?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In recent years, low power consumption and portable devices have been developed that will be connected and used everywhere. However, this explosion will bring critical challenges to electricity consumption. In this work, copper and zinc electrodes are embedded in prickly pear plant as an electricity generation has been analysed and evaluated. Many experimental setups have been performed to determine the optimum method to collect the maximum amount of energy from the living plant. They study the influence of distance between electrodes, influence of the electrode’s size, influence of the electrode’s depth, effect of the couple electrodes number, and effect of series and parallel connection to harvested energy. Therefore, a combination of series and parallel connection among the leaves can be installed to harvest higher voltage and current. The experimental results show that 58,8 mW electrical power can be harvested using 18 pairs of electrodes embedded in 6 leaves. The produced energy is used to power up low-power devices such as a calculator or a light emitting diode (LED) and can be stored in a capacitor or a battery.
I. Introduction
One of the challenges for the coming future in the modern world is to increase renewable energy to 32% by 2030 (European Commission. COM Citation2014). To realise this purpose, it is of great necessity to replace traditional electricity generated from fossil fuels by environmental-friendly renewable generation called bioelectricity. In the 2030 vision, the European Union estimates that renewable energy can contribute at about 27% in electricity generation (Ifaei, Farid, and Yoo Citation2018). This objective would reach 20% for China, and 12% for the United States (Ifaei, Farid, and Yoo Citation2018). Globally, the share of renewable energy in the total mix of generation is estimated to reach 45% in 2040 (Yuan et al. Citation2018).
To achieve these purposes many renewable energy production technologies had been invented and explored. It includes solar photovoltaic, biomass, biofuels, wind power systems, geothermal energy, power plants, solar thermal as well as hydrogen and fuel cells (Twidell and Weir Citation2015; Bull Citation2001; Wu et al. Citation2020). Living plants are explored as a new alternative source for renewable green energy since they are abundant and widely distributed in nature. Their harvested energy can be obtained from the mechanical movement of the plants where any motion and vibration of the plants will be converted into electricity using piezoelectric, electrostatic, or electromagnetic transducer (Teng et al. Citation2018a; Vullers et al. Citation2009; Özdemir and Akkaya Oy Citation2016; Akkaya Oy and Özdemir Citation2016; Park et al. Citation2014; Galchev Citation2010; Jie et al. Citation2018). The most used two alternatives to generate bioelectricity from living plants are the plant microbial fuel cell (PMFC) (Kabutey et al. Citation2019; Deng, Chen, and Zhao Citation2012; Goto et al. Citation2015; Mohan, Mohanakrishna, and Chiranjeevi Citation2011; Timmers et al. Citation2010; Nitisoravut and Regmi Citation2017) and the plant-based cell (PBC) (Chong et al. Citation2019).
Different kinds of living plants have been used as green energy sources. Bigleaf maple tree have been studied by Himes et al (Himes et al. Citation2010) using two steel nails, one inserted into the tree trunk and another inserted on the soil 30 cm away from the tree. Tanaka et al (Tanaka et al. Citation2012) used galvanised iron nails inserted into the tree trunk and a stainless steel electrode planted in the soil nearby to study the voltage harvested from Pachira tree. In the case of poplar trees, Hao et al (Hao et al. Citation2015) used two copper electrodes, one inserted into the tree trunk and another inserted on the tamped soil 30 cm away from the tree, to study the energy harvested from this kind of living plants. Konstantopoulos et al (Citation2016) used a zinc anode alloy in a combination with copper cathode as a pair of electrodes inserted into the tree trunk to study the harvested energy from Avocado plants. Choo et al (YY Choo and J.Dayou Citation2013) studied the energy harvested by Pulai and banana trees.
Attention has been also devoted to study the green energy harvested from fruits and vegetables. Citrus fruits including orange, lemons have been used to produce bioelectricity (Jesus, Tenreiro Machado, and Boaventure Cunha Citation2008; Chavan, Singh, and Kaur Citation2018; Guimarães et al. Citation2010). Besides, these citrus fruits and the oil palm fruit have been used by Aliteh et al (Aliteh et al. Citation2020) to generate a battery that is considered as a significant source of electrical energy. In the case of pickled fruits and vegetables such as radish, pumpkin, rhubarb, and so on, electricity is generated due to the salt content of these foods when they soak in brine or in vinegar (Shittu, Ajagbe, and Oloruntola Citation2018).
In the case of vegetables, tomatoes and potatoes were the most studied. Tomato waste is used in a microbial electrochemical cell with a special type of bacteria that breaks down the waste tomatoes (Shrestha et al. Citation2016). Potatoes can act as a conduit for electrical energy as it was shown by Liu et al (Liu et al. Citation2013), and Cai et al (Cai et al. Citation2010).
Living plants are capable to store a large amount of energy that is unused and can be exploited to drive multiple applications (Hill and Govindjee Citation2014; Blankenship et al. Citation2011; Asner, Scurlock, and Hicke Citation2003; Huang et al. Citation2016). Generated from living plants, green energy is considered as a possible way to power up low power consumption and portable devices which can be used to prevent against deforestation activities such as forest fire and diseases. It can be also used to power up systems to control heat and humidity of plants (Teng et al. Citation2018b). Besides, it can be a good way to expand the network coverage in rural zones by incorporating the wireless system using low power with bioelectricity from living plants (Teng et al. Citation2018b). Xu et al (Xu, Lu, and Zhu Citation2021) proposed the use of harvested energy from plants to amplify 5 G signal. Lew et al (Lew et al. Citation2020) have discussed the emergence of Plant Nanobionics and Living Plants as Technology (Lew et al. Citation2019) and for Arsenic detection. Lan et al (Lan et al. Citation2021) proposed the realisation of nanogenerators for one-plant self-powered sustainable agriculture system. Lew et al (Babu et al. Citation2022) discussed examples ranging from environmental monitoring to single cell monitoring in plants via a nanobionic interface.
The aim of the present work is to investigate the characteristics of the prickly pear living plant as a potential renewable green energy source and to determine its optimum setup to harvest a higher amount of green renewable energy from this plant. The present paper is organised as; section II describes the living plant’s structure and the mechanism of producing green renewable energy. Section III explains the various experimental setups to investigate the conditions to harvest the maximum green energy from the plant, section IV discusses the experimental findings in detail, and section V concludes the principle obtained results.
