were distributed evenly in the sample. They were then washed, dried, and fed in the reactor chamber. In BCSIR, the properties of waste tires were analysed, and the results are summarised in . Zeolite ZSM-5 with a pore size of 5.5 ºA and Si/Al ratio of 25 was used as the catalyst. The catalyst was brought from China and manufactured by Xi’an Lvneng Purification Technology Co. Ltd.; it was in powder form, and the surface area was 320–360 m2/g. The flow diagram of the overall experiment is presented in .
Table 1. Properties of Scrap Tire
2.2 Experimental Set-up and Procedure
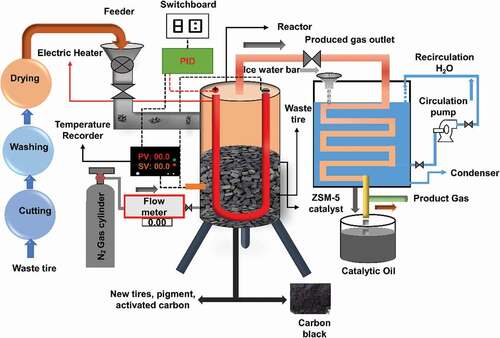
A batch type fixed-bed reactor was used in this experiment. The reactor chamber was 26 cm in length; the circular chamber’s inner and outer diameter was 20 cm and 20.7 cm, correspondingly. shows the schematic diagram of the experimental set-up, which consists of N2 gas cylinder, fixed-bed reactor chamber, digital temperature controller for controlling the temperature within the reactor chamber, condenser chamber for condensing condensable vapour to pyrolytic liquid, liquid collecting pot, and a centrifugal pump for circulating icy water into the condenser chamber. N2 gas was supplied into the lower portion of the reactor chamber for 30 minutes to create an inert atmosphere, and the supply was controlled with a gas control valve. Three tubular electric heaters of 10-inch length were used for providing 1.5 kW of electric power. The heaters were fixed equidistantly from each other at the top part of the reactor. Asbestos rope and glass wool were used for insulating the reactor chamber thermally. Thermocouple sensors were used to determine the inside temperature of the reactor chamber. The heating rate of the experiments was 5°C/min. 1.0 kg of tire was loaded in the reactor of each experiement.
After loading raw materials, powdered zeolite ZSM-5 was taken into the reactor chamber. The power supply was started, and the temperature inside the reactor chamber was permitted to increase. A steady flow rate of 20 mL/min nitrogen was maintained during the operation. The reaction time was 60 minutes for every single operation. After some time, when the decomposition of scrap tires took place, colourless non-condensable gases came out from the bottom part of the condenser chamber, which was flared into the atmosphere. Meanwhile, condensable vapour was condensed and generated pyrolytic oil, which was collected in glass bottles. Three operations were conducted for each temperature of 300°C, 340°C, 380°C, 420°C, 460°C, 500°C, 540°C, and 580°C. The average value of three operations with a standard deviation was noted. The product yields were measured using the following equations:
The yield of pyrolytic oil (%) = X 100
The yield of pyrolytic char (%) = X 100
Gas yield (%) = 100 ‒ {oil yield (%) + char yield (%)}
2.3 Analytical Methods
The pyrolytic liquid was characterised using normal ASTM methods such as CHNS, gas chromatography coupled with mass spectrophotometry (GC–MS), and Fourier transform infrared spectroscopy (FT-IR), as well as the high heating value (HHV) by bomb calorimeter.
For CHNS analysis vario MICRO CHNS analyser was used. Less than 1-millilitre sample can be analysed. The samples were decomposed thermally up to 1200°C for analytical accurateness and 1800°C during combustion. The results are given in baseline resolution of all analytical peaks irrespective of comparative concentration. Analytical time was automatically optimised. All analytical results were automatically calculated and completed, and operating conditions for each sample were stored.
For proximate analysis, 100 gm samples of scrap tires were taken. The moisture content of the sample was measured when it was heated at 110°C for 25 minutes, and the volatile matter was measured by heating the sample at 900°C in an inert environment, while the ash content was measured when the oxidation temperature was 810°C, and fixed carbon was obtained from the following equation, 100 – (ash content + volatile matter).
For GC-MS analysis, Shimadzu GCMS- TQ8040 with an inbuilt database library was used in this experiment. The carrier gas was Helium of 99.99% pure and 5% diphenyl 95% dimethyl polysiloxane (Rxi-5 ms) of 0.25 μm with dimension of 30 m and 0.25 mm ID was used as a column. The obtained pyrolytic oil was filtered through a 0.45 μm membrane syringe filter, and then 1 μl was injected into the instrument. The following programme was adopted for the GC/MS experiment.
The presence of various functional groups in the feedstock and the generated pyrolytic oil was investigated using FT-IR analysis. For FT-IR analysis, Shimadzu IRPrestige-21 ranging from 500–4000 nm equipped with a liquid sample holder with a cell thickness of 0.5 mm with NaCl window was used for the FTIR analysis. The sample was injected into the cell and analysed in the instrument. The Gross calorific value (GCV) of the pyrolytic oils was measured by an Oxygen Bomb Calorimeter.
3. Results and Discussion
Results and discussion of the research work are explained in the following sections.
3.1 Thermal cracking of tire waste for without catalyst
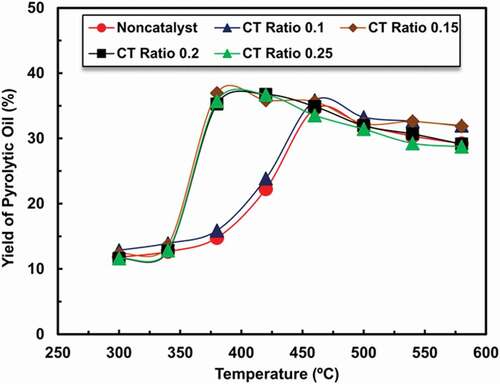
In order to compare the pyrolysis of waste tires with powdered ZSM-5 catalysts to that of waste tires without a catalyst, this paper performed non-catalysed tire pyrolysis. The pyrolysis temperature was increased from 300 to 580°C. The pyrolysis of the waste tire without catalyst results was shown in . It can be seen that a high oil yield of about 35 wt.% can be obtained from the pyrolysis of the waste tire without catalyst at 460°C. This number was because of the thermal cracking effects of the pyrolysis gases from the waste tire.
