ABSTRACT
Over the last 3 decades, monoclonal antibodies have become the most important class of therapeutic biologicals on the market. Development of therapeutic antibodies was accelerated by recombinant DNA technologies, which allowed the humanization of murine monoclonal antibodies to make them more similar to those of the human body and suitable for a broad range of chronic diseases like cancer and autoimmune diseases. In the early 1990s in vitro antibody selection technologies were developed that enabled the discovery of “fully” human antibodies with potentially superior clinical efficacy and lowest immunogenicity.
Antibody phage display is the first and most widely used of the in vitro selection technologies. It has proven to be a robust, versatile platform technology for the discovery of human antibodies and a powerful engineering tool to improve antibody properties. As of the beginning of 2016, 6 human antibodies discovered or further developed by phage display were approved for therapy. In 2002, adalimumab (Humira®) became the first phage display-derived antibody granted a marketing approval. Humira® was also the first approved human antibody, and it is currently the best-selling antibody drug on the market. Numerous phage display-derived antibodies are currently under advanced clinical investigation, and, despite the availability of other technologies such as human antibody-producing transgenic mice, phage display has not lost its importance for the discovery and engineering of therapeutic antibodies.
Here, we provide a comprehensive overview about phage display-derived antibodies that are approved for therapy or in clinical development. A selection of these antibodies is described in more detail to demonstrate different aspects of the phage display technology and its development over the last 25 years.
Antibody phage display
Monoclonal antibodies represent the most important class of recombinant protein therapeutics on the market. As of May 2016, over 50 antibodies and antibody conjugates have been approved by the US. Food and Drug Administration (FDA) or European Medicines Agency (EMA),Citation1 and about 500 antibodies are under clinical investigation. Most therapeutic antibodies are approved for cancer and autoimmune diseases and the annual sales revenues of all therapeutic antibodies exceeded 75 billion US$ in 2013.Citation2 The first approved monoclonal antibody, muromonab-CD (Orthoclone OKT3®), which blocks CD3-mediated activation of T cells to prevent organ rejection after transplantation,Citation3 was produced by hybridoma technology. However, a significant percentage of patients who were administered this murine antibody developed anti-drug antibodies (ADA) and were sensitized to OKT3 therapy.Citation4 In the late 1980s, recombinant DNA technology was used to substitute murine antibody sequences with human antibody sequences to reduce immunogenicity.Citation5,6 First, only murine constant immunoglobulin G (IgG) domains were replaced by human counterparts resulting in chimeric antibodies like rituximab (Rituxan®),Citation7 which lowered the risk of immunogenicity. However, the murine variable regions were still prone to generate antiidiotypic antibodies.Citation8 Therefore, it was also important to substitute the murine framework regions in the variable antibody domains with the closest human framework sequencesCitation9, which results in humanized antibodies like daclizumab (Zynbrita® and formerly Zenapax®)Citation10,11 or bevacizumab (Avastin®).Citation12 Humanization, however, did not eliminate the possibility of an immune responseCitation13,14 because the success and degree of humanization is dependent on the individual antibody, which often requires back mutationsCitation15 and can involve a tremendous amount of antibody engineering effort. Moreover, the complementarity-determining regions (CDRs) mediating most of the interaction with the antigen are still from non-human origin, and, therefore, pose some risk for ADA responses.Citation5,13,14 Therefore, “fully” human antibodies were considered to be the optimal solution for therapy because they are indistinguishable from those in the human body and had the lowest risk of immunogenicity.Citation5
Discovery of human antibodies required new technologies because immunization and hybridoma technology could not be transferred to human donors. Therefore, the isolation of human B cellsCitation16 was limited to such indications, where human donors have developed a natural antibody response to the target antigen like after natural infections. Starting in the 1990s, however, transgenic mice or rats, endowed with the human antibody gene repertoire or parts of it, offered access to human antibodies by in vivo immunization and hybridoma technology.Citation17-22 These technologies were developed by various companies, e.g., Kirin, Medarex, Regeneron, Abgenix (transgenic mice) or OMT (transgenic rats). Despite the tremendous success of this technology, the immunization of transgenic mice does not always result in a successful in vivo antibody response to all types of antigens. Particularly, conserved, toxic, and unstable antigens, proteins with allosteric conformational changes and transmembrane proteins are not well suited for an immunization approach and require an alternative solution for antibody discovery.
In vitro selection technologies like antibody phage display do not depend on the in vivo immune response, and can be used to discover antibodies to almost every type of antigen and to a broader range of epitopes, which may be suppressed by the immune system. Phage display is the first and most widely used in vitro antibody selection technology. The approach is based on the groundbreaking work of George P. Smith on filamentous E. coli phage M13 and the fusion of peptides to the phage envelope proteins, which allows the phenotypic in vitro selection of the corresponding peptide encoding gene fragment packaged in the same phage particle.Citation23 The selection process was called “panning” because of the resemblance to the method used to find gold.Citation24
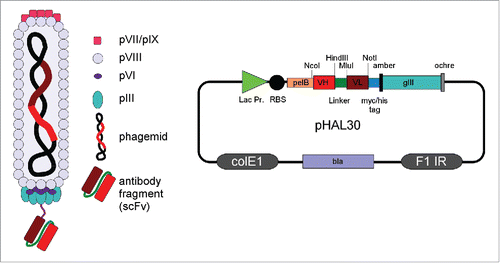
Immediately after the discovery of smaller recombinant antibody formats like single-chain variable fragments (scFv)Citation25 and their secretory periplasmic expression in E. coli,Citation26 antibody phage display technology was independently developed in 3 different places: at the Deutsches Krebsforschungszentrum (DKFZ) in Heidelberg, Germany,Citation27 at the MRC Laboratory of Molecular Biology in Cambridge, United KingdomCitation28,29 and at the Scripps Research Institute in La Jolla, USA.Citation30,31 All major phage display systems were based on antibody::pIII fusion proteins, but they differed in the location of the antibody gene expression cassette. The integration of the antibody::pIII encoding gene into the phage genomeCitation29 was not as successful as the more flexible phagemid system,Citation27 which uncouples antibody::pIII expression from the expression of the phage proteins and from phage replication. The phagemid bears a bacterial origin of replication and antibiotic selection; in addition to the antibody::pIII expression cassette, it behaves more like a normal plasmid in the absence of other phage proteins. Thus, it can also be used for standard genetic manipulation and cloning procedures. For packaging into the phage particles, the phagemid contains an additional morphogenetic signal for replication of single-stranded DNA copies and packaging into phage particles during assembly, which requires the co-expression of the other M13 phage proteins provided by the helper phage.Citation27 This dual antibody surface display and expression system allows the switch between oligovalent and monovalent antibody display by using different helper phage, for example using Hyperphage for oligoclonal display.Citation32,33 After phage packaging, the antibody fragments are displayed as pIII fusion protein on the surface of M13 phage and the corresponding antibody gene fragment is packaged in the same phage particle. The combination of antibody genotype and phenotype in the same phage particle allows for the in vitro selection of antibody genes due to the encoded phenotypic antibody properties like antigen specificity, affinity, or stability. The physical linkage of antibody and phage pIII protein is usually achieved by genetic fusion of antibody and pIII, but there are also other approaches using conjugation via free cysteinesCitation34 or dimerization motifsCitation35 or protein A-derived ZZ domains.Citation36 Small recombinant antibody fragments are commonly used for phage display, e.g., scFvCitation37-39 or fragment antigen-binding (Fab).Citation40,41 The smallest antibody fragments are human (single) domain (VH) antibodies (dAbs) or the variable domains of the naturally occurring camel heavy chain antibodies (VHH).Citation42-44 An antibody (scFv) phage and a corresponding phagemid are illustrated in . More detailed overviews are given in different reviews.Citation41,45,46 Full IgGs have even been displayed on phage,Citation36 but the E. coli's secretion and folding apparatus cannot process complex antibody formats without a very strong bias. The analysis of a new advanced universal human scFv antibody library has not revealed such bias from the E. coli system regarding the frequency of V gene families and amino acid distribution in light and heavy chain CDR3sCitation47. This analysis involved the comparison of more than 800 antibodies after selection to a variety of different antigens with the parental naïve library.
Figure 1. Schema of an antibody (scFv) phage and phage display vector (phagemid) pHAL30 Citation47. Abbreviations: bla = β-lactamase, ampicillin resistance; ColE1 = bacterial origin of DNA replication; F1 IR = intergenic region of phage f1, phagemid packaging signal; gIII = phage gene encoding pIII; Lac Pro = promoter of lacZ; pIII, pVI, pVII, pVIII, pVIX = phage protein III, VI, VII, VIII, VIX; pelB = Erwinia carotovora pectate lyase B leader peptide; scFv = single chain fragment variable; VH = variable domain heavy chain; VL= variable domain light chain.
The antibody libraries are “gold mines” for therapeutic antibodies
Antibody libraries are huge collections (>1010) of antibody genes encoding antibodies with unknown properties. They are the essential source for antibody discovery by phage display and other in vitro selection technologies, and their design is critical to success. Two major types of antibody libraries are distinguished: 1) immune libraries, and 2) universal libraries.
Immune libraries are constructed from blood cell samples from immunized donors, which, in the case of human antibodies, is limited to donors who received vaccination, or patients who have suffered an infection or disease. In medical research, immune libraries are mostly used to discover antibodies against targets from infectious pathogens, e.g., antibodies neutralizing the human immunodeficiency virus (HIV) from long-term non-progressor patients,Citation48 West Nile virusCitation49 or immunized cancer patients.Citation50 Immune libraries can also be generated from immunized animals,Citation51-55 including macaques which have antibodies that are very similar to those of humans.Citation56 Use of transgenic animals containing human antibody gene repertoire allows the discovery of human antibody from such immune libraries.Citation57 Immune libraries already contain affinity-matured antibodies, because they are generated from antibody repertoires that underwent antigen driven in vivo selection, but these extended somatic hypermutations may increase the risk of immunogenicity.
