ABSTRACT
The decarboxylation of 9-cis-octadecenoic (oleic) acid to aromatic and aliphatic hydrocarbons suitable as blend components for aviation fuel applications utilising ruthenium dodecacarbonyl [Ru3(CO)12] with a variety of other catalysts is presented. Due to the different relative rates of decarboxylation and hydrogenation/dehydrogenation, different product distributions are possible when the catalyst is varied. Chloro-1,5-cyclooctadiene iridium (I) dimer [(C8H12IrCl)2] gave a similar conversion to the ruthenium system but provided an altered product distribution. Aromatic contents of up to 35% were achieved in the iridium system, thereby providing a wider range of blending options for fuel producers. Additionally, both catalytic systems were found to decarboxylate high-oleic soybean oil fatty acids, giving a product of similar energy content to that obtained using high grade oleic acid.
Introduction
Natural oil use as feedstocks for various industries has been a topic of interest of recent interest (Biermann et al. Citation2011). The formation of polymer feedstocks (Kraus Citation2014; Kraus and Riley Citation2012; Meier, Metzger, and Schubert Citation2007) or hydrocarbon fuels from natural oils requires the removal of oxygen from the stating material, which has been accomplished by several different methods (Argauer and Landolt Citation1972; Bezergianni and Kalogianni Citation2009; Chandrasekaran et al. Citation2015; Dupont et al. Citation2009; Kang et al. Citation2005; Kimmich Citation2002; Kloprogge, Duong, and Frost Citation2005; Popov and Kumar Citation2015; Quirino et al. Citation2009; Santillan-Jimenez and Crocker Citation2012; Smith, El-Hiti, and Al-Shamali Citation2006; Takemura et al. Citation1985; Tran et al. Citation2010; Twaiq, Zabidi, and Bhatia Citation1999; Vasquez, Eduardo Silva, and Fernanto Castillo Citation2017; Zhao, Bruck, and Lercher Citation2013) dating back 70 years. (Chang and Wan Citation1947). Both homogeneous and heterogenous (Snåre et al. Citation2006) systems are reported. These methods include the use of Metal Organic Frameworks (MOFs) (Kimmich Citation2002; Ranu and Jana Citation1998), zeolites (Foglia and Barr Citation1976; Takemura et al. Citation1985; Vasquez, Eduardo Silva, and Fernanto Castillo Citation2017), and both precious metals, base metals including lead (Kochi, Sheldon, and Lande Citation1969). Further, a binuclear system (Gao et al. Citation1998) and a report into the use of supercritical fluids are also available (Jessop, Ikariya, and Noyori Citation1999). Generally, they can be separated into decarbonylation systems, which liberate carbon monoxide, and decarboxylation systems, which liberate carbon dioxide. The energies of these processes are different, but are both favourable at high temperatures (Gong, Ma, and Wang Citation2007; Immer, Kelly, and Lamb Citation2010; Ohlmann et al. Citation2012). In one interesting report, both are quantified using palladium on alumina (Berenblyum et al. Citation2011). The later, decarboxylation, is the process which is studied in this work.
One of the most promising recent developments in this area is a reaction based on ruthenium catalysis (Murray, Doll, and Liu Citation2018; Murray, Walter, and Doll Citation2014). This process, building on ruthenium carbonyl carboxylates described earlier (Crooks et al. Citation1969; Salvini, Frediani, and Piacenti Citation2000), is most effective on mono-unsaturated carboxylic acid substrates. The mechanism involves isomerisation of the double bond and then a decarboxylation reaction to yield carbon dioxide and an alkene that is has one fewer carbon atom than the original carboxylic acid. The isomerisation aspect of the invention improved the chemical and physical properties of a lubricant additive (Doll et al. Citation2016), but the decarboxylation aspect is considered most useful for the synthesis of polymer additives and fuels.