Table 2. Pyrolysis of Scrap Tire Without Catalyst at Various Temperature
3.2 Influence of Pyrolytic Temperature on Product Yield for various CT Ratio
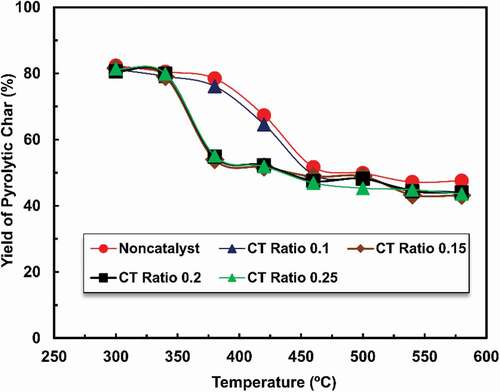
Scrap tire pyrolysis with zeolite ZSM-5 for different CT ratios was performed at various temperatures between 300°C to 580°C. A study on non-catalysed and catalysed scrap tire pyrolysis showed that amount of pyrolytic oil generation is higher in catalysed processes at various temperatures (S. Hossain et al. Citation2017). In the present study, pyrolytic oil, char, and gas generation are compared within different CT ratios for catalysed scrap tire pyrolysis. The catalysts were placed with tire waste in a catalytic reactor. shows the influence of temperature on oil production, which indicates that within the range of 340–460°C, the output of oil is higher for using ZSM- 5 catalysts than non-catalyst between temperature 360–400°C as related CT ratios and then slightly decrease when increasing temperatures. Non-catalysed pyrolysis yield of product at around 34. 5 wt. % whereas CT ratio 0.1 samples showed have similar products, while the CT ratio of 0.15, 0.2, and 0.25 samples showed a comparable trend of oil production at high temperature. However, the maximum yield of oil obtained was 34.54% for non-catalysed moreover, the yield of oil was 35.83%, 36.934%, 36.74%, and 36.68% when changing catalyst to tire ratio, for example, CT ratio 0.1, 0.15, 0.2, and 0.25 for respectively. The oil, gas and char production were remained almost constant between temperatures 450 to 580°C. Among them, non-catalysed and CT ratio 0.1 needed 460°C while CT ratio 0.15, 0.2, and 0.25 needed 380°C, 420°C, and 420°C temperature correspondingly. The same pattern was observed for the ZSM-5 catalyst, with the oil yield decreasing as the catalytic temperature increased above 460°C. When the catalytic temperature was raised from 460 to 480oC, then the yield of oil decreased from 34.5 to 32.1 wt.%, correspondingly. It has been discovered that when the catalyst has been used in waste tire pyrolysis, the oil yield had reduced. The explanation of this phenomenon is that the zeolite catalyst caused more materials to be cracked into un-condensed goods. (Venuto and Habib Citation1979) has found that catalytic cracking of tire pyrolysis yielded large quantities of gaseous materials and (Williams and Brindle Citation2003) also discovered that catalytic cracking of waste tires reduced yield of oil at higher reactor setting temperature.
displays the effect of temperature on char production, which shows that char production has been gradually decreasing with higher temperatures. Although within the range of 300–400°C, the char production was higher for non-catalysed and for CT ratio 0.1 samples. After 400°C, oil samples of CT ratio of 0.15, 0.2 and 0.25 showed nearly constant char production. When the catalytic temperature was raised from 400 to 580°C, then char yield remains steady at 42 wt.%. It was observed that when the catalyst has been used in the waste tire pyrolysis process, the char yield was comparatively lower than the non-catalyst process at high reaction temperature, which indicates that the production of oil expands as well as gas production has been improved.
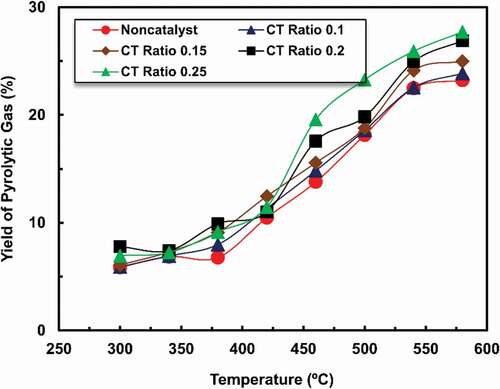
displays the effect of temperature on gas production, indicating that gas production is higher for higher CT ratios. Oil sample for CT ratio 0.25 showed maximum production of gas within the range of 430–580°C. Higher temperature activates secondary reactions, which increases gas generation (Alsaleh and Sattler Citation2014). All other four samples showed similar trends in gas production. At 580°C temperature, all the five oil samples non-catalysed, CT ratio 0.1, 0.15, 0.2, and 0.25 obtained their maximum yield of gas as recorded 23.23%, 23.87%, 24.98%, 26.89%, and 27.71%, respectively.
3.3 Property Comparison of Pyrolytic Oil with Other Commercial Fuels
The density, kinematic viscosity, and gross calorific value of evolved pyrolytic oil samples were measured in the Heat Engine laboratory of the Department of Mechanical Engineering, KUET, Khulna. The measured values are compared with other commercial fuels, as shown in . As illustrated in , the density of evolved pyrolytic oils has been decreasing, and GCV has increased with increasing CT ratio. None of the samples are comparable with diesel in terms of density or GCV. Further increment of CT ratio may decrease the density as well as increase the GCV as like diesel. Kinematic viscosity has also been falling as the CT ratio increases, which makes the oil more superior. This falling viscosity indicates the benefit of using more catalysts.