Universal libraries generated from natural naïve human antibody gene repertoires are relatively close to the human antibody germ line and have low risk of immunogenicity. These also called “single-pot“ libraries contain a very broad repertoire of antigen specificities theoretically covering every possible antigen.Citation45,58 Naïve antibody libraries are constructed from rearranged V genes from B cells of non-immunized donors, i.e., the IgM repertoire. These antibodies have already undergone in vivo selection for functional B cell receptors, including a selection for low immunogenicity and low toxicity. Nevertheless, antibodies to most if not all human antigens with relatively high affinities (low nanomolar to picomolar range) from naïve antibody libraries have been described.Citation59 Affinity engineering, a well-established technology, can also be done by phage display.Citation60 Examples of naïve universal libraries are the human Fab library constructed by de Haard and colleagues at Dyax (now Shire),Citation40 the scFv libraries from Cambridge Antibody Technology (CAT),Citation39,61 scFv and Fab libraries from XOMACitation59 or the HAL scFv libraries.Citation37,47
In addition to naïve universal libraries, which are derived completely from natural human IgM repertoire, there are also synthetic or semi-synthetic universal libraries consisting of only synthetic sequences or a mixture of natural and synthetic antibody sequences, respectively. Semi-synthetic libraries are constructed either from unrearranged V genes from pre-B cellsCitation62 or by using one antibody frameworkCitation63 in which one (at least HCDR3) or several CDRs is endowed with randomized sequences. Semi-synthetic libraries like the Tomlinson I and J libraries consist of the stable VH IGHV3-23 framework and the Vκ IGKV1-39 framework with randomized positions in CDR2 and CDR3.Citation64 Another strategy for semi-synthetic libraries is the amplification of all 6 CDRs of the natural antibody repertoire to create a pool of diverse CDRs, which are cloned into one defined antibody framework by overlap extension polymerase chain reaction (PCR) like the nCoDeR library of BioInvent.Citation65 A combination of naïve and synthetic repertoires was used for the Dyax FAB310 library. Here, entire light chains from autoimmune patients were combined with an Fd with the human IGHV3-23 framework endowed with synthetic CDR1 and CDR2 as well as naïve CDR3 regions from autoimmune patients.Citation41 Fully synthetic libraries developed by MorphoSys employing 7 human VH and 7 VL frameworks endowed with synthetic CDR cassettes generated by trinucleotide synthesis (human combinatorial antibody libraries, HuCAL). Recently, MorphoSys' Ylanthia library was generated based on 36 fixed variable VH and VL chain pairs, which cover a broad range of canonical CDR structures.Citation34,66-68 The theoretical size of universal libraries is now greater than 1010 independent clones.Citation38,47,59,61,68,69
A particular type of antibody library is generated during guided phage display selection of human VH and VL repertoires using the counter chain of a murine monoclonal antibody as template. This strategy has been used for humanization, but often also for the discovery of new “fully” human antibodies with properties similar to the murine antibody template, like for adalimumab, the first approved phage display-derived therapeutic antibody.Citation70
In vitro selection of antibody panning nuggets
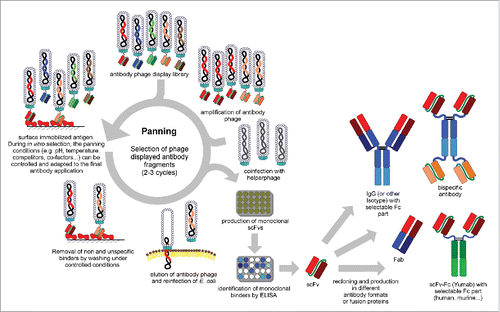
The process of in vitro selection of antibodies from libraries is usually referred to as “panning,” because it resembles the procedure used to extract gold flakes or nuggets from a vast excess of silt by exploiting different physical properties. The situation in antibody phage display is quite similar because the appropriate antibody candidates are very rare in the library. As briefly discussed in the previous section, immune libraries contain a smaller antibody repertoire and often a higher percentage of antigen-specific antibodies, which may be one specific binder in 103–106 non-antigen specific antibodies. In universal libraries the ratio can be one binder in 107–109. Particularly for universal libraries, the In vitro selection process is extremely critical to successfully isolate antigen-specific antibodies. In vitro selection requires the immobilization of the target antigen to a solid surface, such as magnetic beads,Citation71 column matricesCitation27 or plastic surfaces with high protein binding capacity as polystyrene tubes or plates.Citation72 The latter is the most widely used method. Panning in solution, which involves the use of biotinylated antigens followed by a “pull-down” with streptavidin beads,Citation73 can also be done. The vast excess of non-binding antibody phage must be removed by stringent washing. Subsequently, the bound antibody phage will be eluted and reamplified by infection of E. coli and packaging with helper phage to produce a new antibody phage sublibrary, which will be used for another panning round until a significant enrichment of antigen specific antibody phage is achieved. Usually 2-3 panning rounds are required to enrich antibodies even from the largest available libraries. The optimal selection strategy depends on many parameters, including the target antigen, the optimal antigen immobilization strategy, the quality of the library (particularly the frequency of antigen specific antibodies therein) and the binding and washing conditions. The tight control of the in vitro conditions allows the pre-design of antibody properties during the selection including epitope specificity,Citation73,74 and even conformation specificityCitation75,76 and interspecies cross-reactivity.Citation77 Screening for monoclonal antibodies can be performed either by antibody phage using enyzme-linked immunosorbent assay (phage ELISA) or by soluble expression of antibody fragments in E. coli and immunoassays, including ELISA or flow cytometry.Citation78 The antibody fragment can be directly used or converted into other antibody formats for therapeutic development, e.g., IgG, antibody-drug conjugates.Citation41,79-81 shows a schema of the antibody phage display panning procedure.
Figure 2. Schema of antibody (scFv) phage display selection, screening and reformating/production of other antibody formats (modified figure from reference Citation207).
Phage display derived antibodies in clinical development
For this review, we collected data on phage display-derived therapeutic antibodies from different sources. General information about the antibodies and about their clinical development were collected from PubMed-indexed scientific articles and reviews. Additional information was also obtained from the clinical trial database www.clinicaltrials.gov, company websites, international ImMunoGeneTics information system (http://www.imgt.org/mAb-DB/query.action, Development status: Phase M in search field), conference presentations, patents and prescribing drug information and personal communication. Data on phage display-derived human antibodies that are approved for therapy by EMA or FDA, or have been investigated in clinical studies are provided in . Antibodies from discontinued clinical studies are also included. For some antibodies in clinical development, the origin and even the target is not publicly available, and we could not include these in the table. For example, NOV-7 to -11 were probably generated by phage display by MorphoSys in collaboration with Novartis (www.morphosys.com/pipeline/clinical), but the exact source of these antibodies has not been disclosed. We did not include phage display-derived antibodies in preclinical development or antibodies discovered by other technologies. A thorough and annually updated review about therapeutic antibodies in late-stage developments can be found in the “Antibodies to watch” article series from Janice Reichert.Citation1,82-85 A selection of phage display-derived antibodies and other peptide/protein therapeutics are described by Nixon et al.Citation86 A selection of phage display-derived antibodies is described in more detail in the following sections.
Table 1. Phage display-derived antibodies and antibody conjugates evaluated in clinical studies, including those approved by FDA or EMA.
Adalimumab (Humira®)
Adalimumab was the first phage display-derived monoclonal antibody, as well as the first human antibody, approved for therapy. It binds tumor necrosis factor (TNF) with subnanomolar affinityCitation87 and prevents TNF from binding to its receptors. TNF, the key initiator molecule in the proinflammatory cytokine cascade, binds to the receptors TNF-R1 and -R2, which leads to cell activation, inflammation and fever, acute phase responses and sepsis, cell survival and proliferation, as well as apoptosis.Citation88 The cross-talk of the different TNF-signaling pathways interferes on several physiological levels, and made it impossible to develop TNF itself as therapeutic agent. However, use of TNF inhibition with antibodies or soluble receptors to suppress a broad range of inflammatory autoimmune diseases was possible,Citation89 and today 5 different biological TNF antagonists are on the market, i.e., adalimumab (Humira®), infliximab (Remicade®), golimumab (Simponi®), certolizumab pegol (Cimzia®) and etanercept (Enbrel®).
Adalimumab was discovered through a collaboration between BASF Bioresearch Corporation/Knoll and CT in 1993. BASF/Knoll had extensive experience with TNF from a Biogen collaboration, which failed to develop TNF into a commercialized drug product. At that time, BASF also developed the anti-TNF murine monoclonal antibody MAK195 as an F(ab')2 for acute reactions like sepsis (afelimomab, Segard).Citation90 MAK195 mediated potent TNF inhibition, but, as a murine antibody, it was not considered for treatment of chronic autoimmune diseases. The company decided to develop a new human antibody that would have low immunogenicity and long plasma half-life. MAK195 was used as a template for guided selection of human antibody V-domains with CAT's antibody phage display technology, with the goal to achieve binding to a similar epitope on TNF with similar high affinity. Two scFv libraries were constructed, one library with MAK195 VH combined with a human variable light chain repertoire and one library with MAK195 VL and a heavy chain repertoire. Both libraries were used for panning on TNF. From the selected hybrid scFvs, human VH and VL were combined in a third library and reselected on TNF. Subsequently, CDR mutagenesis was performed, resulting in D2E7.Citation70 The final clinical candidate D2E7 was taken through most of the drug development process by Knoll before BASF sold its pharma division, including Knoll, to Abbott Laboratories, which performed further development, manufacturing and marketing. In clinical trials, adalimumab demonstrated long-term efficacy and safety, and its convenient subcutaneous administration. Ready-to-use liquid formulation and every-other-week dosing with the option to increase to weekly also in combination with methotrexate (MTX) were also beneficial for patients.Citation91-94
Adalimumab was first approved by the FDA on December 31, 2002 for treatment of moderate-to-severe forms of rheumatoid arthritis as monotherapy or in combination with MTX or other disease-modifying anti-rheumatic drugs, and then marketed by Abbott Laboratories (AbbVie after the company split in 2013) under the brand name Humira®, which stands for “human monoclonal antibody in rheumatoid arthritis.” When Humira® was launched, it was the third TNF inhibitor on the market, after the chimeric monoclonal antibody infliximab and the TNF-R Fc fusion protein etanercept. Despite this late start in the market, adalimumab developed into the best-selling antibody drug, achieving more than 10 billion USD global sales in 2013.Citation2 At a dose of 40 mg per patient subcutaneously every 2 weeks, adalimumab has a lower discontinuation risk and simpler administration (autoinjector pen)Citation95 compared to infliximab (3–10 mg/kg intravenously every 4–8 weeks). Etanercept (50 mg per patient subcutaneously per week), however, had a better retention rate than adalimumab or infliximab as first-line biotherapy in rheumatoid arthritis, and adalimumab as second-line biotherapy.Citation96
Today, adalimumab is approved in the United states (US), European Union (EU), and Japan (JP) for moderate-to-severe chronic rheumatoid arthritis in adults and polyarticular juvenile idiopathic arthritis in children, psoriasis and psoriatic arthritis, ankylosing spondylitis, pediatric and adult Crohn's disease,Citation97 ulcerative colitis (US and EU only), hidradenitis suppurativa, axial spondyloarthritis (EU only), intestinal Behçet disease (JP only), and it is in clinical testing for treatment of sarcoidosis and uveitis.Citation98 Treatment emergent adverse effects (TEAE) of adalimumab like reactivation of latent infections, such as tuberculosis, or a higher prevalence of opportunistic infections are mainly due to the TNF suppression and also occur with other TNF inhibitors.Citation99 The risk of lymphoma is 3-fold higher compared with the general population according to the Humira® Injection Prescribing Information and Medication Guide (Abbott Laboratories; IL, USA: 2009), but there is no significant difference in tumor risk compared to rheumatoid arthritis patients naïve to anti-TNF therapy.Citation100 Due to rare reports of serious liver injury, demyelinating central nervous system disorders, and cardiac failure, as well as anaphylaxis and serious allergic reactions, physicians are instructed to carefully monitor patients. Adalimumab is indicated for adult Crohn's disease patients who are already intolerant to infliximab, whereas it is not indicated for TNF-blocker resistant ulcerative colitis patients. In 2014, Zydus Cadila Healthcare Ltd., launched in India the first adalimumab biosimilar (Exemptia®) for about a fifth of Humira® price, in the Indian market, where the Humira® patent was not claimed. Humira® is protected by many patents, the main US patent US6090382 will expire in 2016.