While generally, the use of edible feedstocks is controversial the use of technologies such as this have greater possibilities in the potential utilisation of non-edible feedstocks compared to traditional biodiesel, where very specific fatty acid combinations are required to produce fuel that meets specifications (Knothe Citation2011). The fuel properties of the biobased fuel prepared via isomerisation/decarboxylation of fatty acids using ruthenium catalysis were studied in preliminary reports (Doll et al. Citation2017; Moser et al. Citation2016), followed by a more detailed study (Knothe et al. Citation2017). The decarboxylation of 9-cis-octadecenoic (oleic) acid gave an ~75% yield of hexadecene isomers along with saturated hexadecane and a small amount of aromatic components. Further, this fuel mixture was blended with conventional petroleum diesel fuel and the relevant properties were measured. A subsequent study (Knothe et al. Citation2017) took a more detailed look at the isomers produced via this process as well as the aromatic components. Within this work, it was observed that not only did the major decarboxylation and isomerisation reactions occur, but minor amounts of hydrogenation, dehydrogenation, and cyclization/aromatisation could be quantified by the observed product ratios.
It is the products of the minor reaction that are of interest to the current work. The synthesis of synthetic hydrocarbons suitable for aviation fuel has been important to researches in recent years (Vasquez, Eduardo Silva, and Fernanto Castillo Citation2017) where the ideal content of aromatics is an important aspect of aviation fuel. Although beyond the scope of this work, a full and recent review of aviation fuel is available (Vozka and Kilaz Citation2020). Aromatics are beneficial because they alter fuel density and, more importantly, reduce shrinkage in elastomeric seals and reduce seal failure. However, if the content of aromatics is too high, these same seals will over-soften, which may result in failure. A minimum aromatic content of 8% by volume is needed in aviation fuels for density and seal shrinkage issues, and a maximum limit of 25% needed to maintain other desirable fuel properties, as is specified in ASTM standard D1655-12. There is a with a further requirement in the specification that naphthalenes be restricted to a maximum of 3% by volume.
Conventionally produced petrochemically-based aviation fuels contain approximately 16% aromatics, which limits the blending ratio of any non-aromatic containing component to 50%. This effectively caps the amount of bio-based fuels made via the ruthenium catalysed process that can be blended with conventional aviation fuel. Thus, new processes are necessary (Vozka and Kilaz Citation2020), which yield biofuels with higher aromatic content to enhance their blend ratios with conventional aviation fuel beyond 50%.
To form the desired cyclic aromatic components from oleic acid, the substrate must be dehydrogenated (Scheme 1). While ruthenium is known to be strongly active in decarboxylation and isomerisation catalysis, it is not considered highly active for hydrogenation or dehydrogenation reactions. However, iridium, combined with the appropriate ligands, has demonstrated the dehydrogenation of numerous carbon-based substrates (Caulton et al. Citation1976; Maetani et al. Citation2011), specifically in the production of further unsaturation in alkenes (Ray et al. Citation2005). It was hypothesised that combining iridium-based dehydrogenation into the isomerisation and decarboxylation process could lead to increased production of aromatic products.
Scheme 1. The reaction scheme for decarboxylation of oleic acid, which can result in unsaturated, saturated, or aromatic hydrocarbons. Liquid compounds studied in this work could be identified my GC-MS methods, and the gas compounds could be quantified by utilisation of a micro GC, in comparison to known gas standards
This report compares iridium to the ruthenium catalysed decarboxylation of oleic acid, with a specific focus on the production of aromatic components. Further, it was discovered that iridium catalysed aromatisation as well as decarboxylation and isomerisation, even without the presence of ruthenium, which was a previously unreported finding. The systems are compared in this report, with the main difference being in the relative production of aromatic compounds, and the finding that decarboxylation technology disclosed herein can produce a biofuel with aromatic contents of 8% or greater. In other words, these renewable fuels can be blended with conventional aviation fuel in any proportion yet still provide sufficient aromatics in the fuel in contrast to previous methods that do not yield aromatic products. Additionally, there are no observed napthalenes in the bio-based fuel produced by this technology, thereby avoiding that restriction as well.