Table 3. Property Comparison of Pyrolytic Oil Extracted from Different CT Ratios with Other Commercial Fuels
3.4 Analysis of Pyrolytic Char
After each experiment, pyrolytic char was collected from the reactor chamber. GCV analyses of pyrolytic char obtained from different pyrolysis temperatures and CT ratio showed that the gross calorific values were within the range of 19–20 MJ/kg, which could easily be used as a potential source of heat for the pyrolysis process itself and also for other processes of energy production. Used tires can be converted into useful recyclable goods by the pyrolysis method. One of the essential byproducts of tire pyrolysis is pyrolytic char. Char carbonised at 600°C has superior heavy metal adsorption ability to commercial activated carbons (Helleur et al. Citation2001). The investigation of pyrolytic char will be needed for accelerating the practicality of catalytic production of char in the future study.
3.5 Analysis of Pyrolytic Gas
Pyrolytic gas produced in this process was flared into the open air. The yield of gas was within the range of 5–30%. Produced gas contains C1-C4 hydrocarbons. The catalytic pyrolysis process was examined in our laboratory on a small scale. When the temperature was increased in the reactor with an increasing CT ratio, the gas production has increased, and the yield of oil was deceased. The zeolite catalyst caused more materials to be cracked into un-condensed products, according to the explanation. (Venuto and Habib Citation1979) has discovered that catalytic cracking of waste tires creates significant amounts of gaseous materials, and (Williams and Brindle Citation2003) discovered that catalytic cracking of waste tires decreases oil yield at higher reactor setting temperatures. Due to the pyrolysis process’s small scale operation, it could not reuse the product gas for fuel heating source. However, large-scale pyrolytic gas production could easily be used as a source of energy needed to operate the pyrolysis process. The investigation of pyrolytic gas will be needed in future research to speed up catalytically produced gas.
3.6 Carbon, Hydrogen, Nitrogen & Sulphur Elemental Analysis
The elemental analysis of two specific samples of CT ratio 0.1 and 0.15 was carried out in BCSIR, Dhaka. The results are shown below. shows the elemental analysis of the oil samples with a CT ratio of 0.1 and 0.15. The analytical results are summarised in .
Table 4. Elemental Analysis of the Catalysed Pyrolytic Oil for CT Ratio of (a) 0.1 and (b) 0.15
The percentages of the elements in the oil sample with a CT ratio of 0.1 were nitrogen 0.00%, carbon 86.81%, hydrogen 8.25%, and sulphur 1.32%. On the other hand, the percentages of the elements in the oil sample with a CT ratio of 0.15 were nitrogen 0.00%, carbon 88.60%, hydrogen 8.486%, and sulphur 1.06%. By evaluating the results of elemental analysis, it can be considered that the percentage of carbon increases as the percentage of catalyst has also increased. The percentage of carbon increases 1.79% as the CT ratio increases from 0.1 to 0.15. Thus, catalytic pyrolysis increases the carbon percentage as well as the heating value of the pyrolytic oil. The presence of sulphur is corrosive for types of machinery, and increasing the CT ratio decreases sulphur percentage. As the CT ratio increases from 0.1 to 0.15, the sulphur percentage decreases about 0.261%.
3.7 Compositional Group Fourier Transform Infra-Red (FT-IR) Spectroscopy
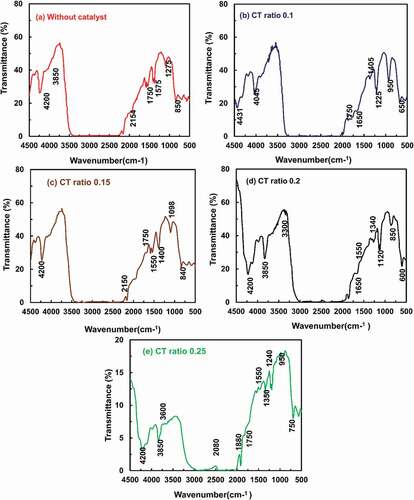
FT-IR spectra of different pyrolytic oil samples produced from scrap tire pyrolysis for different CT ratios and non-catalysed pyrolysis are shown in the following figures. To evaluate saturated, aromatic, and polar elements, the FT-IR is not the best analytical technique. The existence of different functional groups in the derived liquid oils from catalytic and non-catalytic pyrolysis was determined using FT-IR analysis. Absorbance range varies for different oil samples with dissimilar CT ratios. (a), (b), (c), (d), and (e) shows the FT-IR spectra of pyrolytic oil for non-catalysed pyrolysis and catalysed pyrolysis having a CT ratio of 0.1, 0.15, 0.2, and 0.25, respectively. In , using a catalyst with tires during operation, ‒C ≡ C‒ stretching vibrations at frequency 2106.77 cm-1, 2162.22 cm-1, 2229.73 cm-1, and 2247.09 cm-1 indicate the presence of alkynes. The presence of simple aromatic ring compounds is discovered at 3262.02 cm-1 with C‒H stretching and H-bonded vibrations. The presence of alcohols, phenols, and carboxylic acids is identified at 3600.65 cm-1 and 3669.6 cm-1 with O-H stretching vibrations. In the yield liquid oils from catalysed and non-catalysed pyrolysis, the FT-IR study showed several clear peaks ranging from 600 to 4000 cm-1. The produced oil contained a mixture of aliphatic (alkanes, alkenes, aldehydes) and aromatic hydrocarbon compounds, confirming our GC–MS findings. However, liquid oil produced by ZSM-5 catalytic processes produced more aromatic compounds, which could be seen clearly between 1500 and 1700 cm-1. The FT-IR results matched the GC–MS findings. The liquid oil derived from catalysed and non-catalysed scrap tire pyrolysis (Rofiqul et al. Citation2010) published similar findings. According to the report of (Mohammed and Hankish Citation1985), the peak area of the 1650–1550 cm-1 band, which represents the stretching vibration of the C = C bond in the aromatic ring, is also proportional to the aromatic content of the comparative mixtures. The results of the FT-IR study for pyrolytic liquids extracted from waste tire waste are summarising in . As a result, the data indicates that the current liquids mostly contain aliphatic and aromatic compounds.
