ABSTRACT
Paint, tannery and textile manufacturing wastewaters contain highly toxic and organic biorefractory compounds and have adverse effects on human health. These effluents require efficient and environment friendly solutions before being discharged. Jar-test experiments are conducted in order to assess the efficiency of a tannin-based polymer (TBP) on the treatment of these industrial wastewaters.
The results indicate that TBP is more effective than classical coagulant iron chloride. Coagulation flocculation involving TBP does not require any coagulation pH adjustment, reduces more than 96% of colour and achieves 60, 65 and 87% of COD removal from textile, tannery and paint wastewater respectively. Compared to iron chloride, TBP produces the least amount of sludge for a given amount of COD and colour removed. It produces 28 to 60 % less volume of sludge than FeCl3, and generates a good settling sludge with lower values of SVI (31- 80 ml g−1). Furthermore, TBP allows 4 to 6.5% of water recovery more than FeCl3. Utilisation of such polymer represents an important progress in sustainable environmental technology as it is a renewable resource and allows a lower pollution load, a good settling sludge and an adequate water recovery.
1. Introduction
Industrial wastewater may be a hazardous and noxious product which should be adequately treated in order to avoid environmental and health implications (Sahu et al. Citation2018; Olakunle et al. Citation2018). These detrimental effects are more apparent and observable in developing countries due to their less stringent environmental regulations and difficulty in constructing, operating and maintaining proper wastewater treatment systems due to high fixed costs (Montgomery and Elimelech Citation2007). To overcome these constraints, the best immediate option is to use simple and relatively cost-effective point-of-use (POU) technologies such as coagulation (Bhuptawat, Folkard, and Chaudhari Citation2007). In coagulation flocculation treatment we commonly use inorganic coagulants such as aluminium sulphate, ferric chloride and calcium carbonate and synthetic organic polymer (polyaluminium chloride (PACl) polyethylene imine). While the effectiveness of these chemicals as coagulants is well-recognised, there are, nonetheless, disadvantages associated with their usage, such as, ineffectiveness in low-temperature water (Xiao et al. Citation2008), detrimental effects on human health (Flaten Citation2001), production of large sludge volumes and the fact that they significantly affect pH of treated water. Moreover, the sludge generated using aluminium salts leads to disposal problems such as aluminium accumulation in the environment (Abdullah et al. Citation1995). It is therefore desirable to replace these chemical coagulants with natural-based agents to counteract the aforementioned drawbacks. Although many plant-based coagulants have been reported as effective substitutes to inorganic coagulants, only four types are generally well-known within the scientific community, namely, nirmali seeds (Strychnos potatorum) (Sarawgi et al. Citation2009; Gautam et al. Citation2021), Moringa oleifera (Beltrán-Heredia and Sánchez-Martín Citation2009; Shan et al. Citation2017), cactus (Sellami et al. Citation2014; Rachdi, Srarfi, and Slim Shimi Citation2017) and tannin (Aboulhassan et al. Citation2005; Kavitha and Kandasubramanian Citation2020; Azreen, Abu Zahrim, and Junidah Citation2021).
Various studies have been reported concerning the use of tannin as a coagulant in the wastewater treatment. The effect of modified condensed tannins with five distinct shear viscosities (30–430 cP) on colour removal of anionic (Duasyn Direct Red and Acid Black 2) and cationic (Methylene Blue and Crystal Violet) dyes was reported by Grenda et al. (Citation2018). Good decolourisation results (85–96% reduction) were obtained with the simultaneous introduction of biocoagulant and minimal dosages of other additives into the process, which was bentonite and a cationic or anionic polyacrylamide. Mat Yasin et al. (Citation2020) studied the influence of tannin on palm oil refinery effluent treatment. It was found that the maximum removal of BOD, COD, turbidity, and SS was 97.62%, 88.89%, 93.01%, and 90.21%, respectively. According to Grenda et al. (Citation2020), the modified tannins were applied for cosmetic industry wastewater treatment. Good turbidity and decolourisation results (93% and 89% reduction, respectively) were obtained with the simultaneous introduction of 200 ppm of bio-coagulant followed by a minimal dosage (5 ppm) of cationic polyacrylamide.