Experimental
Materials
Triruthenium dodecacarbonyl (Ru3(CO)12, Strem Chemical, Newburyport, MA, 99%), chloro-1,5-cyclooctadiene iridium (I) dimer (Strem Chemical, Newburyport, MA, 99%), bis(1,5-cyclooctadiene) diiridium(I) dichloride (MilliporeSigma, St. Louis, MO 97%), (1,5-cyclooctadiene)(methoxy)iridium(I) dimer (MilliporeSigma, St. Louis, MO), diiodo(pentamethylcyclopentadienyl)iridium(III) dimer (MilliporeSigma, St. Louis, MO), iridium carbonyl (Strem Chemical, Newburyport, MA, 99%), ruthenium on alumina (Strem Chemical, Newburyport, MA, 5% loading), palladium on alumina (MilliporeSigma, St. Louis, MO, 5 wt.% loading), Os3(CO)12 (Strem Chemical, Newburyport, MA, 99%), xylene (MilliporeSigma, St. Louis, MO, reagent grade), 1-octadecene (MilliporeSigma, St. Louis, MO Technical ~90%,), stearic acid (MilliporeSigma, St. Louis, MO 95%), 9-cis-octadecenoic acid (oleic acid, Nu-Chek Prep, Elysian, MN, >99%), 9,12-cis-octadecadienoic acid (linoleic acid, Nu-Chek Prep, Elysian, MN, >99%) were all used as received. Fatty acids from soybean oil (KIC Chemical, New Paltz, NY, RBD) and high oleic soybean oil (Corteva, Wilmington, DE, Plenish 100%) were prepared by literature methods (Theodorou et al. Citation2007), utilising sodium hydroxide at scales yielding 16.9 g and 26.1 g, respectively.
Instrumentation and analysis
In all the examples, conversion was determined using an Agilent (Santa Clara, CA) model 7890A GC-FID with a DB35-MS (30 m × 320 um, 0.25 μm film) column. Relative response factors for alkanes, methyl esters, and carboxylic acids were calculated by comparison of authentic samples. Identities of products were also verified by injection into a similar GC that was equipped with an MS detector. The Nuclear Magnetic Resonance (NMR) spectra were taken in CDCl3 at 500 MHz for 1H and 125 MHz for 13C. It was equipped with a 5 mm dual probe. The gross calorific value of the samples was measured using an IKA C2000 automated bomb calorimeter (Wilmington, NC) with a D-Neslab RTE 7.0 cooler (23.5°C) and paraffin ignition strips, according to ASTM method 5468–02 (2007). Samples were placed in capsules and complete combustion of the samples was observed without the use of any combustion aid. Triplicate measurements gave standard deviations less than 0.6 MJ kg−1 in all cases. The instrument was calibrated with benzoic acid as specified in the method.
Reactions
Samples that were ~2 g were decarboxylated at temperatures of 200–300°C for 4 h in 16 × 150 mm glass tubes. Catalysts, ~20 mg in most cases, were added in an inert-atmosphere glovebox and the tubes were connected to a Schlenk line through a 22-gauge needle and septa-capped lid. Aliquots of the reaction products were analysed as described above.
Results and discussion
A series of many different catalysts, conditions, and additives were utilised in this work. This included use of additive, hydrogen donors, and water scavengers. In all, 73 different reactions were performed, not including the appropriate control experiments. All of the results reported are averages of multiple repeated experiments.
Ruthenium dodecacarbonyl
The decarboxylation of oleic acid was performed in glass tubes using triruthenium dodecacarbonyl [Ru3(CO)12] with a focus on the overall conversion and the aromatic content of the resultant product. Conversion was low with 1 wt. % catalyst at 200°C but was consistently over 90% at 250°C and quantitative at 300°C. The aromatic content at 250°C was 6.2% and <1% at 200°C. These results were similar to the comparable reactions in previous studies (Knothe et al. Citation2017) and showed that 250°C was the optimum temperature for the catalyst survey experiments with regard to overall yield as well as production of aromatic species. The previous work has shown demonstrated the recyclability of the catalyst, and also its ability to perform in a reactive distillation utilising 4 fresh aliquots of substrate (Murray, Walter, and Doll Citation2014).
Other catalysts
The reaction (Scheme 1) requires dehydrogenation to occur in order to form aromatic products. The natural consequence of the presence of this hydrogen is the conversion of some of the substrate or unsaturated product into saturated materials. Thus, higher production of aromatics, leading to increased liberation of hydrogen, should correlate with higher alkane content. This relationship was demonstrated at 250°C when higher aromatic and alkane contents (37% and 6.2%, respectively) were obtained relative to similar reactions conducted at 200°C (19% and <1%, respectively). Other factors are interesting here as well. There is some ambiguity regarding the homogeneous vs heterogeneous nature of the catalyst. It is possible that the liberated hydrogen is changing the highly reducible iridium metal, especially in this heated and pressurised environment. This could also explain the difference between the Ir+1 and Ir0 precatalyst species.