Table 5. FT-IR Functional Groups and Recognised Class of Compounds
3.8 Gas Chromatography-mass Spectrometry
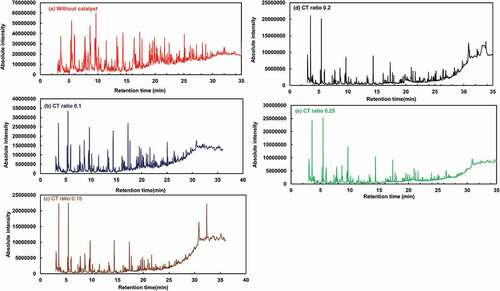
Gas chromatography-mass spectrometry analysis of different oil samples derived from scrap tire pyrolysis for different CT ratios and non-catalysed pyrolysis are discussed below. The GC–MS results of liquid fuels produced from the catalysed and non–catalysed scrap tire pyrolysis are illustrated as a chromatogram in (a) without catalyst, (b) CT ratio 0.1 (c) CT ratio 0.15, (d) CT ratio 0.2 (e) CT ratio 0.25 and most of the compound found after catalytic and non-catalytic pyrolysis are summarised below respectively. The oil’s complex existence, with many aliphatic and aromatic compounds, is revealed by the GC-MS study of tube pyrolytic oil. The table lists the key components and their percentage area compared to the total area of the chromatogram, providing an approximation of their relative oil concentration. It can be seen from the table that the availability of benzene, toluene, o-Xylene, d-limonene, and naphthalene has increased as the CT ratio of the pyrolysis process increases. The highest amount of benzene has been found in the sample of CT ratio 0.2. Toluene reached a peak value of 10 % in the sample of CT ratio 0.2.It seems that the number of peaks as well as their area are lower as the CT ratio increases. Considering using the same volume of sample and with the same dilution, the production visible fraction of the oil decreases as the amount of catalyst is increased because the presence of catalyst could be persuading polymerisation reactions.
Table 6. GC–MS results of probable compound of pyrolytic oil from waste tire over without catalyst
Table 7. GC–MS results of probable compound of pyrolytic oil from waste tire over CT Ratio 0.1
Table 8. GC–MS results of probable compound of pyrolytic oil from waste tire over CT Ratio 0.15
Table 9. GC–MS results of probable compound of pyrolytic oil from waste tire over CT Ratio 0.2
Table 10. GC–MS results of probable compound of pyrolytic oil from waste tire over CT Ratio 0.25
The presence of o-Xylene and d-limonene reached a maximum value of 9.91%and 7.51% in the oil sample of CT ratio 0.25. Limonene, for example, is used in the preparation of synthetic solvents, adhesives, and resins. It is also a pigment dispersing agent, a scent in personal care goods, and used as an environmentally friendly solvent (Cunliffe, P. T. W and Williams Citation1998; Pakdel, Pantea, and Roy Citation2001; Roy, Chaala, and Darmstadt Citation1999) Alkane and alkene groups make up the majority of aliphatic compounds. The majority of aromatic compounds found in the catalytic oil were described. The GC-MS results collaborate with the findings of the FT-IR studies. A small amount of nitrogen and oxygen-containing compounds were found additionally with key hydrocarbons. The waste tire-related liquids also contain other oxygen-containing compounds such as acid and alcohol. Although in the sample of CT ratio 0.1, naphthalene reached 24.62% area concentration.
The presence of aromatic compounds in pyrolytic oils derived from waste tires is a common phenomenon (Czajczyńska et al. Citation2020), and the amount increases significantly with increasing use of a catalyst (Osayi and Osifo Citation2019). Moreover, Pyrolytic oil from tire pyrolysis is a complex mixture that includes both aliphatic and aromatic compounds, with total concentrations of 49.54 and 16.65 %, respectively (M. Rofiqul Islam, Tushar, and Haniu Citation2008).
explains most of the product compound is aromatic and aliphatic hydrocarbon compounds present in pyrolytic oil without catalyst. Those compounds are including 1,2,3,4 trimethyl benzene (9.25%), d-limonene (6.44%), 1,2,3,4,5-tetramethyl benzene (4.10%) and Naphthalene (14.31%). According to (Roy et al. Citation1990), performed non-catalysed scrap tire pyrolysis in a vacuum reactor and recorded similar GC–MS results for generated oil. Pyrolytic oil derived from catalysed pyrolysis with ZSM-5 catalyst has produced more aromatic hydrocarbon compounds, including 1-ethyl-3,4-methyl benzene (5.77%), benzene acetamide (4.65%), o-xylene (1.975%), ethylbenzene (1.83%), d-limonene (4.94%), and naphthalene (24.62%) for CT ratio 0.1. When CT ratio has been increased, concentration area for benzene (18.66%), toluene (10%), phenol (10.57%) for 0.2 CT ratio and benzene (9.91%), toluene (9.07%), o-xylene (9.91%), ethylbenzene (1.87%), d-limonene (7.51%) and Naphthalene (16.71%) for CT ratio 0.25 has been found respectively.