Other studies have been focused on the efficiency of tannin coagulant agents compared to classical coagulant salts. Lopes et al. (Citation2019) discussed the coagulation flocculation of dyehouse effluents using a commercial tannin-derived coagulant compared with the common use of iron salt. Two simulated solutions of textile dyehouse effluents were prepared using Direct Blue 85 dye: E1, containing only dye and salts, and E2, with three supplementary dyeing chemicals. At acidic conditions (pH 4–5), iron (III) sulphate provided decolourisation and easily settleable flocs using much lower dosages than those required for the tannin coagulant. However, at neutral and alkaline conditions, which are of major relevance in practical terms, the tannin-coagulant was much more efficient than the iron coagulant. Findings from the study of Aboulhassan et al. (Citation2016) indicated that a tannin-based coagulant was more effective than FeCl3 and Al2(SO4)3 for the treatment of COD and colour in paint manufacturing wastewater. No pH adjustment was needed and coagulation using the tannin-based coagulant allowed removal of more than 87% COD and 99% colour while producing a lower volume of decanted sludge when compared to metal salts coagulant. In another study, an optimisation was performed to determine the performance of a tannin-based coagulant in treating the stamping industry effluents. The percentages of removal using 400 mg l−1 tannin under optimised conditions were 94.8%, 99.2% and 99.7% of COD, colour and turbidity, respectively which were slightly higher when compared to treatment with 600 mg l−1 Al2(SO4)3 resulting in 93.1%, 99.1% and 99.3% removal of COD, colour, and turbidity, respectively (Osorio Moreira Couto et al. Citation2012).
Tannin is a general name given to polyphenol compounds obtained from natural materials, and traditionally used as a tanning agent in the leather industry, hence their name, but one also finds several of them used as coagulant or coagulant aid for water treatment. Lab scale experiments have demonstrated that it is possible to synthesise tannin-derived coagulant from several tannin feedstocks, through a very simple procedure that involves Mannich base reaction. Mannich reaction can be described as the introduction of a quaternary nitrogen inside the tannin complex structure (Tramontini and Angiolini Citation1994). Tannins undergo Mannich aminomethylation by reaction with an aldehyde and an amine, generating the addition of the carboxyl groups and amines to the tannin structure (Arismendi et al. Citation2018; Lopes et al. Citation2019). As a result, tannin Mannich polymer has a higher molecular weight due to formaldehyde and Mannich base crosslinking, and has also an ampholytic character due to the presence of both cationic amines and anionic phenols on the polymer. However, the chemical complexity of tannins and the fact that they are usually taken from natural matrix without a very thorough purification make knowing their structure a very difficult task. A full study about tannins, chemical structure and properties can be found in previous scientific literature (Pizzi Citation2008).
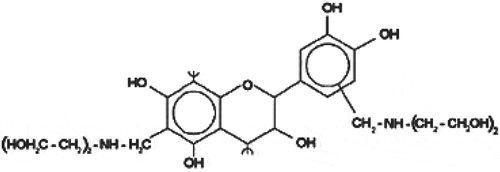
Mannich bases are obtained using NH4Cl or other types of nitrogen compounds, such as mono or diethanolamine, and the reaction is completed by the controlled addition of formaldehyde. The production processes of these coagulants are not completely known, but by following the rules of Mannich reactions one can surely make an approach of their synthesis. In the case of diethanolamine (DEA), formaldehyde and tannin extract mixture, the coagulant chemical formula may respond to tannin–(CH2–NH–(CH2–CH2OH)2)n and its probable expanded chemical structure is shown in (Beltrán-Heredia, Sánchez-Martín, and Gómez-Muñoz Citation2010).
Figure 1. Probable chemical structure of tannin based polymer (Beltrán-Heredia, Sánchez-Martín, and Gómez-Muñoz Citation2010)
In many cases, the natural coagulants can perform at their best when used for treatment of wastewater with less variety of contaminants (synthetic wastewater) (Nakano, Takeshita, and Tsutsumi Citation2001; Zhan and Zhao Citation2003; Palma, Freer, and Baeza Citation2003; Kim and Nakano Citation2005; Beltrán-Heredia et al. Citation2009). However, there are not many scientific references reporting the tannin application to real industrial wastewater treatment. This investigation focuses its interest in wastewater treatment by means of a tannin-based cationic polymer (TBP) with minimum colour, organic matter and suspended solids present in the treated wastewater. The principal goal is to assess the efficacy of TBP on the treatment of real industrial wastewater, especially, textile, paint and tannery effluents. The chemical oxygen demand (COD), colour, sludge volume index (SVI) and water recovery are used as evaluating parameters.
