Abstract
Quality by design (QbD) is an innovative approach to drug development that has started to be implemented into the regulatory framework, but currently mainly for chemical drugs. The recent marketing authorization of the first monoclonal antibody developed using extensive QbD concepts in the European Union paves the way for future further regulatory approvals of complex products employing this cutting-edge technological concept. In this paper, we report and comment on insights and lessons learnt from the non-public discussions in the European Medicines Agency's Biologicals Working Party and Committee for Medicinal Products for Human Use on the key issues during evaluation related to the implementation of an extensive QbD approach for biotechnology-derived medicinal products. Sharing these insights could prove useful for future developments in QbD for biotech products in general and monoclonal antibodies in particular.
Keywords:
Abbreviations
| BWP | = | Biologics Working Party |
| CHMP | = | Committee for Medicinal Products for Human Use |
| EMA | = | European Medicines Agency |
| QbD | = | quality by design |
| QTPP | = | Quality Target Product Profile |
| CQAs | = | critical quality attributes |
| CPPs | = | critical process parameters |
Introduction
Quality by design (QbD) is an innovative product development process approach using both existing knowledge and emerging science to identify key “quality” issues (in regulatory jargon, the chemistry/manufacturing/control (CMC) of a medicine) in order to address or predict their impact on product attributes and ultimately patients’ health. This can enable a certain freedom to manoeuver manufacturing parameters of a product within a pre-approved design space while they happen during manufacture, without consulting regulatory agencies upfront.
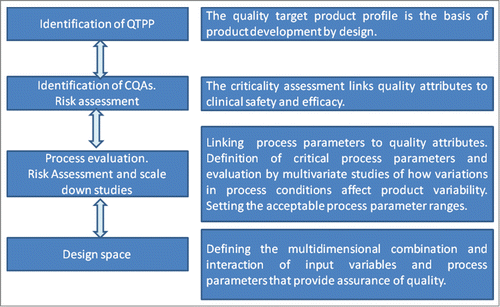
The basis of the product development design, according to QbD, consists of the establishment of the Quality Target Product Profile (QTTP) according to which critical quality attributes (CQAs) and critical process parameters (CPPs) are identified, and appropriate control strategies established and implemented. QbD “builds quality into the product instead of testing it.”Citation1
The main pros and cons of QbD are intrinsic to the process itself, as QbD requires an understanding of clinical characteristics and desired product performance already from early on in the development process; criteria are set as the goal at which product formulation and process development are aimed. A QbD approach increases product/process knowledge and understanding, thereby reducing risk of batch failure, but requires significant investment in resources at very early stages of product development where it is often far from clear if the drug candidate will be safe and efficacious in later clinical trials. Of interest is the possibility, enabled by QbD, to improve manufacturing efficiency of the product and facilitate regulatory flexibility in the post-approval setting – thus having the potential of being faster, more straightforward and potentially cheaper in the long run. The latter is assured, for example, by the identification of a product design space. Movements within an approved design space do not need to be notified to regulatory authorities.
The need of understanding and keeping the complex manufacturing processes for biotech products under control, together with reduced overall costs related to development and maintenance of marketing authorization, is the driver for pharmaceutical companies to apply QbD principles during biopharmaceutical development.Citation2-4 However, implementing QbD for biotechnology products still represents a challenge due to the complexity of both the manufacturing processes and the product itself.
Successful implementation of QbD concepts often presupposes a huge amount of cooperative work, involving not only several sectors within a pharmaceutical company (Research and Development, manufacturing, quality control and regulatory affairs), but also regulatory agencies who need to accept the particular concept. General principles of QbD are outlined in the Q8, Q9, Q10 and Q11 guidelines issued by the “International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use” (ICH),Citation5-8 leaving room for flexibility in the specific approach to be adopted by the different pharmaceutical companies. However, this increases the possibility for lack of harmonisation in the use of definitions, in the overall validation approach, in the application of statistical techniques, and, last but not least, in the information provided in the marketing authorisation application (MAA) dossier.