Belimumab (Benlysta®)
The technologies for DNA sequencing that emerged in the early 1990s led to the discovery of a new member of the tumor necrosis factor (TNF) ligand family, B-lymphocyte stimulator (BLyS, now TNFSF13B) by Human Genome Sciences (HGS). BLyS was suggested to be involved in monocyte-driven B-cell activation.Citation101,102 In 2001, several groups reported a connection between elevated levels of circulating BlyS and systemic lupus erythematosus (SLE).Citation103,104 Patients with SLE generate autoreactive antibodies that deregulate inflammatory responses and form immune complexes, the deposition of which causes target organ failure. Probable inherent abnormalities in the immune system and an environmental trigger, e. g., ultraviolet radiation, infection with the Epstein-Barr virus, may lead to ineffective clearance of apoptotic nuclear fragments, which are processed by antigen-presenting cells (APC) and presented to autoreactive T cells. These T cells activate autoreactive B cells to produce anti-self antibodies. These act as APCs and release proinflammatory cytokines such as interferon-α, interleukin (IL)-6, IL-17, TNF, a proliferation-inducing ligand (APRIL) and BLyS.Citation105-107
BLyS was chosen as a target for the development of an antagonistic antibody that is able to block B-cell activation, but also affects Th1 and/or Th17 cells.Citation108-110 In a collaboration with CT, HGS selected about 1,200 antibodies from a scFv phage display libraryCitation39 that were able to inhibit BLyS activity.Citation111 Affinity maturation lead to the development of LymphoStatB, subsequently named belimumab, which was able to deplete B cells in spleen and lymph nodes of cynomolgus monkeys.Citation112 A Phase 1 clinical study of belimumab in individuals with mild-to-moderate SLE disease was initiated in 2001. Efficacy was not shown in this study due to small number of patients and brief treatment studies. Nevertheless, it was considered successful, and 2 Phase 2 studies in SLE and rheumatoid arthritis patients were initiated.Citation113,114 The Phase 2 study also did not show a significant effect of belimumab in SLE patients, which could have led to the discontinuation of the development of this antibody, but a deeper analysis revealed a deficiency in the study design. A modified analysis revealed that belimumab-treated patients had a significantly better response than patients treated with the standard of care plus placebo.Citation115 The FDA accepted the new interpretation of the results, and a pivotal trial was initiated in 2007.Citation116 Belimumab was ultimately approved for SLE in 2011. The drug was marketed as Benlysta® by GlaxoSmithKline (GSK), which purchased HGS one year later. At the same time the transgenic mouse-derived human antibody tabalumab, which also targets BlyS and was developed by Eli Lilly, was terminated in clinical Phase 3 studies due to lack of efficacy.Citation117
DX-2930
In classical hereditary angioedema (HAE), patients have a deficiency of the acute phase protein C1 esterase inhibitor, which regulates plasma kallikrein (pKal), factor XIIa and other proteases of the plasma contact system. pKal liberates bradykinin involved in vasodilation and inflammation. This results in local edema affecting the skin, gastrointestinal tract, genitourinary tract and larynxCitation118-120. A potential treatment is the inhibition of pKal, which is also involved in the complement system and coagulation cascade. Using phage display, Dyax selected a 66 amino acid Kunitz domain that blocks pKal activity. The Kunitz domain DX-88 (ecallantide) was approved by the FDA in 2009 and marketed by Dyax under the brand name Kalbitor®.Citation121-123 As a follow-on to the peptide program, human antibodies were selected from the Dyax FAB310 phage library using biotinylated pKal immobilized on streptavidin beads. The Fab M0162-A04 inhibits active pKal, but not prekallikrein or any other serine protease and was further affinity matured resulting in the Fab M0199-A08. The human IgG1 variant DX-2930 showed good pharmacokinetic and pharmacodynamic properties in cynomolgus monkey, as well reduced edema in a rat model.Citation124 Subsequently, a successful clinical phase 1 study was performed.Citation119 In October 2015, a Phase 3 study was started (ClinicalTrials.gov Identifier: NCT02586805). In January 2016, Shire acquired Dyax mainly because of DX-2930 and DX-88.
Anti-TRAIL receptor antibodies
Mapatumumab (HGS-ETR1) is a human monoclonal antibody that binds and activates the tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1; also known as, TNFRSF10A, APO2, CD261, and death receptor 4). TRAIL-R1 (and 2) are death receptors that activate the second key signaling mechanism for caspase activation and apoptosis induction. The natural ligand TRAIL (Apo2L) binds and activates both TRAIL-R1 and TRAIL-R2. Overexpression of TRAIL-R1/2 in some tumor types, as well as their distinct, p53-independent mode of apoptosis activation via extracellular ligand binding makes these receptors attractive targets for agonistic ligands and antibodies designed as cancer therapies.Citation125
The agonistic TRAIL-R1 antibody mapatumumab (HGS-ETR1) was discovered by phage display by CAT using the Vaughan libraryCitation39 in collaboration with HGS (now GSK) in 1999.Citation126 It induces cell death in multiple tumor cell lines in vitro and in vivo, which can be enhanced by combination with chemotherapeutic agents like camptothecin, cisplatin, carboplatin, or 5-fluorouracil.Citation126 Mapatumumab demonstrated safety and tolerability in cancer patients with advanced solid tumors or non-Hodgkin's lymphoma (NHL), both as a single agent and in combination with chemotherapy.Citation127-129 Although no clinical activity of single-agent mapatumumab was observed in patients with refractory colorectal cancer in a Phase 2 trial,Citation130 its favorable safety profile and pre-clinical evidence of potential synergy with chemotherapy was the rationale for further clinical testing. Mapatumumab in combination with paclitaxel and carboplatin in unselected patients with advanced non-small-cell lung cancer (NSCLC) did not reveal any clinical benefit compared to chemotherapy alone.Citation131,132 However, a Phase 1b/2 trial conducted in patients with relapsed or refractory non-Hodgkin's lymphoma as monotherapy with 6 doses of either 3 or 10 mg/kg mapatumumab every 21 days achieved clinical responses in 3 patients with follicular lymphoma (FL), 2 of them with complete responses and one with partial response.Citation133 A randomized Phase 2 trial of mapatumumab in combination with the proteasome inhibitor bortezomib for treatment of advanced multiple myeloma did not demonstrate efficacy compared to the control group.Citation134
Lexatumumab (HGSETR2) targets pro-apoptotic TRAIL-receptor 2 (TRAIL-R2, TNFRSF10B, CD253, DR5), and is another phage display derived human antibody from the collaboration of CT and HGS.Citation126 Like mapatumumab, lexatumumab mediated synergistic effects in combination with chemotherapeutics.Citation135 In combination with radiation, lexatumumab showed some clinical activity in pediatric solid tumors.Citation136
Drozitumab (Apomab, PRO95780, Roche/Genentech) was also generated by phage display from a human scFv library against TRAIL-R2.Citation137 A Phase 1 study demonstrated safety in humans at doses up to 20 mg/kg and evidence of activity in several different tumor types at 4 and 10 mg/kg.Citation138 In contrast to these and other agonistic antibodies, soluble recombinant human TRAIL (dulanermin) did not result in objective responses in patients with indolent B-cell Non-Hodgkin lymphoma (NHL)Citation139 or NSCLC.Citation140
Granulocyte-macrophage colony-stimulating factor antibodies
MOR103 is a human monoclonal antibody specific for granulocyte-macrophage colony-stimulating factor (GM-CSF) developed by MorphoSys in collaboration with GSK (GSK3196165). The proinflammatory cytokine GM-CSF is produced by a variety of cells including activated T and B cells, monocytes/macrophages, endothelial cells, and fibroblasts and it stimulates myeloid differentiation of haematopoietic stem cells, proliferation and activation of macrophages, monocytes, neutrophils, eosinophils, dendritic cells, and microglia.Citation141 GM-CSF plays a critical role in autoimmune diseases as shown in a murine collagen-induced arthritis model,Citation142,143 transgenic mice constitutively expressing GM-CSF develop autoimmune gastritisCitation144 and elevated levels of GM-CSF correlate with the active phase of multiple sclerosis (MS).Citation145,146
MorphoSys developed MOR103 with phage display selection from the HuCal Gold libraryCitation67 followed by affinity maturation with tri-nucleotide cassette mutagenesisCitation147 of CDR-L3 and CDR-H2, affinity-driven selection and cross-cloning of the best candidates.Citation148 MOR103 (MOR04357) inhibits human GM-CSF with an IC50 in the low picomolar range and cross-reacts with rhesus and rat GM-CSF.Citation148 MOR103 was tested for treatment of moderate rheumatoid arthritis in a Phase 1b/2a trial using 0.3, 1.0 or 1.5 mg/kg once a week for 4 weeks and with follow-up to 16 weeks. MOR103 was well tolerated and showed preliminary evidence of clinical efficacy.Citation149 In Phase 1b study, MOR103 was well-tolerated in patients with relapsing-remitting MS and secondary progressive MS without any evidence of immunogenicity.Citation150
Namilumab/MT203 is another GM-CSF antagonizing antibody under clinical evaluation by Takeda. MT203 was discovered by Micromet by guided selection using a rat monoclonal antibody as template in combination with technology licensed from CAT. The antibody contains a human VL domain and humanized VH with parental rat HCDR3.Citation151,152
Mavrilimumab, which inhibits the GMCSF receptor, is undergoing evaluation in Phase 2 studies of patients with rheumatoid arthritis.Citation153 This antibody was developed by CAT, which was acquired by AstraZeneca in 2006, using phage display.Citation154
Antibodies targeting epidermal growth factor receptor, vascular endothelial growth factor or vascular endothelial growth factor receptors
Necitumumab (Portrazza®)
The epidermal growth factor receptor (EGFR) plays an important role in many cancers, including NSCLC. In 40 to 80% of lung cancer patients, EGFR is overexpressed and a high copy number can be found in up to 60% of the patients.Citation155-157 Stimulation of the receptor by ligand binding leads to activation of different signaling cascades, such as mitogen-activated protein kinase (MAPK) and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3k)/Akt pathway. This results in cell proliferation, differentiation, invasion and metastasis, which promotes the malignant phenotype.Citation158
Consequently, EGFR is the target of different types of anticancer therapeutics, including small-molecule inhibitors of the kinase domain (e. g., gefitinib, erlotinib) or monoclonal antibodies to the receptor domain (e. g., chimeric cetuximab).Citation159
Necitumumab (clone name IMC-11F8) was developed by ImClone Systems, Eli Lilly and BristolMyers Squibb (BMS) from the “de Haard” Dyax Fab phage display library.Citation40 In this case, epidermal carcinoma cells (A431) were used to screen for antibodies that blocked EGFR activation. Necitumumab binds to the receptor with an affinity of 3.3 ± 0.5 nM.Citation160 The antibody blocks the binding of several relevant ligands and inhibits the proliferation of different cancer cell lines.Citation161 Clinical studies were initiated in 2004, and necitumumab was finally approved by the FDA in 2015 for combinatory therapy with gemcitabine and cisplatin of squamous NSCLC.Citation162 Necitumumab treatment resulted in increased toxicity and mortality in non-squamous NSCLC. Necitumumab is marketed by Eli Lilly under the brand name Portrazza®. Currently, there are ongoing clinical trials to investigate safety and efficacy in other NSCLC-related indications.Citation160 Necitumumab was also tested in combination with chemotherapy (modified chFOLFOX6) in first-line treatment of locally advanced or metastatic colorectal cancer. The combination therapy was active and toxicity was manageable.Citation164
Ranibizumab (Lucentis®)
Endothelial cells can be stimulated by angiogenic factors to form new blood vesselsCitation165 to generate new vascularization. Additionally, angiogenic factors are known to play an important role in diverse cancer diseases, rheumatoid arthritis and macular degeneration.Citation166-168 Bevacizumab (Avastin®, Roche) is a humanized antibody derived from the murine antibody A.4.6.1. which binds to vascular endothelial growth factor (VEGF).Citation169 Avastin® is approved for the treatment of colon, lung, breast, kidney and ovarian cancer. Bevacizumab was affinity maturated using phage display in the Fab format.Citation170 The results of an alanine-scanning study and the crystal structureCitation171 of the Fab-VEGF complex were used to build different antibody gene libraries. For each CDR of the heavy chain a single mutation library was constructed by randomizing only those amino acid positions shown to be important for the interaction with VEGF. Additionally, another mutation library was constructed with mutations at particular sites in the framework 3 of the VH domain. Off-rate selection was used to identify antibody clones with higher affinity. Finally, the beneficial mutations were combined and screened for high affinity antibodies. Clone Y0317 (subsequently named ranibizumab) had an affinity to VEGF of about 0.1 nM and contained only 6 mutations compared to the parental antibody. Ranibizumab demonstrated a 100-fold improvement for inhibition of VEGF-dependent cell proliferation compared to the parental antibody.Citation170 The Fab of ranibizumab is produced in E. coli and was first approved by the FDA in 2010 for the treatment of macular edema following retinal vein occlusion. In 2012, it was approved for the treatment of diabetic macular edema, and in 2015 for treating diabetic retinopathy. Novartis markets ranibizumab under the brand name Lucentis®. The humanized parental IgG bevacizumab and the affinity-matured Fab ranibizumab have equivalent effects on visual acuity for the treatment of neovascular age-related macular degeneration (AMD) when administered according on the same schedule.Citation172 Bevacizumab is not approved for treatment of AMD, but is used off-label for this purpose.