2. Material and methods
2.1. Sampling procedures of industrial wastewaters
Samples of effluents are collected from outfall of paint, tannery and textile manufacturing factories situated in an industrial park of the Casablanca city, Morocco. The generated effluents are discharged into sewer without any treatment. Sampling of the wastewater was carried out according to standard methods for the examination of wastewater. After in-site pH, conductivity, and turbidity measurements, the samples were transferred to the laboratory for further analysis, and treatment.
2.2. Coagulation-flocculation process
Tannin-based polymer is a natural organic polymer extracted from vegetable material. TBP has a viscosity between 10 and 50 cps and a density of 1.1 g cm−3 at 20°C.
Jar test experiments were conducted under controlled laboratory conditions using a standard jar test apparatus. Four equal-volume polyethylene beakers were used to examine the four different dosages of coagulant in each run. The sample bottles were thoroughly shaken for resuspension of possibly settling solids and then the appropriate volumes of sample were transferred to the corresponding jar test beakers. For each test, 1000 ml of wastewaters were taken in a 1000 ml work volume beaker and, after addition of coagulant, mixed for 5 min at 150 rpm to ensure complete dispersion. After rapid mixing the slow mixing stage took place for 15 min at 30 rpm. Finally, the beaker contents were transferred into Imhoff cones for a one hour settling step.
2.3. Analytical procedures
The physicochemical parameters were evaluated in raw and treated wastewater, in order to characterise wastewaters and to assess the efficacy of coagulants on wastewater treatment. Physicochemical parameters, colour, and decanted sludge are determined in our laboratory according to standardised methods.
Physicochemical parameters: chemical oxygen demand (COD), biochemical oxygen demand (BOD), Total Kjeldahl Nitrogen (TKN), total phosphorus (TP), suspended matters (SM), chloride ion (Cl−) and sulphate (SO42-) were measured according to Standard Methods for the Examination of Water and Wastewater APHA (Clescen, Greenberg, and Eaton Citation1998).
Colour measurement: Prior to colour measurement, the sample was filtered through a microporous glass fibre membrane filter (0.45 µm) to prevent turbidity. Colour content was determined using a UV/Visible spectrophotometer (Model 7800 UV/VIS spectrophotometer) by measuring the absorbance at three wavelengths (436, 525 and 620 nm) and taking the sum of these absorbencies (Aysegül and Enis Citation2002).
Sludge volume index, SVI: SVI measurement was carried out in accordance with the Standard Methods for the Examination of Water and Wastewater APHA (Clescen, Greenberg, and Eaton Citation1998). The settled sludge volume was measured using a 1000 ml Imhoff cone and the SVI was calculated accordingly using the value of the settled sludge volume. SVI is the volume in millilitres occupied by 1 g of a suspension after 30 min settling.
3. Results and discussion
3.1. Wastewater characteristics
The quantity and quality of industrial effluent are functions of the ongoing industrial process, raw material utilised, and products manufactured in each industrial unit, and therefore the composition and constitution of wastewater generated are different for different industries.
The characteristics of wastewater have been studied in terms of key parameters like BOD, TDS, COD and colour. These parameters help in gauging the level of potential hazard the effluent can pose to the environment and human health. As seen in wastewater pHs varied from acidic to basic especially for textile rejection (5 to 11). The effluents are characterised by their colour and by including substantial organic matter, suspended solids rich and high salinity. BOD/COD index (Metcalf and Eddy Inc Citation1985) indicates that biological treatment seems to be difficult, and then a physicochemical process is required to overcome the pollution toxicity. The coagulation flocculation process using TBP and iron chloride was used in the treatment of these effluents.