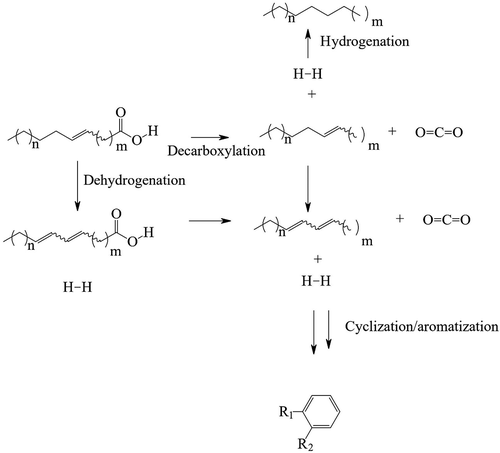
In order to achieve more aromatic content, catalysts known to be more active in dehydrogenation reactions were selected for study. Heterogeneous Pt on alumina, Pd on alumina, and Ru on alumina, or combinations of the three, did not give high reactivity in the decarboxylation reaction. An initial selection of homogenous iridium catalysts was not more fruitful, where iridium carbonyl, (1,5-cyclooctadiene)(methoxy)iridium(I)dimer, bis(1,5-cyclooctadiene) diiridium(I) dichloride, diiodo(pentamethylcyclopentadienyl)iridium(III) dimer, either displayed low conversion, <25%, or displayed obvious metal precipitation early in the reaction. However, chloro-1,5-cyclooctadiene iridium (I) dimer (Ir 1,5 COD dimer) ((C8H12IrCl)2) was found to be an effective catalyst under conditions similar to the ruthenium systems. More importantly for this work, the aromatic content of the products increased dramatically (), with a highest observed value of 34.9%.
Figure 1. A comparison of the observed aromatic content achieved by different catalysts, in the decarboxylation of oleic acid, 1 wt.% catalysts, 250 °C, 24 hours. Conversions for the unsupported species were all high: Ru3(CO)12–92%; (C8H12IrCl2)2–96%; Ir4(CO)12–84%; Os3(CO)12 54%. Of the supported species, the Pd Alumina displayed 14% conversion, whereas the displayed < 2% conversion
Iridium catalyst ((C8H12IrCl)2)
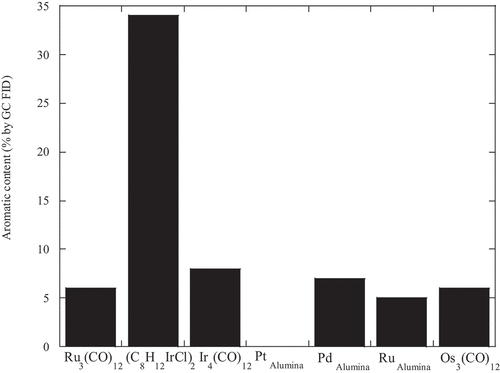
This catalyst was studied in more detail. First, the overall activity of this catalyst, used at 1% wt., at different temperatures, was compared to that of the ruthenium system (). The results showed the expected increase of conversion with increasing temperature. Although the results at 200°C and 250°C were slightly lower than the ruthenium system.
Figure 2. The decarboxylation of oleic acid, 1 wt.% catalyst, 24 hours, Ru3(CO)12 (○), chloro-1,5-cyclooctadiene iridium (I) dimer, (C8H12IrCl)2, (□), at different temperatures
A test reaction on 1-octadecene was performed utilising both systems. Because there is no carboxylate moiety in this substrate, there can obviously be no decarboxylation so the aromatic pathway can be examined directly. Aromatic products were formed in both systems, 1.9% for Ru3(CO)12 and 4.6% for the (C8H12IrCl)2. This was in agreement with the trend found in the decarboxylation systems, where iridium was more effective at aromatisation than ruthenium, albeit at a lower amount. Interestingly, in both systems, hydrogenation to octadecane also occurred at similar levels, 24.7 and 25.9%, respectively. In order to affect the balance of hydrogen, other co-reagents, such as 1-eicosene or 3-hexadecanone, were added. Surprisingly, these reagents had little effect on either aromatic or alkane production from oleic acid, where ruthenium continued to produce ~5.6–6.3% aromatics, and iridium ~ 29.8–30.9%.
