ABSTRACT
Nivolumab, an anti-programmed death (PD)1 IgG4 antibody, has shown notable success as a cancer treatment. Here, we report that nivolumab was susceptible to aggregation during manufacturing, particularly in routine purification steps. Our experimental results showed that exposure to low pH caused aggregation of nivolumab, and the Fc was primarily responsible for an acid-induced unfolding phenomenon. To compare the intrinsic propensity of acid-induced aggregation for other IgGs subclasses, tocilizumab (IgG1), panitumumab (IgG2) and atezolizumab (aglyco-IgG1) were also investigated. The accurate pH threshold of acid-induced aggregation for individual IgG Fc subclasses was identified and ranked as: IgG1 < aglyco-IgG1 < IgG2 < IgG4. This result was cross-validated by thermostability and conformation analysis. We also assessed the effect of several protein stabilizers on nivolumab, and found mannitol ameliorated the acid-induced aggregation of the molecule. Our results provide valuable insight into downstream manufacturing process development, especially for immune checkpoint modulating molecules with a human IgG4 backbone.
Abbreviations
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| CDC | = | complement-dependent cytotoxicity |
| LC-MS | = | liquid chromatography-mass spectrometry |
| DSC | = | differential scanning calorimetry |
| CD | = | circular dichroism |
| CEX | = | cation-exchange chromatography |
| HMW | = | high molecular weight |
| QbD | = | quality by design |
| CPP | = | critical process parameter |
| CQA | = | critical quality attributes |
Introduction
Nivolumab (MDX-1106, BMS-936558, ONO-45338), a human hinge-modified (S228P) IgG4 monoclonal antibody (mAb) that targets programmed cell death protein 1 (PD1) receptor,Citation1 was recently approved in the United States (US) and European Union (EU) for treatment of cancer patients. Nivolumab can elicit potential antitumor immune response of anergy or exhausted T cells, which was inhibited by PD1 receptor binding to its ligand. Clinic trials demonstrated that nivolumab had promising activity, particularly in advanced melanoma, non-small cell lung cancer and renal cell carcinoma, with tolerable toxicity.Citation2,3 In addition, anti-PD1 pembrolizumab, with the same mechanism of action and IgG4 backbone structure, has also been approved in the US and EU.
When developing therapeutic antibodies, the IgG subclass needs to be carefully chosen according to whether effector functions are necessary for the mechanism of action. In general, IgG1 and IgG3 have greater ability to activate antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) than IgG2 and IgG4. Accordingly, IgG1 has been the most popular choice by far when the effector function is required to eliminate target cells, while IgG2 and IgG4 backbones have been chosen specifically in cases when only blocking function was required.Citation4 To date, IgG3 format has not been used for therapeutic antibodies because it exhibits a short half-life in circulation. The clinically relevant subclasses of human IgG, which differ in their heavy chain sequences, hinge region lengths and disulfide bonding patterns, exhibit different conformational preferencesCitation5 and thermodynamic stability. For instance, it was reported that the order of aggregation propensity under thermal stress was IgG1 < IgG2< IgG4.Citation6
The instability of protein molecules is often manifested by the formation of aggregates during processing and storage. Generally, protein aggregates exhibit either reduced or no biological activity and, more importantly, might show high immunogenicity in the clinic. Consequently, aggregate levels and properties of therapeutic protein products need to be controlled.Citation7 Recently, formation of antibody aggregates had been described under a variety of circumstances, including freeze/thaw, agitation, thermal stress, and changes in formulation buffer, that occur in the manufacturing process.Citation8 In particular, exposure to low pH during purification processes (e.g., rProtein A chromatography, low-pH virus inactivation) was shown to contribute to IgG aggregation.Citation9,10
Previous studies have shown that IgG1 has a lower propensity to aggregate compared with IgG2,Citation11,12 and IgG4 is susceptible to aggregation in acidic condition.Citation10,13 In contrast, Arosio et al studied an IgG2 and 2 IgG1 antibodies and reported that aggregation propensity of antibodies did not correlate with subclass.Citation14 Studies on the underlying mechanism and kinetics of acid-induced aggregation have shown that lower pH led to larger net repulsive colloidal interactions of IgG1, decreased thermal stability of Fc and Fab regions and accelerated formation of aggregates.Citation15 Additionally, subclass-specific aggregation-prone motifs on Fc fragments led to 2 distinct aggregation pathways,Citation16 and changing the pH significantly altered the growth kinetics of acid-induced aggregation.Citation17 Furthermore, Seiki Yageta et al reported CH3 domain plays the most critical role in driving intact IgG aggregation under acidic conditions.Citation18
Here, we report nivolumab was prone to aggregation during a purification process conventionally used for mAbs. Aggregate and monomer fractions of nivolumab bulk substance were characterized in-depth by liquid chromatography-mass spectrometry (LC-MS). After excluding disulfide bond mismatches and post-translational modification changes, we concluded that exposure to acidic conditions accounted for the excessive aggregation of nivolumab. Subsequently, we analyzed the effect of pH on human monoclonal antibodies and Fcs from representative molecules of 4 subclasses (aglyco-IgG1, IgG1, IgG2, and IgG4). To gain further insight into the underlying mechanism of the acid-induced aggregation, the thermostability and conformational state of the 4 Fc subclasses at acidic solution were also characterized by differential scanning calorimetry (DSC) and circular dichroism (CD). Lastly, the acid-induced aggregation of nivolumab was ameliorated by addition of mannitol, which is pharmacologically inert and could be easily removed during further downstream processing, to the rProtein A elution buffer.