Figure 9. GC–MS results of probable compound of pyrolytic oil from waste tire (a), (b, (c), (d) and (e)
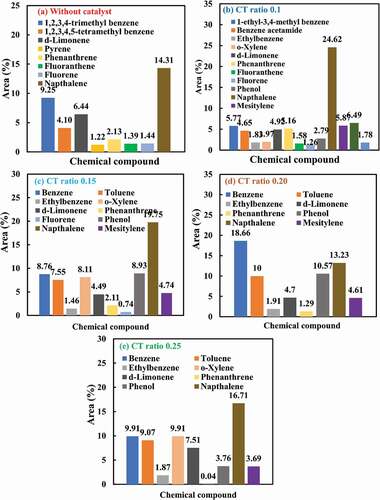
: GC–MS results of probable compound of pyrolytic oil from waste tire
Different researchers used different forms of zeolite catalysts to catalytically pyrolyse tire waste and obtained comparable outcomes for pyrolytic oil composition (Qu et al. Citation2006; Shen et al. Citation2006; Williams and Brindle Citation2003) Because of their wide surface area, and high catalytic activity, oil generated by catalysed pyrolysis of synthetic zeolites produced a high amount of aromatic compounds (Williams and Brindle Citation2003). According to (Miguel et al. Citation2006), zeolite catalysts favoured the processing of aromatic compounds during catalytic pyrolysis of scrap tires. These aromatic compounds are highly valuable in terms of raw materials for various chemical industries. Some of the vital usages, namely are making of plastic materials, synthetic fibres, resins, rubber lubricants, detergents, dyes, pesticides, and drugs.
4. Conclusion
In this research work, scrap tire pyrolysis with a different catalyst to tire ratio was carried out. The influence of pyrolysis temperature and different CT ratios indicates that within a specific range of operating temperature, oil and gas production has increased, and generation of char has decreased for higher CT ratios. Density, kinematic viscosity, and GCV of high CT ratio pyrolytic oil produced from scrap tire pyrolysis are quite favourable to be used in various industrial applications. Elemental analysis showed an increment of carbon and reduction of sulphur in produced oil. FT-IR tests suggest enhancement of aromatic and aliphatic compounds by increasing the CT ratio. GC-MS analysis showed that generated pyrolytic oils are rich in various monocyclic and polycyclic aromatic hydrocarbons. The concentration of benzene, toluene, o-Xylene, naphthalene, and d-limonene was increased with the increasing catalyst to tire ratio. Upgraded scrap tire-generated fuel oil using zeolite ZSM-5 can be suggested as an alternative source of energy that could make a clean environment. On the other hand, the utilisation of scrap tire can fulfill the goals of sustainable waste tire management and reduce environmental degradation in developing countries like Bangladesh.
Highlights
Zeolite ZSM-5 catalyst pyrolyzes scrap tire in a fixed-bed reactor.
Scrap tire pyrolysis using zeolite ZSM-5 decreases oil yield but increases the gas output.
The scrap tire catalytic pyrolysis increases the aromatic compound contents.
Acknowledgments
The authors acknowledge the University Grants Commission’s (UGC) funded research project to conduct this research work. The authors are also grateful to the Vice-Chancellor of Khulna University of Engineering & Technology (KUET) for permitting piloting the project. Technical help for chemical analysis provided by BCSIR is thankfully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Adnan Abedeen
Md Shameem Hossain is currently a Ph.D. candidate in the Graduate School of Science and Technology (GSST), Department of Environment and Energy Systems, Shizuoka University, Hamamatsu, Japan. Previously, he worked as an Assistant Professor, Department of Energy Science and Engineering (ESE), Khulna University of Engineering & Technology (KUET), Khulna-9203, Bangladesh. He has completed his Bachelor of Engineering degree in Chemical Engineering and Polymer Science from Shahjalal University of Science and Technology (SUST), Sylhet, Bangladesh in 2007 (passing year 2009). After that, he completed his M. Sc. Engineering degree in Energy Technology from Khulna University of Engineering & Technology (KUET), Khulna-9203 in 2015. In the meantime, he has joined as a lecturer in the Department of Energy Science and Engineering (ESE) since 01 Dec 2014, and later on, he was promoted as an Assistant Professor and has been serving in the same post from Feb 2017 to till date. He has about 10 years of experience, including in the energy industry, teaching, and research areas. Before joining as an academician, he was working as a shift supervisor in Pertomax Refinery Ltd, which is the largest Natural Gas Condensate Refinery Plant in the private sector in Bangladesh. His areas of research are renewable energy, biogas, biofuel, waste to energy, and alternative fuel. His areas of research interest are clean energy, structured catalyst, methanation, utilization of CO2, fuel cell, and sustainable energy.
Md Shameem Hossain
Adnan Abedeen is currently working as a lecturer in the Institute of Environment and Power Technology, Khulna University of Engineering & Technology (KUET), Khulna-9203, Bangladesh from 2020. He has received his M.Sc. Engineering degree in Energy Science and Engineering from Khulna University of Engineering & Technology in 2019 and B.Sc. Engineering degree in Petroleum and Mining Engineering from Jashore University of Science and Technology in 2015. He has presented several scientific papers in international conferences and worked as research assistant in two research projects. His area of research are renewable energy, biofuel, waste to energy and alternative fuel.
Uday Som
Mr. Uday Som is studying as a master’s degree student in the department of Applied Chemistry and Biochemical Engineering, Shizuoka University, Japan. He completed his B.Sc. in Chemical Engineering from Jashore University of Science and Technology (JUST), Bangladesh in 2016. He has about 3 years of experience in industry and research. Before study of master’s program as a student in Shizuoka University, he was working as a production engineer in Samuda Chemical Complex Ltd., is the largest hydrogen peroxide (H2O2)plant on private sector in Bangladesh. His area of research is Catalyst, biofuel, waste to energy and alternative fuel. His area of research interest are catalyst and sustainable energy.