II. Living plants: structure and mechanism of producing green renewable energy
This section describes the living plants structure in relation with harvesting green energy as well as the mechanism of producing green renewable energy from them.
II-1 Living plants structure
Plants have possessed certain structures for energy harvesting and conversion from the environment. They used their vast root network structure to extract minerals and water from the soil which serves as an autosampler of the underground environment. Via their roots, stem, and leaves which are made up of a natural circuitry consisting of vascular bundles they are capable to enable rapid and efficient transport of water, peptides, nutrients, and ions between the roots and the shoots. Their leaves are made of specialised tissues such as the palisade mesophyll, spongy mesophyll, and the epidermis layer. Both upper and the lower epidermis contain stomata pores flanked by guard cells that regulate the opening and closing of the stomata. These cells are responsible for the gaseous exchange between plant and its environment, and for controlling the rate of transpiration that can provide a driving force for water and nutrients to be transported from the roots via capillary action. The plant leaf cells contain chloroplasts which endow plants with a unique ability to fix carbon via photosynthesis. Carbohydrate molecules can be synthetised by living plants from carbon dioxide and water with the help of light energy from the sun causing electrons transfer phenomena inside the living plants from the leaves to down the roots creating a potential difference between them. In dark conditions i.e. absence of light, respiration takes over in plants where it generates energy by the conversion of carbohydrates molecules produced during photosynthesis into bioelectricity. In both process, electrons and ions transfer exists enabling energy to be harvested from living plants (Clark Citation1935; Elongation, The, and Coleoptile Citation1925).
II-2 Harvesting green energy mechanism from living plants
As it was mentioned above, the two alternatives to generate green energy from living plants are the PMFC and the PBC techniques.
In the PBC system, living plants have been proven to have a potential for renewable green energy source. It consists of embedding pairs of electrodes into it to allow flow of ions and electrons and hence generate bioelectricity. Different type of electrodes and plants have been used to suggest that voltages are produced to greater or lesser extents where combination of copper (Cu)-zinc (Zn) and Aloe Vera produces the highest voltage output. In this context, Chong et al (Chong et al. Citation2019) showed that 1111.55 µW electrical power can be harvested from Aloe Barbadensis Miller (Aloe Vera) leaves with 24 pairs of Cu-Zn electrodes. The harvested energy has the potential to be stored in a capacitor for storage. Labady et al (Labady et al. Citation2002) harvested electricity by inserting half an inch of aluminium roofing nail to tree and copper water pipe seven inches into the ground. These voltage differences have been originally aimed to be used in attempts to monitor plant activity and have been hypothesised to be due to various sources, most well-known of which appears to be the streaming potential mechanism (Y. Y. Choo and J.Dayou Citation2013). By embedding electrodes into the plants, an electrochemistry process happens where it will convert the chemical energy to electrical energy via an oxidisation-reduction reaction (Choo, Dayou, and Surugau Citation2014; Ying and Dayou Citation2014). The oxidation process takes place at the anode electrode whereas the reduction happens at the cathode electrode. This causes the electron flow from anode to cathode to produce electricity. Inside, the plant’s organic matter is functioning as an electrolyte between the two electrodes. This method provides a direct way to harvest DC current and voltage from the living plants.
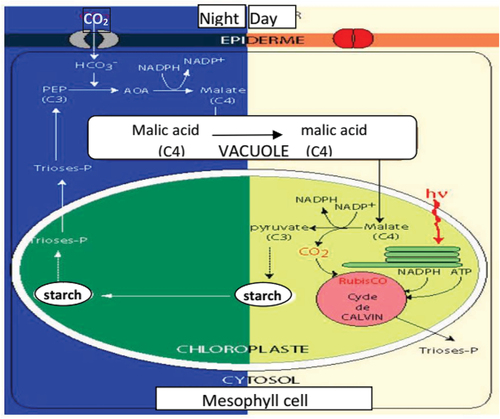
presents that the prickly pear inner flesh gel contains different ions coming from photosynthesis phenomenon during night and day indicating that energy can be harvested at the day and at the night. The prickly pear is a plant that has CAM-type photosynthesis (Crassulacean Acid Metabolism). CAM plants are specialised in the fixation of CO2 during the night. This fixation is carried out by phosphoenolpyruvate carboxylase (PEP), which comes from the degradation of starch and sucrose produced in the chloroplast during the day. This fixation makes it possible to form oxaloacetate, which will be immediately reduced to malate, then stored in a vacuole in the form of malic acid, hence the name of plant with an acid metabolism. CAM plants need the light energy of the day to complete the Calvin cycle and thus complete photosynthesis (Boutakiout Citation2015).
Living plants can harvest energy autonomously from sunlight, water, and carbon dioxide with self-repair capabilities. Under sun illumination, only 53% of photons fall into the absorption range of plants. Due to the incomplete absorption, around 30% of the photons are lost, with additional 24% of energy lost due to wavelength-mismatch degradation compared to the 700 nm energy used in photosynthesis (Blankenship et al. Citation2011).
The photosynthesis process can be described by the following reaction.
III. Setup experiments and materials
Experiments under different conditions are performed to study different aspects that influence the harvested energy from the prickly pear (Opuntia ficus-indica and Opuntia megacantha) plant. The selected plants are old than 3 years and have many branches. Experiments are performed under natural condition where the plant is located in the field where the temperature is between 30 and 40 °C, and the humidity is between 50% and 65%. Also, experiments are carried at laboratory to study the cat-off leaves where the temperature is between 25 and 30 °C. The copper and zinc electrode pair used in all experiments are chosen based on studies performed in many bibliographic references that are producing the high voltage and current magnitude from plants. Electrode pairs are embedded into a leaf to measure current and voltage generated by the plant. The current and voltage are measured by a high precision multi-metre connected to the electrodes. Many electrode pairs are used to harvest energy from living plants such as coper-zinc, copper-nickel, aluminium-zinc, nickel-aluminium, zinc-nickel and copper-aluminium. The best combination to harvest the highest energy from the plant is copper and zinc electrodes compared to others such as studied by P-L. Chang et all (Chong et al. Citation2019) and Y-T. Choo et all (Choo, Dayou, and Surugau Citation2014).