Results
Characterization of non-native aggregate and monomer fractions of nivolumab
As shown in , aggregated nivolumab reached more than 30% of bulk substance after purification-based rProtein A capture chromatography and 2 polishing chromatography steps, which is a platform purification method that has been successfully used for several biosimilar candidates produced in our lab. We are aware that aggregation of IgG2 occurs as a consequence of free thiols and mismatched disulfide bonds.Citation19,20 Potential contributors to aggregation, such as structural variations or post-translational modifications, were thus taken into consideration.
Figure 1. In-depth characterization of nivolumab aggregate and monomer fractions by LC-MS. (A) SEC-HPLC chromatogram of nivolumab bulk substance after rProtein A capture chromatography, which contained 36% aggregate. (B) SEC-HPLC chromatogram of nivolumab aggregates and monomer fractions isolated by strong IEC. (C) Deconvolution MS spectrum of aggregate and monomer fractions at intact protein level. (D) Modification abundance difference for aggregate and monomer fragments assessed by LC-MS in subunit level.
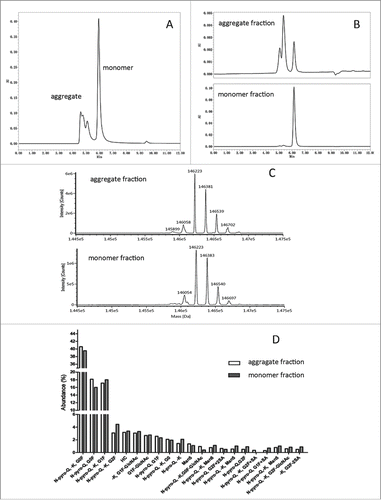
Aggregate and monomer fractions of nivolumab bulk substance were isolated and enriched by strong cation-exchange chromatography (strong CEX). As shown in , the high molecular weight (HWM) species were 70% of the aggregate fraction, while 1% of the monomer fraction. The aggregate and monomer fractions were then characterized by LC-MS. As illustrated in , the intact protein molecular weights of the aggregate fraction (146058, 146223, 146381, 146539, 146702 Da) were highly similar with those of the monomer fraction (146054, 146223, 146383, 146540, 146697 Da), although some deviation (<5 Da) can be attributed to systematic error. After reduction of disulfide bonds, no obvious differences in the abundance of major post-translation modifications, including Fc glycosylation, C-terminal lysine truncation, and N-terminal pyroglutamination, were observed at the subunit level (). Importantly, peptide mapping analysis also verified the correct formation of disulfide bonds in the aggregate fractions (data not shown). Taken together, the results demonstrated that aggregates of nivolumab were not associated with post-translation modifications or disulfide shuffling in the protein primary structure, unlike a previous study that showed IgG2 covalent dimer formation via disulfide shuffling.Citation20
Acid-induced aggregation of nivolumab depends on the Fc
Acid-induced aggregates of Campath-1H exposed to rProtein A elution buffer (0.1 M sodium citrate, pH3.2) have been shown to reach 25%.Citation21 When nivolumab was eluted by 25 mM citrate (pH3.0) from an rProtein A column, the elution pool pH was ∼3.5 or even lower. To verify whether overexposure to acidic condition could result in aggregation, 1 mg/mL nivolumab sample was incubated in 25 mM citrate buffer at various pH values for 1 hour. Triplicate runs reproducibly revealed that monomer decreased from 98% at pH4.0 to 70% at pH3.5 ().
Figure 2. Acid-induced aggregation of nivolumab was attributed to pH threshold of Fc fragment. (A) SEC-HPLC chromatogram of nivolumab bulk substance in 25 mM citrate buffer at pH4.0 or 3.5. (B) SEC-HPLC chromatogram of nivolumab digested with IdeS incubated at pH4.0 and 3.5.