Ramucirumab (Cyramza®)
Angiogenesis is one of the most important steps for cancer progression.Citation167 The VEGFs and their receptors are involved in this process. To date, 4 human VEGFsCitation173 and 3 receptorsCitation174-178 have been identified. The most important receptor for angiogenesis is thought to be VEGFR2 (also known as kinase insert domain receptor (KDR)), which forms a homodimer,Citation179,180 but also heterodimers that include other receptors involved in signal transduction.Citation181,182 The importance of the VEGF angiogenesis pathway for cancer progression led to the development of humanized antibodies against VEGF, such as bevacizumabCitation183 and ranibizumab,Citation170 as well as VEGF-binding recombinant fusion proteins. These consist of peptides derived from the extracellular domains of human VEGF receptors 1 and 2 (i.e. VEGF-trap) fused to human IgG1 Fc discovered by Regeneron and named aflibercept (Eylea®, approved for AMD in 2011; co-development with Bayer) or ziv-aflibercept (Zaltrap®, approved in 2012 for colorectal cancer; co-development with Sanofi).Citation184 Another strategy was the inhibition of the VEGF receptors.
Ramucirumab was screened from the de Haard Fab phage display libraryCitation40 against recombinantly expressed kinase insert domain-containing receptor (KDR or VEGFR2) fused to alkaline phosphatase.Citation185 Here, 4 different antibody candidates were identified that were able to block the interaction between VEGF and human VEGF-R2, inhibit VEGF-induced proliferation of human endothelial cells and the migration of VEGF-R2 positive leukemia cells. Three of the 4 clones contained the same heavy chain sequence. Therefore, this VH was used for the generation of a new phage display library, in which this heavy chain was paired with the variable light chain repertoire of the naïve library.Citation186 The new library was screened under modified conditions to enrich antibodies with higher affinities, and clone 1121, which showed a more than 30-fold increase in affinity (100 pM), better receptor ligand binding inhibition and better inhibition of receptor phosphorylation by ligand binding, was identified.Citation187 Clone 1121 (later named ramucirumab) had an ∼8-fold higher affinity to the receptor compared to that of the natural ligand, VEGF.Citation188
The results of a Phase 1 clinical dose-escalation trial to evaluate safety, maximum-tolerated dose, pharmacokinetics and pharmacodynamics of ramucirumab appeared very promising with regard to its anti-cancer activity in patients with different kinds of cancers.Citation189 Starting in 2009, Phase 3 clinical trials were carried out with patients suffering from advanced gastric or gastro-esophageal junction adenocarcinoma. The results showed that ramucirumab was the first biological treatment given as a single drug that provided survival benefits in these patients.Citation190
Ramucirumab was approved by the FDA in 2014 for the treatment of advanced gastric or gastro-esophageal junction adenocarcinoma and metastatic non-small-cell carcinoma. The antibody is marketed by Eli Lilly under the brand name Cyramza®. In 2013, Eli Lilly announced that Phase 3 studies of ramucirumab for the treatment of breast and liver cancer were terminated due to lack of efficacy.
Antibodies for infectious diseases
Raxibacumab (Abthrax®)
Anthrax is an infectious disease caused by Bacillus anthracis, an aerobic, Gram-positive, spore-forming bacterium that is found in soils around the world. The Centers for Disease Control and Prevention (CDC) classified anthrax as category A agent, indicating that it has a high risk of potential use as bioweapon.Citation191 B. anthracis secrets 2 toxins, the lethal toxin (LT) and the edema toxin (ET).Citation192 Both toxins are composed of 2 subunits. The LT consists of the lethal factor (LF) and the protective antigen (PA); the ET is formed by the edema factor (EF) and PA. PA is necessary for the cellular uptake of both toxins.Citation193 For this reason, PA was chosen as the target for the development program that yielded raxibacumab, which neutralizes PA with an IC50 of 0.21 nM. Binding kinetics of raxibacumab to PA revealed an equilibrium binding constant (Kd) of 2.78 nM. Raxibacumab was selected for PA neutralization by HGS (now part of GSK) using a naïve human scFv phage display library licensed from CAT. The final format is an IgG1λ mAb. In 2012, the FDA approved raxibacumab to treat inhalational anthrax. The use of raxibacumab in combination with antibiotics is advised.Citation194,195 An alternative to targeting PA is the inhibition of the lethal factor complex formation.Citation196
Foravirumab
Rabies is caused by the rabies virus, which infects the central nervous system. Before Louis Pasteur developed a rabies vaccination, the disease was always fatal. The current post-exposure therapy is based on vaccination, as performed by Pasteur in 1885, and polyclonal anti-rabies immunoglobulins.Citation197-199 Crucell selected a panel of recombinant antibodies against the rabies glycoprotein from an immune scFv library.Citation200 The neutralization of 27 wild type rabies viruses (“representative street rabies viruses”) was tested in vitro, and the best neutralizing antibodies were tested in vivo in a hamster rabies infection model. Here, the antibody CR4098 showed 100% post-exposure protection with 40 IU/kg.Citation201 This antibody was further analyzed in combination with another human antibody, CR57, derived by somatic cell hybridization technique,Citation202 in in vitro and in vivo models.Citation199 The safety of this cocktail, named CL184, was tested in a clinical Phase 1 study,Citation203 and subsequently in 2 Phase 2 studies (ClinicalTrials.gov Identifier: NCT00708084, NCT00656097). The antibodies were named foravirumab (CR4098) and rafivirumab (CR57). This program demonstrated that antibody phage display using immune libraries is an efficient technology for the discovery of neutralizing antibodies to infectious pathogens and toxins.
Summary and outlook
The large number of phage display-derived antibodies in clinical development demonstrates the value of this in vitro selection technology for the discovery of therapeutic antibodies. Phage display has not only proven to be a versatile and reliable platform technology for the discovery of “fully” human antibodies for over 2 decades, but it has been successfully used for the development of other types of peptide and protein therapeutics, such as ecallantide (Kalbitor®, Dyax/Shire),Citation122 Xyntha purification peptideCitation204 and the peptibody trebananib (AMG-386, Amgen)Citation205 (for review see Nixon et al.Citation86).
The changing intellectual property landscape will further affect development. For many years access to antibody phage display was controlled by a few companies, based on their technology patents. But these, namely the Breitling/Dübel (EP0440147) and McCafferty/Winter (EP0774511, EP0589877) families, expired in 2011 in Europe. In the US the patent US5969108 (filed 1990, granted 1991, published 1999), US6172197 (filed 1991, granted 1995, published 2001) and US6806079 (filed 1990, granted 2000, published 2004) are most relevant. The expiration of US patents is more complex and depends on variables such as the filling date, the grant date, patent term adjustments and extensions. Together with lower pricing driven by the competition with biosimilar products, the patent expiry should spur innovation and allow more companies to advance human antibodies made by phage display into the clinic.
Antibody phage display has advantages over newer technologies like yeast or mammalian cell display, including library size (>10Citation10), robust and well-established E. coli expression system, scalability, short panning cycles and cost-efficiency, but also in vitro selection conditions and the ability to pan on antigen-expressing cells. Issues with E. coli expression and phage display of full size IgGs can be addressed with efficient expression platforms and screening technologies.Citation206 The power of phage display lies in the choice of the optimal in vitro selection conditions, which allows pre-definition of many biophysical and biochemical antibody properties at a very early step of the discovery process. For example, it is possible to target distinct antigen epitopes, even specific conformations, address interspecies cross-reactivity, and reduce off-target binding using phage display. Moreover, the technique can be combined with immunization and immune libraries to get the best of these antibody discovery approaches.
Disclosure of potential confllicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Stefan Dübel for writing a summary of the phage display IP situation and Luciano Puzer for discussion and corrections on the manuscript. We are very grateful for corrections and numerous improvements on the manuscript by Janice Reichert.
