ABSTRACT
Antibody therapeutics against different target antigens are widely used in the treatment of different malignancies including ovarian carcinomas, but this disease still requires more effective agents. Improved understanding of the biological features, signaling pathways, and immunological escape mechanisms involved in ovarian cancer has emerged in the past few years. These advances, including an appreciation of the cross-talk between cancer cells and the patient's immune system, have led to the identification of new targets. In turn, potential antibody treatments with various mechanisms of action, including immune activation or toxin-delivery, that are directed at these targets have been developed. Here, we identify established as well as novel targets for antibodies in ovarian cancer, and discuss how they may provide fresh opportunities to identify interventions with enhanced therapeutic potential.
Introduction
It is estimated that ∼22,280 American women will receive new diagnoses of ovarian cancer in 2016, and an estimated 14,240 deaths from the disease will occur in this year.Citation1 Standard therapy is a combination of maximal surgical cytoreductive and taxane- and platinum-based chemotherapeutic agents. Nevertheless, relapse is common after first-line treatment. Despite investigations of novel chemotherapeutic regimes, targeted and other therapies, there have been no significant improvements in clinical outcomes or cure rates, with current 5-year overall survival rates at only 45%.Citation2
Given that ovarian cancer is known to be immunogenic and high numbers of infiltrating immune cells, including effector cells such as T cells and macrophages, are associated with improved survival rates,Citation3 antibody-based therapies are thought to offer promise. Monoclonal antibody immunotherapies may redirect these effector cells against cancer and mediate specific and potent anti-tumor immune responses, with the aim of restricting tumor growth and improving disease course. Here, we focus on established and emerging new targets for antibody treatments in ovarian carcinoma, and we discuss monoclonal antibodies that have been studied in patients with this disease.
Targets for antibody treatments
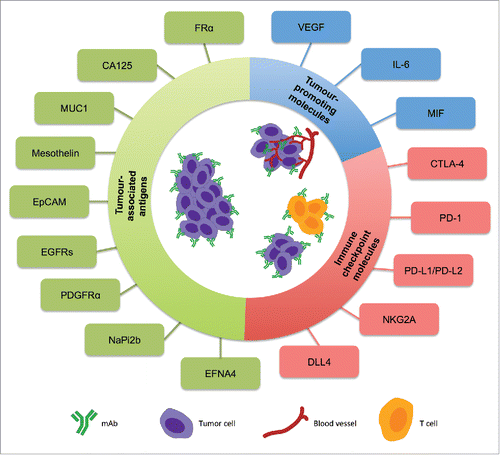
Tumor-associated antigens
Tumor-associated antigens (TAAs) are surface-associated molecules or receptors expressed by tumor cells, which have limited or no expression on normal cells. Often TAAs are involved in the activation of signaling transduction pathways that support unregulated growth or division of cancer cells. This specificity in expression and role in pro-tumoral functions make TAAs promising antibody targets, allowing tumor cells to be specifically marked for immune cell destruction or blockade of tumor-associated signaling, which impedes malignant function, invasiveness and survival.
CA125
CA125 (MUC16), an extremely large, 2500–5000 kDa, mucin-like surface glycoprotein, is expressed in greater than 95% of non-mucinous stage III/IV epithelial ovarian cancers (EOCs) and in 50–80% of ovarian tumors overall. CA125 is thought to support tumor-associated immune escape in the tumor microenvironment (TME).Citation4,5 High CA125 expression correlates with protection against cytolytic killing by natural killer (NK) cells, which is linked to reduced activating immune synapses between NK and target cells, and thus decreased cell adhesion.Citation5 This may be because the NK synaptic cleft requires a distance of 10–50 nm between NK and cancer cells, which is thought to be disrupted by the large (up to 24,000 amino acid) protein backbone of CA125 that can protrude from ovarian tumor cells by up to 1–5μm. Citation5 However, given the heterogeneity of the size of CA125 reported, which may be a result of the biological source of the molecules studied or differing biological methods used to characterize them,Citation6 or significant variation in the extent of protein glycosylation,Citation5 the degree of immune escape as well as other biological functions of CA125-expressing tumor cells may vary.
It has also been suggested that inhibition may be due to a CA125-induced reduction in NK cell expression of the Fc activating receptor, CD16.Citation4 In fact, NK cells from patients with EOCs have shown significant reduction in CD16 expression compared to NK cells from healthy donors. A down-regulation of activatory receptors, such as CD16, leads to relative predominance of NK cell inhibitory receptors, and thus NK cells fail to respond to tumor cells, allowing tumor evasion of the innate immune response.Citation4 Similarly, a downregulation of CD16 may also lead to ovarian tumor cell evasion of the adaptive immune system, by prevention of CD16 binding to host tumor-specific immunoglobulins.Citation4 These immunoediting mechanisms are likely to potentiate the progression and proliferation of ovarian tumors.
CA125 is also thought to facilitate pro-tumor cell-cell interactions in an N-linked glycan dependent manner.Citation7 CA125 on the surface of ovarian tumor cells binds to the glycoprotein mesothelin, expressed on epithelial cells (described below), with a high Kd of 5–10 nM. This cell adhesion is likely to occur in the peritoneum of patients with ovarian cancer, and may provide the first step to metastasis of tumor cells, likely reinforced by recruitment of CD44, β-1 integrins and other adhesion molecules.Citation7
CA125 is shed from ovarian cancer cells into the blood and peritoneal cavity upon proteolytic cleavage. CA125 serum levels are known to correlate with tumor progression and recurrence. Thus, monitoring serum CA125 levels is a well-established and useful surrogate for evaluating response to conventional chemotherapeutic and surgical treatments, and is routinely used for surveillance in follow-up.Citation8
MUC1
MUC1 is an epithelial mucin, comprising a heavily glycosylated transmembrane glycoprotein, overexpressed in many carcinomas, including 90% of EOC cells.Citation9 The subunit, MUC1-C, is thought to contribute to malignant cellular transformation by regulating gene transcription, blocking stress-induced apoptosis and necrosis, and attenuating death receptor activation. The extracellular domain consists of mucin-like tandem repeats, which are differentially glycosylated between malignant and normal cells, resulting in the exposure of differing peptide epitopes. MUC1 expression in cancer facilitates tumor invasive growth and metastasis. It can act in both an anti-adhesion manner, resulting in the release of cells from tumor nests and increased metastasis, and a pro-adhesion manner, leading to malignant spread.Citation9,10 Cancer cells also exploit MUC1 interactions with a number of growth receptors to promote survival. High expression of MUC1 by tumor cells leads to reduced cytotoxic killing by T and NK cells. This may be due to the large and complex nature of this TAA, which results in masking of extra-cellular domains for immunosurveillance and mediates tumor cell escape.Citation9
Humoral and cellular responses against MUC1 have been observed in cancer patients.Citation11 Anti-MUC1 antibodies have been shown to inversely correlate with ovarian carcinoma risk factors,Citation12 thus supporting MUC1 as a promising target for therapy.
EpCAM
EpCAM (CD326) is a type I transmembrane glycoprotein that is highly expressed across all ovarian cancer subtypes. Its expression is maintained in metastatic disease,Citation13 and EpCAM overexpression is prognostic of reduced overall survival (OS) in ovarian cancer.Citation14 Expression on epithelial carcinomas is at the cell surface, whereas in normal epithelial tissues, EpCAM is expressed basolaterally and is protected by tight junctions, making this an attractive target for anti-cancer therapy.
However, EpCAM is reported to have mixed functions in tumor formation, motility and metastasis. EpCAM-expressing cells make homophilic interactions, forming aggregated clusters, which may be important in the structure of a tumor, but may also impede invasion and metastasis. In contrast, EpCAM inhibits E-cadherin mediated cell-cell interactions, and therefore, in this manner, may promote cell motility and metastasis.Citation15 It is likely that these contrasting functions may be induced differentially depending on the TME, and there is little evidence to ascertain how these dynamics affect ovarian cancer.