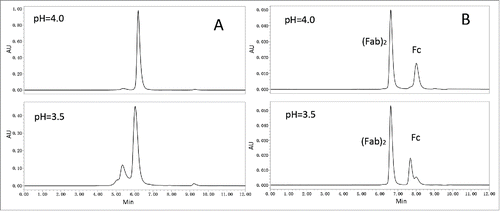
To identify the potential aggregation-prone regions, nivolumab samples were digested with IdeS and incubated at pH4.0 or pH3.5 for 1 hour. Compared to nivolumab fragments in pH4.0, the chromatographic peak of the Fc in pH3.5 () was obviously changed, while the peak shape of (Fab)2 remained in its native state. The results suggested that the Fc fragment of nivolumab was more sensitive to acidic condition than the (Fab)2, and IgG4Fc may be the cause of the acid-induced aggregation of nivolumab observed in the rProtein A elution pool.
pH thresholds of different IgG subclasses in acidic conditions
Because the 3 natural isotypes (IgG1, IgG2 and IgG4) and mutated aglyco-IgG1 (N297A) are now commonly used as therapeutic antibody formats, the effects of pH on tocilizumab, panitumumab, nivolumab and atezolizumab, which are approved antibody therapeutics of the IgG1, IgG2, IgG4 and aglyco-IgG1 subclasses, respectively, were comparatively investigated. In the pH titration experiment, the pH values of 25 mM citrate solution were adjusted to 3.0–4.0, which mimic the pH fluctuation range of Protein A elution buffer; extremely acidic conditions (pH2.0, 2.5) were also investigated in our study. The incubation time was set to 1 hour, which was the time required for the rProtein A chromatography elution step during large-scale manufacturing of a commercial antibody.
As shown in , nivolumab (IgG4) exhibited a particularly high aggregation propensity, which resulted in a loss of 30% monomer at pH3.5. In contrast, there was less aggregation of atezolizumab (5%) or panitumumab (13%) under the same acid-exposure conditions. Tocilizumab exhibited the greatest stability to acid in our study, even no obvious aggregation formation until the pH was below 3.0. The acid-induced aggregation of the Fc or (Fab)2 was also investigated. As shown in , the Fcs had a greater acid-induced aggregation tendency than (Fab)2 at the same pH value. In the case of nivolumab, Fc aggregates reached 40% at pH3.75, while (Fab)2 aggregates were only 7%. The thresholds of acid stability for 4 antibodies and their fragments were determined (). The results suggest that the acid-induced aggregation propensities of the 4 subclass IgGs and Fcs could be ranked as: IgG1 < aglycosylated IgG1 < IgG2 < IgG4.
Figure 3. The effect of pH value on monomer stability for 4 antibody subclasses and their fragments. Monomer of 4 antibody subclasses (A), Fc (B) and (Fab)2 (C) of tocilizumab (circle), panitumumab (square), nivolumab (diamond), and atezolizumab (triangle) were monitored using SEC-HPLC. Each data point represents the mean of triplicate measure and error bars represent SD.
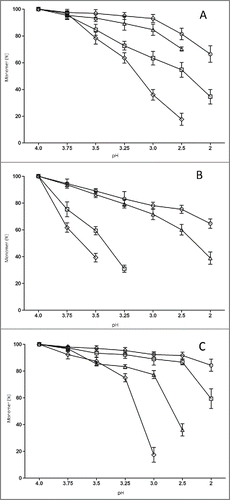
Table 1. The acid-induced aggregation pH thresholds of 4 therapeutic antibodies.
Since different antibodies in the same subclass category differ in their complementarity-determining regions, the (Fab)2 thresholds determined in the study may not be relevant to other antibodies. However, thresholds for Fcs can be used to understand the behavior of acid-induced aggregation of various subclasses of IgG, and provide clues to optimize manufacturing processes at low pH conditions.
Thermo-stability of 4 subclasses of mAbs and Fc
It has been reported that thermal stability of IgGs is strongly dependent on sample pH.Citation22 To address how the Fcs of different IgG subclasses affected the molecule thermodynamic stability in acidic conditions, the 4 model antibodies and their Fcs were concentrated to 0.5 mg/mL in 25 mM citrate at pH6.0 or pH3.5 for 1 hour. Then, DSC measurements were performed to identify apparent mid-point unfolding temperature (Tm) and calculate the enthalpy of major unfolding transitions (ΔH), which appear as significant endothermic peaks in the DSC profile.
As depicted in , the DSC profile of tocilizumab contained a small peak attributed to the CH2 domain at the lowest Tm and a large peak attributed to the Fab and CH3 domain. For other 3 antibodies, only one large peak was observed in the profile because the 2 peaks overlapped. All thermodynamic parameters of the 4 antibodies are summarized in . We compared the individual Tm and ΔH of the 4 antibodies at pH3.5 with their counterparts at pH6.0, and found that the Tm value was decreased almost 15°C and the ΔH value was reduced by half. For instance, for nivolumab, the Tm was shift from 69.9°C at pH6.0 to 54.6°C at pH3.5, and the ΔH value decreased from 859 to 445 KJ/mol. Similarly, the same thermodynamic tendency of individual Fc subclasses was also observed in data shown in . It was worth noting that an atypical DSC peak of nivolumab Fc was observed at pH3.5, owing to the unfolding status of IgG4 Fc below its pH threshold. Based on the data shown in , the thermostability of Fc subclasses was ranked as: IgG1 > aglyco-IgG1 > IgG2 > IgG4, which was in agreement with the pH thresholds of IgGs mentioned above.
Figure 4. Thermal unfolding curves of 4 therapeutic antibodies and Fc fragments in acidic solution. Temperatures inducing melting of tocilizumab (dotted line), panitumumab (dashed line), nivolumab (thick solid line) and atezolizumab (thin solid line) was recorded at pH 6.0 (A) or pH3.5 (B), and related Fc at pH6.0 (C) or pH3.5 (D).