References
- Reichert JM. Therapeutic monoclonal antibodies approved or in review in the European Union or the United States [Internet]. The Antibody Society 2016 [cited 2016 Jun 24]; Available from:http://www.antibodysociety.org/news/approved-antibodies/
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs 2015; 7:9-14; PMID:25529996; http://dx.doi.org/10.4161/19420862.2015.989042
- Emmons C, Hunsicker LG. Muromonab-CD3 (Orthoclone OKT3): the first monoclonal antibody approved for therapeutic use. Iowa Med 1987; 77:78-82; PMID:3557906
- Kimball JA, Norman DJ, Shield CF, Schroeder TJ, Lisi P, Garovoy M, O'Connell JB, Stuart F, McDiarmid SV, Wall W. OKT3 antibody response study (OARS): a multicenter comparative study. Transplant Proc 1993; 25:558-60; PMID:8438413
- Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods 2005; 36:3-10; PMID:15848070; http://dx.doi.org/10.1016/j.ymeth.2005.01.001
- Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev 2006; 58:640-56; PMID:16904789; http://dx.doi.org/10.1016/j.addr.2006.01.026
- Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 1997; 90:2188-95; PMID:9310469
- Khazaeli MB, Saleh MN, Liu T, Kaladas PM, Gilman SC, LoBuglio AF. Frequent anti-V-region immune response to mouse B72.3 monoclonal antibody. J Clin Immunol 1992; 12:116-21; PMID:1373150; http://dx.doi.org/10.1007/BF00918141
- Güssow D, Seemann G. Humanization of monoclonal antibodies. Meth Enzymol 1991; 203:99-121; PMID:1762576; http://dx.doi.org/10.1016/0076-6879(91)03007-4
- Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med 1998; 338:161-5; PMID:9428817; http://dx.doi.org/10.1056/NEJM199801153380304
- Bielekova B, Richert N, Howard T, Blevins G, Markovic-Plese S, McCartin J, Frank JA, Würfel J, Ohayon J, Waldmann TA, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci USA 2004; 101:8705-8; PMID:15161974; http://dx.doi.org/10.1073/pnas.0402653101
- Chen HX, Gore-Langton RE, Cheson BD. Clinical trials referral resource: Current clinical trials of the anti-VEGF monoclonal antibody bevacizumab. Oncology (Williston Park, NY) 2001; 15:1017, 1020, 1023-6; PMID:11548974
- Stephens S, Emtage S, Vetterlein O, Chaplin L, Bebbington C, Nesbitt A, Sopwith M, Athwal D, Novak C, Bodmer M. Comprehensive pharmacokinetics of a humanized antibody and analysis of residual anti-idiotypic responses. Immunology 1995; 85:668-74; PMID:7558164
- Weinblatt ME, Maddison PJ, Bulpitt KJ, Hazleman BL, Urowitz MB, Sturrock RD, Coblyn JS, Maier AL, Spreen WR, Manna VK. CAMPATH-1H, a humanized monoclonal antibody, in refractory rheumatoid arthritis. An intravenous dose-escalation study. Arthritis Rheum 1995; 38:1589-94; PMID:7488279; http://dx.doi.org/10.1002/art.1780381110
- Lee Y-H, Iijima M, Kado Y, Mizohata E, Inoue T, Sugiyama A, Doi H, Shibasaki Y, Kodama T. Construction and characterization of functional anti-epiregulin humanized monoclonal antibodies. Biochem Biophys Res Commun 2013; 441:1011-7; PMID:24239549; http://dx.doi.org/10.1016/j.bbrc.2013.11.014
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 2009; 458:636-40; PMID:19287373; http://dx.doi.org/10.1038/nature07930
- Fishwild DM, O'Donnell SL, Bengoechea T, Hudson DV, Harding F, Bernhard SL, Jones D, Kay RM, Higgins KM, Schramm SR, et al. High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat Biotechnol 1996; 14:845-51; PMID:9631008; http://dx.doi.org/10.1038/nbt0796-845
- Ishida I, Tomizuka K, Yoshida H, Tahara T, Takahashi N, Ohguma A, Tanaka S, Umehashi M, Maeda H, Nozaki C, et al. Production of human monoclonal and polyclonal antibodies in TransChromo animals. Cloning Stem Cells 2002; 4:91-102; PMID:12006160; http://dx.doi.org/10.1089/153623002753632084
- Jakobovits A. Production of fully human antibodies by transgenic mice. Curr Opin Biotechnol 1995; 6:561-6; PMID:7579668; http://dx.doi.org/10.1016/0958-1669(95)80093-X
- Lonberg N, Huszar D. Human antibodies from transgenic mice. Int Rev Immunol 1995; 13:65-93; PMID:7494109; http://dx.doi.org/10.3109/08830189509061738
- Ma B, Osborn MJ, Avis S, Ouisse L-H, Ménoret S, Anegon I, Buelow R, Brüggemann M. Human antibody expression in transgenic rats: comparison of chimeric IgH loci with human VH, D and JH but bearing different rat C-gene regions. J Immunol Methods 2013; 400–401:78-86; PMID:24184135; http://dx.doi.org/10.1016/j.jim.2013.10.007
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9:767-74; PMID:20811384; http://dx.doi.org/10.1038/nrd3229
- Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 1985; 228:1315-7; PMID:4001944; http://dx.doi.org/10.1126/science.4001944
- Parmley SF, Smith GP. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 1988; 73:305-18; PMID:3149606; http://dx.doi.org/10.1016/0378-1119(88)90495-7
- Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science 1988; 242:423-6; PMID:3140379; http://dx.doi.org/10.1126/science.3140379
- Skerra A, Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science 1988; 240:1038-41; PMID:3285470; http://dx.doi.org/10.1126/science.3285470
- Breitling F, Dübel S, Seehaus T, Klewinghaus I, Little M. A surface expression vector for antibody screening. Gene 1991; 104:147-53; PMID:1916287; http://dx.doi.org/10.1016/0378-1119(91)90244-6
- Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature 1991; 352:624-8; PMID:1907718; http://dx.doi.org/10.1038/352624a0
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 1990; 348:552-4; PMID:2247164; http://dx.doi.org/10.1038/348552a0
- Barbas CF, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci USA 1991; 88:7978-82; PMID:1896445; http://dx.doi.org/10.1073/pnas.88.18.7978
- Burton DR, Barbas CF, Persson MA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA 1991; 88:10134-7; PMID:1719545; http://dx.doi.org/10.1073/pnas.88.22.10134
- Rondot S, Koch J, Breitling F, Dübel S. A helper phage to improve single-chain antibody presentation in phage display. Nat Biotechnol 2001; 19:75-8; PMID:11135557; http://dx.doi.org/10.1038/83567
- Soltes G, Hust M, Ng KKY, Bansal A, Field J, Stewart DIH, Dübel S, Cha S, Wiersma EJ. On the influence of vector design on antibody phage display. J Biotechnol 2007; 127:626-37; PMID:16996161; http://dx.doi.org/10.1016/j.jbiotec.2006.08.015
- Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, Hoess A, Wölle J, Plückthun A, Virnekäs B. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol 2000; 296:57-86; PMID:10656818; http://dx.doi.org/10.1006/jmbi.1999.3444
- Paschke M, Zahn G, Warsinke A, Höhne W. New series of vectors for phage display and prokaryotic expression of proteins. BioTechniques 2001; 30:720-4, 726; PMID:11314251
- Mazor Y, Van Blarcom T, Carroll S, Georgiou G. Selection of full-length IgGs by tandem display on filamentous phage particles and Escherichia coli fluorescence-activated cell sorting screening. FEBS J 2010; 277:2291-303; PMID:20423457; http://dx.doi.org/10.1111/j.1742-4658.2010.07645.x
- Hust M, Meyer T, Voedisch B, Rülker T, Thie H, El-Ghezal A, Kirsch MI, Schütte M, Helmsing S, Meier D, et al. A human scFv antibody generation pipeline for proteome research. J Biotechnol 2011; 152:159-70; PMID:20883731; http://dx.doi.org/10.1016/j.jbiotec.2010.09.945
- Schofield DJ, Pope AR, Clementel V, Buckell J, Chapple SD, Clarke KF, Conquer JS, Crofts AM, Crowther SRE, Dyson MR, et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol 2007; 8:R254; PMID:18047641; http://dx.doi.org/10.1186/gb-2007-8-11-r254
- Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, McCafferty J, Hodits RA, Wilton J, Johnson KS. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol 1996; 14:309-14; PMID:9630891; http://dx.doi.org/10.1038/nbt0396-309
- de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, de Bruïne AP, Arends JW, Hoogenboom HR. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem 1999; 274:18218-30; PMID:10373423; http://dx.doi.org/10.1074/jbc.274.26.18218
- Hoet RM, Cohen EH, Kent RB, Rookey K, Schoonbroodt S, Hogan S, Rem L, Frans N, Daukandt M, Pieters H, et al. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol 2005; 23:344-8; PMID:15723048; http://dx.doi.org/10.1038/nbt1067
- Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM. Domain antibodies: proteins for therapy. Trends Biotechnol 2003; 21:484-90; PMID:14573361; http://dx.doi.org/10.1016/j.tibtech.2003.08.007
- Muyldermans S. Single domain camel antibodies: current status. J Biotechnol 2001; 74:277-302; PMID:11526908; http://dx.doi.org/10.1016/S1389-0352(01)00021-6
- Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, Leonhardt H, Magez S, Nguyen VK, Revets H, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol 2009; 128:178-83; PMID:19026455; http://dx.doi.org/10.1016/j.vetimm.2008.10.299
- Dübel S, Stoevesandt O, Taussig MJ, Hust M. Generating recombinant antibodies to the complete human proteome. Trends Biotechnol 2010; 28:333-9; PMID:20538360; http://dx.doi.org/10.1016/j.tibtech.2010.05.001
- Bradbury ARM, Sidhu S, Dübel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol 2011; 29:245-54; PMID:21390033; http://dx.doi.org/10.1038/nbt.1791
- Kügler J, Wilke S, Meier D, Tomszak F, Frenzel A, Schirrmann T, Dübel S, Garritsen H, Hock B, Toleikis L, et al. Generation and analysis of the improved human HAL9/10 antibody phage display libraries. BMC Biotechnol 2015; 15:10; PMID:25888378; http://dx.doi.org/10.1186/s12896-015-0125-0
- Trott M, Weiβ S, Antoni S, Koch J, von Briesen H, Hust M, Dietrich U. Functional characterization of two scFv-Fc antibodies from an HIV controller selected on soluble HIV-1 Env complexes: a neutralizing V3- and a trimer-specific gp41 antibody. PLoS ONE 2014; 9:e97478; PMID:24828352; http://dx.doi.org/10.1371/journal.pone.0097478
- Duan T, Ferguson M, Yuan L, Xu F, Li G. Human Monoclonal Fab Antibodies Against West Nile Virus and its Neutralizing Activity Analyzed in vitro and in vivo. J Antivir Antiretrovir 2009; 1:36-42; PMID:20505850; http://dx.doi.org/10.4172/jaa.1000005
- Thie H, Toleikis L, Li J, von Wasielewski R, Bastert G, Schirrmann T, Esteves IT, Behrens CK, Fournes B, Fournier N, et al. Rise and fall of an anti-MUC1 specific antibody. PLoS ONE 2011; 6:e15921; PMID:21264246; http://dx.doi.org/10.1371/journal.pone.0015921
- Hülseweh B, Rülker T, Pelat T, Langermann C, Frenzel A, Schirrmann T, Dübel S, Thullier P, Hust M. Human-like antibodies neutralizing Western equine encephalitis virus. MAbs 2014; 6:717-26; http://dx.doi.org/10.4161/mabs.28170
- Miethe S, Rasetti-Escargueil C, Liu Y, Chahboun S, Pelat T, Avril A, Frenzel A, Schirrmann T, Thullier P, Sesardic D, et al. Development of neutralizing scFv-Fc against botulinum neurotoxin A light chain from a macaque immune library. MAbs 2014; 6:446-59; PMID:24492304; http://dx.doi.org/10.4161/mabs.27773