Table 1. Characteristics of paint, tannery and textile wastewaters
3.2. Effect of coagulation pH
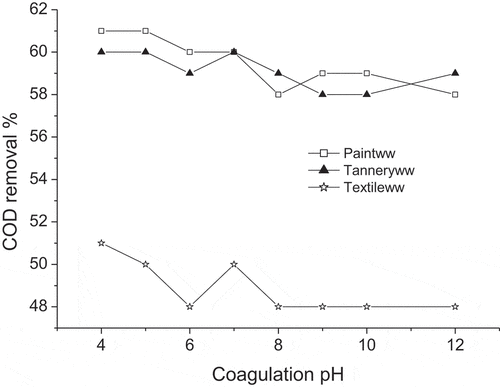
Classical salt coagulants have some drawbacks as the efficiency of the coagulation flocculation process strongly depends on pH. The chemical speciation of the coagulant, the surface charge of colloid and sometimes the charge of soluble molecule depend on pH. Moreover, the process is not always efficient enough because at different environmental conditions such as at extreme pH and at very low or very high temperature, it may produce very sensitive and fragile flocs, which result in poor sedimentation. These flocs may rupture under any type of physical forces. To select the coagulation pH, wastewaters adjusted to desirable pH values were introduced in a serial of 1000 ml work volume beakers. Then a known volume of prepared TBP solution was added to each jar and placed in jar test apparatus. The effect of coagulation pH on COD removal from jar tests for coagulation of textile, paint and tannery wastewaters is shown in . It can be seen that Initial pH did not clearly affect TBP effect on COD removal from industrial wastewaters. At pH values ranged from 4 to 12, COD removal were 48–51% from textile wastewater and 58–61% from paint and tannery wastewaters. Therefore, the process implicating TBP does not require coagulation pH adjustment. This non dependence on pH is an advantage in the coagulation–flocculation process.
3.3. Effect of TBP on pollutant removal
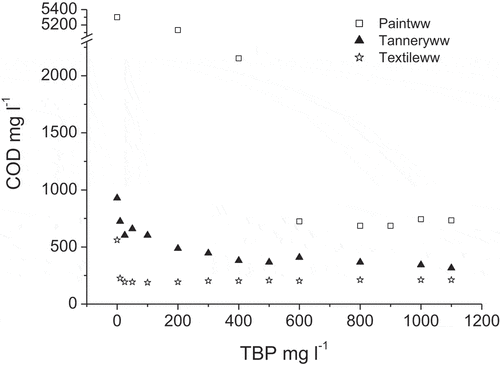
The study of the TBP effect on COD and colour removal has been undertaken by varying the amount of the tannin agent in wastewaters while keeping other conditions constant. indicates that for the quantitative removal of 87, 60 and 65% of COD from paint, tannery and textile wastewaters, minimum TBP dosages of 600, 400 and 25 mg l−1 are required, respectively. Further increase in the polymer dosage does not generate better removal and does not causes the restabilisation of particles phenomenon as the removal efficiency still almost constant. This stability may be due to the sweep coagulation mechanism linked to chemical flocculation, which occurred as the structure of TBP is large.
The difference on COD removal efficiency may be attributed to the nature of wastewater, as well as to the large difference in initial suspended solid content (). The process allows less removal of COD from textile and tannery wastewaters (60–65%); which may be explained by the solubility of a large part of COD, consequently it cannot be removed by decantation.
Colour and COD removals corresponding to the optimal dosage of TBP for each industrial wastewater are shown in . It can be seen that the optimal dosage of TBP and the corresponding COD and colour removals depend on the type of industrial wastewater. Additionally, the process removes more colour than COD and leads to the reduction of 60–87% of COD and 96–99% of colour. As the COD is linked to particular and soluble fractions, the removal efficiency of COD may be explained by the fact that in the soluble COD fraction, only some macromolecules are integrated in the flocculation process by sorption into the floc structure. However, colourants in aqueous solutions are often present in the form of colloids and the flocculated decolourisation seems to be the main mechanism responsible for the decolourisation process. The process is essentially attributed to the interaction between the TBP and colourant, but not to the effluent nature like COD. Through energetic and hydrophobic interactions, the molecules producing colour, colloidal metallic hydroxides or organic compounds, intensively bind with the TBP (Ying et al. Citation2003), leading finally to colour removal.