Couple copper-zinc electrodes generate the higher amount of electricity from prickly pear plant because they are more reactive than other cited couples. Zinc electrode undergoes an oxidisation causes electrons release by the change of the Zn atom to Zn2+ ion (Zn ➡ Zn2+ + 2e-). Electrons flow from the external wire to the copper electrode where reduction occurs. The prickly pear inner semi-solid flesh acts as the electrolyte solution of the oxidisation-reduction phenomenon. Hence, the zinc electrode acts as the anode (negative terminal) and the copper as the cathode (positive terminal) in this system which operates like photovoltaic cell. So, the current flows from copper electrode to zinc electrode causes a potential difference between them. Experiments are simple, less expensive and carried at natural condition compared to other materials to starve green energy from living organisms such as plants and bacteria. Measurement results show that with a single pair of zinc and copper electrode embedded in a prickly pear leaf, the harvested voltage value varies from 0.9 V to 1.1 V, and the current value varies from 0.9 mA to 9.1 mA. Also many measurements are repeated in different experiments giving the same values. The uncertainty in the measurement it should be less than 2%.
IV. Results and discussion
IV.1 Influence of distance between electrodes to the harvested energy
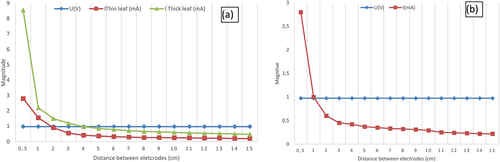
The experimental setup investigates the effect of the distance between the two electrodes to the harvested energy from prickly pear plant. The same copper and zinc electrodes are immersed in the leaf, their depth is fixed to 1 cm, but the distance between them is varied from 0.5 cm to 15 cm to determine the optimum distance to harvest maximum energy from the plant. This experiment is performed using two types of leaf, a thin leaf, and a thick leaf. A thin leaf is a leaf that has a thickness less than or equal to 1 cm, however a thick leaf which has a thickness greater than 1 cm. shows that the current value gathered from a thin leaf is greater than the current value from a thick leaf. Measurement results presented in show that, the voltage value is approximately equal to 0.975 V. But the current magnitude decreases from 8.5 mA to 0.4 mA for a thick leaf and the current values decreases from 2.9 mA to 0.2 mA for a thin leaf when the distance between embedded electrodes increases from 0.5 cm to 15 cm. Thus, the harvested energy from the prickly pear plant is higher when the distance between zinc and copper electrodes is smaller and immersed in a thick leaf. This result is explained based on electrochemistry theory that only maximum electron transfer occurs when electrodes are immersed at a close distance (Mayé Citation2010). Thus, when this distance is smaller, the resistance also is smaller which enables an easier transfer of electrons that gives a higher current magnitude. To harvest maximum amount of energy, electrodes should be immersed in a thick leaf at close distance.
IV.2. Influence of the electrode’s size to the harvested energy
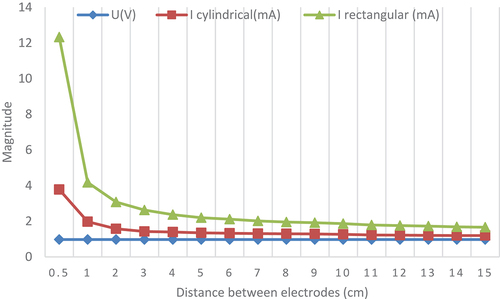
presents the experiment results that study the electrode size effect to the voltage and current values. Electrodes are immersed in the thick leaf at a fixed depth which equal to 1 cm. Two size’s type of copper and zinc electrodes are used in this experiment. Firstly, a rectangular electrode with 3 cm length, 2 cm width and 0.1 cm thickness is used. Secondly, a cylindrical electrode with 0.4 cm diameter and 5 cm length is also used. All electrode types are immersed in a prickly pear leaf separated by a distance increased from 1 cm to 15 cm to perform the gathered energy. Measurement results shown in indicate that the current harvested from the plant using rectangular electrodes is higher than the current collected using cylindrical electrodes. However, the voltage magnitude of cylindrical electrodes is equal to the rectangular electrodes voltage values This result suggests that the contact area between electrodes and the inner flesh gel is greater for the rectangular type (contact area = 2*3*1*2 = 12 cm2) than the cylindrical electrodes (contact area = 2*3.14*0.4*1 = 2.51 cm2). The inner flesh gel acts as an electrolyte at the position where the electrode is embedded reduces volume of the conducting medium between the electrodes. As the amount of the contacts area rises the value of the harvested current increases. The harvested energy from prickly pear plant using rectangular electrodes gives the higher quantity but, cylindrical electrodes are simple and less expensive. We suggest to use rectangular electrodes to gather energy from living plants.
IV.3. Influence of the electrode’s depth to current and voltage magnitude
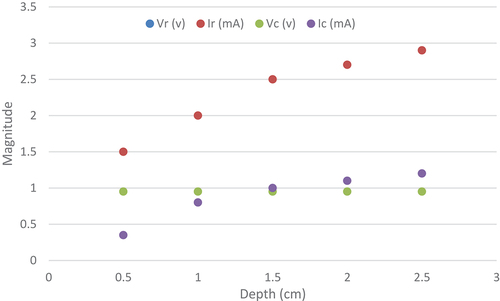
Experimental setup aims to study the effect of embedded electrodes depth in the leaf to the energy harvested. This experiment is carried out to fix the optimum depth of electrodes to harvest energy. Copper and zinc electrodes are penetrated into the leaf separated by a distance maintained to 1 cm with various depths (from 0.5 cm to 2.5 cm) to study the magnitude of current and voltage produced by the living plant. Based on design of experiments (DOE), results measurement presented in show that the current rose discernably with increasing electrodes immersing depth. However, the voltage values remain constant during this experiment (Vr = Vc = 0.95). The measured current magnitude increases when electrodes depth increases (Ir increases from 1.5 mA to 2.9 mA, and Ic varies from 0.35 mA to 1.2 mA) which rises the contact area with the prickly pear inner semi-solid flesh. This result is conform to electrochemistry theory that for an electrochemical reaction the current production rate is proportional to the contact area (Mayé Citation2010). The optimum amount of energy is harvested when electrodes pair are embedded at the maximum depth that’s suggest to use thick leaves.