- Miethe S, Rasetti-Escargueil C, Avril A, Liu Y, Chahboun S, Korkeala H, Mazuet C, Popoff M-R, Pelat T, Thullier P, et al. Development of Human-Like scFv-Fc Neutralizing Botulinum Neurotoxin E. PLoS ONE 2015; 10:e0139905; PMID:26440796; http://dx.doi.org/10.1371/journal.pone.0139905
- Rasetti-Escargueil C, Avril A, Chahboun S, Tierney R, Bak N, Miethe S, Mazuet C, Popoff MR, Thullier P, Hust M, et al. Development of human-like scFv-Fc antibodies neutralizing Botulinum toxin serotype B. MAbs 2015; 7:1161-77; PMID:26381852; http://dx.doi.org/10.1080/19420862.2015.1082016
- Rülker T, Voß L, Thullier P, O’ Brien LM, Pelat T, Perkins SD, Langermann C, Schirrmann T, Dübel S, Marschall H-J, et al. Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo. PLoS ONE 2012; 7:e37242; PMID:22666347; http://dx.doi.org/10.1371/journal.pone.0037242
- Pelat T, Hust M, Thullier P. Obtention and engineering of non-human primate (NHP) antibodies for therapeutics. Mini Rev Med Chem 2009; 9:1633-8; PMID:20105119; http://dx.doi.org/10.2174/138955709791012283
- Rossant CJ, Carroll D, Huang L, Elvin J, Neal F, Walker E, Benschop JJ, Kim EE, Barry ST, Vaughan TJ. Phage display and hybridoma generation of antibodies to human CXCR2 yields antibodies with distinct mechanisms and epitopes. MAbs 2014; 6:1425-38; PMID:25484064; http://dx.doi.org/10.4161/mabs.34376
- Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol 1994; 12:433-55; PMID:8011287; http://dx.doi.org/10.1146/annurev.iy.12.040194.002245
- Schwimmer LJ, Huang B, Giang H, Cotter RL, Chemla-Vogel DS, Dy FV, Tam EM, Zhang F, Toy P, Bohmann DJ, et al. Discovery of diverse and functional antibodies from large human repertoire antibody libraries. J Immunol Methods 2013; 391:60-71; PMID:23454004; http://dx.doi.org/10.1016/j.jim.2013.02.010
- Steinwand M, Droste P, Frenzel A, Hust M, Dübel S, Schirrmann T. The influence of antibody fragment format on phage display based affinity maturation of IgG. MAbs 2014; 6:204-18; PMID:24262918; http://dx.doi.org/10.4161/mabs.27227
- Lloyd C, Lowe D, Edwards B, Welsh F, Dilks T, Hardman C, Vaughan T. Modelling the human immune response: performance of a 1011 human antibody repertoire against a broad panel of therapeutically relevant antigens. Protein Eng Des Sel 2009; 22:159-68; PMID:18974080; http://dx.doi.org/10.1093/protein/gzn058
- Griffiths AD, Williams SC, Hartley O, Tomlinson IM, Waterhouse P, Crosby WL, Kontermann RE, Jones PT, Low NM, Allison TJ. Isolation of high affinity human antibodies directly from large synthetic repertoires. The EMBO Journal 1994; 13:3245-60; PMID:8045255
- Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, Neri D. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. The Journal of Biological Chemistry 1998; 273:21769-76; PMID:9705314; http://dx.doi.org/10.1074/jbc.273.34.21769
- de Wildt RM, Mundy CR, Gorick BD, Tomlinson IM. Antibody arrays for high-throughput screening of antibody-antigen interactions. Nat Biotechnol 2000; 18:989-94; PMID:10973222; http://dx.doi.org/10.1038/79494
- Söderlind E, Strandberg L, Jirholt P, Kobayashi N, Alexeiva V, Aberg AM, Nilsson A, Jansson B, Ohlin M, Wingren C, et al. Recombining germline-derived CDR sequences for creating diverse single-framework antibody libraries. Nat Biotechnol 2000; 18:852-6; PMID:10932154; http://dx.doi.org/10.1038/78458
- Prassler J, Thiel S, Pracht C, Polzer A, Peters S, Bauer M, Nörenberg S, Stark Y, Kölln J, Popp A, et al. HuCAL PLATINUM, a synthetic Fab library optimized for sequence diversity and superior performance in mammalian expression systems. J Mol Biol 2011; 413:261-78; PMID:21856311; http://dx.doi.org/10.1016/j.jmb.2011.08.012
- Rothe C, Urlinger S, Löhning C, Prassler J, Stark Y, Jäger U, Hubner B, Bardroff M, Pradel I, Boss M, et al. The human combinatorial antibody library HuCAL GOLD combines diversification of all six CDRs according to the natural immune system with a novel display method for efficient selection of high-affinity antibodies. J Mol Biol 2008; 376:1182-200; PMID:18191144; http://dx.doi.org/10.1016/j.jmb.2007.12.018
- Tiller T, Schuster I, Deppe D, Siegers K, Strohner R, Herrmann T, Berenguer M, Poujol D, Stehle J, Stark Y, et al. A fully synthetic human Fab antibody library based on fixed VH/VL framework pairings with favorable biophysical properties. MAbs 2013; 5:445-70; PMID:23571156; http://dx.doi.org/10.4161/mabs.24218
- Glanville J, Zhai W, Berka J, Telman D, Huerta G, Mehta GR, Ni I, Mei L, Sundar PD, Day GMR, et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc Natl Acad Sci USA 2009; 106:20216-21; PMID:19875695; http://dx.doi.org/10.1073/pnas.0909775106
- Osbourn J, Groves M, Vaughan T. From rodent reagents to human therapeutics using antibody guided selection. Methods 2005; 36:61-8; PMID:15848075; http://dx.doi.org/10.1016/j.ymeth.2005.01.006
- Moghaddam A, Borgen T, Stacy J, Kausmally L, Simonsen B, Marvik OJ, Brekke OH, Braunagel M. Identification of scFv antibody fragments that specifically recognise the heroin metabolite 6-monoacetylmorphine but not morphine. J Immunol Methods 2003; 280:139-55; PMID:12972195; http://dx.doi.org/10.1016/S0022-1759(03)00109-1
- Hust M, Maiss E, Jacobsen H-J, Reinard T. The production of a genus-specific recombinant antibody (scFv) using a recombinant potyvirus protease. J Virol Methods 2002; 106:225-33; PMID:12393153; http://dx.doi.org/10.1016/S0166-0934(02)00166-0
- Schütte M, Thullier P, Pelat T, Wezler X, Rosenstock P, Hinz D, Kirsch MI, Hasenberg M, Frank R, Schirrmann T, et al. Identification of a putative Crf splice variant and generation of recombinant antibodies for the specific detection of Aspergillus fumigatus. PLoS ONE 2009; 4:e6625; PMID:19675673; http://dx.doi.org/10.1371/journal.pone.0006625
- Zhou M, Meyer T, Koch S, Koch J, von Briesen H, Benito JM, Soriano V, Haberl A, Bickel M, Dübel S, et al. Identification of a new epitope for HIV-neutralizing antibodies in the gp41 membrane proximal external region by an Env-tailored phage display library. Eur J Immunol 2013; 43:499-509; PMID:23180650; http://dx.doi.org/10.1002/eji.201242974
- Droste P, Frenzel A, Steinwand M, Pelat T, Thullier P, Hust M, Lashuel H, Dübel S. Structural differences of amyloid-β fibrils revealed by antibodies from phage display. BMC Biotechnol 2015; 15:57; PMID:26084577; http://dx.doi.org/10.1186/s12896-015-0146-8
- Nizak C, Monier S, del Nery E, Moutel S, Goud B, Perez F. Recombinant antibodies to the small GTPase Rab6 as conformation sensors. Science 2003; 300:984-7; PMID:12738866; http://dx.doi.org/10.1126/science.1083911
- Liang W-C, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber H-P, Ferrara N, et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 2006; 281:951-61; PMID:16278208; http://dx.doi.org/10.1074/jbc.M508199200
- Frenzel A, Kügler J, Wilke S, Schirrmann T, Hust M. Construction of human antibody gene libraries and selection of antibodies by phage display. Methods Mol Biol 2014; 1060:215-43; PMID:24037844; http://dx.doi.org/10.1007/978-1-62703-586-6_12
- Dübel S. Recombinant therapeutic antibodies. Appl Microbiol Biotechnol 2007; 74:723-9; PMID:17225094; http://dx.doi.org/10.1007/s00253-006-0810-y
- Jäger V, Büssow K, Wagner A, Weber S, Hust M, Frenzel A, Schirrmann T. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol 2013; 13:52; PMID:23802841; http://dx.doi.org/10.1186/1472-6750-13-52
- Romani C, Cocco E, Bignotti E, Moratto D, Bugatti A, Todeschini P, Bandiera E, Tassi R, Zanotti L, Pecorelli S, et al. Evaluation of a novel human IgG1 anti-claudin3 antibody that specifically recognizes its aberrantly localized antigen in ovarian cancer cells and that is suitable for selective drug delivery. Oncotarget 2015; 6:34617-28; PMID:26416446
- Reichert JM. Which are the antibodies to watch in 2013? MAbs 2013; 5:1-4; PMID:23254906; http://dx.doi.org/10.4161/mabs.22976
- Reichert JM. Antibodies to watch in 2014. MAbs 2014; 6:5-14; PMID:24284914; http://dx.doi.org/10.4161/mabs.27333
- Reichert JM. Antibodies to watch in 2015. MAbs 2015; 7:1-8; PMID:25484055; http://dx.doi.org/10.4161/19420862.2015.988944
- Reichert JM. Antibodies to watch in 2016. MAbs 2016; 8:197-204; PMID:26651519; http://dx.doi.org/10.1080/19420862.2015.1125583
- Nixon AE, Sexton DJ, Ladner RC. Drugs derived from phage display: from candidate identification to clinical practice. MAbs 2014; 6:73-85; PMID:24262785; http://dx.doi.org/10.4161/mabs.27240
- Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian DK, Salfeld J, Sasso EH. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol 2009; 131:308-16; PMID:19188093; http://dx.doi.org/10.1016/j.clim.2009.01.002
- Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996; 334:1717-25; PMID:8637518; http://dx.doi.org/10.1056/NEJM199606273342607
- Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet 1989; 2:244-7; PMID:2569055; http://dx.doi.org/10.1016/S0140-6736(89)90430-3
- Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 2004; 32:2173-82; PMID:15640628; http://dx.doi.org/10.1097/01.CCM.0000145229.59014.6C
- Kempeni J. Preliminary results of early clinical trials with the fully human anti-TNFalpha monoclonal antibody D2E7. Ann Rheum Dis 1999; 58 Suppl 1:I70-72; PMID:10577977; http://dx.doi.org/10.1136/ard.58.2008.i70
- van de Putte LBA, Atkins C, Malaise M, Sany J, Russell AS, van Riel PLCM, Settas L, Bijlsma JW, Todesco S, Dougados M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 2004; 63:508-16; PMID:15082480; http://dx.doi.org/10.1136/ard.2003.013052
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003; 48:35-45; PMID:12528101; http://dx.doi.org/10.1002/art.10697
- Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, Teoh LS, Velagapudi RB, Noertersheuser PA, Granneman GR, et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther 2003; 25:1700-21; PMID:12860493; http://dx.doi.org/10.1016/S0149-2918(03)80164-9
- Borrás-Blasco J, Gracia-Pérez A, Rosique-Robles JD, Casterá MD-E, Abad FJ. Acceptability of switching adalimumab from a prefilled syringe to an autoinjection pen. Expert Opin Biol Ther 2010; 10:301-7; http://dx.doi.org/10.1517/14712590903530633
- Frazier-Mironer A, Dougados M, Mariette X, Cantagrel A, Deschamps V, Flipo RM, Logeart I, Schaeverbeke T, Sibilia J, Le Loët X, et al. Retention rates of adalimumab, etanercept and infliximab as first and second-line biotherapy in patients with rheumatoid arthritis in daily practice. Joint Bone Spine 2014; 81:352-9; PMID:24721422; http://dx.doi.org/10.1016/j.jbspin.2014.02.014
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda APM. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013; 72:517-24; PMID:22562972; http://dx.doi.org/10.1136/annrheumdis-2011-201244