Beyond 9-cis-octadecenoic acid
Earlier work (Knothe et al. Citation2017) had shown an increase in aromatic products when polyunsaturated 9,12-cis-ocatadecadienoic (linoleic) acid was used as a substrate. It was of interest to see if the same observation was possible with the (C8H12IrCl)2. Unfortunately, these reactions did not lead to aviation fuel-range products. Obvious increases in viscosity were observed, which was probably due to unwanted polymerisation of the polyunsaturated acids.
The problem of increased viscosity implicating polymerisation occurred when either Ru3(CO)12 or (C8H12IrCl)2 was used with a mixture of fatty acids derived from soybean oil. However, when high oleic soybean oil was used to make the fatty acids, high conversions were again possible. The aromatic contents for these were 18.4 and 31.8% for the two catalysts, respectively. Such a result demonstrated that commodity oils, at least of high oleic content, are viable substrates for the production of aviation fuels by this method.
Energy content
Because these materials are being considered for fuel use, the energy from combustion of the products is of interest (). The heat of combustion of oleic acid was ~39 MJ kg−1, agreeing with that reported in the literature (Levine et al. Citation2014). By comparison, hydrocarbons have a higher combustion energy, with paraffinic hydrocarbons being slightly greater than the aromatics. This was confirmed in our studies by comparing the energy contents of xylene (~42 MJ kg−1) and octadecene (~46 MJ kg−1). As expected, for both oleic acid and high-oleic soybean oil fatty acids, the decarboxylated product had increased energy content compared to the starting materials. Those decarboxylated with Ru3(CO)12 had a detectably higher energy than those from (C8H12IrCl)2, as expected due to the lower aromatic content produced by the ruthenium. Additionally, comparison of the products from oleic acid were quite similar to those from high oleic soybean oil fatty acids. This is also expected, as oleic acid is the main component of high-oleic soybean oil. The authors here also recognise that a precious metal catalyst is not ideal for this application but we hope to encourage further work to bring about this reaction with earth-abundant catalysts. In addition, the nature of the true catalytic species for both the dehydrogenation, the aromatisation, and the decarboxylation, parts of the manuscript are of interest. Studies utilising advanced techniques, such as XRD and SEM are being considered.
Table 1. The measured heats of combustion of products obtained in this study, and relevant comparisons
Conclusion
This work focuses on the conversion of oleic acid into a fuel component by decarboxylation. The use of an iridium catalyst instead of the precedented ruthenium gave similar conversion and also gave a product with higher desirable aromatic content. An appropriate level of these aromatic molecules are beneficial because they alter fuel density and, more importantly, they are proven to reduce shrinkage in elastomeric seals and reduce seal failure. A new iridium-based decarboxylation technology reported here, produces fuel aromatic contents greater than prior technologies, so that this new renewable fuel can be blended with conventional jet fuel in any proportion and still leave sufficient aromatic molecules in the fuel. The new technology has been studied under a variety of conditions and compares well with a previously invented method. This research will benefit the producers of vegetable oils suitable for fuel use, such as soybean oil and high oleic soybean oil, as well as the renewable fuel industry. This work also reported the use of fatty acids from high-oleic soybean oil and showed that the energy content of the products from that mixture are similar to that obtained using high grade oleic acid.
Acknowledgments
The authors thank Karl L. Vermillion for acquisition of NMR spectra and Erin L. Walter for technical assistance and performing the reactions reported herein. This work was supported by the U.S. Department of Agriculture, Agricultural Research Service.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Kenneth M. Doll
Kenneth Doll received a B.S. in chemistry in 1995 from the University of Wyoming, and a Ph.D. from Colorado State University in 2003, the same year that Dr. Doll joined the USDA in Peoria Illinois. Dr. Doll currently works in the Bio-Oils research unit with an objective to use biologically derived oils as a chemical building blocks for fuels, industrial applications and consumer products.
Bryan R. Moser
Bryan Moser is an organic chemist with expertise in oleochemistry and analytical chemistry. He was awarded a B.S. in chemistry from the New Mexico Institute of Mining and Technology in 1997 and a Ph.D. in organic synthesis in 2004 from Arizona State University. Bryan joined the Bio-Oils Research Unit at the National Center for Agricultural Utilization Research in Peoria, IL in 2005. The Moser lab is primarily focused on non-fuel, non-food industrial applications of vegetable oils as replacements or substitutes for existing petroleum-derived products.