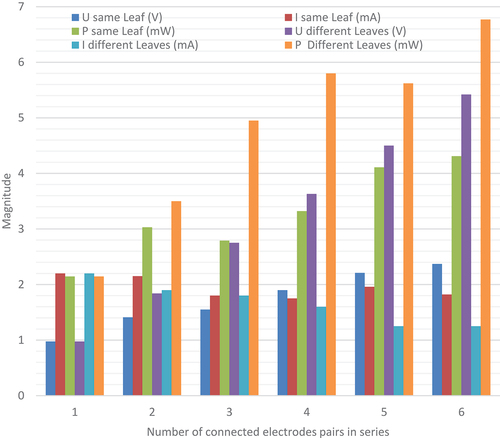
IV.4. Effect of the couple electrodes number connected in series to the harvested energy
The experiment is carried out to determine the effect of the number of electrode pairs connected in the same and different leaves to harvest energy. Copper and zinc pairs are embedded at a constant depth equal to 1 cm and are maintained at distance between them equal to 1 cm. The number of electrode pairs is varied from 1 to 6 and immersed in series in the same and different leaves to study the variation of current and voltage magnitude. summarises measured results which indicate that increasing the number of electrode pairs in series leading the harvested power from the prickly pear plant to rise. In addition, the voltage output varies proportional to the number of electrode pairs in accordance with the Kirchhoff voltage law when electrodes are embedded in different leaves (Udifferent leaves increases from 0.975 V to 5.42 V). But, when electrodes are immersed in the same leaf, the voltage rises less than the Kirchhoff law due to resistance of the inner flesh solid of prickly pear plant (Usame leaf rises from 0.975 V to 2.37 V). Each electrode pair acts as a voltage source such as a cell or a bio-electrochemical cell when connecting them in series increasing the voltage magnitude. However, the current decreases when the number of electrode pairs connected in series increases due to the introduction of higher internal resistance between electrodes (Idifferent leaves deceases from 2.2 mA to 1.25 mA and Isame leaf decreases from 2.2 mA to 1.82 mA). The maximum gathered power is Pdifferent leaves = 6.77 mW when using six electrode pairs immersed in series at different leaves of prickly pear plant compared to Psame leaf = 4.31 mW. Thus, a great number of cells should be connected in series at different leaves to harvest the maximum of power.
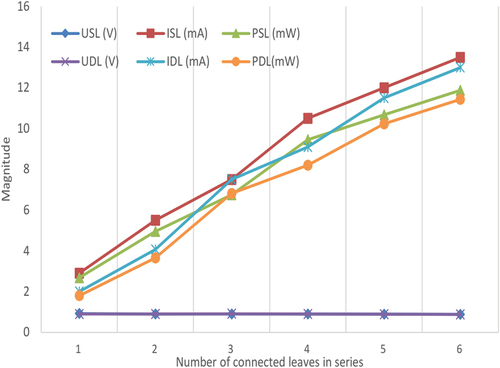
IV.5. Effect of parallel connection among prickly pear plant to the harvested energy
This experiment presents the effect of parallel connection of electrodes pairs embedded in prickly pear leaves on the current and voltage values gathered from the plant under no load. Each electrodes pair is immersed having the same size separated by a distance equal to 1 cm and depth equal to 1 cm. The number of immersed electrode pairs in parallel is varied from one to six firstly at the same leaf, secondly at different leaves in order to determine the optimum manner to harvest energy. Measured results presented in indicate that the voltage value remains constant (Vsame leaf = Vdifferent leaves = 0.975 V) when the number of electrode pairs connected in parallel increases. Moreover, current magnitude rises (ISL rises from 2.9 mA to 13.5 mA and IDL rises from 2 mA to 13 mA) proportional to the increase of the number of connected electrode pairs in accordance with the Kirchhoff law of electric current. Thus, each electrode pair (cell) appears as an electric current source like an electrochemical cell when they are connected in parallel increases the current magnitude. The great amount of gathered power energy is equal to PSL = 11.88 mW, when 6 electrode pairs are connected in parallel at the same leaf, proves that the current value is proportional to the number of connected cells. The difference between power values gathered from connected electrodes pairs in parallel at the same and different leaves (PSL = 11.88 mW and PDL = 11.44 mW) is due to the internal resistance between embedded electrodes pairs.
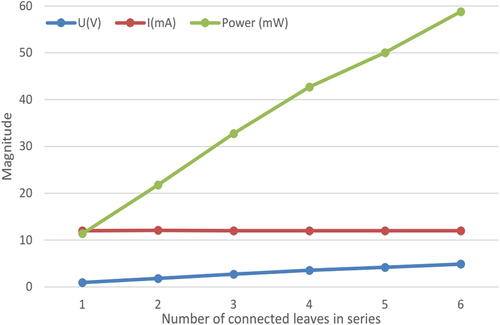
IV.6. Influence of series and parallel connections in different leaves to the harvested energy
The experiment shown in presents the effect of series and parallel connection of electrodes pairs between the prickly pear leaves on the current and voltage values gathered from the plant under no load. Many dispositions of series and parallel connection are tested. Each electrode pair is immersed having the same parameters (distance = 0.5 cm, depth = 1 cm) and same size. Previous experiments demonstrate that the parallel connection among the same leaf produces a higher amount of current when more cells are connected in parallel. On the other hand, the series connection among the leaves produces a higher amount of voltage when more leaves are connected in series. Therefore, a combination of series and parallel connection among the leaves can be installed to harvest either higher voltage or current. It is worth to note that I = 12 mA can be harvested when 3 cells are connected in parallel in the same leaf while U = 4.9 V can be harvested from 6 leaves when they are connected in series. summarises the measurement results of harvested energy when 3 electrodes pairs are connected in parallel in the same leaf with increasing the number of leaves connecting electrodes pairs in series. Note that P = 58.8 mW is the maximum amount of collected power using a combination of connected 18 cells in series and parallel. In order to increase the harvested power it should rise the number of connected cells, for example 54 cells can harvest a power more than 150 mW that will be used to charge a smart phone.