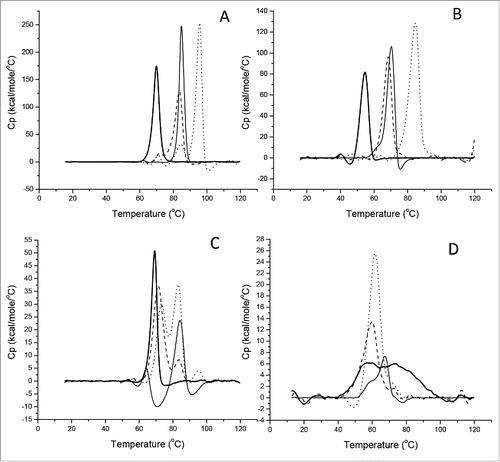
Table 2. The melting transitions of 4 antibodies and their Fcs.
Conformational changes of acid-induced IgG Fc aggregation
To investigate the conformational changes of various Fcs after exposure to acidic solutions, far-UV and and near-UV CD were used to monitor the secondary structure and tertiary structure changes, respectively. After the purified Fcs were exchanged into phosphate-buffered saline (PBS; pH7.0), the buffer pH was adjusted to the pH threshold determined above. The sample was then concentrated to 0.2 mg/mL for far-UV and 1.0 mg/ml for near-UV CD spectra.
As can be seen in the overlay of the far-UV CD spectra (), the Fcs at their own pH threshold had very similar amplitude distribution compared with native structures in PBS at pH7.0, indicating the secondary structures were retained at the pH threshold. Moreover, the 4 predominant secondary structure elements of Fcs, helix, sheet, turn and random coil, were calculated (), and few apparent changes between the Fc monomer and their acid-induced aggregates were observed.
Figure 5. Near- and far-UV CD spectra of Fc monomer and aggregation recorded in PBS. Tocilizumab (A,B), panitumumab (C,D), nivoluamb (E,F) and atezolizumab Fcs (G,H) were investigated. Spectra recorded in 25 mM citrate, adjusted to pH7.0 (solid line) or their own pH threshold (dotted line).
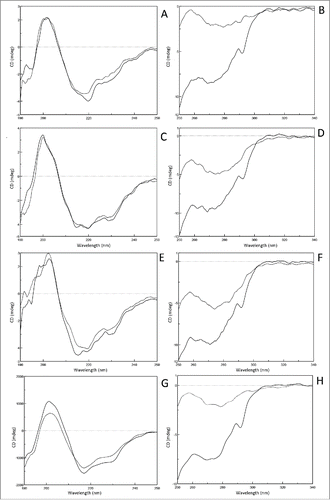
Table 3. Secondary structure change of 4 subclass Fcs after exposure to acidic solution.
In the near-UV CD spectrum of Fcs in PBS at pH7.0 (), the main minimum was at 273 nm and a second minimum was observed at 290 nm, which were consistent with the typical profile of IgGs reported previously.Citation23 However, the curve of the Fc at its own pH threshold was dramatically shifted from 250 nm to 300 nm compared with its counterpart at pH7.0, showing that the tertiary structures of Fc were clearly distinguishable from the native state. Furthermore, the tendency and magnitude of changes in the tertiary conformation of the Fcs at their pH thresholds were very similar. Therefore, all our observations supported the conclusion that adjusting the Fcs' solutions to their own pH threshold values induced limited conformational changes of the secondary structures, but the tertiary structures were destroyed.
The effects of pH on the conformation of nivolumab in pH3.5 were also investigated. CD was used to monitor the conformational changes of nivolumab at pH3.5 and 7.0. The spectra showed that aggregation of nivolumab at pH3.5 was accompanied by the loss of tertiary structure (Fig. S1).
The effect of protein stabilizers on acid-induced aggregation
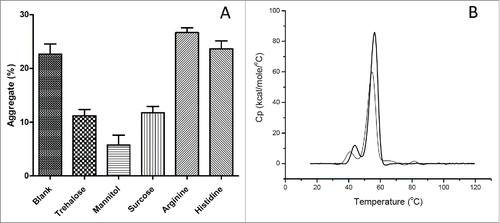
To overcome the instability barrier, several common protein stabilizers (trehalose, mannitol, sucrose, arginine, and histidine) were evaluated as additives to the elution buffer in our study. As shown in , the level of aggregates was 6% vs 23% in nivolumab solutions containing 10% or 0% (control) w/v mannitol. Addition of histidine or arginine provided negligible or negative stabilizing effects, although these basic amino acids were reported to prevent aggregation during the rProtein A chromatography purification process. Citation13,24, 25 In addition, the thermal scan of nivolumab in 25 mM citrate (pH3.5) with 10% w/v mannitol showed that the Tm increased from 40.75, 54.76°C to 43.94, 56.29°C, and the ΔH value increased from 452 to 599 KJ/mol (). Taken together, our results show that the addition of mannitol can effectively minimize acid-induced aggregation of nivolumab.