Folate receptor-α
Folate receptor-α (FRα) is a 38 kDa glycosylphosphatidylinositol (GPI)-anchored membrane protein, which can transport folate via receptor-mediated endocytosis. It is overexpressed in 80–90% of EOCs.Citation16 The level of FRα expression on cancer cells, has been shown to correlate with the histologic grade and stage of ovarian cancer.Citation17-19 Overexpression is significantly higher in serous ovarian carcinoma than in mucinous carcinomas and other epithelial histotypes. FRα expression is thus considered a marker of tumor aggressiveness. Although there is conflicting data reported in the literature, when all histotypes of ovarian cancer are studiedCitation18 elevated tumor expression of FRα is associated with lower disease-free interval (DFI) and poor OS in patients with serous ovarian carcinoma.Citation19
A role for FRα in tumor cell survival and progression is demonstrated by the preservation of FRα expression on recurrent tumors and metastatic foci.Citation18 It is thought that over-expression of FRα by cancer cells may support tumor progression by modulating folate uptake and regulatory signals, resulting in increased proliferation of malignant cells through enhanced DNA synthesis.Citation20 Knockdown of FRα expression on the SKOV-3 ovarian carcinoma cells resulted in inhibition of folate-mediated cell proliferation, migration and invasion.Citation17 FRα may also facilitate chemo-resistance in ovarian carcinoma.Citation19-21 Higher tissue FRα expression correlates with poor chemotherapeutic response in patients with serous ovarian carcinoma. Lower levels of apoptosis in vitro were induced by cisplatin, paclitaxel or topotecan treatment of OVACR-3 cells expressing high levels of FRα, compared to low-expressing control cells. The mechanism of this apoptotic escape is thought to be via FRα-mediated down-regulation of caspase 3 and 7 apoptosis pathways, increased expression of the anti-apoptotic molecule, Bcl-2 and decreased expression of the pro-apoptotic molecule, Bax.Citation19 Furthermore, inhibition of the tumor suppressor gene, caveolin-1, by FRα is thought to support ovarian carcinoma progression.Citation20 In vitro studies using IGROV1 and SKOV3 ovarian cancer cells demonstrated that FRα expression levels correlate with the downregulation of caveolin-1 expression, allowing tumor-progression by promoting cell cycle progression and anchorage-independent growth; functions which are normally inhibited by caveolin-1.Citation22
FRα is considered to be a promising target for anti-cancer therapiesCitation23, since it is highly expressed on carcinoma cells, but has limited expression on normal tissues; expression is found only at low levels on the retina, placenta, intestine and choroid plexus, at the luminal surface of the kidney and lung, which are not accessible to the bloodstream.Citation24 Furthermore, it has been reported that patients with ovarian cancer demonstrate increased immunity to FRα compared to healthy controls, suggesting that this is a sensible target for immunotherapies.Citation25
Mesothelin
Mesothelin is a 40 kDa immunogenic cell surface protein that is highly expressed, particularly in non-mucinous subtypes of ovarian cancer cells, with normal tissue expression restricted to mesothelial cells.Citation26 As described above, an interaction between CA125 on tumor cells and mesothelin in the peritoneum may be involved in the metastatic process. Furthermore, co-expression of mesothelin and CA125 on tumor cells is thought to lead to clustering of tumor cells at metastatic sites, as CA125-expressing cells have demonstrated significantly higher homotypic adhesion interactions compared to CA125-negative tumor cells.Citation7 Such multicellular spheroids have been observed in the ascitic fluid of patients with ovarian cancer, and these have been shown to adhere to human mesothelial cell layers. This suggests a role for CA125-mesothelin mediated cell clusters in peritoneal dissemination of disease.Citation27 Although adhesion is partly reduced by β-1 integrin inhibition, molecules such as mesothelin are also likely to be important.Citation7
Consistent with its proposed role in tumor metastasis, high expression of mesothelin in ovarian cancer has been associated with more advanced disease, and worse progression-free survival (PFS) and OS.Citation28 Additionally, high levels of mesothelin expression correlate with resistance to chemotherapy.Citation28
Epidermal growth factor receptor family
The epidermal growth factor receptor (EGFR) family comprises 4 structurally-related tyrosine kinase receptors expressed on the apical surface of epithelial cells; ErbB1/HER1 (commonly known as EGFR or HER1), ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4. Upon ligand binding, receptor dimerization occurs, followed by tyrosine auto-phosphorylation and subsequent activation of EGFR signaling. Downstream signaling is known to augment cellular responses, including cancer cell proliferation, motility, invasion, and survival.Citation29
HER1 is the most widely studied EGFR family member involved in ovarian cancer. Increased HER1 expression is an early event in ovarian cancer development, and thus may be involved in neoplasia.Citation29 HER1 amplification analysis in tissue microarrays showed that HER1, but not HER2, expression was greater in higher grade ovarian carcinomas.Citation30 EGFR pathway activation is also involved in tumor growth and survival. Stimulation by HER1 ligands has been demonstrated in vitro by inhibition of ovarian tumor cell growth upon HER1 activation blockadeCitation31 and in vivo by the ablation of ovarian tumor cell xenograft growth when mice are depleted of EGF.Citation32 HER1 may support cancer cell invasion and migration through the regulation of cell adhesion proteins.Citation33,34 When HER1 expression was decreased in OVCAR-8 ovarian cancer cells, a selective decrease in cell clustering and adhesion to laminin-1 was observed. Cells with inhibited HER1 expression also lost MMP-9 activity and had impaired migratory capacity.Citation33 Depleted HER1 expression resulted in decreased E-cadherin expression by tumor cells.Citation34 Furthermore, overexpression of HER1 by tumor cells is associated with resistance to hormone therapies, chemotherapy and radiotherapy.Citation29,35-37 One proposed mechanism for this tumor cell escape is that HER1 activation leads to the up-regulation of the apoptosis inhibitor protein, survivin, which is found at high levels in many tumor types.Citation35
Since members of the EGFR family are overexpressed on many solid tumors, including ovarian carcinomas, and expression is associated with poorer patient outcomes,Citation37 they present promising targets for therapy. Specifically, HER1 is expressed in 25–50% of ovarian cancersCitation21 and HER2 expression is thought to range from 5 to 66%, but expression levels are low in peripheral normal tissues.
PDGFRα
Platelet-derived growth factor receptor-α (PDGFRα) is a 170 kDa member of the class III receptor tyrosine kinase family. Upon binding of platelet-derived growth factor (PDGF), receptor dimerization occurs, leading to downstream events including stimulation of tumor cell growth and inhibition of apoptosis, and regulation of stroma and angiogenesis.Citation38
Elevated PDGF and PDGFRα have been detected in ovarian cancer tissue across serous, mucinous and endometrioid histologies and different stage of disease.Citation38-40 In ovarian cancer cell cultures, an autocrine mechanism of PDGF/PDGFRα-mediated tumor cell growth was identified.Citation41 Furthermore, associations between prognosis and tumor cell PDGFRα expression have also been observed.Citation39,40
NaPi2b
NaPi2b (SLC34A2) is a multi-transmembrane, sodium-dependent phosphate transporter (also termed the TAA, MX35). This TAA is expressed in ∼90% of ovarian cancers; overexpression of NaPi2b was detected in specimens from all histological types of EOC, but not mucinous tumors. Expression is restricted in normal tissues, making NaPi2b a potential target of immunotherapeutic and diagnostic approaches for EOC. In addition, NaPi2b levels tend to be higher in well-differentiated EOC, suggesting that expression may be a marker of low grade tumors and better prognosis.Citation42
EFNA4
Ephrin-A4 (EFNA4), a member of the Ephrin (Eph) family of receptors, is a tumor antigen that is overexpressed by a number of different tumors, including primarily triple negative breast cancers and ovarian cancers. Recently EFNA4 has also been identified as a novel tumor-initiating cell (TIC)-associated target.Citation43 TICs are a subpopulation of tumor cells that drive tumor growth, resistance to treatment, and disease recurrence. These characteristics could render Ephrin-A4 a promising target for antibody therapies.
Tumor-promoting molecules
Tumor promoting molecules are secreted by cancer cells in order to support tumor growth and suppress or subvert anti-tumoral immune responses (). These have also become targets for antibody therapeutic strategies. Two key examples are discussed here.
Figure 1. Schematic representation of the monoclonal antibody targets for ovarian cancer. Antibodies for treatment of ovarian cancer can be targeted against tumor-associated antigens, tumor-promoting molecules produced and secreted in tumor environments, or against immune checkpoint molecules to engender immune cell activation and overcome immune tolerance supported by tumors.
VEGF
Vascular endothelial growth factor (VEGF) is a pro-angiogenic cytokine that binds specific tyrosine kinase receptors (VEGFRs). This leads to the activation of downstream pathways that enhance endothelial cell proliferation and survival, increase migration and invasion of endothelial cells, increase permeability of existing vessels, and enhance homing of vascular precursor cells from the bone marrow. All of these aid the formation of new blood vessels; a critical part of the survival, proliferation, invasion and metastasis of cancer cells.Citation44 VEGF can also have autocrine effects on tumor cells, directly influencing their survival, migration and invasion, and immune suppression.Citation44 VEGF and VEGFR, both expressed in EOC, form an autocrine loop that protects EOC cells from apoptosis in anchorage free growth conditions (anoikis). In vitro studies, using a number of ovarian carcinoma cell lines, have demonstrated that blockade of VEGF-VEGFR engagement results in an abolishment of receptor auto-phosphorylation and subsequent increases in apoptosis of single-cell tumor cultures.Citation45 These conditions reflect those of tumor cells in ascitic fluid, implying a role for VEGF in ascites development.
Furthermore, secretion of VEGF by tumor cells is thought to create an immunosuppressive microenvironment, where maturation of dendritic cells is ablated, and T cell responses are inhibited.Citation46 VEGF may also influence homing of bone marrow-derived haematopoietic progenitors to ‘prepare’ an organ for metastasis.Citation44 In mice, VEGF mediates the homing of VEGFR-positive haematopoietic precursor cells to form clusters at tumor-specific pre-metastatic sites before the migration of tumor cells.Citation47
In ovarian cancer, VEGF overexpression correlates with increased microvascular density, advanced stage of disease, disease recurrence and decreased survival.Citation48 VEGF serum levels also correlate with advanced disease, poorly-differentiated ovarian cancer, the presence of ascites and metastases, and poor patient survival.Citation49,50 Therefore, it is suggested that VEGF is associated with more aggressive clinical behavior in ovarian cancer, and measurement of VEGF in the serum is a proposed prognostic biomarker in all stages of ovarian carcinoma.Citation50,51
IL-6
IL-6 is a 20 kDa cytokine that is involved in normal follicle development in the ovary.Citation52 However, elevated IL-6 concentrations have been measured in the serum and ascitic fluid of patients with ovarian cancer, and are thought to correlate with volume of disease. Furthermore, high serum levels of IL-6 correlate with shorter survival and are associated with resistance to chemotherapy.Citation53,54
Elevated IL-6 may support tumor growth by a number of mechanisms. Although this cytokine does not appear to directly promote tumor cell proliferation, it may influence the migration and invasion of ovarian cancer cells.Citation52,54-56 When NOM1 and SKOV ovarian cancer cells were cultured with IL-6, they showed enhanced chemotactic and chemokinetic activity, as well as increased invasiveness.Citation56 Invasion of tumor cells is often mediated by the action of extracellular matrix (ECM)-degrading proteinases, such as metalloproteinases. Incubation with IL-6 blocking antibodies resulted in a decrease in the secretion of MMP-9 and MMP-2 by SKOV3 cells in vitro, suggesting that IL-6 acts in an autocrine manner on ovarian carcinoma cells to up-regulate tumorigenic mediators.Citation52
Angiogenesis is also enhanced by IL-6. Endothelial cells in both normal ovary and carcinoma specimens have been shown to express functional IL-6 receptors, and thus IL-6 secreted by ovarian carcinoma cells is thought to act on the microvascular endothelium, to trigger a significant angiogenic response. IL-6 enhanced endothelial cell migration in vitro and the degree of angiogenesis in BALB/c mice; however there is mixed evidence for whether this process is dependent on IL-6-mediated VEGF upregulation.Citation57
IL-6 acts through its hexameric receptor, which contains the common cytokine receptor signal-transducing subunit, gp130. When IL-6 binds, it triggers signal transduction pathways such as JAK/STAT, Ras/MEK/Erk and PI3K/Akt.Citation58,59 These pathways have been implicated in the chemo-resistance of ovarian cancer.Citation60
Taken together, IL-6 has a multi-faceted role in the progression of ovarian cancer, and is thus considered to be a potential target for therapy.
MIF
Macrophage migration-inhibitory factor (MIF) is a cytokine secreted by granulosa cells that is found in follicular fluids of normal ovarian tissue, but it has critical roles in malignancy, e.g., increasing angiogenesis and tumor cell migration, suppressing p53 activity and activating cyclin D1 and E2F transcription factors. MIF is also involved in tumor immune escape: MIF has been shown to down-regulate natural killer group 2D receptor (NKG2D), an activating receptor on NK cells and a co-stimulatory receptor on CD8+ T cells, leading to inhibition of anti-tumoral NK and CD8+ T cell cytotoxicity.Citation61
In ovarian cancer tissue, MIF is strongly overexpressed and significantly higher levels of MIF mRNA have been measured in ovarian carcinoma tissue compared to normal ovarian tissue. Furthermore, elevated MIF protein levels were detected in ascitic fluids from ovarian cancer patients compared to non-malignant ascites and MIF expression levels were significantly higher in patients who had developed ascites compared to those that had not.Citation61 Elevated MIF is also detectable in the serum of ovarian carcinoma patients.Citation62 Therefore, it has been suggested that MIF may be associated with poor prognosis in ovarian cancer, and may be a promising target for ovarian cancer therapy.