Although great effort has been invested by ICH to facilitate a common understanding of the QbD concepts, improvement of QbD knowledge through scientific discussions involving regulatory agencies, industry and Academia is still needed in order to gain a common background and to enable implementation of the opportunities of QbD. As of 1 April 2014, the European Medicines Agency (EMA) and the United States Food and Drug Administration (US FDA) have agreed on a 2-year extension of their joint pilot program for the parallel evaluation of QbD applications,Citation9 underlining the great potential that this approach bears. Collaboration between industry and regulators is ongoing (e.g., a joint EMA-Industry QbD workshop held at the European Medicines Agency in early 2014-) in order to reach agreement on implementation of QbD principles, on terminology, definitions, and filing requirements. At present, many issues still need to be addressed and, to date, marketing approval based on a QbD dossier has essentially been granted to only a few products, mainly chemicals.
Biotechnology-derived medicinal products, however, are now emerging on the QbD stage. Recently, a marketing authorization, valid throughout the European Union, was issued for an anti-human epidermal growth factor receptor (HER)2 monoclonal antibody (mAb), for use in combination with trastuzumab and docetaxel for the treatment of adult patients with HER2-positive metastatic or locally recurrent un-resectable breast cancer, who have not received previous anti-HER2 therapy or chemotherapy for their metastatic disease.Citation10 In this paper, we discuss lessons learnt from the evaluation of this pioneer dossier, which should rather be seen as an exemplifying case study than the discussion of an individual dossier. The MAA was filed to regulatory agencies making use of QbD concepts, on the basis of previous knowledge acquired in the manufacturing of other authorised mAbs with different specificities, and adopting risk assessment and decision tools to establish CQAs, CPPs, acceptable process parameter ranges, as well as the drug substance and drug product control systems and process monitoring.
Although substantial effort was made by the Applicant to explain and justify their approaches and rationales, the evaluation of the data presented in the dossier triggered extensive discussion among members of the EMA's Biologics Working Party (BWP), its QbD Core Group, and the quality (CMC) assessors (Rapporteur and Co-Rapporteur, respectively). A number of issues were identified that required further clarification and discussion prior approval.
Identification of Critical Quality Attributes
The first issue raised in the review process of the MAA dossier concerned the identification of CQAs, defined as “a physical, chemical, biological or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality.”Citation5 Defining criticality of a specific attribute is a matter of risk assessment; it should be evaluated by counterbalancing the known or potential product quality impact on efficacy and safety taking into account the degree of uncertainty. Although a conservative approach had been adopted for CQA designation in the filed dossier, compliant with current guidelines, regulators required some further clarifications and refinement. For example, initially antibody-dependent cell-mediated cytotoxicity (ADCC) had not been identified as a CQA, based on the characterization studies that demonstrated that ADCC has no role in the mechanism of action of the mAb molecule. However, the review process highlighted the potential impact of ADCC on safety, and led to the inclusion of a test for fucosylation pattern, as this strongly correlated to ADCC activity, into the control strategy of the product. This was considered important for the efficacy and safety of therapeutic antibodies, especially in the oncology field; ADCC enhancement technology, including the modification of N-glycans attached to the constant (Fc) region of the antibody, has become a focus of attention for the biopharmaceutical industry.Citation11 According to QbD principles, the rationale for the selection of a specific CQA can be built up considering data from literature and from similar molecules, along with existing product knowledge.
Although the use of prior knowledge from similar products, when available and soundly grounded, is encouraged in the QbD approach, limitations may apply. For example, when using existing data from related mAbs that target different ligands, it has to be taken into account that the same product-related variants (e.g., dimers) may have different impacts on the efficacy and safety profile of related mAbs, mainly due to differences in the target population and specific characteristics of the underlying disease. Thus, in filing a MAA for a biotech product based on a QbD approach, the clinical context should be considered to identify, on a case-by-case basis, whether (and which) data from clinical batches or previous knowledge would more appropriately drive the setting of acceptance criteria for CQAs (CQA-ACs). A comparability exercise and justification of quality differences may support, in some cases, the acceptance of CQA-ACs primarily based on data from similar products.