Table 2. COD and colour removal efficiencies using TBP
3.4. TBP compared to classical coagulants
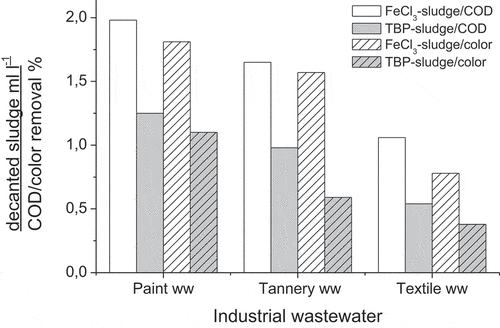
As an approach to the importance of this natural agent, tests comparing effectiveness of the classical coagulant iron chloride and TBP has been carried out. To compare the results, we consider ratios between the produced sludge and the removal of organic and colouring matters (). Clearly the ratios obtained with TBP are markedly less than those obtained using iron chloride, and indicate that TBP produces the least amount of sludge for a given amount of COD and colour removed than FeCl3. The fact that this natural coagulant is more efficient than iron chloride may be due to its ability to contribute to the three-dimensional network as a cross-linking agent. While iron chloride is able to destabilise colloidal materials only by a coagulation mechanism, the TBP polymer is involved in a second mechanism of flocculation (Graham et al. Citation2008). TBP is macro-molecular structure with variety of functional groups which can either work as coagulant by destabilising the charged stable particles mainly through the process of adsorption and neutralisation, and can work as coagulant aid by attaching the destabilised particles with the functional groups by interparticle bridging. The amine groups attached to the first and the last aromatic rings (-CH2-NH-(CH2-CH2OH)2), the oxygen atoms in the middle and the two N-H bonds, provide the polar propriety to this natural coagulant molecule (). Anionic colloid particles can then react with the coagulant’s positively charged nitrogen atom to form a more complex molecule, and subsequently the colloidal structure is destabilised and if the size is large enough, settling operates (Kim Citation1995). Following this initial stage of floc formation, free active centres on the floc surface can appear because of the large structure of the TBP. The adsorption process occurs via electrostatic attraction between suspended colloids and the floc surface. The flocs begin to grow, and colloids removal increases (Bulatovic Citation2007). Furthermore, the intermolecular interaction between π-electron system from colourant molecule and the OH group (electron-deficient hydrogen atom) from TBP (hydrogen bonding) may contribute to substantive adsorption of colourants to highly solvated polymers in aqueous solution due to the delocalised π-electron clouds of dye (Yoshida, Osawa, and Oda Citation1964). TBP’s action leads to the formation of flocs, so that these two effects, coagulation and adsorption onto flocs, work synergistically.
3.5. Sludge production and water recovery
The stability of generated flocs and the effluent quality as well as the water recovery are crucial in solid liquid separation process. In general, the amount and characteristics of the sludge produced during the coagulation–flocculation process depend on the coagulants used and on the operating conditions. To examine the settling characteristics of the sludge produced, SVI was determined. mentions SVI and water recovery versus the industrial wastewater type and the applied treatment agent. Coagulation flocculation using TBP generates 31, 46.27 and 80.50 ml g−1 of SVI for paint, tannery and textile wastewaters respectively, which reflect a good settling sludge. Ahmad et al. (Ahmad et al. Citation2007) report that the SVI values below 100 are desired to assure that the produced sludge in the coagulation–flocculation process has sufficient settling characteristics. TBP allowed highest dewatering efficiency of sludge and produced 28 to 60 % less sludge volume compared to FeCl3. In fact, the classical coagulant generated more volume of sludge without sufficient settling characteristics especially for textile and tannery wastewaters as the SVI values are beyond than 100 ().
Table 3. SVI and water recovery using TBP and iron chloride
The SVI in coagulation–flocculation process is generally governed by three factors: high polymer effect, osmotic pressure effect and hydration effect (Ives Citation1978). TBP as a cationic polymer could destabilise particles by neutralising the negative charge and bridging. However, the iron chloride ability of removing pollutants is achieved mainly by the adsorption of the amor polymer chain. After the polymer was added into the wastewater, the colloidal particles were aggregated into highly dense flocs, which precipitated more readily, and then were effectively separated. At the same time and as the TBP was positively charged, the osmotic effect could be decreased and the high polymer effect could become neglected. Therefore, TBP was favourable for minimising the SVI. Furthermore, the effect of FeCl3 on SVI can be explained by the production of iron hydroxide as a precipitate. While, if TBP is used, only initial suspended particles are agglomerated into larger and settleable flocs, but no additional precipitate is formed. As a result, better quality and higher water quantity could be obtained.
One aspect to be considered for the choice of a coagulant, in addition to pollutant removal, is how much water it will recover (Al-Mutairi, Hamoda, and Al-Ghusain Citation2004). shows that TBP allowed high water recovery and produced less volume of sludge. Depending on the type of industrial effluent, TBP allowed 4 to 6.5% of water recovery more than FeCl3.
According to Bolto and Gregory (Bolto and Gregory Citation2007), natural polymeric coagulants form large and steady flocs via bridging effect with higher resistance to shear forces in a turbulent flow compared to non-polymeric coagulants such as iron chloride. This implies that TBP can be used within a batch stirred tank setup to treat industrial wastewater since bridging linkages are more resistant to breakage at high shear levels.