Immune checkpoint molecules
A number of molecules or receptors that are expressed by immune cells have roles in either suppressing or activating their functions (). By specifically targeting these checkpoint molecules, novel therapies aim to upregulate anti-tumor immune responses, down-regulate pro-tumor functions or depress regulator pathways utilized by tumor cells to subvert anti-tumor responses.
Natural immune responses have been observed in ovarian cancer. Infiltration of immune cells into ovarian tumors was first observed over 30 y ago, and since then an association between T cell infiltration and improved survival in ovarian cancer has been established.Citation3,63-66 However, ovarian tumor cells take advantage of a number of mechanisms to evade or suppress this anti-tumor immunity. Targeting checkpoint molecules has shown clinical benefits in other solid tumors such as melanoma and some types of lung carcinomas, prompting studies to test this strategy in ovarian cancer.
PD-1 and PD-L1
The programmed cell death 1 (PD-1) co-inhibitory receptor is expressed on activated T cells, and binding of its ligands, PD-L1 and PD-L2, regulates T cell proliferation and immune repsonses.Citation21 Ovarian cells are thought to evade anti-tumor immune responses by expressing PD-L1, and thus suppressing T cell activation.Citation67 In fact, an inverse correlation has been observed between PD-L1 expression by tumor cells and the number of intra-epithelial CD8+ T lymphocytes in ovarian patient specimens, and high PD-L1 expression by ovarian carcinoma cells is associated with poor prognosis. Therefore, blockade of this receptor/ligand interaction is thought to be a promising therapeutic avenue, which may release the immune blockade to improve anti-tumor immunity. In contrast, there is little evidence to-date to support raised PD-L2 expression in ovarian tumors, with weak or no expression of PD-L2 detected in the same cohort of ovarian cancer patient tissues.Citation68
CTLA-4
A similar immunoregulatory target, CTLA-4 is a CD28:B7 immunoglobulin superfamily ligand expressed on activated lymphocytes. CD28 signals are known to stimulate T cell activation and survival, whereas the T cell-APC interaction via CTLA-4 inhibits T cell responses, and can lead to T cell arrest and inhibition of effector functions.Citation69 Although the activity of CTLA-4 as part of immune evasion in ovarian cancer progression is unclear, therapeutic approaches to prevent its immunosuppressive activity may be beneficial and are being explored.
NKG2A
NKG2A is an inhibitory receptor that controls both innate immunity (via NK cells) as well as adaptive immunity (via cytotoxic T cells). Binding of NKG2A to its ligand, HLA-E, which is expressed on tumor cells, results in inhibition of NK and cytotoxic T cells, and thus tumor cells are able to escape destruction by NKG2A+ immune cells. Ovarian cancer is a suitable indication for an NKG2A receptor inhibitor, since HLA-E is upregulated in ∼80% of tumorsCitation70 and appears to be prognostic.Citation71
DLL4
Delta-like ligand 4 (DLL4), is an activatory ligand of the Notch signaling pathway, which is an intracellular signaling system that plays a critical role in cell survival, as well as in embryonic development, tissue repair, hematopoiesis, and the maintenance of endothelial and gut epithelial stem cell homeostasis.Citation72 Alterations in the Notch pathway can therefore result in the development of malignancies. DLL4 is expressed at sites of angiogenesis, and is the specific ligand of this pathway in endothelial cells. Therefore, DLL4 blockade can result in both ineffective angiogenesis and inhibition of tumor growth.Citation73
Monoclonal antibodies for the treatment of ovarian cancer
Monoclonal antibodies have been developed as vaccines and to target tumor-associated antigens, tumor-promoting molecules and immune checkpoint molecules. Examples of such agents in ovarian cancer are detailed below. Agents that have reached Phase 3 clinical trials are summarized in . Agents approved for ovarian cancer treatment by the US Food and Drug Administration (FDA), European Medicines Agency (EMA) and UK National Institute for Health and Care Excellence (NICE) are summarized in .
Table 1. Monoclonal Antibodies for treatment of ovarian cancer
Table 2. Monoclonal antibodies approved for ovarian cancer treatment
Monoclonal antibody vaccinations
Abagovomab
Abagovomab is a murine monoclonal anti-idiotypic antibody that mimics parts of CA125. It is designed to act as an active immunogen aimed at breaking immune tolerance to the antigen. A Phase 1 study to evaluate the safety and efficacy of abagovomab in patients with recurrent EOC, individuals with detectable anti-CA125 antibodies demonstrated prolonged survival in contrast to patients who did not show a positive immune response to the vaccination. In a Phase 1b/2 trial, 68% of patients developed a specific anti-idiotypic antibody response, and cytotoxicity against CA125-positive cancer cells was detected in 27% of patients. Moreover a correlation between the detection of anti-anti-idiotypic antibodies (Ab3) and survival time was observed.Citation74
Long-term efficacy of abagovomab was evaluated in a Phase 3 study (NCT00418574) in ovarian cancer patients who were in complete clinical remission after primary surgery and platinum-based chemotherapy. Monthly maintenance injections of abagovomab were considered to be safe and induced a robust immune response; however, treatment did not prolong PFS or OS.Citation75
Oregovomab
The murine, anti-CA125 IgG1 antibody, oregovomab, readily binds antigen-presenting cells (APCs) when complexed with CA125, leading to the production of anti-CA125 antibodies, and subsequent B and T cell immune responses.Citation76 Clinical benefit of this antibody was first observed when used as a technetium-99m-labeled radiodiagnostic, and it has been evaluated in a number of Phase 2 and 3 trials.
In patients with recurrent ovarian carcinoma, oregovomab was well-tolerated, and both human anti-mouse antibody (HAMA) and anti-idotype antibody immune responses were measured.Citation77,78 Increases in T cell immune responses to CA125, autologous tumor and oregovomab were measured in 39%, 63% and 50%, respectively, of patients undergoing continued oregovomab and chemotherapy treatment.Citation77 Patients who mounted immune responses to CA125 or autologous tumor demonstrated significantly improved survival compared to those who did not. However, oregovomab monotherapy did not induce such significant clinical outcomes in Phase 2 studies in patients following initial first-line surgical and chemotherapy treatment. A subpopulation of patients with optimal surgical outcomes appeared to demonstrate a possible clinical response to oregovomab; however, these results were not repeated in a larger multi-center Phase 3 placebo-controlled trial.Citation79 This may be due to the significant reduction in circulating CA125 in these post-surgery patients, resulting in a relative depression of immune response augmented by oregovomab. Given that the most promising outcomes were observed in patients undergoing concomitant chemotherapy,Citation77 current clinical trials aim to evaluate oregovomab in combination with carboplatin and paclitaxel (NCT01616303). Oregovomab was granted US and EU orphan designations for treatment of ovarian cancer.
HMFG1
HMFG1 is a murine monoclonal IgG1 antibody that recognizes a specific epitope (PDTR) on human MUC1. The first Phase 1 clinical study in patients with EOC, who had undergone at least one regimen of platinum-based therapy, demonstrated the safety and tolerability of repeated intradermal injections of HMFG1. Furthermore, anti-idiotypic antibodies were detected in all patients after 3 vaccinations.Citation80 A large Phase 3 trial in patients with EOC who had undergone a surgically defined complete remission of the disease, was then carried out to compare yttrium-90 labeled murine HMFG1 (90Y-muHMFG1), plus standard therapy, with standard therapy alone. However, no difference in time to relapse was detected between the 2 arms of the study, and a single injection of 90Y-muHMFG1 did not improve OS.Citation81
Monoclonal antibodies targeting tumor-associated antigens
PankoMab-GEX
PankoMab-GEX is a glycoengineered humanized IgG1 mAb specifically targeting the PDTRP epitope of MUC1. The optimized glycosylation of PankoMab-GEX leads to enhanced antibody-dependent cell-mediated cytotoxicity and phagocytosis (ADCC/ADCP).Citation82 In a Phase 1 study (NCT01222624) in patients with advanced MUC1-expressing solid tumors, PankoMab-GEX was well tolerated; no maximum tolerated dose (MTD) was reached and AEs were mainly mild to moderate infusion-related reactions. Clinical benefit was observed in 47% of patients, including 1 complete response (CR) in a patient with ovarian carcinoma, and confirmed stable disease (SD) in 19 patients.Citation82 There is now an ongoing placebo-controlled Phase 2 study (NCT01899599) of PankoMab-GEX as maintenance therapy in advanced ovarian, fallopian tube or primary peritoneal cancer.
Catumaxomab
Catumaxomab is a bispecific trifunctional antibody (trAb) specific to both EpCAM and CD3, which is expressed on T cells. The unique hybrid Fc portion of the antibody is composed of mouse IgG2a and rat IgG2b and selectively binds type I, IIa and III human Fcγ receptors expressed on accessory immune cells such as macrophages, NK cells and dendritic cells,Citation83 leading to ADCC and ADCP, and activation of T-cell mediated tumor cell cytotoxicity.Citation84
In a Phase 1/2 study of catumaxomab in ovarian cancer patients with ascites containing EpCAM-positive tumor cells, significant ascites reduction was observed and minimal adverse events (AEs) occurred.Citation83 A subsequent Phase 2/3 trial comparing paracentesis plus catumaxomab with paracentesis alone showed that addition of catumaxomab significantly prolonged puncture-free survival and time to next paracentesis. Furthermore, patients given catumaxomab suffered fewer symptoms from ascites and had a positive trend in OS.Citation85 Catumaxomab has EMA and NICE approval for use in EpCAM-positive ascites treatment (see ).
chMOv18 IgG
chMOv18 IgG is a human chimeric IgG1 antibody specific for FRα, cloned from a murine MOv18 IgG antibody that was generated by immunization of mice with a surgical specimen of ovarian cancer.Citation86 Following studies showing effective tumor uptake and anti-tumor efficacy of radiolabeled murine MOv18 IgG,Citation87 radiolabeled chMOv18 IgG was shown to localize well into the tumor tissue of ovarian cancer patients.Citation88 Results of a Phase 1 trial, in which chMOv18 IgG given intravenously (i.v.) at increasing doses, indicated that the antibody was safe with no significant hematological or biochemical changes.Citation89
The route of administration for radiolabeled chMOv18 IgG was evaluated in 2 studies comparing i.v administration with locoregional delivery via intraperitoneal (i.p.) administration.Citation90,91 Radiolabeled chMOv18 IgG given either i.p or i.v. was considered to give no normal organ toxicity, with no AEs observed. One study suggested that that better accumulation of antibody occurred after locoregional i.p administration, compared to i.v.,Citation90 but the other study did not measure any advantage. Use of the i.p route of administration was recommended with respect to bone marrow toxicity, as the area under the blood activity vs. time curve (AUC) was significantly lower for the i.p. route.Citation91
Farletuzumab
Farletuzumab (MORab003) is a humanized IgG1 antibody specific for FRα. Following in vitro studies showing a number of mechanisms of anti-tumor activity,Citation92,93 a Phase 1 study, in patients with platinum-resistant EOC was carried out to establish the MTD.Citation94 The safety of farletuzumab in combination with carboplatin and pegylated liposomal doxorubicin in patients with platinum-sensitive EOC was then demonstrated in a Phase 1b study.Citation95
Promising overall response rates were observed in a Phase 2 study in patients with platinum-sensitive ovarian cancer given farletuzumab/carboplatin/taxane combination therapy, followed by farletuzumab maintenance therapy. Furthermore, farletuzumab did not lead to additive toxicity when combined with chemotherapy.Citation96 Subsequently, a Phase 3 trial was carried out in platinum-sensitive patients with recurrent ovarian cancer compared the carboplatin/taxane treatment alone with the addition of farletuzumab. Here, improved PFS was observed in patients with lower CA125.Citation97 A second Phase 3 study in patients with platinum-resistant ovarian cancer compared paclitaxel alone, or in combination with farletuzumab (NCT00738699); however this trial was discontinued when the pre-specified criteria for continuation were not met.