Process Evaluation: Linking Process Parameters to Quality Attributes
CQAs are central for process evaluation as they allow for analysis of the impact of process parameters on the final achievement of defining the QTPP. Process parameters are variables or conditions of the manufacturing process, typically physical or chemical conditions (e.g., temperature, pH, process time, column flow rate, column wash volume, reagent concentration). If the variability of a process parameter has a significant impact on a CQA, the process parameter is identified as critical (CPP), and it should be monitored or controlled (e.g., granulation end point) to ensure that the product is of the desired quality.Citation5
The achievement of an objective quantification of the absolute impact of the process parameters on the CQAs represents one of the central challenges of an extensive QbD approach. This process passes through 2 main steps: 1) the estimate of the severity of the parameter's effect on a product QA, by a risk assessment tool, to guide on the minimum level of complexity with which parameters should be characterized in further studies (i.e., decision to perform multivariate studies evaluating how multiple variations in process conditions affect CQAs and thus product variability, univariate studies, or no further evaluation); and 2) the quantification of the PP impact on the CQA based on the characterization studies and on the output of statistical and mathematical analysis. In this regard, the setting of appropriate ranges for CQA-ACs, i.e., the identification of tolerance limits within which the measured result of a particular attribute of a manufacturing batch should fall, is of pivotal importance. The width of a CQA-AC was used in the normalization of the measure (e.g., impact ratio) that quantifies the impact of a process parameter on a CQA, so that all the parameters can be evaluated by applying the same criteria/approach. Regulators intensively discussed how to balance the fact that, if a narrow process parameter range is studied, the process impact on a CQA might not be seen due to the narrow range investigated and, as a consequence, a critical process influence might be undetected. In contrast, if the commercial production process will always be run under these narrow process parameter settings, no impact on a CQA is foreseen. The latter case, however, implies a lack of knowledge for assessors at regulatory agencies (and the manufacturer) about how close the commercial process is to the edge of failure. The rationale adopted for setting the different thresholds used in the whole approach (risk assessment, statistical significance of the effects and cut-off values to classify the PPs), to identify the impact of the effects of a given parameter on a CQA was considered crucial and required a detailed discussion and justification.
The Validity of Small and Pilot Scale Observations for Process Evaluation and Validation
The complete process evaluation and verification project may include studies conducted using a combination of scale-down models and full-scale equipment. Meaningful assessment of study results performed using scale-down models relies on the ability of the scale-down model to predict performance at manufacturing scale. Scale-down models can be used for the multivariate analysis conducted to identify how all involved parameters, within their full ranges, in each process step may influence the CQAs. Finally, based on the multivariate studies a design space may be defined (Fig. 1). It is therefore important that the scale-down models are reliable in predicting performance at manufacturing scale. For complex unit operations, such as chromatography steps, the qualification of scale-down models is properly accomplished by comparing process performance at manufacturing versus small/pilot scale when they are all operated at the normal operating targets. For a given CQA, the “scaling problem” may be addressed by performing statistical tests for equivalence of means on CQA results obtained on small and full scale data. CQAs whose level can be considered equivalent are identified as non-scale-dependent and can be used to reliably support an extensive QbD approach in the building of a design space.
Equivalence test outcomes were in this case study descriptively summarized in 4 categories: “equivalence/probable equivalence,” “non-equivalence/probable non-equivalence.” Regulators favored a cautious approach in the interpretation of the equivalence test results. In fact, only the “fully equivalent” output was considered indicative of scale-independence, while all the other cases were considered as scale-dependent. The statistical equivalence analysis adopted in the MAA to compare 2 different scales output was based on the definition of “equivalence bands,” i.e., intervals over which a difference between test groups was not considered significant. Although it should be pre-defined, proper selection of the bands width is difficult to achieve and can be challenging. In fact, it depends on the assay precision, process variability or capability, and adjustments may be needed. Prior knowledge and experience can help in setting these parameters. In the case of the dossier discussed in this paper, most of the scale-down models proposed had been used by the Applicant for many years in both the development and validation of the commercial manufacturing processes for other approved antibody products. This was considered as solid support for the qualification of the scales, and resulted in the acceptance by regulators of the bands width set as the 10% of the manufacturing scale average or the 10% of the allowed variation of the CQA (defined by the difference between the manufacturing scale average and the CQA-Target Range). Although the adequacy and acceptability of equivalence bands width is product-dependent and no generalization may be applied, approaches taking into account existing knowledge as well as assay and process specificity appear sound and are encouraged.