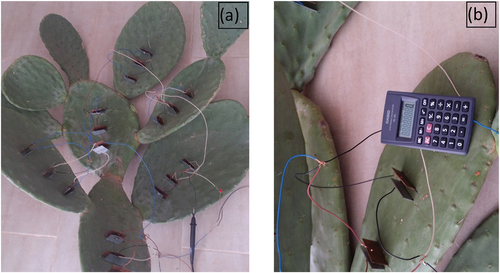
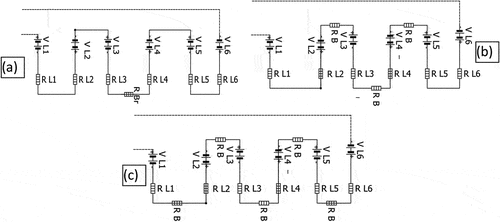
Figure 8. Three embedded electrodes pairs are connected in parallel in the same leaf where (a) leaves around same branche, (b) leaves aroud different branches are connected in series.
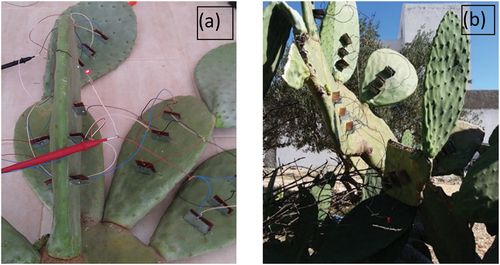
It is observed that the series connections among leaves of the same branch in the prickly pear produce minimum voltage (U = 3.5 V) compared to the series of connection among leaves around the same branch (U = 4.3 V). In addition, voltage magnitude (U = 4.9 V) produced by a series connection around different branches are the higher. However, the collected current remains the same at all cases of series connections of prickly pear leaves. These results show that the same variation of gathered voltage and current also occurs in the result of electrodes pairs connected in series at the same and different leaves due to the high resistance of the electrolyte. presents the equivalent circuit for series connection among leaves from different branches, same branches and around same branches. Hence, to harvest maximum energy from prickly pear plant, it should connect electrode pairs in parallel at the same leaf which are connected in series around different branches. The gathered energy can be used to aliment low power devices, such as presented in ) calculator alimented by the harvested energy from prickly pear plant. Also, ) shows the LED lights powered by the collected energy.
IV.7. Effect of current and voltage magnitude delivered by the plant under load
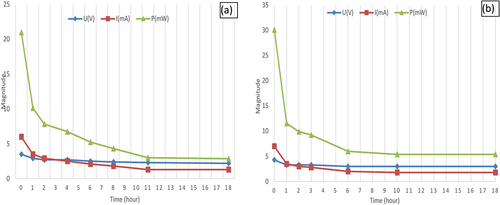
The experiment presented in studies the effect of load to harvested energy from living plant using a Led lighting. Three electrodes’ pairs are connected in parallel and embedded in a leaf, where six separated leaves are connected in series to gather maximum energy. This energy is used to emit light using an LED which is connected to the embedded electrodes at the prickly pear plant. The power consumption of the used LED is P = 60 mW (12 mA and 5 V), so it consumes all harvested energy from the plant. presents measured results that indicates at the beginning of the experiment the produced power is equal to P = 30.1 mW, the voltage value is U = 4.3 V and the current value is I = 7 mA. After 10 hours of emitting light (plant under load), the measured voltage becomes U = 3 V and the current magnitude I = 1.8 mA. So, the power of harvested energy is decreased from P = 30.1 mW to P = 5.4 mW. The delivered power is equal to 20 mWh at the first 10 hours of consuming the produced energy, then the delivered power is 5.5 mWh. It is concluded that low power consumption devices can be powered from prickly pear plants for a long period. The number of connected series and parallel cells is fixed based on the power consumption of each device to produce the needed power. An important implication of our study derives from our finding is to use energy harvested from prickly pear plant to power up low power devices such as IoT(Internet of Things) systems installed at urban areas. This devices should be powered continuously. However photovoltaic cells can produce energy only at the day. In this sense, we believe that our research is especially used in the night or continuously. The second implication is the association of prickly pear cells in series or/and in parallel behave like photovoltaic cells and electrochemical battery where gathered current and voltage rises in accordance to the Kirchhoff law as it was mentioned previously.
IV.8. Effect on charging capability of different capacitors
However, renewable energy systems suffer from the discredit of intermittency, for which surplus energy obtained can be stored in several ways, and later utilised during periods of intermittencies or shortages. Thus, battery energy storage systems are likely to have a significant impact in the small-scale integration of renewable energy sources into IoT systems, wireless system to expand the network coverage in rural zones and other portable low power devices. Battery energy storage systems as one of the potential solutions, with advantages such as fast response capability, sustained power delivery, and geographical independence. During the implementation of battery energy storage systems, one of the most crucial issues is to optimally determine the size of the battery. Studies should be performed to optimise battery sizing for different renewable energy systems using a range of criteria and methods. We have tested the Effect on charging capability of different capacitors.
Experiment shown in presents new method to harvest energy and charging different capacitors as an battery energy storage system. We have used only three cut-off leaves, each leaf is divided in two slices where three electrode pairs are embedded in parallel at each slice. All slices are connected in series to harvest maximum amount of energy which is used to test the capability to charge different capacitors. shows the measured values of produced energy by cut-off half-leaves of prickly pear plant. It is observed that all tested capacitors can be charged up to 5 V many times such as shown in with a charge time Ꞇ = RC, where R = 1KΩ. Though, the produced energy can be used to charge a battery, the value of the battery must be chosen accurately to the requirement of operating output load proportional to its efficiency and operating period (Mensah-Darkwa et al. Citation2019; Yanga et al. Citation2018). This method proves that the same energy can be harvested from three or two portable leaves where each leaf can be divided into three or four slices. Every slice connects three parallel couple electrodes, however slices are connected in series.
Figure 14. Recharging and discharging of capacitors (a) 330 µF, (b) 2200µ F and (c)3300µF) using harvested energy.