Figure 6. Effect of protein stabilizer on acid-induced aggregation of nivolumab. (A) Aggregates of nivolumab in 25 mM citrate buffer with 10% trehalose, mannitol, sucrose, or 50 mM arginine, histidine. Each data point represents the mean of triplicate experiments and error bars represent SD. (B) DSC thermal scans of nivolumab in 25 mM citrate at pH3.5 (thin solid line), plus 10% w/v mannitol (thick solid line).
Based on the results mentioned above, we concluded that acid-induced aggregates of nivolumab could be decreased by rProtein A elution buffer optimization. After adjusting the elution buffer pH to 3.8 and adding mannitol, aggregates were decreased to less than 2% (Fig S2).
Discussion
Aggregates of biotherapeutics are thought to pose a risk of immunogenicity, and they are classed as impurities that must be reduced to an acceptable level.Citation7 Minimizing aggregation, while maximizing a biotherapeutic drug product's shelf-life, remains a substantial challenge for the biotechnology industry. Citation8 Although the acid-induced aggregation of IgG is well-known, Citation13,16,21,26 we report for the first time that nivolumab, an IgG4(S228P) mAb, is susceptible to aggregation at pH3.5. To our knowledge, the majority of commercial therapeutic antibodies retain native-folded structure in acidic conditions, even at pH3.0 after elution from a rProtein A column. Nevertheless, in our experiments, acid-induced aggregates of nivolumab reached levels of almost 30% at pH3.5, and the mAb was even substantially aggregated at pH 3.75. Moreover, we found the IgG4 Fc domain was responsible for nivolumab's tendency to aggregate in acidic conditions. These findings are generally consistent with previous reports that IgG4 is prone to aggregation in acidic condition,Citation27,28 and IgG4 aggregation is dependent on the Fc region, which has some hydrophobic aggregation motifs.Citation29
To provide context for the results of our experiments with nivolumab, we examined the pH-dependent stability of 3 other IgG subclass antibodies and their Fcs. The pH range investigated in our study is wider (pH2.0–4.0) than that typically used in industrial manufacturing processes. Four marketed antibodies (tocilizumab, panitumumab, nivolumab, atezolizumab) were used as model molecules. Our study revealed that the acid-induced aggregation propensity of IgGs could be ranked as: IgG4 > IgG2 > aglyco-IgG1 > IgG1, which corroborated previous reports that IgG2 Citation12, 19 and IgG4 Citation27 were less stable than IgG1 and aggregated more readily. More importantly, the pH threshold, as an intrinsic attribute of various IgG subclasses, was identified accurately for the model antibodies. These results were cross-validated by thermostability analysis. A decrease of Tm and ΔH values with decreasing pH indicated that the flexibility of amino acid residues was enhanced under certain solvent conditions. As expected, aglycosylated IgG1 (atezolizumab) had a lower Tm compared to glycosylated IgG1 (tocilizumab), consistent with the critical role of CH2 glycans in stabilizing the overall structure, as reported previously.Citation12 The conformations of the Fcs at their pH thresholds were also assessed by CD, and it was evident that the secondary structure remained unchanged, while the tertiary structure was damaged. Our findings provide important insight into the stability of these 4 subclasses of IgG antibodies, and a better understanding of the acid-induced aggregation process.
Our results clearly demonstrated that IgG4 Fc was more prone to aggregation than other subclasses when exposed to the low-pH conditions. The threshold of the IgG4 Fc was pH 3.75, which is higher than the pH of standard Protein A chromatography elution buffers. The results indicate that downstream manufacturing processes should be customized for IgG4 subclass antibodies to avoid acid-induced aggregation.
Protein A chromatography is a universal mAb capture step for commercial antibody manufacturing. The use of low pH buffer for elution from rProtein A was known to contribute to aggregate production.Citation8,10 Recently, Mazzer et al reported that the formation of aggregation at low pH followed pH-dependent exponential decay kinetics.Citation10 The addition of urea at concentrations of 0.5 M to 1 M was found to be effective in reducing on-column and in-solution aggregation.Citation30 In other work, arginine (0.5–2 M) was found to prevent protein aggregation on elution from rProtein A.Citation9, 31 Additionally, mitigating aggregation by optimization of column operation temperature, manipulation of the pH during the transition from washing to elution, and increasing salt concentration has also been reported.Citation32 In our study, the addition of mannitol could substantially decrease acid-induced aggregation of nivolumab, and the stabilizing effect of mannitol was also proved by DSC.
Although only 4 typical antibodies (tocilizumab, panitumumab, nivolumab and atezolizumab) were investigated in our study, our findings and conclusions may be relevant for other therapeutic mAbs with the same Fcs (IgG1, IgG2, IgG4, and aglyco-IgG1). As the pharmaceutical industry embraces the concept of quality by design (QbD), the critical process parameters (CPP), the variability of which has major effects on critical quality attributes (CQA), should be monitored and strictly controlled. The manufacturer should conduct experiments to set acceptable range limit of CPPs. We recommended that the different susceptibilities of the 4 IgGs subclass to acid conditions be used as a helpful guideline for IgG subclass selection. Moreover, the thresholds provide an operating range or window for manufacturing processes, especially when determining the CPPs of rProtein A capture chromatography and viral inactivation.