The manufacturer of farletuzumab has since announced the development of a diagnostic assay to identify patients with high FRα expression, suggesting that farletuzumab trials in the future may stratify patients according to tumor target expression.Citation98 The suggestion that farletuzumab may be most beneficial in patients with low CA125 serum levels is being addressed in a Phase 2 study comparing farletuzumab, combined with carboplatin and paclitaxel, or with carboplatin and pegylated liposomal doxorubicin (NCT02289950).
Amatuximab
Amatuximab (MORAb-009) is a high-affinity chimeric IgG1 mAb targeting mesothelin. Following promising preclinical studies,Citation99 a Phase 1 study of amatuximab in 24 patients with treatment-refractory, mesothelin-expressing solid tumors was initiated. In this study, amatuximab was well tolerated, an MTD of 200 mg/ml was determined and 11 patients experienced SD, with no observed objective responses. The most common AE was drug hypersensitivity consisting of infusion reactions/cytokine release syndrome and allergic reactions.Citation100 To date, no Phase 2 trials in ovarian cancer have been planned for this antibody.
Cetuximab
Cetuximab is a chimeric IgG1 antibody specific for the extracellular domain of EGFR (i.e., HER1) that is approved for use in colorectal and head and neck cancers. Upon binding, the antibody prevents signaling via EGFR, and accelerates internalization of the receptor.Citation101 Cetuximab demonstrated anti-tumor activity and augmentation of the efficacy of chemotherapies such as cisplatin, doxorubicin, paclitaxel, and topotecan both in vitro studies and in human xenograft animal models.Citation102 In a Phase 2 trial of cetuximab as a single agent in patients with persistent/recurrent ovarian cancer, minimal clinical benefit was observed, with 9 patients achieving SD and only 1 patient demonstrating a partial response (PR).Citation103 Furthermore, in a Phase 2 study of cetuximab, combined with paclitaxel and carboplatin, in patients with stage III/IV ovarian cancer, this combination therapy was adequately tolerated, but did not demonstrate prolonged PFS compared to historical data.Citation104 More promising results were observed in another Phase 2 study, where cetuximab was combined with carboplatin in platinum-sensitive ovarian cancer patients. Here 9 of 29 patients had an objective response and 8 patients had SD. As previously observed, cetuximab therapy was associated with an acneiform rash in the majority of patients, and occasional serious hypersensitivity reactions were observed.Citation105
Although meaningful efficacy data is lacking for the use of cetuximab in ovarian cancer, future developments may include the use of pharmacogenetic data (such as K-RAS mutation status) to select appropriate patients for treatment, as has been carried out for colorectal cancer, or cetuximab may be used in combination VEGF inhibitors such as bevacizumab.Citation103,104
Panitumumab
Panitumumab is a human recombinant IgG2 mAb approved for use in metastatic colorectal cancer.Citation106 It binds to the extracellular domain of EGFR/HER1, preventing its activation.Citation107 In a Phase 2 study, platinum-resistant, K-RAS wild-type, EOC patients were given panitumumab in combination with pegylated liposomal doxorubicin. Overall response as determined by CA125 levels was observed in 18.6% of the intent-to-treat population. Using RECIST evaluation, 9.3% of patients had a PR, 18.6% had SD and 39.5% progressed on treatment. The most common treatment-related grade 3 toxicities were skin toxicity, fatigue and vomiting, and skin toxicities were the major reason for dose reductions. Overall, although a moderate response rate was observed, the occurrence of skin toxicities was considered to have a substantial effect on the quality-of-life of patients.Citation108 The K-RAS mutation status of patients may be a useful molecular marker for selection of patients to treat with panitumumab.
Panitumumab in combination with everolimus and bevacizumab was also evaluated in a Phase 1 study in patients with advanced solid tumors.Citation107 Three of the 32 subjects had ovarian cancer, and these individuals showed prolonged disease control ranging from 11 to >40 months. The recommended combination dose regimen showed acceptable safety and tolerability and moderate clinical activity, particularly in gynecological cancers.Citation107 More recently, a Phase 2 trial of panitumumab combined with gemcitabine in patients with relapsed ovarian cancer was terminated because newer technologies are available and the study had poor accrual (NCT01296035).
Matuzumab
Matuzumab (EMD72000) is a humanized IgG1 mAb that binds to EGFR/HER1 at the ligand-binding region. It is thought that matuzumab acts by inhibiting EGFR binding and signaling, and by triggering ADCC against EGFR-expressing tumor cells.Citation109 Results of a Phase 1 studies in colon cancer patients showed matuzumab was well tolerated, with the predominant toxicities being those of the skin.Citation110 A Phase 2 trial in patients with recurrent, EGFR-positive ovarian cancer demonstrated that the therapy was reasonably well tolerated, although no formal responses of matuzumab efficacy were observed.Citation109
Trastuzumab
Trastuzumab is a humanized IgG1 antibody that binds to the extracellular domain IV of HER2, preventing activation of the intracellular tyrosine kinase, and thus inhibiting proliferation of cells overexpressing HER2.Citation111 It is approved for breast cancer and adenocarcinoma of the stomach. On the basis of the observed safety of trastuzumab in breast cancer, a single-arm Phase 2 trial of trastuzumab was carried out in persistent or recurrent ovarian cancer patients selected on the basis of high levels of HER2 tumor expression. Only a 7.3% response rate and 39% disease stabilization rate was observed in these patients. However, several patients that responded or showed SD continued to receive trastuzumab therapy for more than 1 year, demonstrating the overall tolerability of treatment; reported drug related toxicities tended to be mild and not dose-limiting. Furthermore, data from this study suggested that there was no correlation between the level of HER2 expression and response to trastuzumab treatment.Citation111 Another Phase 2 study of trastuzumab combined with a carboplatin-paclitaxel regimen was also initiated in patients with ovarian cancer that had previously relapsed within 6 months of carboplatin-paclitaxel treatment and had high levels of tumor HER2 expression. This trial has since been terminated with no reason reported (NCT00189579).
A Phase 3 trial (NCT00011986) of trastuzumab in triplet or doublet treatment combinations with paclitaxel and carboplatin in patients with EOC has been reported as complete, although results have not yet been published. A number of other Phase 1 studies evaluating trastuzumab in combined with interleukin-12 have also been carried out, with results yet to be reported (NCT00028535 and NCT00004074).
Pertuzumab
Pertuzumab (rhuMAb 2C4) is an IgG1 humanized mAb that binds to the extracellular domain II of HER2, preventing hetero-dimerization with HER1 or HER3. It is approved for use in breast cancer treatment,Citation112 but has shown efficacy against ovarian cancer cells in vitro.Citation113-116 In the Phase 1 study in patients with advanced solid tumors, pertuzumab was well tolerated, and 2 of the 21 patients, including one with ovarian cancer, achieved a PR.Citation117 In a Phase 2 trial, pertuzumab alone was evaluated in platinum-resistant ovarian cancer. Overall, 14.5% of participants were considered to have achieved any clinical benefit.Citation118 In a larger Phase 2 trial, pertuzumab combined with gemcitabine was compared to gemcitabine plus placebo in platinum-resistant patients. The objective response rate was 13.8% in patients receiving the combination, compared to 4.6% in those receiving gemcitabine and placebo. This increased benefit of pertuzumab was shown to occur more in patients with lower HER3 mRNA expression, whereas patients with high HER3 tumor expression were less likely to benefit from pertuzumab addition to treatment. In this study, an increased incidence of grade 3 and 4 neutropenia, thrombocytopenia, back pain and diarrhea were observed in the combination arm.Citation115 The efficacy of pertuzumab was also evaluated in a Phase 2 trial in combination with carboplatin and paclitaxel or gemcitabine in platinum-sensitive patients; however no significant differences in PFS or other secondary endpoints were observed between the cohorts.Citation119
Although HER2 expression by ovarian cancer and breast cancer cells is comparable, efficacy of pertuzumab in ovarian cancer is not equivalent to that seen in treating breast cancer.Citation112 This may be dependent on HER3 expressionCitation115 or HER2 activation.Citation114 Therefore, future pertuzumab studies are likely to include stratification of patients using such biomarkers. There is an ongoing Phase 3 study combining pertuzumab and standard chemotherapy in platinum-resistant ovarian cancers with low HER3 mRNA expression (NCT01684878), and another Phase 2 trial evaluating HER2 activation on pertuzumab efficacy in advanced ovarian cancer has just been completed, with results yet to be reported (NCT00058552).
Seribantumab
Seribantumab (MM-121, SAR256212) is a human HER3-targeting IgG2 mAb that prevents the dimerization of HER2 and HER3 and inactivates downstream signaling.Citation120 This antibody has recently been evaluated in combination with paclitaxel in a randomized Phase 2 study of platinum resistant advanced ovarian and fallopian tube cancer (NCT01447706). Most reported AEs were mild to moderate; however PFS was not prolonged by combination of seribantumab with paclitaxel.Citation121
Olaratumab
Olaratumab (IMC-3G3), a human IgG1 mAb that selectively binds PDGFRα, prevents ligand bindingCitation122 and significantly enhances the efficacy of chemotherapy against ovarian tumor cells in vitro and in vivo.Citation123 In a Phase 1 study in patients with solid tumors olaratumab was well tolerated and no DLTS were observed so the MTD was not identified. No CR or PR was measured, but SD was measured in 12 of the 19 evaluable patients and RP2Ds of 16 mg/kg weekly and 20 mg/kg biweekly were determined. Citation122 A Phase 2 study (NCT00913835) evaluating the efficacy of PLD, with or without olaratumab, in platinum-refractory or resistant advanced ovarian cancer has been conducted and results are awaited.