The identification of suitable approaches to manage those CQA levels that do not result as fully equivalent is a key issue that still needs extensive discussion between pharmaceutical companies and regulators as no appropriate validated strategies/tools are at present available. A possible way forward could be the introduction of “correction factors” to adapt results from the small scale and make them predictive of the performance obtained for full manufacturing scale. In the MAA dossier discussed here, the absolute difference between the CQA mean values obtained at the small (pilot) and manufacturing scales was proposed as a “correction factor.” The latter was thus introduced in the normalization process for quantification of the impact of a process parameter on a CQA (e.g., impact ratio). Regulators, however, at present intensively debate the use and acceptance of “correction factors” with contrasting views.
Strategies aimed at minimizing potentially occurring risks in the prediction of the manufacturing scale, such as, for instance, the attribution of “scale-dependence” to those test outputs on CQA levels resulting only as “probably equivalent,” need to be proposed and discussed among industry and regulators to meet an agreement.
The objective of the correct prediction of the manufacturing scale by the qualification of small scales is at present still considered a major challenge by both industry and regulators. An added value could be represented by the use of supportive approaches for the qualification of scale-down models, which can range from the use of nonparametric techniques for mean values comparison to the adoption of more complex statistical multivariate analysis (e.g., “Principal Component Analysis”) that could ensure higher sensitivity than the univariate comparison of mean values. The investigation and identification of new strategies aimed at overcoming the “scaling problem” are encouraged for the future implementation in an MAA based on QbD. Innovative strategies to adequately qualify the small scale and to manage all the cases that are not found to be fully equivalent should be identified in parallel, validated and discussed. Any effort of the manufacturers in this sense is strongly encouraged by regulators.
Definition of the Design Space
Once high- and low-impact CPPs are defined (on the basis of the magnitude of their effect on a CQA) and sufficient knowledge of process performance when operated under worst-case conditions for each CQA is acquired, a design space for the overall process can be defined. The design space is thus the multidimensional combination and interaction of input variables and process parameters that has been demonstrated to provide assurance of quality. Working within the approved design space is not considered as a change in the regulatory approval because it has already been assured that it does not significantly affect quality. Movement outside of the design space would result in the initiation of a regulatory “variation” process that requires regulatory approval.Citation5 When defining a design space, “safe” ranges for several parameters that may vary simultaneously should be taken into account. Hence, one of the most critical points in the definition of a design space is whether critical parameters should be included alone or in combination with non-critical ones. According to the ICH Q8 guideline, the design space is defined as the multidimensional combination of all CPPs for a process, with the acceptable degree of variation described by the Multivariate Acceptable Range defined for each CPP.
During the MAA reviewing process of the first mAb dossier using a QbD approach, the inclusion of non-CPPs to define the boundaries of the design space was considered of pivotal importance by regulators in order to gain further confidence that any post-authorisation process change within the design space would not significantly affect the QTPP. This conservative approach was based on the consideration that the simultaneous change on more than one non-CPP could result in an increased impact on QAs that would require adequate control.
Questions around an operation area comprising all relevant process parameters and tolerance ranges had been identified, along with remaining issues on the consistency of the designation of process of parameter criticality, as well as the demonstration of equivalence between the small-scale models and manufacturing scale, and this led to a complex discussion during the review process highlighting the need to further investigate and address the robustness of the design space approach.
Control Strategy
As defined in the ICH Q10 guideline, the control strategy consists of “a planned set of controls, derived from current product and process understanding that assures process performance and product quality.” The peculiarity of the QbD approach allows framing of control to critical elements. Establishment of the control strategy typically includes risk assessment of identified process criticalities and capability to meet the QTPP.
Controls can include drug substance and drug product materials and components, drug product specifications, facility and equipment operating conditions, in-process control, batch release and stability testing, comparability evaluation, in case of a change in the manufacturing process or as further process knowledge is gained, as well as the associated analytical methods and frequency of monitoring and control.
Soundness and reliability of the planned control strategy is likely to increase if the robustness of the testing strategy is evaluated. This was accomplished in the MAA evaluation by taking into consideration the type of test performed (e.g., direct/indirect measure) and its sensitivity and specificity.