Materials and methods
Protein preparation
The tocilizumab, panitumumab, nivolumab and atezolizumab in this study were manufactured by Shanghai Zhangjiang Biotechnology Co. The bulk substance was stored in 4°C at a protein concentration of 20 mg/ml. The bulk substance was buffer exchanged with 25 mM citrate (pH4.0) using Millipore Amicon Ultracel 30 K MWCO regenerated cellulose filter at 3000 rpm in Beckman centrifuge. The protein concentration was determined by UV-absorption at 280 nm.
Aggregates and monomer fractions purification
The nivolumab bulk substance was isolated by Fractogel® EMD SO3 (S) strong ion exchange chromatography (Merck KGaA). The binding buffer was 20 mM acetate buffer (pH5.0), and the elution buffer was composed of 20 mM acetate buffer (pH5.0) with 1 M NaCl. The first peak was collected as the monomer fraction, and the later elution peak was collected as the aggregate fraction.
Preparation of Fc and Fab
Intact mAbs bulk substance was digested by IdeS (Shanghai Zhangjiang Biotechnology Co.) at 37°C for 2 hours, the Fc was isolated by rProtein A (Shanghai Zhangjiang Biotechnology Co.) chromatography (tocilizumab, panitumumab, nivolumab) or rProtein L (GE USA) chromatography (atezolizumab). The purity of Fc and (Fab)2 fragments assessed by SEC and non-reduced SDS-PAGE was more than 95%. All samples were further concentrated with a 10 K MWCO filter and buffer exchanged with 25 mM citrate to a final concentration of 1 mg/mL.
pH titration
The pH of samples were adjusted to the desired value (2.0, 2.5, 3.0, 3.25, 3.5, 3.75, 4.0), using hydrochloric acid (10% v/v). The amount of acid solution required to achieve the desired pH was determined in separate preliminary experiments. The acid solution was quickly mixed to minimize protein exposure to local regions of extreme acidity.
Screening of protein stabilizers
One mg/mL nivolumab was dissolved in 25 mM citrate buffer (pH3.5), containing 50 mM of the tested amino acids or 10% w/v sugars. The percentage of protein aggregation was determined by analytical SEC, and thermodynamic parameters of nivolumab in 25 mM citrate with 10% w/v mannitol were measured by DSC.
Size-exclusion chromatography
The amounts of aggregates (high molecular weight species, HMWS) and monomer were analyzed by UPLC-SEC with BEH200 SEC column (1.7 μm, 4.6 ×150 mm, Waters), as described previously. Citation33 Purity was calculated as the area of monomer peak divided by the total area of all peaks.
Reversed-phase chromatography and Mass spectrometry
After the nivolumab monomer and aggregate fractions were reduced with dithiothreitol, the intact and reduced samples were analyzed by reverse-phase LC-MS. The reverse-phase desalting separations of intact samples were performed on a Waters MassPREP™ Micro Desalting Column (2.1 × 5 mm) using a gradient (6–7 min, 10–95% B). The reverse-phase separations of reduced samples were performed on a Waters C4 Column (2.1 × 50 mm, 1.7 µm) using a gradient (3–9 min, 20–35% B). The mobile phase A was water with 0.1% formic acid; mobile phase B was acetonitrile with 0.1% formic acid. The collected LC-MS/MS data were processed by BiopharmaLynx 1.3 software as described previously.Citation34
Differential scanning calorimetry
DSC experiments were performed using VP-DSC (Microcal, Northampton, MA). The antibodies or their Fcs were tested individually at a protein concentration of 0.5 mg/mL; a buffer control without protein was used as reference. The samples were scanned from 10 to 120°C at a rate of 100°C/hour following an initial 10 min equilibration at 10°C. At least 5 buffer-buffer scans were performed to obtain baseline values and establish thermal history. A filtering period of 16 sec was used, and the data were analyzed using Origin 7.0 (OriginLab Corp., Northampton, MA) to obtain apparent midpoint temperatures (Tm) of unfolding and apparent enthalpy of unfolding (ΔH). The resulting thermograms were corrected by subtraction of buffer control scans. The corrected thermograms were normalized for protein concentration.
Circular Dichroism
CD spectroscopy was performed with a JASCO J-810 spectropolarimeter (JASCO Corp., Tokyo). Far-UV spectra (190–260 nm) were obtained with highly purified IgG at a concentration of 0.2 mg/mL using a quartz cuvette with a path length of 0.1 cm. Near-UV spectra (250–350 nm) were obtained with antibodies or Fcs at a concentration of 1.0 mg/mL using a quartz cuvette with a path length of 1.0 cm. For both, 32 scans were accumulated with a scan rate of 100 nm/min and time constant of 0.125 sec. All spectra were corrected by subtracting the buffer baseline and averaged 32 times. All experiments were conducted at room temperature. Relative amounts of random coil, α-helix, and β-sheet were calculated using K2D2 software.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_Data.doc
Download MS Word (209 KB)Acknowledgments
The authors thank Jingya Xue, Yunping Wen for IEX chromatography, and Qing Zhu, Yuhui Shi and Yanfeng Ma for SEC-HPLC analysis.