Monoclonal antibodies targeting tumor-promoting molecules
Bevacizumab
Bevacizumab, a recombinant humanized monoclonal IgG1 antibody specific to all biologically active forms of VEGF-A, has demonstrated anti-angiogenic activity and inhibition of ovarian cancer progression in in vitro studies and animal models.Citation124 In a Phase 2 study in patients with recurrent EOC and primary peritoneal cancer (PPC), 3% and 18% of patients showed CR and PR, respectively, and 40% of patients showed at least 6 months PFS following treatment.Citation125
A number of Phase 3 trials of bevacizumab have been completed. In patients with EOC, PPC and fallopian tube cancer, paclitaxel/carboplatin treatment combined with placebo was compared to concurrent bevacizumab, and concurrent plus maintenance bevacizumab. Although concurrent bevacizumab treatment showed no improvement in PFS compared to the chemotherapy arm, patients who also received concurrent plus maintenance bevacizumab had an increased PFS of 3.8 months. However, no difference in OS was observed. Bevacizumab treatment was well tolerated, with the major AEs attributed to chemotherapy.Citation126 In another Phase 3 trial, patients with EOC were treated with carboplatin and paclitaxel alone or in combination with concurrent bevacizumab. Here, improved PFS was observed in those receiving chemotherapy combined with bevacizumab.Citation127 The efficacy of bevacizumab in combination with chemotherapy has also been studied in a Phase 3 trial in patients with platinum-resistant ovarian cancer. Patients were treated with paclitaxel, pegylated liposomal doxorubicin (PLD) or topotecan alone, or combined with bevacizumab. Those receiving bevacizumab in combination with chemotherapy showed significantly improved PFS.Citation128 The observed improvements in these studies may not only be due to the anti-angiogenic effect of bevacizumab, but may also be attributed to bevacizumab–related normalization of tumor vasculature, which may lead to enhanced delivery of chemotherapeutic drugs. Bevacizumab is approved for ovarian cancer treatment by the FDA, EMA and NICE (see ).
Volociximab
Volociximab is a first-in-class chimeric IgG4 mAb that targets human α5β1 integrin with high affinity. Preclinical studies of this antibody, which showed inhibition of VEGF- and bFGF-induced neovascularization, associated reduction in tumor burden, ascites and number of metastases, and increased survival in animal models of ovarian cancer,Citation129 were followed by a number of clinical trials. In a Phase 1 study, no DLTs were observed and dose reduction was not required. Of the 20 patients evaluable for efficacy, 1 patient with renal cell carcinoma had a minor response and 5 others had durable SD. No CRs or PRs were observed.Citation130 Subsequently, a multicenter Phase 2 single-arm, 2-stage study in platinum-resistant advanced EOC or primary peritoneal cancer was carried out. Of the 14 patients evaluable for efficacy, 1 had SD at 8 weeks, but the remaining 13 patients all demonstrated PD. This lack of efficacy may have been caused by the chemotherapy-resistant study population. In addition, 75% of patients experienced AEs, primarily headache and fatigue.Citation131 In a second Phase 2 study, patients with EOC were stratified by platinum sensitivity and treated with PLD or volociximab alone, or the combination.Citation132 AEs and response rates were similar across treatment groups.Citation132
Siltuximab
Siltuximab (CNTO 328, cCLB8) is a chimeric mAb that binds to IL-6, neutralizing its effects in a number of human malignancies including ovarian cancer. Following in vitro analyses showing that the intensity of IL-6 immunohistochemistry (IHC) staining of ovarian cancer tissues was associated with poor patient prognosis, and that siltuximab had tumor growth efficacy, a single arm, Phase 2 study of siltuximab in patients with recurrent, platinum-resistant ovarian cancer was conducted. Although the response rate to siltuximab, the primary endpoint of the study, was modest, prolonged periods of disease stabilization were observed. Median OS was similar to that of large Phase 2 trials of conventional chemotherapy in platinum-resistant ovarian cancer.Citation133 Plasma cytokine/chemokine levels were also almost unchanged following 3 siltuximab infusions; however, patients who received at least 6 months of infusions showed lower levels of IL-6-regulated chemokines, CCL2 and CXCL12, and the angiogenic molecule VEGF. Efficacy of siltuximab was also evaluated in a Phase 1/2 study in patients with advanced solid tumors, including EOC. This study was successful in demonstrating that siltuximab monotherapy is well tolerated, but it did not demonstrate clinical activity.Citation134 No additional studies have been initiated in ovarian cancer.
Imalumab
Imalumab (Bax69) is a human IgG1 mAb that targets oxidized MIF (oxMIF) to reduce tumorigenesis. Interim reports of a Phase 1 study in solid tumors (NCT01765790), report that imalumab was well tolerated and no MTD had been reached. SD was measured in 7 heavily pretreated patients and a recommended Phase 2 dose (RP2D) of weekly 10 mg/kg i.v. was determined.Citation135 A Phase 1/2a study of imalumab is now recruiting patients with ovarian carcinoma and recurrent malignant ascites (NCT02540356). In this study, imalumab monotherapy will be administered i.p. (study single-route arm) and compared to i.v. followed by i.p. infusions (double-route arm).
Monoclonal antibodies specific for immune targets
Nivolumab
Nivolumab is a human anti-PD-1 IgG4 mAb. A Phase 1 study in patients with advanced tumors demonstrated clinical responses to nivolumab. The greatest rate of response was observed in melanoma patients; of the 17 ovarian cancer patients treated, 6% had a PR and 18% had SD.Citation136 In a Phase 2 trial in platinum-resistant ovarian cancer the overall response rate to nivolumab was 15%, and the disease control rate was 45%. The median OS for the cohort was 20 months.Citation137 Strategies for patient stratification are still unclear as no significant correlation between clinical response and patient tissue PD-L1 expression was identified.Citation137
Pembrolizumab
Recently, interim results were presented from a Phase 1b study of pembrolizumab, a humanized anti-PD-1 IgG4 mAb. Of the 26 patients with PD-L1-expressing advanced ovarian cancer who were treated with pembrolizumab, 1 patient had a CR, 2 achieved PR, and 6 had SD, resulting in a disease control rate of 34.6%.Citation138
BMS-936559
A human anti-PD-L1 IgG4 mAb, BMS-936559, has been evaluated in a Phase 1 study that included patients with ovarian cancer. In this study, 6% of patients receiving the MTD of 10 mg/kg achieved PR and 18% had SD lasting more than 24 weeks.Citation139
Avelumab
A human IgG1 mAb targeting PD-L1, avelumab (also known as MSB0010718C) is unlike other PD-L1 targeting mAbs as it is able to mediate ADCC of tumor cells (due to its functional IgG1 Fc region). In a Phase 1 study in patients with refractory cancers, including ovarian carcinoma, significant inhibition of the PD-L1 receptor on peripheral blood lymphocytes (PBLs) was measured following avelumab treatment. To-date, the data from this study are still being collected. However, preliminary results from an ongoing Phase 1b study in patients with recurrent or refractory ovarian cancer (NCT01772004) have shown that 17.4% of patients had a PR and 47.8% had SD.Citation140 A Phase 3 study to evaluate avelumab alone, compared to avelumab combined with PLD, and PLD alone, is now recruiting patients with platinum-resistant or refractory ovarian cancer (NCT02580058).
Ipilimumab
Ipilimumab, a human IgG1 targeting CTLA-4, was approved by the FDA in 2011 for treatment of metastatic or unresectable melanoma. The mAb was well tolerated in a Phase 1/2 trial in patients with stage IV ovarian carcinoma, who had previously either received chemotherapy or granulocyte-macrophage colony-stimulating factor modified irradiated autologous tumor cells (GVAX).Citation141,142 One ovarian cancer patient had a significant decrease in serum CA125 levels and tumor regression, and an additional 5 patients showed stabilization of CA125 levels.Citation142 Furthermore, the therapeutic effects of ipilimumab were associated with anti-NY-ESO-1 antibody responses. Tumor regression also correlated with the CD8+/Treg ratio, suggesting that other Treg-depleting therapies may be combined with ipilimumab for a synergistic effect. Another Phase 2 study (NCT01611558) of ipilimumab monotherapy in patients with platinum-sensitive ovarian cancer is ongoing.
Tremelimumab
Tremelimumab is a human IgG2 mAb that targets CTLA-4. An ongoing Phase 1/2 trial (NCT02571725) is evaluating the combination of tremelimumab with the PARP-inhibitor olaparib in patients with BRCA-deficient recurrent ovarian cancer. Phase 1 studies (NCT02261220, NCT01975831) of tremelimumab combined a PD-1 inhibitor, MEDI4736, in patients with solid tumors including ovarian cancer are also now recruiting.Citation143
Monalizumab
Monalizumab (IPH2201) is a humanized IgG4 mAb that blocks the binding of NKG2A to HLA-E, allowing activation of NK and cytotoxic T cell responses. IPH2201 may also enhance the cytotoxic potential of other therapeutic antibodies. In a Phase 1 dose-escalation study, monalizumab was safe and well-tolerated.Citation144 It is currently under evaluation in a Phase 1/2 trial (NCT02459301) of platinum-resistant or sensitive patients with high grade ovarian cancer.
Demcizumab
Demcizumab (OMP-21M18) is a humanized IgG2 mAb targeting DLL4. The Phase 1 study of demcizumab demonstrated initial single-agent activity, including early evidence of durable anti-tumor responses. This study included one patient with an ovarian granulosa cell carcinoma who demonstrated SD as best response, and remained on treatment for 17 months.Citation145 Overall, therapy was generally well tolerated; however, the prolonged exposure to demcizumab, as with the patient with ovarian granulosa cell carcinoma, resulted in delayed cardiac toxicity and tumor-associated bleeding consistent with dysfunctional angiogenesis, necessitating termination of the study.Citation145 A Phase 1b/2 study (NCT01952249) of demcizumab, plus paclitaxel, is now recruiting patients with platinum-resistant ovarian, primary peritoneal or fallopian tube cancer.