- Reichert JM. Adalimumab (Humira). In: Handbook of therapeutic antibodies. Weinheim: Wiley Blackwell; 2014. page 1309-22; http://dx.doi.org/10.1002/9783527682423
- Salliot C, Gossec L, Ruyssen-Witrand A, Luc M, Duclos M, Guignard S, Dougados M. Infections during tumour necrosis factor-alpha blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology (Oxford) 2007; 46:327-34; PMID:16880188; http://dx.doi.org/10.1093/rheumatology/kel236
- Schiff MH, Burmester GR, Kent JD, Pangan AL, Kupper H, Fitzpatrick SB, Donovan C. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis 2006; 65:889-94; PMID:16439435; http://dx.doi.org/10.1136/ard.2005.043166
- Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999; 285:260-3; PMID:10398604; http://dx.doi.org/10.1126/science.285.5425.260
- Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 1999; 189:1747-56; PMID:10359578; http://dx.doi.org/10.1084/jem.189.11.1747
- Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 2001; 44:1313-9; PMID:11407690; http://dx.doi.org/10.1002/1529-0131(200106)44:6%3c1313::AID-ART223%3e3.0.CO;2-S
- Zhang J, Roschke V, Baker KP, Wang Z, Alarcón GS, Fessler BJ, Bastian H, Kimberly RP, Zhou T. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001; 166:6-10; PMID:11123269; http://dx.doi.org/10.4049/jimmunol.166.1.6
- Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther 2011; 13:228; PMID:21745419; http://dx.doi.org/10.1186/ar3349
- Kamal A, Khamashta M. The efficacy of novel B cell biologics as the future of SLE treatment: a review. Autoimmun Rev 2014; 13:1094-101; PMID:25149393; http://dx.doi.org/10.1016/j.autrev.2014.08.020
- Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet 2014; 384:1878-88; PMID:24881804; http://dx.doi.org/10.1016/S0140-6736(14)60128-8
- Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, Wang J, Gao H-X, Putterman C, Koss MN, et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol 2009; 182:2532-41; PMID:19201910; http://dx.doi.org/10.4049/jimmunol.0802948
- Sutherland APR, Ng LG, Fletcher CA, Shum B, Newton RA, Grey ST, Rolph MS, Mackay F, Mackay CR. BAFF augments certain Th1-associated inflammatory responses. J Immunol 2005; 174:5537-44; PMID:15843552; http://dx.doi.org/10.4049/jimmunol.174.9.5537
- Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol 2009; 183:3160-9; PMID:19657089; http://dx.doi.org/10.4049/jimmunol.0900385
- Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, Lappin PB, Riccobene T, Abramian D, Sekut L, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum 2003; 48:3253-65; PMID:14613291; http://dx.doi.org/10.1002/art.11299
- Halpern WG, Lappin P, Zanardi T, Cai W, Corcoran M, Zhong J, Baker KP. Chronic administration of belimumab, a BLyS antagonist, decreases tissue and peripheral blood B-lymphocyte populations in cynomolgus monkeys: pharmacokinetic, pharmacodynamic, and toxicologic effects. Toxicol Sci 2006; 91:586-99; PMID:16517838; http://dx.doi.org/10.1093/toxsci/kfj148
- Stohl W, Merrill JT, McKay JD, Lisse JR, Zhong ZJ, Freimuth WW, Genovese MC. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging Study. J Rheumatol 2013; 40:579-89; PMID:23547209; http://dx.doi.org/10.3899/jrheum.120886
- Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum 2009; 61:1168-78; PMID:19714604; http://dx.doi.org/10.1002/art.24699
- Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS-lupus connection. Nat Biotechnol 2012; 30:69-77; PMID:22231104; http://dx.doi.org/10.1038/nbt.2076
- Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK-M, Thomas M, Kim H-Y, León MG, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:721-31; PMID:21296403; http://dx.doi.org/10.1016/S0140-6736(10)61354-2
- Schiff M, Combe B, Dörner T, Kremer JM, Huizinga TW, Veenhuizen M, Gill A, Komocsar W, Berclaz P-Y, Ortmann R, et al. Efficacy and safety of tabalumab, an anti-BAFF monoclonal antibody, in patients with moderate-to-severe rheumatoid arthritis and inadequate response to TNF inhibitors: results of a randomised, double-blind, placebo-controlled, phase 3 study. RMD Open 2015; 1:e000037; PMID:26535134; http://dx.doi.org/10.1136/rmdopen-2014-000037
- Bork K. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol 2010; 6:15; PMID:20667118; http://dx.doi.org/10.1186/1710-1492-6-15
- Chyung Y, Vince B, Iarrobino R, Sexton D, Kenniston J, Faucette R, TenHoor C, Stolz LE, Stevens C, Biedenkapp J, et al. A phase 1 study investigating DX-2930 in healthy subjects. Ann Allergy Asthma Immunol 2014; 113:460-466.e2; PMID:24980392; http://dx.doi.org/10.1016/j.anai.2014.05.028
- Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood 1997; 90:3819-43; PMID:9354649
- Duffey H, Firszt R. Management of acute attacks of hereditary angioedema: role of ecallantide. J Blood Med 2015; 6:115-23; PMID:25931832; http://dx.doi.org/10.2147/JBM.S66825
- Lehmann A. Ecallantide (DX-88), a plasma kallikrein inhibitor for the treatment of hereditary angioedema and the prevention of blood loss in on-pump cardiothoracic surgery. Expert Opin Biol Ther 2008; 8:1187-99; PMID:18613770; http://dx.doi.org/10.1517/14712598.8.8.1187
- Zuraw B, Yasothan U, Kirkpatrick P. Ecallantide. Nat Rev Drug Discov 2010; 9:189-90; PMID:20190785; http://dx.doi.org/10.1038/nrd3125
- Kenniston JA, Faucette RR, Martik D, Comeau SR, Lindberg AP, Kopacz KJ, Conley GP, Chen J, Viswanathan M, Kastrapeli N, et al. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J Biol Chem 2014; 289:23596-608; PMID:24970892; http://dx.doi.org/10.1074/jbc.M114.569061
- Yerbes R, Palacios C, López-Rivas A. The therapeutic potential of TRAIL receptor signalling in cancer cells. Clin Transl Oncol 2011; 13:839-47; PMID:22126726; http://dx.doi.org/10.1007/s12094-011-0744-4
- Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M, Mahoney A, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer 2005; 92:1430-41; PMID:15846298; http://dx.doi.org/10.1038/sj.bjc.6602487
- Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res 2008; 14:3450-5; PMID:18519776; http://dx.doi.org/10.1158/1078-0432.CCR-07-1416
- Mom CH, Verweij J, Oldenhuis CNAM, Gietema JA, Fox NL, Miceli R, Eskens FALM, Loos WJ, de Vries EGE, Sleijfer S. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study. Clin Cancer Res 2009; 15:5584-90; PMID:19690193; http://dx.doi.org/10.1158/1078-0432.CCR-09-0996
- Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol 2007; 25:1390-5; PMID:17416859; http://dx.doi.org/10.1200/JCO.2006.08.8898
- Trarbach T, Moehler M, Heinemann V, Köhne C-H, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer 2010; 102:506-12; PMID:20068564; http://dx.doi.org/10.1038/sj.bjc.6605507
- Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, Lo L, Gallant G, Klein J. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer 2008; 61:82-90; PMID:18255187; http://dx.doi.org/10.1016/j.lungcan.2007.12.011
- Stanovnik L, Logonder-Mlinsek M, Erjavec F. The effect of compound 48/80 and of electrical field stimulation on mast cells in the isolated mouse stomach. Agents Actions 1988; 23:300-3; PMID:2456001; http://dx.doi.org/10.1007/BF02142570
- Younes A, Vose JM, Zelenetz AD, Smith MR, Burris HA, Ansell SM, Klein J, Halpern W, Miceli R, Kumm E, et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma. Br J Cancer 2010; 103:1783-7; PMID:21081929; http://dx.doi.org/10.1038/sj.bjc.6605987
- Kapoor P, Ramakrishnan V, Rajkumar SV. Bortezomib combination therapy in multiple myeloma. Semin Hematol 2012; 49:228-42; PMID:22726546; http://dx.doi.org/10.1053/j.seminhematol.2012.04.010
- Engesæter B, Engebraaten O, Flørenes VA, Mælandsmo GM. Dacarbazine and the agonistic TRAIL receptor-2 antibody lexatumumab induce synergistic anticancer effects in melanoma. PLoS ONE 2012; 7:e45492; http://dx.doi.org/10.1371/journal.pone.0045492
- Merchant MS, Geller JI, Baird K, Chou AJ, Galli S, Charles A, Amaoko M, Rhee EH, Price A, Wexler LH, et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol 2012; 30:4141-7; PMID:23071222; http://dx.doi.org/10.1200/JCO.2012.44.1055
- Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ 2008; 15:751-61; PMID:18219321; http://dx.doi.org/10.1038/sj.cdd.4402306
- Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Sager J, Ashkenazi A, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res 2010; 16:1256-63; PMID:20145186; http://dx.doi.org/10.1158/1078-0432.CCR-09-1267
- Geng C, Hou J, Zhao Y, Ke X, Wang Z, Qiu L, Xi H, Wang F, Wei N, Liu Y, et al. A multicenter, open-label phase II study of recombinant CPT (Circularly Permuted TRAIL) plus thalidomide in patients with relapsed and refractory multiple myeloma. Am J Hematol 2014; 89:1037-42; PMID:25092564; http://dx.doi.org/10.1002/ajh.23822
- Soria J-C, Márk Z, Zatloukal P, Szima B, Albert I, Juhász E, Pujol J-L, Kozielski J, Baker N, Smethurst D, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol 2011; 29:4442-51; PMID:22010015; http://dx.doi.org/10.1200/JCO.2011.37.2623
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 2008; 8:533-44; PMID:18551128; http://dx.doi.org/10.1038/nri2356
- Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis 1997; 56:364-8; PMID:9227165; http://dx.doi.org/10.1136/ard.56.6.364
- Cook AD, Pobjoy J, Steidl S, Dürr M, Braine EL, Turner AL, Lacey DC, Hamilton JA. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther 2012; 14:R199; PMID:22995428; http://dx.doi.org/10.1186/ar4037
- Biondo M, Nasa Z, Marshall A, Toh BH, Alderuccio F. Local transgenic expression of granulocyte macrophage-colony stimulating factor initiates autoimmunity. J Immunol 2001; 166:2090-9; PMID:11160260; http://dx.doi.org/10.4049/jimmunol.166.3.2090
- Carrieri PB, Provitera V, De Rosa T, Tartaglia G, Gorga F, Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol Immunotoxicol 1998; 20:373-82; PMID:9736442; http://dx.doi.org/10.3109/08923979809034820
- McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med 2001; 194:873-82; PMID:11581310; http://dx.doi.org/10.1084/jem.194.7.873
- Virnekäs B, Ge L, Plückthun A, Schneider KC, Wellnhofer G, Moroney SE. Trinucleotide phosphoramidites: ideal reagents for the synthesis of mixed oligonucleotides for random mutagenesis. Nucleic Acids Res 1994; 22:5600-7; http://dx.doi.org/10.1093/nar/22.25.5600
- Steidl S, Ratsch O, Brocks B, Dürr M, Thomassen-Wolf E. in vitro affinity maturation of human GM-CSF antibodies by targeted CDR-diversification. Mol Immunol 2008; 46:135-44; PMID:18722015; http://dx.doi.org/10.1016/j.molimm.2008.07.013
- Behrens F, Tak PP, Østergaard M, Stoilov R, Wiland P, Huizinga TW, Berenfus VY, Vladeva S, Rech J, Rubbert-Roth A, et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis 2015; 74:1058-64; PMID:24534756; http://dx.doi.org/10.1136/annrheumdis-2013-204816