MD Moniruzzaman
Md Moniruzzaman is currently Senior Scientific Officer of Bangladesh Reference Institute for Chemical Measurements (BRiCM), Ministry of Science & Technology, Bangladesh. Previously he worked as Scientific Officer and Senior Scientific Officer at Bangladesh Council of Scientific & Industrial Research (BCSIR), Bangladesh from the year 2011. Before that he also worked as Process Engineer at Samuda Chemical Complex, Munshigonj, Bangladesh and Graduate Trainee Engineer at Karnaphuli Fertilizer Company (KAFCO), Chittagong, Bangladesh. Moniruzzaman obtained his B.Sc degree in Engineering in Chemical Engineering and Polymer Science from Shahjalal University of Science & Technology (SUST), Sylhet, Bangladesh in 2007 (passing year 2009). Md. Moniruzzaman is actively involved in the field of chemical measurement like calibration, method development & validation and proficiency testing. He is the first in organizing chemical proficiency testing for testing laboratories in Bangladesh. Adsorption, Renewable Energy, Method Validation, Measurement Uncertainties is his research interests. He has published more the 10 research articles. Currently he is working on the efficacy of PVP-I oro- nasal solution to reduce SARS-CoV-2 load on oro and nasopharings.
References
- Ahamed, R., S. Hossain, M. Haque, and A. Iqbal. 2020. “Recovery of Hydrocarbon Fuel from a Mixture of Municipal Waste Plastics Using a Catalyst.” International Journal of Sustainable Engineering 13 (4): 252–263. doi:https://doi.org/10.1080/19397038.2020.1773565.
- Alsaleh, A., and M. L. Sattler. 2014. “Waste Tire Pyrolysis: Influential Parameters and Product Properties.” Current Sustainable/Renewable Energy Reports 1 (4): 129–135. doi:https://doi.org/10.1007/s40518-014-0019-0.
- Barbooti, M. M., T. J. Mohamed, A. A. Hussain, and F. O. Abas. 2004. “Optimization of Pyrolysis Conditions of Scrap Tires under Inert Gas Atmosphere.” Journal of Analytical and Applied Pyrolysis 72 (1): 165–170. doi:https://doi.org/10.1016/j.jaap.2004.05.001.
- BBS. 2008. “Bangladesh Bureau of Statistics, Government of People’s Republic of Bangladesh.” Statistical Year Book of Bangladesh, 24th.
- Benallal, B., C. Roy, H. Pakdel, S. Chabot, and M. A. Poirier. 1995. “Characterization of Pyrolytic Light Naphtha from Vacuum Pyrolysis of Used Tyres Comparison with Petroleum Naphtha.” Fuel 74 (11): 1589–1594. doi:https://doi.org/10.1016/0016-2361(95)00165-2.
- Cunliffe, P. T. W, A. M., and P. T. Williams. 1998 “Composition of Oils Derived from the Batch Pyrolysis of Tyres.” Journal of Analytical and Applied Pyrolysis 44(2)131–152 doi:https://doi.org/10.1016/S0165-2370(97)00085-5.
- Czajczyńska, D., K. Czajka, R. Krzyżyńska, and H. Jouhara. 2020. “Waste Tyre Pyrolysis – Impact of the Process and Its Products on the Environment.” Thermal Science and Engineering Progress 20: 100690. doi:https://doi.org/10.1016/j.tsep.2020.100690.
- Czajczyńska, D., R. Krzyżyńska, H. Jouhara, and N. Spencer. 2017. “Use of Pyrolytic Gas from Waste Tire as A Fuel: A Review.” Energy 134: 1121–1131. doi:https://doi.org/10.1016/j.energy.2017.05.042.
- Czernik, S., and A. V. Bridgwater. 2004. “Overview of Applications of Biomass Fast Pyrolysis Oil.” Energy and Fuels 18 (2): 590–598. doi:https://doi.org/10.1021/ef034067u.
- De Marco Rodriguez, I., M. F. Laresgoiti, M. A. Cabrero, A. Torres, M. J. Chomón, and B. Caballero. 2001. “Pyrolysis of Scrap Tyres.” Fuel Processing Technology 72 (1): 9–22. doi:https://doi.org/10.1016/S0378-3820(01)00174-6.
- Ding, K., Z. Zhong, B. Zhang, J. Wang, A. Min, and R. Ruan. 2016. “Catalytic Pyrolysis of Waste Tire to Produce Valuable Aromatic Hydrocarbons: An Analytical Py-GC/MS Study.” Journal of Analytical and Applied Pyrolysis 122: 55–63. doi:https://doi.org/10.1016/j.jaap.2016.10.023.
- Felisa, L. M., I. De Marco, A. Torres, B. Caballero, M. A. Cabrero, and M. J. Chomón. 2000. “Chromatographic Analysis of the Gases Obtained in Tyre Pyrolysis.” Journal of Analytical and Applied Pyrolysis 55 (1): 43–54. doi:https://doi.org/10.1016/S0165-2370(99)00073-X.
- Franck, H.-G., J. W. Stadelhofer, H.-G. Franck, and J. W. Stadelhofer. 1988. “Production and Uses of Benzene Derivatives.” In Industrial Aromatic Chemistry, 132–235. Springer Berlin Heidelberg.1988 doi:https://doi.org/10.1007/978-3-642-73432-8_5.
- Han, R. A. K., M. D. T. A. I. Haon, and S. M. M. Oniruzzaman. 2014. Energy Recovery from Waste Tyres and Its Recycling Pattern in Khulna City.“ International Journal of Renewable Energy and Environmental Engineering 2 (4): 262-265.
- He, Z., Q. Jiao, Z. Fang, T. Li, C. Feng, H. Li, and Y. Zhao. 2018. “Light Olefin Production from Catalytic Pyrolysis of Waste Tires Using nano-HZSM-5/Γ-Al2O3 Catalysts.” Journal of Analytical and Applied Pyrolysis 129 (December): 66–71. doi:https://doi.org/10.1016/j.jaap.2017.12.002.