Conclusions
Using a QbD approach allows the capture of key information from prior knowledge and scientific data to perform risk assessments aimed at selecting straightforward studies and testing, thus avoiding redundant investigations. If a robust QbD program is put in place, this may result in developmental relief, and it may well be an attractive investment in the long-run. Experience in risk assessment of biotech products may facilitate future development of complex drugs with similar biophysical and biochemical properties, ensuring at the same time standards of quality and manufacturing flexibility, and eventually resulting in a simplified post-approval product lifecycle management plan. However, the QbD approach in pharmaceutical development is a multi-step process consisting of highly interconnected assumptions, definitions and selections of criteria, attributes and relative ranges. Failing to appropriately address only one part of the whole process inevitably results in loss of reliability of the entire system. A common language and a core of shared principles and rules to enable consistency of approach and judgment throughout the whole QbD development process therefore needs to be agreed by industry and regulators.
The presented case scenario demonstrated that implementing QbD for complex biotechnology products is feasible, but challenging, for both industry and regulators. Reviewing the MAA gave the European regulators an opportunity to review an innovative strategy and accumulate experience in the field, further fuelling the QbD approach in biotech product development in general. Lessons learnt during the review process of the first MAA involving a biotech product produced by a QbD strategy point out the need to strengthen in future MAAs the data packages supporting selection of CQAs and relative tolerance limits, prompt investment in scale-down model design, and foster the identification of the most suitable approaches to define and verify the proposed design space. In fact, in 2014 a marketing authorization was granted for another mAb from the same Applicant, with lessons learnt from the first case already implemented.Citation12
Disclosure of Potential Conflicts of Interest
The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
Acknowledgments
The authors wish to thank all members of the Biologicals Working Party for fruitful discussion.
References
- Rathore AS, Winkle H. Quality by design for biopharmaceuticals. Nature Biotech 2009; 27:26-34; http://dx.doi.org/10.1038/nbt0109-26
- Kozlowski S, Swann P. Current and future issues in the manufacturing and development of monoclonal antibodies. Adv Drug Deliv Rev 2006; 58:707-722; PMID:16828921; http://dx.doi.org/10.1016/j.addr.2006.05.002
- Martin-Moe S, Lim FJ, Wong RL, Sreedhara A, Sundaram J, Sane SU. A new roadmap for biopharmaceutical drug product development: Integrating development, validation, and quality by design. J Pharm Sci 2011; 100:3031-3043; PMID:21425164; http://dx.doi.org/10.1002/jps.22545
- Rathore AS. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol 2009; 27:546-553; PMID:19647883; http://dx.doi.org/10.1016/j.tibtech.2009.06.006
- International Conference of Harmonisation (ICH) Harmonised Tripartite Guideline: Q8(R1-R2) Pharmaceutical Development. 2008. Available at: www.ich.org/products/guidelines/quality/article/quality-guidelines.html
- International Conference of Harmonisation ICH Harmonised Tripartite Guideline: Q9 Quality Risk Management. 2005. Available at: www.ich.org/products/guidelines/quality/article/quality-guidelines.html
- International Conference of Harmonisation ICH Harmonised Tripartite Guideline: Q10 Pharmaceutical Quality Systems. 2008. Available at: www.ich.org/products/guidelines/quality/article/quality-guidelines.html
- International Conference of Harmonisation ICH Harmonised Tripartite Guideline: Q11 Development and manufacture of drug substances (chemical entities and biotechnological/biological entities). 2012. Available at: www.ich.org/products/guidelines/quality/article/quality-guidelines.html
- EMA and FDA extend pilot programme for parallel assessment of quality-by-design applications. 2014. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/03/news_detail_002035.jsp&mid=WC0b01ac058004d5c1
- European Medicines Agency (EMA). European public assessment report for Perjeta. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002547/WC500141004.pdf
- Shade K-T C, Anthony RM. Antibody glycosylation and inflammation. Antibodies 2013; 2:392-414; http://dx.doi.org/10.3390/antib2030392
- European Medicines Agency (EMA). european public assessment report for Gazyvaro. 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002799/WC500171596.pdf