Antibody-drug conjugates
An antibody-drug conjugate (ADCs) directs the potent activity of a small molecule cytotoxic agent to the appropriate tumor cells by covalently attaching the agent to a tumor antigen-specific mAb. Most cytotoxic agents used as payloads in ADCs are potent microtubule inhibitors or DNA damaging agents, and do not have a therapeutic index when administered as free drugs.Citation146
DMUC4064A
DMUC4064A is an ADC composed of a CA125-targeting humanized IgG1 conjugated to an anti-mitotic agent, MMAE. A Phase 1 study (NCT02146313) of DMUC4064A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer is ongoing.
IMGN853
IMGN853 (mirvetuximab soravtansine) is a FRα-targeting ADC composed of a humanized FRα-binding antibody attached to a highly potent maytansinoid, DM4, that induces cell-cycle arrest and cell death by targeting microtubules.Citation147 A Phase 1 study in patients with EOC or other FRα-positive solid tumors, demonstrated encouraging clinical activity in heavily pretreated patients, with a manageable AE profile.Citation148 Clinical benefit (PR or CR, CA125 response, SD ≥ 6 cycles) was observed in 11/44 patients.Citation148 In another trial, the RP2D showed clinical benefit in 5/10 evaluable patients with platinum-resistant EOC. A Phase 1b study of IMGN853 administered with bevacizumab, carboplatin, or PLD, in patients with FRα-positive advanced EOC, primary peritoneal cancer, fallopian tube cancer or endometrial cancer is open to recruitment (NCT02606305). Furthermore, a Phase 2 study (NCT02631876) is recruiting patients with FRα-positive advanced EOC, primary peritoneal cancer or fallopian tube cancer to compare IMGN853 with the investigator's choice of chemotherapy.
DNIB0600A
DNIB0600A (lifastuzumab vedotin) is a humanized IgG1 anti-NaPi2b mAb conjugated to an anti-mitotic agent, MMAE, that has shown anti-proliferative activity in xenograft models.Citation149 In a Phase 1 study of DNIB0600A in patients with non-small cell lung cancer or platinum-resistant ovarian cancer, archival tumor NaPi2b expression was evaluated by IHC.Citation150 At the recommended Phase 2 dose, 41% of IHC 2/3+ patients with ovarian cancer had confirmed PRs. No patient with an IHC score of 0 showed a clinical response.Citation151 DNIB0600A in combination with carboplatin, and with or without bevacizumab is now being evaluated in a Phase 1b study (NCT01995188) in patients with platinum-sensitive ovarian cancer. A Phase 2 randomized trial (NCT01991210) of DNIB0600A, in comparison to PLD, in patients with platinum-resistant ovarian cancer is also ongoing.
PF-06647263
PF-06647263 is a first-in-class, humanized IgG2 mAb (huE22) specific for EFNA4, and linked to calicheamicin, a DNA-damaging cytotoxic agent.Citation43 Initial results from a Phase 1 study in patients with advanced solid tumors unselected for EFNA-4 expression show a DLT in only 1 patient, and 2 patients (1 with ovarian cancer and one with triple negative breast cancer) have experienced a PR.Citation151
SC-003
SC-003 is an ADC composed of a proprietary TAA-specific mAb conjugated to an undisclosed cytotoxic agent with unpublished mechanism of action.Citation152 A Phase 1 study (NCT02539719) of SC-003 is now recruiting patients with platinum-resistant, refractory ovarian cancer.
New directions for ovarian cancer immunotherapy
IMAB027
A proportion of advanced stage ovarian cancers express high levels of Claudin 6 (CLDN6), a carcino-embryonic transmembrane protein that is not found on normal adult human tissue. IMAB027 is a mAb that binds to CLDN6. Pre-clinically, this antibody was shown to inhibit tumor growth and to kill cancer cells by ADCC and complement-dependent cytotoxicity (CDC). A Phase 1/2 trial (NCT02054351) of IMAB027 in patients with recurrent advanced ovarian cancer is currently underway. Early data suggests that IMAB027 was well tolerated, with no DLTs observed, and the MTD was not reached.Citation153
MOv18 IgE
MOv18 IgE is a FRα-specific chimeric IgE antibody engineered from MOv18 IgG1.Citation154 Following trial results of farletuzumab and chMOv18 IgG1, where FRα was shown to be a promising target and chMOv18 IgG1 was well tolerated (described above), MOv18 IgE was developed to investigate the hypothesis that antibodies engineered with Fc regions of the IgE class may offer advantages over their IgG counterparts.Citation155 MOv18 IgE afforded superior protection compared with its IgG1 counterpart in a number of in vivo models of ovarian cancer,Citation156,157 and is now undergoing evaluation in a Phase 1 clinical study (NCT02546921).
Conclusion and future perspectives in antibody design
Improved understanding of the contribution of tumor-associated molecules to ovarian cancer growth and spread has helped identify known and novel targets for therapy. Alongside novel emerging targets involved in immune regulation, these insights are now yielding a range of different treatment approaches involving monoclonal antibodies.
One area likely to receive increasing attention may be antibody engineering strategies aimed at enhancing Fc-mediated antibody mechanisms of action and bioavailability.Citation158 These may include: 1) engineering of antibodies or antibody fragments with specificity for more than one target (e.g., bispecific antibodies), with the aim of simultaneously recruiting immune effector cells to the tumor target; 2) engineering of differentially glycosylated Fc regions in order to enhance antibody bioavailability and augment the capacity of antibodies to trigger ADCC; or 3) mutational engineering of antibody Fc regions with the aim of improving both ADCC and CDC. Beyond antibody engineering techniques, attempts at augmenting Fc-mediated responses have been made, by strategies to increase the activation, expansion and recruitment of effector cells such as by co-administering the antibody with recombinant cytokines.
All currently approved mAbs are of the IgG isotypeCitation159 due to favorable pharmacokinetic properties and the pioneering experiments by Neuberger and colleagues, which demonstrated that IgG, and in particular IgG1, was more effective than other antibody isotypes in activating complement and promoting ADCC and CDC against tumor cells in vitro. Different IgG antibody subclasses, however, have been employed therapeutically, such as IgG1, IgG2 and IgG4, depending on the desired role of the Fc-mediated mechanisms of action. The IgG1 Fc region generally has the highest affinity of all of the IgG antibody subclasses for Fcγ receptors (FcγRs) on immune effector cells, leading to more potent effector functions.Citation160 On the other hand, IgG4 has weaker affinity for FcγRs, which translates into weaker or ineffective effector functions. Therefore, IgG4 mAbs are used therapeutically when Fc-mediated effector functions are not desirable.Citation136 Furthermore, there has been some exploration of the use of mAbs of alternate Ig classes such as IgA and IgE in cancer immunotherapy, with the aim of enhancing Fc-mediated effector functions at anatomic locations where tumors may be located, and through the engagement of cognate FcRs on immune effector cells such as FcαRI, FcεRI, and CD23.Citation154-157
Another area likely to expand will be the identification of effective combinatory treatments. Single-agent immunotherapies have produced promising clinical responses in ovarian carcinoma and other tumors. However, to achieve the maximal anti-tumor immune response, it is likely that combination therapeutic strategies will be required. For many tumors, several ‘tumor escape mechanisms’ acting in parallel allow tumors to evade detection by the immune system.Citation161 The concept behind a combinational immunotherapy strategy is to target tumor escape mechanisms at different stages, thereby creating the possibility of synergistic effects between agents and potentially benefiting a greater proportion of patients.
An example of a combination antibody immunotherapy strategy in ovarian cancer involves the combination of multiple immune checkpoint blockades. In preclinical studies, almost half of the tumor-infiltrating lymphocytes in an in vivo model of ovarian carcinoma were positive for both CTLA-4 and PD-1, and demonstrated an attenuated capacity to proliferate and produce cytokines.Citation162 Treating these mice with both anti-PD-1 and anti-CTLA-4 antibodies resulted in reversal of the tumor-infiltrating lymphocyte dysfunction, and induced tumor regression in 50% of the mice compared to 25% with either agent as a monotherapy.Citation162 A Phase 1/2 trial evaluating nivolumab and the IDO inhibitor INCB24360 in patients with ovarian cancer is currently underway (NCT02327078). Since the IDO pathway leads to the expansion of Tregs, combining an agent that enhances effector T cell function with an agent that restricts immunosuppressive elements such as myeloid-derived suppressor cell (MDSC), Tregs, and macrophage subtypes in the tumor microenvironment may be more efficacious than either agent as monotherapy. In another study, a dendritic cell-based autologous whole-tumor lysate vaccine combined with bevacizumab was evaluated in patients with ovarian cancer. Four of 6 patients demonstrated a clinical benefit, in addition to an increase in tumor-reactive T cells (as measured by IFN-γ secretion).Citation163
Although it is likely that the interest in novel combination immunotherapy studies in ovarian cancer will increase, simultaneous application of multiple immunotherapies requires careful consideration of: 1) the potential for overlapping toxicities; 2) the need to optimize the timing of administration of each agent (sequential vs. concurrent); and 3) the critical issue of patient stratification, e.g., which patients need combination strategies and what combinations are best in any given patient. A proportion of patients treated with monotherapy demonstrate prolonged responses, suggesting that some patients may not need combination immunotherapy. There is an urgent need to develop biomarkers to identify such patients to preselect them for each monotherapy in order to avoid the toxicities associated with combination immunotherapy. If all of these considerations can be managed, it is likely that combination immunotherapy, and our improved understanding of mechanisms of immune modulation in ovarian carcinoma, may dramatically improve the clinical outcomes of ovarian cancer patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge support by Cancer Research UK (C30122/A11527; C30122/A15774); the Academy of Medical Sciences; CR UK//NIHR in England/DoH for Scotland, Wales and Northern Ireland Experimental Cancer Medicine Center (C10355/A15587); the Medical Research Council (MR/L023091/1); CR UK/EPSRC/MRC/NIHR KCL/UCL Comprehensive Cancer Imaging Center (C1519/A10331). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Center based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors are solely responsible for the decision to publish, and preparation of the manuscript.
References
- Statistics S. http://seer.cancer.gov/statfacts/html/ovary.html. 2016.