TBP presents technical and environmental advantages. Technologically, the process involving TBP is much easier than the one implicating metal salts. Coagulation pH adjustment and further flocculant agents are not needed (as it occurs with iron chloride).
TBP as a natural coagulant can be a potential substitute for synthetic products on industrial wastewaters treatment. It can avoid the negative effects from residual metal salts and may produce biodegradable sludge. Therefore, the effluent after natural polymer treatment can be treated by biological process, if required. This effluent will not pose any harm to the biological organisms, as is offered, if it is treated by means of synthetic coagulants. Not only this, the sludge generated by the natural polymers can further be treated biologically or can be disposed off safely as soil conditioners because of their non-toxicity. Furthermore, tannins are available and easy to store, and they only require a simple chemical modification. In addition, they can be a social change factor as they allow water treatment without coagulants and flocculants exterior dependence.
4. Conclusion
The efficiency of the tannin-based polymer (TBP) on the treatment of wastewater from paint, tannery and textile industries was evaluated. Coagulation flocculation has demonstrated that it is an effective method and TBP can be used as a substitute to classical coagulant agents in industrial wastewater treatment.
TBP as a natural coagulant did not require any coagulation pH adjustment and allowed more than 60% and 96% of COD and colour removals from industrial wastewaters respectively. Compared to iron chloride, TBP produced the least amount of sludge for a given amount of COD and colour removed. Furthermore, TBP had the highest dewatering efficiency, generated a good settling sludge and allowed a raiser rate of water recovery compared to FeCl3.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
M. A. Aboulhassan
M. A. Aboulhassan: Full Professor, department of process, energy and environmental engineering, National School of Applied Sciences, Ibn Zohr University, Agadir Morocco.
S. Harif
S. Harrif: PhD student, department of process, energy and environmental engineering, National School of Applied Sciences, Ibn Zohr University, Agadir Morocco.
S. Souabi
S. Souabi: Full Professor, department of process engineering, Faculty of sciences and technics, Hassan II Mohammedia University, Mohammedia Morocco.
A. Yaacoubi
A. Yaacoubi : Full Professor, department of chimestry, Faculty of sciences Semlalia, Cadi Ayyad University, Marrakech Morocco.
References
- Abdullah, M. P., I. Baba, S. Sarmani, and Erdawati. 1995. “Distribution of Aluminium from Alum Sludge in Water and Sediment.” Marine Freshwater Research 1995 (46): 159–165.
- Aboulhassan, M. A., S. Souabi, A. Yaacoubi, and M. Baudu. 2005. “Treatment of Textile Wastewater Using a Natural Flocculants.” Environmental Technology 26: 705–711.
- Aboulhassan, M. A., S. Souabi, A. Yaacoubi, and M. Baudu. 2016. “Coagulation Efficacy of a Tannin Coagulant Agent Compared to Metal Salts for Paint Manufacturing Wastewater Treatment.” Desalination and Water Treatment 57 (41): 1–7.
- Ahmad, A. L., S. S. Wong, T. T. Teng, and A. Zuhairi. 2007. “Optimization of Coagulation–flocculation Process for Pulp and Paper Mill Effluent by Response Surface Methodological Analysis.” Journal of Hazardous Materials 145: 162–168.
- Al-Mutairi, N. Z., M. F. Hamoda, and I. Al-Ghusain. 2004. “Coagulant Selection and Sludge Conditioning in a Slaughterhouse Wastewater Treatment Plant.” Bioresource Technology 95: 115–119.
- Arismendi, W. A., A. E. Ortiz-Ardila, C. V. Delgado, L. Lugo, L. G. Sequeda-Castañeda, and C. A. Celis-Zambrano. 2018. “Modified Tannins and Their Application in Wastewater Treatment.” Water Science and Technology 78: 1115–1128.
- Aysegül, P., and T. Enis. 2002. “Color Removal from Cotton Textile Industry Wastewater in an Activated Sludge System with Various Additives.” Water Research 36: 2920–2925.
- Azreen, I., Y. Abu Zahrim, and L. Junidah. 2021. “Synthesising Tannin-based Coagulants for Water and Wastewater Applications: A Review.” Journal of Environmental Chemical Engineering 9: 1. doi:https://doi.org/10.1016/j.jece.2020.105007.
- Beltrán-Heredia, J., and J. Sánchez-Martín. 2009. “Removal of Sodium Lauryl Sulphate by Coagulation Flocculation with Moringa Oleifera Seed Extract.” Journal of Hazardous Materials 164 (2–3): 713–719.