- Helleur, R., N. Popovic, M. Ikura, M. Stanciulescu, and D. Liu. 2001. “Characterization and Potential Applications of Pyrolytic Char from Ablative Pyrolysis of Used Tires.” Journal of Analytical and Applied Pyrolysis 58-59: 813–824. doi:https://doi.org/10.1016/S0165-2370(00)00207-2.
- Hijazi, A., A. H. Al-Muhtaseb, S. Aouad, M. N. Ahmad, and J. Zeaiter. 2019. “Pyrolysis of Waste Rubber Tires with Palladium Doped Zeolite.” Journal of Environmental Chemical Engineering 7 (6): 6. doi:https://doi.org/10.1016/j.jece.2019.103451.
- Hooshmand Ahoor, A., and N. Zandi-Atashbar. 2014. “Fuel Production Based on Catalytic Pyrolysis of Waste Tires as an Optimized Model.” Energy Conversion and Management 87: 653–669. doi:https://doi.org/10.1016/j.enconman.2014.07.033.
- Hossain, M., Uday Som, Jinnah Hossain, M. W. Rahman, and S. A. Iqbal. “Recovery of Alternative Fuel from Thermal Pyrolysis of Medical Wastes.” In Proceedia of the ICERIE International. Conference on Engineering Research, Innovation and Education, pp. 683–688. 2017.Sylhet, Bangladesh
- Hossain, M. S., and A. N. M. M. Rahman. 2017. “Catalytic Pyrolysis of Tire Wastes for Liquid Fuel.” Iranica Journal of Energy and Environment 8 (1): 88–94.
- Hossain, M. S., and D. A. N. M. M. Rahman. 2015. “Production of Liquid Fuel from Pyrolysis of WasteTires.” International Journal of Scientific & Engineering Research 6 (11): 1224–1229. doi:https://doi.org/10.14299/ijser.2015.11.013.
- Hossain, M. S., Abedeen, A.,Karim, M. R., Moniruzzaman, M.,& Juwel Hosen, M., 2017 “Catalytic Pyrolysis of Waste Tires: the influence of ZSM-catalyst/tire ratio on Product. Iranian (Iranica).” Journal of Energy & Environment 8 (3): 189–193.
- Ilkiliç, C., and H. Aydin. 2011. “Fuel Production from Waste Vehicle Tires by Catalytic Pyrolysis and Its Application in a Diesel Engine.” Fuel Processing Technology 92 (5): 1129–1135. doi:https://doi.org/10.1016/j.fuproc.2011.01.009.
- Islam, M. R., M. N. Islam, N. N. Mustafi, M. A. Rahim, and H. Haniu. 2013. “Thermal Recycling of Solid Tire Wastes for Alternative Liquid Fuel: The First Commercial Step in Bangladesh.” Procedia Engineering 56: 573–582. doi:https://doi.org/10.1016/j.proeng.2013.03.162.
- Islam, M. R., M. S. H. K. Tushar, and H. Haniu. 2008. “Production of Liquid Fuels and Chemicals from Pyrolysis of Bangladeshi Bicycle/rickshaw Tire Wastes.” Journal of Analytical and Applied Pyrolysis 82 (1): 96–109. doi:https://doi.org/10.1016/j.jaap.2008.02.005.
- Islam, M. R., M. U. H. Joardder, S. M. Hasan, K. Takai, and H. Haniu. 2011. “Feasibility Study for Thermal Treatment of Solid Tire Wastes in Bangladesh by Using Pyrolysis Technology.” Waste Management 31 (9–10): 2142–2149. doi:https://doi.org/10.1016/j.wasman.2011.04.017.
- Kar, Y. 2011. “Catalytic Pyrolysis of Car Tire Waste Using Expanded Perlite.” Waste Management 31 (8): 1772–1782. doi:https://doi.org/10.1016/j.wasman.2011.04.005.
- Khalil, U., J. Vongsvivut, M. Shahabuddin, S. P. Samudrala, S. C. Srivatsa, and S. Bhattacharya. 2020. “A Study on the Performance of Coke Resistive Cerium Modified Zeolite Y Catalyst for the Pyrolysis of Scrap Tyres in A Two-stage Fixed Bed Reactor.” Waste Management 102: 139–148. doi:https://doi.org/10.1016/j.wasman.2019.10.029.
- Kordoghli, S., M. Paraschiv, M. Tazerout, B. Khiari, and F. Zagrouba. 2017. “Novel Catalytic Systems for Waste Tires Pyrolysis: Optimization of Gas Fraction.” Journal of Energy Resources Technology, Transactions of the ASME 139 (3): 22–24. doi:https://doi.org/10.1115/1.4034979.
- Laresgoiti, M. F., B. M. Caballero, I. De Marco, A. Torres, M. A. Cabrero, and M. J. Chomón. 2004. “Characterization of the Liquid Products Obtained in Tyre Pyrolysis.” Journal of Analytical and Applied Pyrolysis 71 (2): 917–934. doi:https://doi.org/10.1016/j.jaap.2003.12.003.
- Luo, S., and Y. Feng. 2017. “The Production of Fuel Oil and Combustible Gas by Catalytic Pyrolysis of Waste Tire Using Waste Heat of Blast-furnace Slag.” Energy Conversion and Management 136: 27–35. doi:https://doi.org/10.1016/j.enconman.2016.12.076.
- Miguel, G. S., J. Aguado, D. P. Serrano, and J. M. Escola. 2006. “Thermal and Catalytic Conversion of Used Tyre Rubber and Its Polymeric Constituents Using Py-GC/MS.” Applied Catalysis B: Environmental 64 (3–4): 209–219. doi:https://doi.org/10.1016/j.apcatb.2005.12.004.
- Mohammed, A. H. A. K., and K. Hankish. 1985. “Determination of Aromatic Hydrocarbons in Petroleum Fractions by Infrared Spectroscopy.” The Analyst 110 (12): 1477–1480. doi:https://doi.org/10.1039/AN9851001477.