- Huang L, Cronin KA, Johnson KA, Mariotto AB, Feuer EJ. Improved survival time: what can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer 2008; 112:2289-300; PMID:18393325; http://dx.doi.org/10.1002/cncr.23425
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:12529460; http://dx.doi.org/10.1056/NEJMoa020177
- Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol 2005; 99:704-13; PMID:16126266; http://dx.doi.org/10.1016/j.ygyno.2005.07.030
- Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer 2010; 9:11; PMID:20089172; http://dx.doi.org/10.1186/1476-4598-9-11
- Bouanene H, Miled A. Conflicting views on the molecular structure of the cancer antigen CA125/MUC16. Dis Markers 2010; 28:385-94; PMID:20683153; http://dx.doi.org/10.1155/2010/918457
- Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer 2006; 5:50; PMID:17067392; http://dx.doi.org/10.1186/1476-4598-5-50
- Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol 2001; 19:4054-7; PMID:11600607
- Deng J, Wang L, Chen H, Li L, Ma Y, Ni J, Li Y. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev 2013; 32:535-51; PMID:23609751; http://dx.doi.org/10.1007/s10555-013-9423-y
- Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 1995; 129:255-65; PMID:7698991; http://dx.doi.org/10.1083/jcb.129.1.255
- Rughetti A, Turchi V, Ghetti CA, Scambia G, Panici PB, Roncucci G, Mancuso S, Frati L, Nuti M. Human B-cell immune response to the polymorphic epithelial mucin. Cancer Res 1993; 53:2457-9; PMID:8495404
- Oei AL, Moreno M, Verheijen RH, Sweep FC, Thomas CM, Massuger LF, von Mensdorff-Pouilly S. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int J Cancer 2008; 123:1848-53; PMID:18661524; http://dx.doi.org/10.1002/ijc.23725
- Bellone S, Siegel ER, Cocco E, Cargnelutti M, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapy. Int J Gynecol Cancer 2009; 19:860-6; PMID:19574774; http://dx.doi.org/10.1111/IGC.0b013e3181a8331f
- Spizzo G, Went P, Dirnhofer S, Obrist P, Moch H, Baeuerle PA, Mueller-Holzner E, Marth C, Gastl G, Zeimet AG. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol 2006; 103:483-8; PMID:16678891; http://dx.doi.org/10.1016/j.ygyno.2006.03.035
- van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis 2010; 31:1913-21; PMID:20837599; http://dx.doi.org/10.1093/carcin/bgq187
- Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005; 338:284-93; PMID:15745749; http://dx.doi.org/10.1016/j.ab.2004.12.026
- Siu MK, Kong DS, Chan HY, Wong ES, Ip PP, Jiang L, Ngan HY, Le XF, Cheung AN. Paradoxical impact of two folate receptors, FRalpha and RFC, in ovarian cancer: effect on cell proliferation, invasion and clinical outcome. PLoS One 2012; 7:e47201; PMID:23144806; http://dx.doi.org/10.1371/journal.pone.0047201
- Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, Hartmann LC. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol 2008; 108:619-26; PMID:18222534; http://dx.doi.org/10.1016/j.ygyno.2007.11.020
- Chen YL, Chang MC, Huang CY, Chiang YC, Lin HW, Chen CA, Hsieh CY, Cheng WF. Serous ovarian carcinoma patients with high alpha-folate receptor had reducing survival and cytotoxic chemo-response. Mol Oncol 2012; 6:360-9; PMID:22265591; http://dx.doi.org/10.1016/j.molonc.2011.11.010
- Vergote IB, Marth C, Coleman RL. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev 2015; 34:41-52; PMID:25564455; http://dx.doi.org/10.1007/s10555-014-9539-8
- Coward JI, Middleton K, Murphy F. New perspectives on targeted therapy in ovarian cancer. Int J Womens Health 2015; 7:189-203; PMID:25678824; http://dx.doi.org/10.2147/IJWH.S52379
- Cerezo A, Guadamillas MC, Goetz JG, Sanchez-Perales S, Klein E, Assoian RK, del Pozo MA. The absence of caveolin-1 increases proliferation and anchorage- independent growth by a Rac-dependent, Erk-independent mechanism. Mol Cell Biol 2009; 29:5046-59; PMID:19620284; http://dx.doi.org/10.1128/MCB.00315-09
- Cheung A, Bax HJ, Josephs DH, Ilieva KM, Pellizzari G, Opzoomer J, Bloomfield J, Fittall M, Grigoriadis A, Figini M, et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016; PMID:27248175; http://dx.doi.org/10.18632/oncotarget.9651
- Buist MR, Molthoff CF, Kenemans P, Meijer CJ. Distribution of OV-TL 3 and MOv18 in normal and malignant ovarian tissue. J Clin Pathol 1995; 48:631-6; PMID:7560169; http://dx.doi.org/10.1136/jcp.48.7.631
- Knutson KL, Krco CJ, Erskine CL, Goodman K, Kelemen LE, Wettstein PJ, Low PS, Hartmann LC, Kalli KR. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J Clin Oncol 2006; 24:4254-61; PMID:16908932; http://dx.doi.org/10.1200/JCO.2006.05.9311
- Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014; 74:2907-12; PMID:24824231; http://dx.doi.org/10.1158/0008-5472.CAN-14-0337
- Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Jr., Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol 2004; 93:170-81; PMID:15047232; http://dx.doi.org/10.1016/j.ygyno.2003.12.034
- Cheng WF, Huang CY, Chang MC, Hu YH, Chiang YC, Chen YL, Hsieh CY, Chen CA. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer 2009; 100:1144-53; PMID:19293794; http://dx.doi.org/10.1038/sj.bjc.6604964
- Hudson LG, Zeineldin R, Silberberg M, Stack MS. Activated epidermal growth factor receptor in ovarian cancer. Cancer Treat Res 2009; 149:203-26; PMID:19763438; http://dx.doi.org/10.1007/978-0-387-98094-2_10
- Dimova I, Zaharieva B, Raitcheva S, Dimitrov R, Doganov N, Toncheva D. Tissue microarray analysis of EGFR and erbB2 copy number changes in ovarian tumors. Int J Gynecol Cancer 2006; 16:145-51; PMID:16445625; http://dx.doi.org/10.1111/j.1525-1438.2006.00286.x
- Casamassimi A, De Luca A, Agrawal S, Stromberg K, Salomon DS, Normanno N. EGF-related antisense oligonucleotides inhibit the proliferation of human ovarian carcinoma cells. Ann Oncol 2000; 11:319-25; PMID:10811499; http://dx.doi.org/10.1023/A:1008350811639
- Kurachi H, Morishige K, Adachi H, Adachi K, Tasaka K, Sawada M, Miyake A. Implantation and growth of epidermal growth factor (EGF) receptor expressing human ovarian cancer xenografts in nude mice is dependent on EGF. Cancer 1994; 74:2984-90; PMID:7954262; http://dx.doi.org/10.1002/1097-0142(19941201)74:11%3c2984::AID-CNCR2820741115%3e3.0.CO;2-M
- Alper O, Bergmann-Leitner ES, Bennett TA, Hacker NF, Stromberg K, Stetler-Stevenson WG. Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J Natl Cancer Inst 2001; 93:1375-84; PMID:11562388; http://dx.doi.org/10.1093/jnci/93.18.1375
- Alper O, De Santis ML, Stromberg K, Hacker NF, Cho-Chung YS, Salomon DS. Anti-sense suppression of epidermal growth factor receptor expression alters cellular proliferation, cell-adhesion and tumorigenicity in ovarian cancer cells. Int J Cancer 2000; 88:566-74; PMID:11058872; http://dx.doi.org/10.1002/1097-0215(20001115)88:4%3c566::AID-IJC8%3e3.0.CO;2-D
- Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem 2006; 281:25903-14; PMID:16847054; http://dx.doi.org/10.1074/jbc.M603414200
- Wang F, Liu R, Lee SW, Sloss CM, Couget J, Cusack JC. Heparin-binding EGF-like growth factor is an early response gene to chemotherapy and contributes to chemotherapy resistance. Oncogene 2007; 26:2006-16; PMID:17001310; http://dx.doi.org/10.1038/sj.onc.1209999
- Anticancer researchAm J, Pathol Psyrri A, Kassar M, Yu Z, Bamias A, Weinberger PM, Markakis S, Kowalski D, Camp RL, Rimm DL, Dimopoulos MA. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res 2005; 11:8637-43; PMID:16361548; http://dx.doi.org/10.1158/1078-0432.CCR-05-1436
- Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer 2010; 116:1018-26; PMID:20127943; http://dx.doi.org/10.1002/cncr.24788
- Henriksen R, Funa K, Wilander E, Backstrom T, Ridderheim M, Oberg K. Expression and prognostic significance of platelet-derived growth factor and its receptors in epithelial ovarian neoplasms. Cancer Res 1993; 53:4550-4; PMID:8402626
- Madsen CV, Dahl Steffensen K, Waldstrom M, Jakobsen A. Immunohistochemical expression of platelet-derived growth factor receptors in ovarian cancer patients with long-term follow-up. Patholog Res Int 2012; 2012:851432; PMID:23094199; http://dx.doi.org/10.1155/2012/851432
- Matei D, Emerson RE, Lai YC, Baldridge LA, Rao J, Yiannoutsos C, Donner DD. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene 2006; 25:2060-9; PMID:16331269; http://dx.doi.org/10.1038/sj.onc.1209232
- Gryshkova V, Goncharuk I, Gurtovyy V, Khozhayenko Y, Nespryadko S, Vorobjova L, Usenko V, Gout I, Filonenko V, Kiyamova R. The study of phosphate transporter NAPI2B expression in different histological types of epithelial ovarian cancer. Exp Oncol 2009; 31:37-42; PMID:19300415
- Damelin M, Bankovich A, Park A, Aguilar J, Anderson W, Santaguida M, Aujay M, Fong S, Khandke K, Pulito V, et al. Anti-EFNA4 calicheamicin conjugates effectively target triple-negative breast and ovarian tumor-initiating cells to result in sustained tumor regressions. Clin Cancer Res 2015; 21:4165-73; PMID:26015513; http://dx.doi.org/10.1158/1078-0432.CCR-15-0695
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 2008; 8:579-91; PMID:18596824; http://dx.doi.org/10.1038/nrc2403
- Sher I, Adham SA, Petrik J, Coomber BL. Autocrine VEGF-A/KDR loop protects epithelial ovarian carcinoma cells from anoikis. Int J Cancer 2009; 124:553-61; PMID:19004006; http://dx.doi.org/10.1002/ijc.23963
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996; 2:1096-103; PMID:8837607; http://dx.doi.org/10.1038/nm1096-1096
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438:820-7; PMID:16341007; http://dx.doi.org/10.1038/nature04186
- Kassim SK, El-Salahy EM, Fayed ST, Helal SA, Helal T, Azzam Eel D, Khalifa A. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem 2004; 37:363-9; PMID:15087251; http://dx.doi.org/10.1016/j.clinbiochem.2004.01.014
- Li L, Wang L, Zhang W, Tang B, Zhang J, Song H, Yao D, Tang Y, Chen X, Yang Z, et al. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res 2004; 24:1973-9; PMID:15274387