- Beltrán-Heredia, J., J. Sánchez-Martín, A. Delgado-Regalado, and C. Jurado-Bustos. 2009. “Removal of Alizarin Violet 3R (Anthraquinonic Dye) from Aqueous Solutions by Natural Coagulants.” Journal of Hazardous Materials 170 (1): 43–50.
- Beltrán-Heredia, J., J. Sánchez-Martín, and M. C. Gómez-Muñoz. 2010. “New Coagulant Agents from Tannin Extracts: Preliminary Optimization Studies.” Chemical Engineering Journal 162: 1019–1025.
- Bhuptawat, H., G. K. Folkard, and S. Chaudhari. 2007. “Innovative Physico-chemical Treatment of Wastewater Incorporating Moringa Oleifera Seed Coagulant.” Journal of Hazardous Materials 142: 477–482.
- Bolto, B., and J. Gregory. 2007. “Organic Polyelectrolytes in Water Treatment.” Water Research 41: 2301–2324.
- Bulatovic, S. 2007. Dispersion, Coagulation and Flocculation In: Handbook of Flotation Reagents. Amsterdam: Elsevier.
- Clescen, L. S., A. E. Greenberg, and A. D. Eaton. 1998. Standard Methods for the Examination of Water and Wastewater. twentieth ed. Washington, DC, USA: APHA.
- Couto, O. M., J. Maria Ang´elica, B. Sim˜oes Dornelas, and P. Nehemias Curvelo. 2012. “Study on Coagulation and Flocculation for Treating Effluents of Textile Industry.” Acta Scientiarum. Technology 35 (1): 83–88. doi:https://doi.org/10.4025/actascitechnol.v35i1.11685.
- Flaten, T. P. 2001. “Aluminium as a Risk Factor in Alzheimer’s Disease, with Emphasis on Drinking Water.” Brain Research Bulletin 55 (2): 187–196.
- Gautam, S., A. S. Arora, A. K. Singh, P. Ekka, H. Daniel, B. Gokul, S. Toppo, P. Chockalingam, H. Kumar, and J. F. Lyngdoh. 2021. “Coagulation Influencing Parameters Investigation on Textile Industry Discharge Using Strychnos Potatorum Seed Powders.” Environment, Development and Sustainability 23: 5666–5673. doi:https://doi.org/10.1007/s10668-020-00836-5.
- Graham, N., F. Gang, G. Fowler, and M. Watts. 2008. “Characterisation and Coagulation Performance of A Tannin-based Cationic Polymer: A Preliminary Assessment. Colloids and Surfaces A.” Physicochemical and Engineering Aspects 327: 9–16.
- Grenda, K., J. Arnold, J. A. F. Gamelas, and M. G. Rasteiro. 2020. “Up-scaling of Tannin-based Coagulants for Wastewater Treatment: Performance in a Water Treatment Plant.” Environmental Science and Pollution Research 27: 1202–1213. doi:https://doi.org/10.1007/s11356-018-2570-5.
- Grenda, K., J. Arnold, D. Hunkeler, J. A. F. Gamelas, and M. G. Rasteiro. 2018. “Tannin-based Coagulants from Laboratory to Pilot Plant Scales for Coloured Wastewater Treatment.” Bioresources 13 (2): 2727–2747. doi:https://doi.org/10.15376/biores.13.2.2727-2747.
- Ives, K. J. 1978. The Scientific Basis of Flocculation, Sijthoff & Noordhoff. The Netherlands: Alphen aan den Rijn.
- Kavitha, V. U., and B. Kandasubramanian. 2020. “Tannins for Wastewater Treatment.” SN Applied Sciences 2: 1081. doi:https://doi.org/10.1007/s42452-020-2879-9.
- Kim, Y. H., Ed. 1995. Coagulants and Flocculants; Theory and Practice. Littleton, USA: Tall Oak Publishing.
- Kim, Y. H., and Y. Nakano. 2005. “Adsorption Mechanism of Palladium by Redox within Condensed-tannin Gel.” Water Research 39: 1324–1330.
- Lopes, E. C., S. C. R. Santos, A. M. A. Pintor, R. A. R. Boaventura, and C. M. S. Botelho. 2019. “Evaluation of a Tannin-based Coagulant on the Decolorization of Synthetic Effluents.” Journal of Environmental Chemical Engineering 7 (3): 103125. doi:https://doi.org/10.1016/j.jece.2019.103125.