- Moniruzzaman, S. M., Q. H. Bari, and T. Fukuhara. 2011. “Recycling Practices of Solid Waste in Khulna City, Bangladesh.” Journal of Solid Waste Technology and Management 37 (1): 1–15. doi:https://doi.org/10.5276/JSWTM.2011.1.
- Namchot, W., and S. Jitkarnka. 2016. “Catalytic Pyrolysis of Waste Tire Using HY/MCM-41 Core-shell Composite.” Journal of Analytical and Applied Pyrolysis 121: 297–306. doi:https://doi.org/10.1016/j.jaap.2016.08.009.
- Osayi, J. I., and P. Osifo. 2019. “International Journal of Chemical Engineering.” Utilization of Synthesized Zeolite for Improved Properties of Pyrolytic Oil Derived from Used Tire 2019. doi:https://doi.org/10.1155/2019/6149189.
- Pakdel, H., D. M. Pantea, and C. Roy. 2001. “Production of Dl-limonene by Vacuum Pyrolysis of Used Tires.” Journal of Analytical and Applied Pyrolysis 57 (1): 91–107. doi:https://doi.org/10.1016/S0165-2370(00)00136-4.
- Qu, W., Q. Zhou, Y. Z. Wang, J. Zhang, W. W. Lan, Y. H. Wu, J. W. Yang, and D. Z. Wang. 2006. “Pyrolysis of Waste Tire on ZSM-5 Zeolite with Enhanced Catalytic Activities.” Polymer Degradation and Stability 91 (10): 2389–2395. doi:https://doi.org/10.1016/j.polymdegradstab.2006.03.014.
- Rejeb, H., E. Berrich, M. H. Chahbani, and F. Aloui. 2020. “Energetic Valourisation of Waste Tyres by Pyrolysis: Catalyst Effect on Tyres Derived Oil and Gases.” International Journal of Global Warming 20 (3): 187–202. doi:https://doi.org/10.1016/j.molcata.2015.09.008.
- Rofiqul, I. M., M. Parveen, H. Haniu, and M. R. I. Sarker. 2010. “Innovation in Pyrolysis Technology for Management of Scrap Tire: A Solution of Energy and Environment.” International Journal of Environmental Science and Development, Vol. 1, No. 1, April 2010 1 (1): 89-96.
- Roy, C., A. Chaala, and H. Darmstadt. 1999. “The Vacuum Pyrolysis of Used Tires.” Journal of Analytical and Applied Pyrolysis 51 (1–2): 201–221. doi:https://doi.org/10.1016/s0165-2370(99)00017-0.
- Roy, C., B. Labrecque, and B. de Caumia. 1990. “Recycling of Scrap Tires to Oil and Carbon Black by Vacuum Pyrolysis.” Resources, Conservation and Recycling 4(3): 203–213.
- Sahouli, B., S. Blacher, F. Brouers, H. Darmstadt, C. Roy, and S. Kaliaguine 1996. “Surface Morphology and Chemistry of Commercial Carbon Black and Carbon Black from Vacuum Pyrolysis of Used Tyres.” Fuel 75 (10): 1244–1250
- Sainz-Diaz, C. I., and A. J. Griffiths. 2000. “Activated Carbon from Solid Wastes Using a Pilot-scale Batch Flaming Pyrolyzer.” Fuel 79 (15): 1863–1871. doi:https://doi.org/10.1016/S0016-2361(00)00052-1.
- Shah, J., M. R. Jan, and F. Mabood. 2009. “Recovery of Value-added Products from the Catalytic Pyrolysis of Waste Tyre.” Energy Conversion and Management 50 (4): 991–994. doi:https://doi.org/10.1016/j.enconman.2008.12.017.
- Sharma, V. K., F. Fortuna, M. Mincarini, M. Berillo, and G. Cornacchia. 2000. “Disposal of Waste Tyres for Energy Recovery and Safe Environment.” Applied Energy 65 (1–4): 381–394. doi:https://doi.org/10.1016/S0306-2619(99)00085-9.
- Shen, B., C. Wu, R. Wang, B. Guo, and C. Liang. 2006. “Pyrolysis of Scrap Tyres with Zeolite USY.” Journal of Hazardous Materials 137 (2): 1065–1073. doi:https://doi.org/10.1016/j.jhazmat.2006.03.040.
- Tang, Z., Q. Huang, Y. Yang, Z. Nie, J. Cheng, J. Yang, Y. Wang, and M. Chai. 2016. “Polybrominated Diphenyl Ethers (Pbdes) and Heavy Metals in Road Dusts from a Plastic Waste Recycling Area in North China: Implications for Human Health.” Environmental Science and Pollution Research 23 (1): 625–637. doi:https://doi.org/10.1007/s11356-015-5296-7.
- Venuto, P. B., and J. E. T. Habib. 1979. “Fluid Catalytic Cracking with Zeolite Catalysts.” United States.
- Wang, F., N. Gao, C. Quan, and G. López. 2020. “Journal of Analytical and Applied Pyrolysis.” Investigation of Hot Char Catalytic Role in the Pyrolysis of Waste Tires in a Two-step Process 146 (October2019): 104770. doi:https://doi.org/10.1016/j.jaap.2019.104770.
- Williams, P. T., and A. J. Brindle. 2003. “Aromatic Chemicals from the Catalytic Pyrolysis of Scrap Tyres.” Journal of Analytical and Applied Pyrolysis 67 (1): 143–164. doi:https://doi.org/10.1016/S0165-2370(02)00059-1.
- Williams, P. T., and R. P. Bottrill. 1995. “Sulfur-polycyclic Aromatic Hydrocarbons in Tyre Pyrolysis Oil.” Fuel 74 (5): 736–742. doi:https://doi.org/10.1016/0016-2361(94)00005-C.