- Constantinescu CS, Asher A, Fryze W, Kozubski W, Wagner F, Aram J, Tanasescu R, Korolkiewicz RP, Dirnberger-Hertweck M, Steidl S, et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015; 2:e117; PMID:26185773; http://dx.doi.org/10.1212/NXI.0000000000000117
- Krinner E-M, Hepp J, Hoffmann P, Bruckmaier S, Petersen L, Petsch S, Parr L, Schuster I, Mangold S, Lorenczewski G, et al. A highly stable polyethylene glycol-conjugated human single-chain antibody neutralizing granulocyte-macrophage colony stimulating factor at low nanomolar concentration. Protein Eng Des Sel 2006; 19:461-70; PMID:16868004; http://dx.doi.org/10.1093/protein/gzl031
- Krinner E-M, Raum T, Petsch S, Bruckmaier S, Schuster I, Petersen L, Cierpka R, Abebe D, Mølhøj M, Wolf A, et al. A human monoclonal IgG1 potently neutralizing the pro-inflammatory cytokine GM-CSF. Mol Immunol 2007; 44:916-25; PMID:16697465; http://dx.doi.org/10.1016/j.molimm.2006.03.020
- Burmester GR, Weinblatt ME, McInnes IB, Porter D, Barbarash O, Vatutin M, Szombati I, Esfandiari E, Sleeman MA, Kane CD, et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis 2013; 72:1445-52; PMID:23234647; http://dx.doi.org/10.1136/annrheumdis-2012-202450
- Burmester GR, Feist E, Sleeman MA, Wang B, White B, Magrini F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann Rheum Dis 2011; 70:1542-9; PMID:21613310; http://dx.doi.org/10.1136/ard.2010.146225
- Hirsch FR, Dziadziuszko R, Thatcher N, Mann H, Watkins C, Parums DV, Speake G, Holloway B, Bunn PA, Franklin WA. Epidermal growth factor receptor immunohistochemistry: comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo-controlled study in advanced nonsmall-cell lung cancer. Cancer 2008; 112:1114-21; PMID:18219661; http://dx.doi.org/10.1002/cncr.23282
- Rusch V, Baselga J, Cordon-Cardo C, Orazem J, Zaman M, Hoda S, McIntosh J, Kurie J, Dmitrovsky E. Differential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lung. Cancer Res 1993; 53:2379-85; PMID:7683573
- Rusch V, Klimstra D, Venkatraman E, Pisters PW, Langenfeld J, Dmitrovsky E. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res 1997; 3:515-22; PMID:9815714
- Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, Caponigro F, Salomon DS. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets 2005; 6:243-57; PMID:15857286; http://dx.doi.org/10.2174/1389450053765879
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 2006; 12:5268-72; PMID:17000658; http://dx.doi.org/10.1158/1078-0432.CCR-05-1554
- Li S, Kussie P, Ferguson KM. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure 2008; 16:216-27; PMID:18275813; http://dx.doi.org/10.1016/j.str.2007.11.009
- Schmitz KR, Ferguson KM. Interaction of antibodies with ErbB receptor extracellular regions. Exp Cell Res 2009; 315:659-70; PMID:18992239; http://dx.doi.org/10.1016/j.yexcr.2008.10.008
- Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy G, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015; 16:763-74; PMID:26045340; http://dx.doi.org/10.1016/S1470-2045(15)00021-2
- Garnock-Jones KP. Necitumumab: First Global Approval. Drugs 2016; 76:283-9; PMID:26729188; http://dx.doi.org/10.1007/s40265-015-0537-0
- Elez E, Hendlisz A, Delaunoit T, Sastre J, Cervantes A, Varea R, Chao G, Wallin J, Tabernero J. Phase II study of necitumumab plus modified FOLFOX6 as first-line treatment in patients with locally advanced or metastatic colorectal cancer. Br J Cancer 2016; 114(4):372-80; PMID:26766738; http://dx.doi.org/10.1038/bjc.2015.480
- Folkman J, Klagsbrun M. Angiogenic factors. Science 1987; 235:442-7; PMID:2432664; http://dx.doi.org/10.1126/science.2432664
- Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 1999; 237:1-30; PMID:9893343
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1:27-31; PMID:7584949; http://dx.doi.org/10.1038/nm0195-27
- Iruela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost 1997; 78:672-7; PMID:9198237
- Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997; 57:4593-9; PMID:9377574
- Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, de Vos AM, Lowman HB. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol 1999; 293:865-81; PMID:10543973; http://dx.doi.org/10.1006/jmbi.1999.3192
- Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure 1998; 6:1153-67; PMID:9753694; http://dx.doi.org/10.1016/S0969-2126(98)00116-6
- CATT Research Group, Martin DF, Maguire MG, Ying G, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364:1897-908; PMID:21526923; http://dx.doi.org/10.1056/NEJMicm1005605
- Roskoski R. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol 2007; 62:179-213; PMID:17324579; http://dx.doi.org/10.1016/j.critrevonc.2007.01.006
- Pajusola K, Aprelikova O, Korhonen J, Kaipainen A, Pertovaara L, Alitalo R, Alitalo K. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res 1992; 52:5738-43; PMID:1327515
- Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990; 5:519-24; PMID:2158038
- Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene 1991; 6:1677-83; PMID:1656371
- Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 1992; 187:1579-86; PMID:1417831; http://dx.doi.org/10.1016/0006-291X(92)90483-2
- de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992; 255:989-91; PMID:1312256; http://dx.doi.org/10.1126/science.1312256
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25:581-611; PMID:15294883; http://dx.doi.org/10.1210/er.2003-0027
- Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 2007; 19:2003-12; PMID:17658244; http://dx.doi.org/10.1016/j.cellsig.2007.05.013
- Huang K, Andersson C, Roomans GM, Ito N, Claesson-Welsh L. Signaling properties of VEGF receptor-1 and -2 homo- and heterodimers. Int J Biochem Cell Biol 2001; 33:315-24; PMID:11312102; http://dx.doi.org/10.1016/S1357-2725(01)00019-X
- Neagoe P-E, Lemieux C, Sirois MG. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J Biol Chem 2005; 280:9904-12; PMID:15637071; http://dx.doi.org/10.1074/jbc.M412017200
- Hsu JY, Wakelee HA. Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. BioDrugs 2009; 23:289-304; PMID:19754219; http://dx.doi.org/10.2165/11317600-000000000-00000
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 2002; 99:11393-8; PMID:12177445; http://dx.doi.org/10.1073/pnas.172398299
- Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer 2002; 97:393-9; PMID:11774295; http://dx.doi.org/10.1002/ijc.1634
- Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P, Witte L, Zhu Z. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem 2003; 278:43496-507; PMID:12917408; http://dx.doi.org/10.1074/jbc.M307742200
- Miao H-Q, Hu K, Jimenez X, Navarro E, Zhang H, Lu D, Ludwig DL, Balderes P, Zhu Z. Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochem Biophys Res Commun 2006; 345:438-45; PMID:16682007; http://dx.doi.org/10.1016/j.bbrc.2006.04.119
- Aprile G, Bonotto M, Ongaro E, Pozzo C, Giuliani F. Critical appraisal of ramucirumab (IMC-1121B) for cancer treatment: from benchside to clinical use. Drugs 2013; 73:2003-15; PMID:24277700; http://dx.doi.org/10.1007/s40265-013-0154-8
- Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O'Bryant C, Chow LQM, Serkova NJ, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010; 28:780-7; PMID:20048182; http://dx.doi.org/10.1200/JCO.2009.23.7537
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383:31-9; PMID:24094768; http://dx.doi.org/10.1016/S0140-6736(13)61719-5
- Froude JW, Stiles B, Pelat T, Thullier P. Antibodies for biodefense. MAbs 2011; 3:517-27; PMID:22123065; http://dx.doi.org/10.4161/mabs.3.6.17621
- Liu S, Moayeri M, Leppla SH. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 2014; 22:317-25; PMID:24684968; http://dx.doi.org/10.1016/j.tim.2014.02.012
- Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002; 287:2236-52; PMID:11980524; http://dx.doi.org/10.1001/jama.287.17.2236
- Kummerfeldt CE. Raxibacumab: potential role in the treatment of inhalational anthrax. Infect Drug Resist 2014; 7:101-9; PMID:24812521; http://dx.doi.org/10.2147/IDR.S47305
- Mazumdar S. Raxibacumab. MAbs 2009; 1:531-8; PMID:20068396; http://dx.doi.org/10.4161/mabs.1.6.10195
- Pelat T, Hust M, Laffly E, Condemine F, Bottex C, Vidal D, Lefranc M-P, Dübel S, Thullier P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob Agents Chemother 2007; 51:2758-64; PMID:17517846; http://dx.doi.org/10.1128/AAC.01528-06
- Briggs DJ. The role of vaccination in rabies prevention. Curr Opin Virol 2012; 2:309-14; PMID:22503445; http://dx.doi.org/10.1016/j.coviro.2012.03.007
- Crowcroft NS, Thampi N. The prevention and management of rabies. BMJ 2015; 350:g7827; PMID:25589091; http://dx.doi.org/10.1136/bmj.g7827
- Goudsmit J, Marissen WE, Weldon WC, Niezgoda M, Hanlon CA, Rice AB, Kruif J de, Dietzschold B, Bakker ABH, Rupprecht CE. Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal anti-rabies immune globulin. J Infect Dis 2006; 193:796-801; PMID:16479514; http://dx.doi.org/10.1086/500470
- Kramer RA, Marissen WE, Goudsmit J, Visser TJ, Clijsters-Van der Horst M, Bakker AQ, de Jong M, Jongeneelen M, Thijsse S, Backus HHJ, et al. The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur J Immunol 2005; 35:2131-45; PMID:15971273; http://dx.doi.org/10.1002/eji.200526134
- Bakker ABH, Marissen WE, Kramer RA, Rice AB, Weldon WC, Niezgoda M, Hanlon CA, Thijsse S, Backus HHJ, de Kruif J, et al. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J Virol 2005; 79:9062-8; PMID:15994800; http://dx.doi.org/10.1128/JVI.79.14.9062-9068.2005
- Dietzschold B, Gore M, Casali P, Ueki Y, Rupprecht CE, Notkins AL, Koprowski H. Biological characterization of human monoclonal antibodies to rabies virus. J Virol 1990; 64:3087-90; PMID:2335829
- Bakker ABH, Python C, Kissling CJ, Pandya P, Marissen WE, Brink MF, Lagerwerf F, Worst S, van Corven E, Kostense S, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine 2008; 26:5922-7; PMID:18804136; http://dx.doi.org/10.1016/j.vaccine.2008.08.050
- Kelley B, Jankowski M, Booth J. An improved manufacturing process for Xyntha/ReFacto AF. Haemophilia 2010; 16:717-25; PMID:20041957; http://dx.doi.org/10.1111/j.1365-2516.2009.02160.x
- Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 2004; 6:507-16; PMID:15542434; http://dx.doi.org/10.1016/j.ccr.2004.09.030
- Jostock T, Vanhove M, Brepoels E, Van Gool R, Daukandt M, Wehnert A, Van Hegelsom R, Dransfield D, Sexton D, Devlin M, et al. Rapid generation of functional human IgG antibodies derived from Fab-on-phage display libraries. J Immunol Methods 2004; 289:65-80; PMID:15251413; http://dx.doi.org/10.1016/j.jim.2004.03.014
- Kuhn P, Fühner V, Unkauf T, Moreira GMSG, Frenzel A, Miethe S, Hust M. Recombinant antibodies for diagnostics and therapy against pathogens and toxins generated by phage display. Proteomics Clin Appl 2016;; PMID:27198131, http://dx.doi.org/10.1002/prca.201600002