Gerhard Knothe
Gerhard Knothe obtained M.S. and Ph.D. degrees in chemistry at the University of Bremen, Germany. After a brief postdoctoral appointment, he was affiliated with the National Center for Agricultural Utilization Research of the U.S. Department of Agriculture in Peoria, IL, from 1989 until 2018. Over the years, his research focused on vegetable oil-derived diesel fuels (biodiesel) and some oleochemistry, which has included the use of NMR and MS. He has many publications and awards to his credit including the Industrial Uses of Soybeans Award sponsored by the USB/AOCS. He edited (together with two co-editors) The Biodiesel Handbook, and has lectured many in courses on biodiesel.
References
- Argauer, R. J., and G. R. Landolt. 1972. “Crystalline Zeolite ZSM-5 and Method of Preparing the Same.” 3702886.
- Berenblyum, A. S., T. A. Podoplelova, R. S. Shamsiev, E. A. Katsman, and V. Y. Danyushevsky. 2011. “On the Mechanism of Catalytic Conversion of Fatty Acids into Hydrocarbons in the Presence of Palladium Catalysts on Alumina.” Petroleum Chemistry 51 (5): 336–341. doi:https://doi.org/10.1134/S0965544111050069.
- Bezergianni, S., and A. Kalogianni. 2009. “Hydrocracking of Used Cooking Oil for Biofuels Production.” Bioresource Technology 100 (17): 3927–3932. doi:https://doi.org/10.1016/j.biortech.2009.03.039.
- Biermann, U., U. Bornscheuer, M. A. R. Meier, J. O. Metzger, and H. J. Schafer. 2011. “Oils and Fats as Renewable Raw Materials in Chemistry.” Angewandte Chemie International Edition 50 (17): 3854–3871. doi:https://doi.org/10.1002/anie.201002767.
- Caulton, K. G., M. G. Thomas, B. A. Sosinsky, and E. L. Muetterties. 1976. “Metal Clusters in Catalysis: Hydrocarbon Reactions.” Proceedings of the National Academy of Sciences 73 (12): 4274–4276. doi:https://doi.org/10.1073/pnas.73.12.4274.
- Chandrasekaran, S. R., B. Kunwar, B. R. Moser, N. Rajagopalan, and B. K. Sharma. 2015. “Catalytic Thermal Cracking of Postconsumer Waste Plastics to Fuels. 1. Kinetics and Optimization.” Energy & Fuels 29 (9): 6068–6077. doi:https://doi.org/10.1021/acs.energyfuels.5b01083.
- Chang, -C.-C., and S.-W. Wan. 1947. “China’s Motor Fuels from Tung Oil.” Industrial and Engineering Chemistry 39 (12): 1543–1548. doi:https://doi.org/10.1021/ie50456a011.
- Crooks, G. R., B. F. G. Johnson, J. Lewis, I. G. Williams, and G. Gamlen. 1969. “Chemistry of Polynuclear Compounds. Part XVII. Some Carboxylate Complexes of Ruthenium and Osmium Carbonyls.” Journal of the Chemical Society A: Inorganic, Physical, Theoretical 2761–2766. doi:https://doi.org/10.1039/j19690002761.
- Doll, K. M., G. B. Bantchev, E. L. Walter, R. E. Murray, M. Appell, J. C. Lansing, and B. R. Moser. 2017. “Parameters Governing Ruthenium Sawhorse-Based Decarboxylation of Oleic Acid.” Industrial & Engineering Chemistry Research 56: 864–871.
- Doll, K. M., E. L. Walter, G. B. Bantchev, M. A. Jackson, R. E. Murray, and J. O. Rich. 2016. “Improvement of Lubricant Materials Using Ruthenium Isomerization.” Chemical Engineering Communications 203: 901–907.
- Dupont, J., P. A. Z. Suarez, M. R. Meneghetti, and S. M. P. Meneghetti. 2009. “Catalytic Production of Biodiesel and Diesel-like Hydrocarbons from Triglycerides.” Energy & Environmental Science 2: 1258–1265.