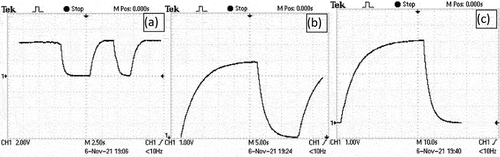
Table 1. Harvested energy with increasing the number of half-leaves connected in series where embedded 3 electrodes pairs.
IV.9. Harvested energy from agave americana (Aloe American) plant
This experiment investigates the effect of series and parallel connections on the agave americana plant on the magnitude of the harvested voltage and current in order to be compared with the prickly pear plant. Agave Americana presented in is a perennial herbaceous succulent plant, with succulent, fleshy, tooth-edged leaves arranged in a rosette. Two types of electrodes are used which are the rectangular and cylindrical electrodes. The copper and zinc electrodes are immersed at 1 cm of depth and a distance between them of 1 cm. Measurement results presented in show that harvested energy (U = 0.92 V, I = 1 mA) using one pair of rectangular electrodes from agave americana. Immersed electrodes pairs act as electrochemical cell, also when they are associated in series and in parallel produce current and voltage values according to Kirchhoff low. indicates the harvested current (I = 3.02 mA) using three cells associated in parallel, and when using three cell connected in series the voltage magnitude U = 2.46 V. Moreover, It is concluded that gathered energy from agave americana plant varies in accordance to harvested energy from prickly pear plant when varying the number of electrodes pairs connected in series and parallel at the same and different leaves. The harvested power from agave americana (P = 31,5 mW) is less than the harvested energy from prickly pear plant (P = 58,8 mW) using the same number of connected cells (18 cells) in series and parallel manner. In this context, Chong et al (Labady et al. Citation2002) showed that P = 1.111 mW electrical power can be harvested from Aloe Barbadensis Miller (Aloe Vera) leaves with 24 pairs of Cu-Zn electrodes. We conclude that our method is the best to harvest energy from living plants which gives the higher amount of produced renewable green energy.
Figure 15. (a) agave americana, (b) current value gathered from 3 immersed rectangular electrodes pairs connected in parallel at the agave plant.

Table 2. Voltage and current magnitude using rectangular electrodes pairs embedded in series and in parallel at the agave americana plant.
V. Conclusion
Efficiently exploiting renewable, sustainable, and green energy resources is one of the most critical challenges facing our world today. Living plant power generation exhibited a promising performance with broad applicability and low cost and be environmentally friendly. It is observed that cooper and zinc electrodes immersed in a prickly pear leaf at a closed distance can produce energy and having the same behaviour as an electrochemical cell. Compared to other studied plants, the prickly pear has the great amount of energy can be harvested from living plants. Measurement results show that for the highest contact area between electrodes pair and inner flesh gel of the plant produce the highest current magnitude. Also the shortest distance between embedded electrodes gives the maximum current value. Moreover, to increase the current magnitude a number of electrodes pairs immersed in prickly pear plant are connected in parallel. A series connection of electrodes pairs embedded in the living plant can generate the higher voltage magnitude. Hence, a combination of series and parallel connection of immersed electrodes pairs at the leaves of prickly pear plant can generate the optimum amount of energy (58.8 mW using 18 electrodes pairs connected in series and parallel). This study demonstrates that the optimum method used to harvested energy is to connect copper and zinc electrodes pairs in parallel at the same leaf to get the maximum current then the leaves from different branches are connected in series to get the maximum voltage. Also, the same amount of energy can be harvested using cut-off leaves of prickly pear plant (P = 45.3 mW). Embedded electrodes pairs in a cut-off leaf or half-leaf are connected in parallel then cut-off leaves or half-leaves are connected in series can produce the highest energy. The harvested energy can be used to power up low power consumption devices or to charge a battery.
We suggested that our work can contribute to the development of renewable green energy sources produced by prickly pear plant. Despite, the amount of the harvested energy can be used to power up portable and low power consumption devices that will be connected and used in everywhere. Our future study is to enhance the amount of harvest energy to be stored in a rechargeable battery and we propose to power up an IoT smart farm monitoring system using living plants and cut-off plants.
Acknowledgments
The authors gratefully acknowledge the Scientific Research Deanship, King Khalid University (KKU), Abha, Asir, Kingdom of Saudi Arabia for funding this research work under the grant number RGP2-10-43
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akkaya Oy, S., and A. E. Özdemir, “Usage of Piezoelectric Material and Generating Electricity,” in Proceeding. 2016 IEEE International Conference on Renewable Energy Research and Applications, ICRERA 2016, 2016, Vol. 5, pp. 63–66.
- Aliteh, N. A., K. Minakata, K. Tashiro, H. Wakiwaka, K. Kobayashi, H. Nagata, and N. Misron. 2020. “« Fruit Battery Method for Oil Palm Fruit Ripeness Sensor and Comparison with Computer Vision Method ».” Sensors 20 (3): 637. doi:10.3390/s20030637.
- Asner, G. P., J. M. O. Scurlock, and J. A. Hicke. 2003. “Global Synthesis of Leaf Area Index Observations: Implications for Ecological and Remote Sensing Studies.” Global Ecology and Biogeography 12 (3): 191. doi:10.1046/j.1466-822X.2003.00026.x.
- Babu, A., D. Rakesh, P. Supraja, K. U. K. Siju Mishra, R. Rakesh Kumar, M. Estari, E. Mamidala, R. Nagapuri, and R. Nagapuri. 2022. “« Plant-based Triboelectric Nanogenerator for Biomechanical Energy Harvesting ».” Results in Surfaces and Interfaces 8: 100075. doi:10.1016/j.rsurfi.2022.100075.
- Blankenship, R. E., D. M. Tiede, J. Barber, G. W. Brudvig, G. Fleming, M. Ghirardi, M. R. Gunner, et al. 2011. “Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement.” Science 332 (6031): 805. doi:10.1126/science.1200165.