- Mat Yasin, N. M. F., S. H. Md., K. H. P. S. Abdul, M. Zulkifli, A. J. A. Adel Al-Gheethi, and N. A. Y. Ahmad. 2020. “Treatment of Palm Oil Refinery Effluent Using Tannin as a Polymeric Coagulant: Isotherm, Kinetics, and Thermodynamics Analyses.” Polymers 12 (10): 2353. doi:https://doi.org/10.3390/polym12102353.
- Metcalf and Eddy Inc. 1985. Wastewater Engineering: Treatment, Disposal and Reuse. third ed. New York, USA: McGraw-Hill.
- Montgomery, M., and M. Elimelech. 2007. “Water and Sanitation in Developing Countries: Including Health in the Equation.” Environmental Science and Technology 41 (1): 17–24.
- Nakano, Y., K. Takeshita, and T. Tsutsumi. 2001. “Adsorption Mechanism of Hexavalent Chromium by Redox within Condensed-Tannin Gel.” Water Research 35: 496–500.
- Olakunle, M. O., A. A. Inyinbor, A. O. Dada, and O. S. Bello. 2018. “Combating Dye Pollution Using Cocoa Pod Husks: A Sustainable Approach.” International Journal of Sustainable Engineering 11 (1): 4–15.
- Palma, G., J. Freer, and J. Baeza. 2003. “Removal of Metal Ions by Modified Pinus Radiata Bark and Tannins from Water Solutions.” Water Research 37 (20): 4974–4980.
- Pizzi, A. 2008. Tannins: Major Sources, Properties and Applications, Monomers, Polymers and Composites from Renewable Sources. Amsterdam: Elsevier.
- Rachdi, R., F. Srarfi, and N. Slim Shimi. 2017. “Cactus Opuntia as Natural Flocculant for Urban Wastewater Treatment.” Water Science and Technology 76 (7): 1875–1883. doi:https://doi.org/10.2166/wst.2017.370.
- Sahu, O., D. G. Rao, A. Thangavel, and S. Ponnappan. 2018. “Treatment of Sugar Industry Wastewater Using a Combination of Thermal and Electrocoagulation Processes.” International Journal of Sustainable Engineering 11 (1): 16–25.
- Sarawgi, G., K. Kamra, N. Suri, A. Kaur, and I. P. Sarethy. 2009. “Effect of Strychnos Potatorum Linn. Seed Extracts on Water Samples from Different Sources and with Diverse Properties.” Asian Journal of Water, Environment and Pollution 6: 13–17.
- Sellami, M., Z. Zarai, M. Khadhraoui, N. Jdidi, R. Leduc, and F. B. Rebah. 2014. “Cactus Juice as Bioflocculant in the Coagulation-flocculation Process for Industrial Wastewater Treatment: A Comparative Study with Polyacrylamide.” Water Science and Technology 70 (7): 1175–1181.
- Shan, T. C., M. A. Matar, E. A. Makky, and N. A. Eman. 2017. “The Use of Moringa Oleifera Seed as a Natural Coagulant for Wastewater Treatment and Heavy Metals Removal.” Applied Water Science 7: 1369–1376. doi:https://doi.org/10.1007/s13201-016-0499-8.
- Tramontini, M., and L. Angiolini. 1994. Mannich Bases-Chemistry and Uses. Vol. 5. CRC Press: Boca Raton.
- Xiao, F., J. Ma, P. Yi, and J. C. Huang. 2008. “Effects of Low Temperature on Coagulation of Kaolinite Suspensions.” Water Research 42 (12): 2983–2992.
- Ying, Y., Y.-Y. Zhuang, L. Ying, and M.-Q. Qiu. 2003. “Effect of Dye Structure on the Interaction between Organic Flocculant PAN-DCD and Dye.” Industrial & Engineering Chemistry Research 42 (26): 1589–1596.
- Yoshida, Z. I., E. Osawa, and R. Oda. 1964. “Intermolecular Hydrogen Bond Involving a -base as the Proton Acceptor. I. Detection by the Refractive Index Method.” Journal of Physical Chemistry 68: 2895–2898.
- Zhan, X. M., and X. Zhao. 2003. “M Echanism of Lead Adsorption from Aqueous Solutions Using an Adsorbent Synthesized from Natural Condensed Tannin.” Water Research 37 (16): 3905–3912.