Funding
This work was supported by grants from the Natural Science Foundation of China (81330061), Ministry of Science and Technology of China (973 projects 2010CB833605 and 863 projects 2011AA020114, 2014AA021004), State Key Project for New Drug Development (2013ZX09101021; 2013ZX09401303), National Key Project for Infectious Diseases (2012ZX10002012-009), Shanghai Key Laboratory of Cell Engineering (14DZ2272300), Shanghai Rising-star Program (16QB1404300), Shanghai Key technologies R&D Program of Biological medicine (15431906100; 16431901200; 16431904700; 16431904100) and Shanghai Excellent technical leader (13XD1424000).
References
- Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014; 2:846-56; PMID:24872026; http://dx.doi.org/10.1158/2326-6066.CIR-14-0040
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/10.1056/NEJMoa1302369
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320-30; PMID:25399552; http://dx.doi.org/10.1056/NEJMoa1412082
- Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol 2007; 25:1369-72; PMID:18066027; http://dx.doi.org/10.1038/nbt1207-1369
- Tian X, Vestergaard B, Thorolfsson M, Yang Z, Rasmussen HB, Langkilde AE. In-depth analysis of subclass-specific conformational preferences of IgG antibodies. IUCrJ 2015; 2:9-18; PMID:25610623; http://dx.doi.org/10.1107/S205225251402209X
- Ito T, Tsumoto K. Effects of subclass change on the structural stability of chimeric, humanized, and human antibodies under thermal stress. Protein Sci 2013; 22:1542-51; PMID:23963869; http://dx.doi.org/10.1002/pro.2340
- FDA U. Guidance for Industry: Immunogenicity assessment for Therapeutic protein products. 2014.
- Vazquez-Rey M, Lang DA. Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng 2011; 108:1494-508; PMID:21480193; http://dx.doi.org/10.1002/bit.23155
- Shukla AA, Gupta P, Han X. Protein aggregation kinetics during Protein A chromatography. Case study for an Fc fusion protein. J Chromatogr A 2007; 1171:22-8; PMID:17920607; http://dx.doi.org/10.1016/j.chroma.2007.09.040
- Mazzer AR, Perraud X, Halley J, O'Hara J, Bracewell DG. Protein A chromatography increases monoclonal antibody aggregation rate during subsequent low pH virus inactivation hold. Journal of Chromatography A 2015; 1415:83-90; PMID:26346187; http://dx.doi.org/10.1016/j.chroma.2015.08.068
- Hari SB, Lau H, Razinkov VI, Chen S, Latypov RF. Acid-induced aggregation of human monoclonal IgG1 and IgG2: molecular mechanism and the effect of solution composition. Biochemistry 2010; 49:9328-38; PMID:20843079; http://dx.doi.org/10.1021/bi100841u
- Latypov RF, Hogan S, Lau H, Gadgil H, Liu D. Elucidation of acid-induced unfolding and aggregation of human immunoglobulin IgG1 and IgG2 Fc. J Biol Chem 2012; 287:1381-96; PMID:22084250; http://dx.doi.org/10.1074/jbc.M111.297697
- Ejima D, Tsumoto K, Fukada H, Yumioka R, Nagase K, Arakawa T, Philo JS. Effects of acid exposure on the conformation, stability, and aggregation of monoclonal antibodies. Proteins 2007; 66:954-62; PMID:17154421; http://dx.doi.org/10.1002/prot.21243
- Arosio P, Rima S, Morbidelli M. Aggregation Mechanism of an IgG2 and two IgG1 Monoclonal Antibodies at low pH: From Oligomers to Larger Aggregates. Pharmaceutical Research 2013; 30:641-54; PMID:23054090; http://dx.doi.org/10.1007/s11095-012-0885-3
- Brummitt RK, Nesta DP, Chang L, Chase SF, Laue TM, Roberts CJ. Nonnative aggregation of an IgG1 antibody in acidic conditions: part 1. Unfolding, colloidal interactions, and formation of high-molecular-weight aggregates. J Pharm Sci 2011; 100:2087-103; PMID:21213308; http://dx.doi.org/10.1002/jps.22448
- Skamris T, Tian X, Thorolfsson M, Karkov HS, Rasmussen HB, Langkilde AE, Vestergaard B. Monoclonal Antibodies Follow Distinct Aggregation Pathways During Production-Relevant Acidic Incubation and Neutralization. Pharm Res 2016; 33:716-28; PMID:26563206; http://dx.doi.org/10.1007/s11095-015-1821-0
- Brummitt RK, Nesta DP, Chang L, Kroetsch AM, Roberts CJ. Nonnative aggregation of an IgG1 antibody in acidic conditions, part 2: nucleation and growth kinetics with competing growth mechanisms. J Pharm Sci 2011; 100:2104-19; PMID:21213307; http://dx.doi.org/10.1002/jps.22447