- Foglia, T. A., and P. A. Barr. 1976. “Decarbonylation Dehydration of Fatty Acids to Alkenes in the Presence of Transition Metal Complexes.” Journal of the American Oil Chemists’ Society 53: 737–741.
- Gao, Y., J. Kuncheria, G. P. A. Yap, and R. J. Puddephatt. 1998. “An Efficient Binuclear Catalyst for Decomposition of Formic Acid.” Chemical Communications 34: 2365–2366.
- Gong, J., X. Ma, and S. Wang. 2007. “Phosgene-free Approaches to Catalytic Synthesis of Diphenyl Carbonate and Its Intermediates.” Applied Catalysis. A, General 316: 1–21.
- Immer, J. G., M. J. Kelly, and H. H. Lamb. 2010. “Catalytic Reaction Pathways in Liquid-phase Deoxygenation of C18 Free Fatty Acids.” Applied Catalysis. A, General 375: 134–139.
- Jessop, P. G., T. Ikariya, and R. Noyori. 1999. “Homogeneous Catalysis in Supercritical Fluids.” Chemical Reviews 99: 475–493.
- Kang G-f, Z.-F. Qin, H.-Q. Zhu, Z.-H. Li, and J.-G. Wang. 2005. “Synthesis of Carbonates via Acid-promoted Alcoholysis of Carbamates.” Journal of Fuel Chemistry and Technology 33: 396–398.
- Kimmich, R. 2002. “Strange Kinetics, Porous Media, and NMR.” Chemical Physics 284: 253–285.
- Kloprogge, J. T., L. V. Duong, and R. L. Frost. 2005. “A Review of the Synthesis and Characterisation of Pillared Clays and Related Porous Materials for Cracking of Vegetable Oils to Produce Biofuels.” Environmental Geology 47: 967–981.
- Knothe, G. 2011. “A Technical Evaluation of Biodiesel from Vegetable Oils Vs. Algae. Will Algae-derived Biodiesel Perform?” Green Chemistry 13: 3048–3065.
- Knothe, G., K. R. Steidley, B. R. Moser, and K. M. Doll. 2017. “Decarboxylation of Fatty Acids with Triruthenium Dodecacarbonyl: Influence of the Compound Structure and Analysis of the Product Mixtures.” ACS Omega 2: 6473–6480.
- Kochi, J. K., R. A. Sheldon, and S. S. Lande. 1969. “Rates of Photochemical and Thermal Decarboxylation of Acids by Lead (IV) Tetraacetate.” Tetrahedron 25: 1197–1207.
- Kraus, G. A. 2014. “Method for Producing Olefins.” 8629312.
- Kraus, G. A., and S. Riley. 2012. “A Large-Scale Synthesis of alpha-Olefins and alpha-omega-Dienes.” Synthesis 44: 3003–3005.
- Levine, F., R. V. Kayea Iii, R. Wexler, D. J. Sadvary, C. Melick, and J. La Scala. 2014. “Heats of Combustion of Fatty Acids and Fatty Acid Esters.” Journal of the American Oil Chemists’ Society 91: 235–249.
- Maetani, S., T. Fukuyama, N. Suzuki, D. Ishihara, and I. Ryu. 2011. “Efficient Iridium-Catalyzed Decarbonylation Reaction of Aliphatic Carboxylic Acids Leading to Internal or Terminal Alkenes.” Organometallics 30: 1389–1394.
- Meier, M. A. R., J. O. Metzger, and U. S. Schubert. 2007. “Plant Oil Renewable Resources as Green Alternatives in Polymer Science.” Chemical Society Reviews 36: 1788–1802.
- Moser, B. R., G. Knothe, E. L. Walter, R. E. Murray, R. O. Dunn, and K. M. Doll. 2016. “Analysis and Properties of the Decarboxylation Products of Oleic Acid by Catalytic Triruthenium Dodecacarbonyl.” Energy & Fuels 30: 7443–7451.
- Murray, R. E., K. M. Doll, and Z. Liu. 2018. “Process for Isomerization and Decarboxylation of Unsaturated Organic Compounds with a Metal Catalyst or Catalyst Precursor.” 9868679.
- Murray, R. E., E. L. Walter, and K. M. Doll. 2014. “Tandem Isomerization-Decarboxylation for Converting Alkenoic Fatty Acids into Alkenes.” ACS Catalysis 4: 3517–3520.