- Boutakiout, A. 2015. “Etude physico-chimique, biochimique et stabilité d’un nouveau produit: Jus de cladode du figuier de Barbarie marocain (Opuntia ficus-indica et Opuntia megacantha)”. Agronomie. Université d’Angers, 2015. Français. ffNNT, ANGE0004ff. fftel–01471785
- Bull, R. 2001. “Renewable Energy Today and Tomorrow.” Proceedings of the IEEE 89 (8): 1216–1226. doi:10.1109/5.940290.
- Cai, X. B., Y. Yang, Y. P. Sun, L. Zhang, Y. Xiao, and H. Zhao. 2010. “« Electricity Generation from Sweet Potato Fuel Ethanol Waste Water Using Microbial Fuel Cell Technology ».” Huan Jing Ke Xue Huanjing Kexue 31 (10): 2512–2517.
- Chavan, P., A. K. Singh, and G. Kaur. 2018. “« Recent Progress in the Utilization of Industrial Waste and by-products of Citrus Fruits: A Review ».” Journal of Food Process Engineering 41 (8): e12895. doi:10.1111/jfpe.12895.
- Chong, P. L., A. K. Singh, S. L. Kok, and D.-V. N. Vo. 2019. “Characterization of Aloe Barbadensis Miller Leaves as a Potential Electrical Energy Source with Optimum Experimental Setup Conditions.” PLoS One 14 (6): 1–21. doi:10.1371/journal.pone.0218758.
- Choo, Y. Y., and J. Dayou. 2013. “A Method to Harvest Electrical Energy from Living Plants.” Journal of Science and Technology 5: 79–90.
- Choo, Y. Y., and J. Dayou. 2013. “A Method to Harvest Electrical Energy from Living Plants.” Journal of Science and Technology 5 (1): 79–90.
- Choo, Y. Y., J. Dayou, and N. Surugau. 2014. “Origin of Weak Electrical Energy Production from living-plants.” International Journal of Energy Research 4 (1): 198–203.
- Clark, W. G. 1935. “Note on the Effect of Light on the Bioelectric Potentials in the Avena Coleoptile.” Proceedings of the National Academy of Sciences 21 (12): 681–684. doi:10.1073/pnas.21.12.681.
- Deng, H., Z. Chen, and F. Zhao. 2012. “Energy from Plants and Microorganisms: Progress in plant-microbial Fuel Cells.” ChemSusChem 5 (6): 1006. doi:10.1002/cssc.201100257.
- Elongation, O. F., O. F. The, and A. Coleoptile. 1925. “The Effect of Light on the Electrical Polarity”. (17): 467–475.
- European Commission. COM. 2014. “A Policy Framework for Climate and Energy in the Period from 2020 to 2030.” 2012: 1–18.
- Galchev, T. V. 2010. Energy Scavenging from low-frequency Vibrations. USA: University of Michigan.
- Goto, Y., N. Yoshida, Y. Umeyama, T. Yamada, R. Tero, and A. Hiraishi. 2015. “Enhancement of Electricity Production by Graphene Oxide in Soil Microbial Fuel Cells and Plant Microbial Fuel Cells.” Frontiers in Bioengineering and Biotechnology 3: 42. doi:10.3389/fbioe.2015.00042.
- Guimarães, R., L. Barros, J. C. Barreira, M. J. Sousa, A. M. Carvalho, and I. C. Ferreira. 2010. “« Targeting Excessive Free Radicals with Peels and Juices of Citrus Fruits: Grapefruit, Lemon, Lime and Orange ».” Food and Chemical Toxicology 48 (1): 99–106. doi:10.1016/j.fct.2009.09.022.
- Hao, Z., G. W. Z, W. Li, J. Zhang, J. Kan, and B.-Y. Cao. 2015. “« Effects of Electrode Material on the Voltage of a Tree-Based Energy Generator ».” PLoS ONE 10 (8): e0136639. PMID: 26302491 10.1371/journal.pone.0136639
- Hill, J. F., and Govindjee. 2014. “The Controversy over the Minimum Quantum Requirement for Oxygen Evolution.” Photosynthesis Research 122 (1): 97. doi:10.1007/s11120-014-0014-8.
- Himes, C., E. Carlson, R. J. Ricchiuti, B. P. Otis, and B. A. Parviz. 2010. “Ultralow Voltage Nanoelectronics PoweredDirectly, and Solely, from a Tree.” IEEE Transactions on Nanotechnology 9: 2–5. https://doi.org/10.1109/TNANO.2009.2032293.
- Huang, S. R., C. H. Chung, H. J. Leu, C. Y. D. Sim, and C. Y. Lin. 2016. “The Living Banana Plant as a long-lasting Battery Cell.” International Journal of Green Energy 13 (7): 2016.
- Ifaei, P., A. Farid, and C. Yoo. 2018. “An Optimal Renewable Energy Management Strategy with and without Hydropower Using a Factor Weighted Multicriteria Decision Making Analysis and nation-wide Big data-Case Study in Iran.” Energy 158: 357–372. doi:10.1016/j.energy.2018.06.043.
- Jesus, I. S., J. Tenreiro Machado, and J. Boaventure Cunha. 2008. “« Fractional Electrical Impedances in Botanical Elements ».” Journal of Vibration and Control 14 (9–10): 1389–1402. doi:10.1177/1077546307087442.
- Jie, Y., X. Jia, J. Zou, Y. Chen, N. Wang, Z.-L. Wang, and X. Cao. 2018. “Natural Leaf Made Triboelectric Nanogenerator for Harvesting Environmental Mechanical Energy.” Advanced Energy Materials 8 (12): 1703133. doi:10.1002/aenm.201703133.
- Kabutey, F. T., Q. Zhao, L. Wei, J. Ding, P. Antwi, F. K. Quashie, and W. Wang. 2019. “An Overview of Plant Microbial Fuel Cells (Pmfcs): Configurations and Applications.” Renewable and Sustainable Energy Reviews 110: 402. doi:10.1016/j.rser.2019.05.016.
- Konstantopoulos, C., E. Koutroulis, N. Mitianoudis, and A. Bletsas. 2016. “« Converting a Plant to a Battery and Wireless Sensor with Scatter Radio and Ultra-Low Cost ».” IEEE Transactions on Instrumentation and Measurement 65 (2): 388–398. doi:10.1109/TIM.2015.2495718.