- Yageta S, Lauer TM, Trout BL, Honda S. Conformational and Colloidal Stabilities of Isolated Constant Domains of Human Immunoglobulin G and Their Impact on Antibody Aggregation under Acidic Conditions. Mol Pharm 2015; 12:1443-55; PMID:25871775; http://dx.doi.org/10.1021/mp500759p
- Franey H, Brych SR, Kolvenbach CG, Rajan RS. Increased aggregation propensity of IgG2 subclass over IgG1: role of conformational changes and covalent character in isolated aggregates. Protein Sci 2010; 19:1601-15; PMID:20556807; http://dx.doi.org/10.1002/pro.434
- Luo Q, Joubert MK, Stevenson R, Ketchem RR, Narhi LO, Wypych J. Chemical modifications in therapeutic protein aggregates generated under different stress conditions. J Biol Chem 2011; 286:25134-44; PMID:21518762; http://dx.doi.org/10.1074/jbc.M110.160440
- Phillips J, Drumm A, Harrison P, Bird P, Bhamra K, Berrie E, Hale G. Manufacture and quality control of CAMPATH-1 antibodies for clinical trials. Cytotherapy 2001; 3:233-42; PMID:12171730; http://dx.doi.org/10.1080/146532401753174061
- Ishikawa T, Ito T, Endo R, Nakagawa K, Sawa E, Wakamatsu K. Influence of pH on heat-induced aggregation and degradation of therapeutic monoclonal antibodies. Biol Pharm Bull 2010; 33:1413-7; PMID:20686240; http://dx.doi.org/10.1248/bpb.33.1413
- Wozniak-Knopp G, Stadlmann J, Ruker F. Stabilisation of the Fc fragment of human IgG1 by engineered intradomain disulfide bonds. PLoS One 2012; 7:e30083; PMID:22272277; http://dx.doi.org/10.1371/journal.pone.0030083
- Ejima D, Yumioka R, Tsumoto K, Arakawa T. Effective elution of antibodies by arginine and arginine derivatives in affinity column chromatography. Anal Biochem 2005; 345:250-7; PMID:16125126; http://dx.doi.org/10.1016/j.ab.2005.07.004
- Falconer RJ, Chan C, Hughes K, Munro TP. Stabilization of a monoclonal antibody during purification and formulation by addition of basic amino acid excipients. J Chem Technol Biotechnol 2011; 86:942-8; http://dx.doi.org/10.1002/jctb.2657
- Mazzer AR, Perraud X, Halley J, O'Hara J, Bracewell DG. Protein A chromatography increases monoclonal antibody aggregation rate during subsequent low pH virus inactivation hold. J Chromatogr A 2015; 1415:83-90; PMID:26346187; http://dx.doi.org/10.1016/j.chroma.2015.08.068
- Neergaard MS, Nielsen AD, Parshad H, Van De Weert M. Stability of monoclonal antibodies at high-concentration: head-to-head comparison of the IgG1 and IgG4 subclass. J Pharm Sci 2014; 103:115-27; PMID:24282022; http://dx.doi.org/10.1002/jps.23788
- Garber E, Demarest SJ. A broad range of Fab stabilities within a host of therapeutic IgGs. Biochem Biophys Res Commun 2007; 355:751-7; PMID:17321501; http://dx.doi.org/10.1016/j.bbrc.2007.02.042
- Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, Sutton BJ. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol 2014; 426:630-44; PMID:24211234; http://dx.doi.org/10.1016/j.jmb.2013.10.039
- Shukla AA, Hubbard B, Tressel T, Guhan S, Low D. Downstream processing of monoclonal antibodies–application of platform approaches. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 848:28-39; PMID:17046339; http://dx.doi.org/10.1016/j.jchromb.2006.09.026
- Arakawa T, Philo JS, Tsumoto K, Yumioka R, Ejima D. Elution of antibodies from a Protein-A column by aqueous arginine solutions. Protein Expr Purif 2004; 36:244-8; PMID:15249046; http://dx.doi.org/10.1016/j.pep.2004.04.009
- Shukla AA, Hinckley PJ, Gupta P, Yigzaw Y, Hubbard BR. Strategies to address aggregation during protein A chromatography. BioProcess International 2005:36-44
- Fekete S, Ganzler K, Guillarme D. Critical evaluation of fast size exclusion chromatographic separations of protein aggregates, applying sub-2 μm particles. J Pharm Biomed Anal 2013; 78–79:141-9; PMID:23499912; http://dx.doi.org/10.1016/j.jpba.2013.02.013
- Zhu L, Guo Q, Guo H, Liu T, Zheng Y, Gu P, Chen X, Wang H, Hou S, Guo Y. Versatile characterization of glycosylation modification in CTLA4-Ig fusion proteins by liquid chromatography-mass spectrometry. MAbs 2014; 6:1474-85; PMID:25484062; http://dx.doi.org/10.4161/mabs.36313