- Ohlmann, D. M., N. Tschauder, J.-P. Stockis, K. Gooßen, M. Dierker, and L. J. Gooßen. 2012. “Isomerizing Olefin Metathesis as a Strategy to Access Defined Distributions of Unsaturated Compounds from Fatty Acids.” Journal of the American Chemical Society 134: 13716–13729.
- Popov, S., and S. Kumar. 2015. “Rapid Hydrothermal Deoxygenation of Oleic Acid over Activated Carbon in a Continuous Flow Process.” Energy and Fuels 29: 3377–3384.
- Quirino, R. L., A. P. Tavares, A. C. Peres, J. C. Rubim, and P. A. Z. Suarez. 2009. “Studying the Influence of Alumina Catalysts Doped with Tin and Zinc Oxides in the Soybean Oil Pyrolysis Reaction.” Journal of the American Oil Chemists’ Society 86: 167–172.
- Ranu, B. C., and U. Jana. 1998. “Indium(III) Chloride-promoted Rearrangement of Epoxides: A Selective Synthesis of Substituted Benzylic Aldehydes and Ketones.” Journal of Organic Chemistry 63: 8212–8216.
- Ray, A., K. Zhu, Y. V. Kissin, A. E. Cherian, G. W. Coates, and A. S. Goldman. 2005. “Dehydrogenation of Aliphatic Polyolefins Catalyzed by Pincer-ligated Iridium Complexes.” Chemical Communications 41: 3388–3390.
- Salvini, A., P. Frediani, and F. Piacenti. 2000. “Alkene Isomerization by Non-hydridic Phosphine Substituted Ruthenium Carbonyl Carboxylates.” Journal of Molecular Catalysis A, Chemical 159: 185–195.
- Santillan-Jimenez, E., and M. Crocker. 2012. “Catalytic Deoxygenation of Fatty Acid Derivatives to Hydrocarbon Fuels via Decarboxylation/decarbonylation.” Journal of Chemical Technology and Biotechnology 87: 1041–1050.
- Smith, K., G. A. El-Hiti, and M. Al-Shamali. 2006. “Rearrangement of Epoxides to Carbonyl Compounds in the Presence of Reusable Acidic Zeolite Catalysts under Mild Conditions.” Catalysis Letters 109: 77–82.
- Snåre, M., I. Kubičková, P. Mäki-Arvela, K. Eränen, and D. Y. Murzin. 2006. “Heterogeneous Catalytic Deoxygenation of Stearic Acid for Production of Biodiesel.” Industrial & Engineering Chemistry Research 45: 5708–5715.
- Takemura, Y., A. Nakamura, H. Taguchi, and K. Ouchi. 1985. “Catalytic Decarboxylation of Benzoic Acid.” Industrial and Engineering Chemistry Product Research and Development 24: 213–215.
- Theodorou, V., K. Skobridis, A. G. Tzakos, and V. Ragoussis. 2007. “A Simple Method for the Alkaline Hydrolysis of Esters.” Tetrahedron Letters 48: 8230–8233.
- Tran, N. H., J. R. Bartlett, G. S. K. Kannangara, A. S. Milev, H. Volk, and M. A. Wilson. 2010. “Catalytic Upgrading of Biorefinery Oil from Micro-algae.” Fuel 89: 265–274.
- Twaiq, F. A., N. A. M. Zabidi, and S. Bhatia. 1999. “Catalytic Conversion of Palm Oil to Hydrocarbons: Performance of Various Zeolite Catalysts.” Industrial & Engineering Chemistry Research 38: 3230–3237.
- Vasquez, M. C., E. Eduardo Silva, and E. Fernanto Castillo. 2017. “Hydrotreatment of Vegetable Oils: A Review of the Technologies and Its Developments for Jet Biofuel Production.” Biomass & Bioenergy 105: 197–206.
- Vozka, P., and G. Kilaz. 2020. “A Review of Aviation Turbine Fuel Chemical Composition-property Relations.” Fuel 268: 117391.
- Zhao, C., T. Bruck, and J. A. Lercher. 2013. “Catalytic Deoxygenation of Microalgae Oil to Green Hydrocarbons.” Green Chemistry 15: 1720–1739.