- Labady, A., D. Thomas, T. Shvetsova, and A. G. Volkov. 2002. “Plant Bioelectrochemistry: Effects of CCCP on Electrical Signaling in Soybean.” Bioelectrochemistry 57 (1): 47–53. doi:10.1016/S1567-5394(01)00175-X.
- Lan, L., J. Xiong, D. Gao, Y. Li, J. Chen, J. Lv, J. Ping, Y. Ying, and P. S. Lee. 2021. “Breathable Nanogenerators for an On-Plant Self-Powered Sustainable Agriculture System.” ACS Nano 15 (3): 5307–5315. doi:10.1021/acsnano.0c10817.
- Lew, T. T. S., V. B. Koman, P. Gordiichuk, M. Park, and M. S. Strano. 2019. “The Emergence of Plant Nanobionics and Living Plants as Technology.” Advanced Materials Technologies 1900657.
- Lew, T. T. S., M. Park, J. Cui, and M. S. Strano. 2020. “Plant Nanobionic Sensors for Arsenic Detection.” Advanced Materials Interfaces 2005683.
- Liu, H., C. Shi, H. Zhang, Z. Wang, and S. Chai. 2013. “« Effects of Potassium on Yield, Photosynthate Distribution, Enzymes’ Activity and Aba Content in Storage Roots of Sweet Potato (’ipomoea Batatas’ Lam.) ».” Australian Journal of Crop Science 7 (6): 735.
- Mayé, P.2010.générateurs électrochimiques piles, accumulateurs et piles à combustibles.Paris: Dunod.
- Mensah-Darkwa, K., C. Z. Pawan, K. Kahol, and K. G. Ram. 2019. “Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy.” Sustainability Journal 11 (2): 414. doi:10.3390/su11020414.
- Mohan, S. V., G. Mohanakrishna, and P. Chiranjeevi. 2011. “Sustainable Power Generation from Floating Macrophytes Based Ecological Microenvironment through Embedded Fuel Cells along with Simultaneous Wastewater Treatment.” Bioresource Technology 102 (14): 7036. doi:10.1016/j.biortech.2011.04.033.
- Nitisoravut, R., and R. Regmi. 2017. “Plant Microbial Fuel Cells: A Promising Biosystems Engineering.” Renewable and Sustainable Energy Reviews 76: 81. doi:10.1016/j.rser.2017.03.064.
- Özdemir, A. E., and S. Akkaya Oy, “Alternative Renewable Energyproducing Systems by Utilizing Piezoelectric Transducers,” in Proceedin. 2016 International Conference on Renewable Energy Research and Application ICRERA 2016, 2016, Vol. 5, pp. 59–62.
- Park, K. I., J. H. Son, G.-T. Hwang, C. K. Jeong, J. Ryu, M. Koo, I. Choi, et al. 2014. “Highly-Efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates.” Advanced Materials 26 (16): 2514–2520. DOI:10.1002/adma.201305659.
- Shittu, S. A., S. A. Ajagbe, and R. F. Oloruntola. 2018. “« Conversion of Fruit to Battery ».” International Journal of Scientific & Engineering Research 9 (1): 1747–1755.
- Shrestha, N., A. Fogg, J. Wilder, D. Franco, S. Komisar, and V. Gadhamshetty. 2016. “Electricity Generation from Defective Tomatoes.” Bioelectrochemistry 112: 67–76. doi:10.1016/j.bioelechem.2016.07.005.
- Tanaka, A., T. Ishihara, F. Utsunomiya, and T. Douseki. 2012. “« Wireless self-powered Plant health-monitoring Sensor System ».” IEEE SENSORS. 1–4. 10.1109/ICSENS.2012.6411369
- Teng, H. C., B. C. Kok, C. Uttraphan, and M. H. Yee. 2018a. “A Review on Energy Harvesting Potential from Living Plants: Future Energy Resource.” International Journal of Energy Research 8 (4): 2398–2414.
- Teng, H. C., B. C. Kok, C. Uttraphan, and M. H. Yee. 2018b. “A Review on Energy Harvesting Potential from Living Plants: Future Energy Resource.” International Journal of Renewable Energy Research 8 (4): 2598.
- Timmers, R. A., D. P. B. T. B. Strik, H. V. M. Hamelers, and C. J. N. Buisman. 2010. “Long-term Performance of a Plant Microbial Fuel Cell with Spartina Anglica.” Applied Microbiology and Biotechnology 86 (3): 973. doi:10.1007/s00253-010-2440-7.
- Twidell, J., and T. Weir. 2015. Renewable Energy Resources. 3rd ed. Routledge.
- Vullers, R. J. M., R. van Schaijk, I. Doms, C. Van Hoof, and R. Mertens. 2009. “Micropower Energy Harvesting.” Solid-State Electronics 53 (7): 684–693. doi:10.1016/j.sse.2008.12.011.
- Wu, H., Z. Chen, G. Xu, J. Xu, Z. Wang, and Y. Zi. 2020. “Fully Biodegradable Water Droplet Energy Harvester Based on Leaves of Living Plants.” ACS Applied Materials & Interfaces 12 (50): 56060–56067. doi:10.1021/acsami.0c17601.
- Xu, Y., Y. Lu, and X. Zhu. 2021. “Toward Plant Energy Harvesting for 5G Signal Amplification.” ACS Sustainable Chemistry & Engineering 9: 1099–1104.
- Yanga, Y., S. Bremnera, C. Menictasb, and M. Kay. August 2018. “Battery Energy Storage System Size Determination in Renewable Energy Systems: A Review.” Renewable and Sustainable Energy Reviews 91: 109–125. doi:10.1016/j.rser.2018.03.047.
- Ying, C. Y., and J. Dayou. 2014. “Increasing the Energy Output from Living Plants Fuel Cells with Natural Photosynthesis.” Advances in Environmental Biology 8 (14): 20–23.
- Yuan, J., C. Li, W. Li, D. Liu, and X. Li. 2018. “Linguistic Hesitant Fuzzy multi-criterion decision-making for Renewable Energy: A Case Study in Jilin.” Journal of Cleaner Production 172: 3201–3214. doi:10.1016/j.jclepro.2017.11.038.