- Hefler LA, Zeillinger R, Grimm C, Sood AK, Cheng WF, Gadducci A, Tempfer CB, Reinthaller A. Preoperative serum vascular endothelial growth factor as a prognostic parameter in ovarian cancer. Gynecol Oncol 2006; 103:512-7; PMID:16750560; http://dx.doi.org/10.1016/j.ygyno.2006.03.058
- Bandiera E, Franceschini R, Specchia C, Bignotti E, Trevisiol C, Gion M, Pecorelli S, Santin AD, Ravaggi A. Prognostic significance of vascular endothelial growth factor serum determination in women with ovarian cancer. ISRN Obstet Gynecol 2012; 2012:245756; PMID:22792477; http://dx.doi.org/10.5402/2012/245756
- Rabinovich A, Medina L, Piura B, Segal S, Huleihel M. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res 2007; 27:267-72; PMID:17352242
- Scambia G, Testa U, Benedetti Panici P, Foti E, Martucci R, Gadducci A, Perillo A, Facchini V, Peschle C, Mancuso S. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer 1995; 71:354-6; PMID:7841052; http://dx.doi.org/10.1038/bjc.1995.71
- Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, Duska LR, Fuller AF, Goodman AK, Nikrui N, et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer 2000; 10:33-41; PMID:11240649; http://dx.doi.org/10.1046/j.1525-1438.2000.00003.x
- Watson JM, Berek JS, Martinez-Maza O. Growth inhibition of ovarian cancer cells induced by antisense IL-6 oligonucleotides. Gynecol Oncol 1993; 49:8-15; PMID:8482564; http://dx.doi.org/10.1006/gyno.1993.1077
- Obata NH, Tamakoshi K, Shibata K, Kikkawa F, Tomoda Y. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Res 1997; 17:337-42; PMID:9066674
- Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res 2005; 65:10794-800; PMID:16322225; http://dx.doi.org/10.1158/0008-5472.CAN-05-0623
- Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev 1997; 8:241-52; PMID:9620640; http://dx.doi.org/10.1016/S1359-6101(98)80005-1
- Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, Li LZ. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett 2010; 295:110-23; PMID:20236757; http://dx.doi.org/10.1016/j.canlet.2010.02.019
- Ohta T, Ohmichi M, Hayasaka T, Mabuchi S, Saitoh M, Kawagoe J, Takahashi K, Igarashi H, Du B, Doshida M, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology 2006; 147:1761-9; PMID:16396982; http://dx.doi.org/10.1210/en.2005-1450
- Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol 2008; 180:7338-48; PMID:18490733; http://dx.doi.org/10.4049/jimmunol.180.11.7338
- Krockenberger M, Kranke P, Hausler S, Engel JB, Horn E, Nurnberger K, Wischhusen J, Dietl J, Honig A. Macrophage migration-inhibitory factor levels in serum of patients with ovarian cancer correlates with poor prognosis. Anticancer Res 2012; 32:5233-8; PMID:23225421
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102:18538-43; PMID:16344461; http://dx.doi.org/10.1073/pnas.0509182102
- Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother 2009; 58:449-59; PMID:18791714; http://dx.doi.org/10.1007/s00262-008-0583-5
- Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009; 4:e6412; PMID:19641607; http://dx.doi.org/10.1371/journal.pone.0006412
- McAlpine JN, Porter H, Kobel M, Nelson BH, Prentice LM, Kalloger SE, Senz J, Milne K, Ding J, Shah SP, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol 2012; 25:740-50; PMID:22282309; http://dx.doi.org/10.1038/modpathol.2011.211
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 2010; 107:7875-80; PMID:20385810; http://dx.doi.org/10.1073/pnas.1003345107
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007; 104:3360-5; PMID:17360651; http://dx.doi.org/10.1073/pnas.0611533104
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23:515-48; PMID:15771580; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115611
- Gooden MJ, van Hall T. Infiltrating CTLs are bothered by HLA-E on tumors. Oncoimmunology 2012; 1:92-3; PMID:22720221; http://dx.doi.org/10.4161/onci.1.1.17961
- Gooden MJ, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, Nijman H, van Hall T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc Natl Acad Sci U S A 2011; 108:10656-61; PMID:21670276; http://dx.doi.org/10.1073/pnas.1100354108
- Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett 2006; 580:2860-8; PMID:16574107; http://dx.doi.org/10.1016/j.febslet.2006.03.024
- Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin Cancer Res 2007; 13:7243-6; PMID:18094402; http://dx.doi.org/10.1158/1078-0432.CCR-07-1393
- Reinartz S, Kohler S, Schlebusch H, Krista K, Giffels P, Renke K, Huober J, Mobus V, Kreienberg R, DuBois A, et al. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: immunological response and survival (phase Ib/II). Clin Cancer Res 2004; 10:1580-7; PMID:15014007; http://dx.doi.org/10.1158/1078-0432.CCR-03-0056
- Sabbatini P, Harter P, Scambia G, Sehouli J, Meier W, Wimberger P, Baumann KH, Kurzeder C, Schmalfeldt B, Cibula D, et al. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a phase III trial of the AGO OVAR, COGI, GINECO, and GEICO–the MIMOSA study. J Clin Oncol 2013; 31:1554-61; PMID:23478059; http://dx.doi.org/10.1200/JCO.2012.46.4057
- Noujaim AA, Schultes BC, Baum RP, Madiyalakan R. Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13–evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother Radiopharm 2001; 16:187-203; PMID:11471484; http://dx.doi.org/10.1089/10849780152389384
- Gordon AN, Schultes BC, Gallion H, Edwards R, Whiteside TL, Cermak JM, Nicodemus CF. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol Oncol 2004; 94:340-51; PMID:15297171; http://dx.doi.org/10.1016/j.ygyno.2004.04.024
- Ehlen TG, Hoskins PJ, Miller D, Whiteside TL, Nicodemus CF, Schultes BC, Swenerton KD. A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer. Int J Gynecol Cancer 2005; 15:1023-34; PMID:16343178; http://dx.doi.org/10.1111/j.1525-1438.2005.00483.x
- Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol 2009; 27:418-25; PMID:19075271; http://dx.doi.org/10.1200/JCO.2008.17.8400
- Nicholson S, Bomphray CC, Thomas H, McIndoe A, Barton D, Gore M, George AJ. A phase I trial of idiotypic vaccination with HMFG1 in ovarian cancer. Cancer Immunol Immunother 2004; 53:809-16; PMID:15127236; http://dx.doi.org/10.1007/s00262-004-0522-z
- Verheijen RH, Massuger LF, Benigno BB, Epenetos AA, Lopes A, Soper JT, Markowska J, Vyzula R, Jobling T, Stamp G, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol 2006; 24:571-8; PMID:16446329; http://dx.doi.org/10.1200/JCO.2005.02.5973
- Fiedler W, DeDosso S, Cresta S, Weidmann J, Tessari A, Salzberg M, Dietrich B, Baumeister H, Goletz S, Gianni L, et al. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur J Cancer 2016; 63:55-63; PMID:27285281; http://dx.doi.org/10.1016/j.ejca.2016.05.003
- Burges A, Wimberger P, Kumper C, Gorbounova V, Sommer H, Schmalfeldt B, Pfisterer J, Lichinitser M, Makhson A, Moiseyenko V, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res 2007; 13:3899-905; PMID:17606723; http://dx.doi.org/10.1158/1078-0432.CCR-06-2769
- Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, Itin C, Prang N, Baeuerle PA. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer 2005; 115:98-104; PMID:15688411; http://dx.doi.org/10.1002/ijc.20908
- Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer 2010; 127:2209-21; PMID:20473913; http://dx.doi.org/10.1002/ijc.25423
- Coney LR, Mezzanzanica D, Sanborn D, Casalini P, Colnaghi MI, Zurawski VR, Jr. Chimeric murine-human antibodies directed against folate binding receptor are efficient mediators of ovarian carcinoma cell killing. Cancer Res 1994; 54:2448-55; PMID:7512887
- Crippa F, Bolis G, Seregni E, Gavoni N, Scarfone G, Ferraris C, Buraggi GL, Bombardieri E. Single-dose intraperitoneal radioimmunotherapy with the murine monoclonal antibody I-131 MOv18: clinical results in patients with minimal residual disease of ovarian cancer. Eur J Cancer 1995; 31A:686-90; PMID:7640039; http://dx.doi.org/10.1016/0959-8049(94)00454-D
- Buist MR, Kenemans P, den Hollander W, Vermorken JB, Molthoff CJ, Burger CW, Helmerhorst TJ, Baak JP, Roos JC. Kinetics and tissue distribution of the radiolabeled chimeric monoclonal antibody MOv18 IgG and F(ab')2 fragments in ovarian carcinoma patients. Cancer Res 1993; 53:5413-8; PMID:8221680
- Molthoff CF, Prinssen HM, Kenemans P, van Hof AC, den Hollander W, Verheijen RH. Escalating protein doses of chimeric monoclonal antibody MOv18 immunoglobulin G in ovarian carcinoma patients: a phase I study. Cancer 1997; 80:2712-20; PMID:9406729; http://dx.doi.org/10.1002/(SICI)1097-0142(19971215)80:12+%3c2712::AID-CNCR50%3e3.3.CO;2-M
- Buijs WC, Tibben JG, Boerman OC, Molthoff CF, Massuger LF, Koenders EB, Schijf CP, Siegel JA, Corstens FH. Dosimetric analysis of chimeric monoclonal antibody cMOv18 IgG in ovarian carcinoma patients after intraperitoneal and intravenous administration. Eur J Nucl Med 1998; 25:1552-61; PMID:9799353; http://dx.doi.org/10.1007/s002590050335
- van Zanten-Przybysz I, Molthoff CF, Roos JC, Verheijen RH, van Hof A, Buist MR, Prinssen HM, den Hollander W, Kenemans P. Influence of the route of administration on targeting of ovarian cancer with the chimeric monoclonal antibody MOv18: i.v. vs. i.p. Int J Cancer 2001; 92:106-14; PMID: 11279613
- Wen Y, Graybill WS, Previs RA, Hu W, Ivan C, Mangala LS, Zand B, Nick AM, Jennings NB, Dalton HJ, et al. Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res 2015; 21:448-59; PMID:25416196; http://dx.doi.org/10.1158/1078-0432.CCR-14-1578
- Kamen BA, Smith AK. Farletuzumab, an anti-folate receptor alpha antibody, does not block binding of folate or anti-folates to receptor nor does it alter the potency of anti-folates in vitro. Cancer Chemother Pharmacol 2012; 70:113-20; PMID:22644798; http://dx.doi.org/10.1007/s00280-012-1890-2
- Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, Vander Els N, Phillips MD, Schweizer C, Weil SC, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res 2010; 16:5288-95; PMID:20855460; http://dx.doi.org/10.1158/1078-0432.CCR-10-0700
- Kim KH, Jelovac D, Armstrong DK, Schwartz B, Weil SC, Schweizer C, Alvarez RD. Phase 1b safety study of farletuzumab, carboplatin and pegylated liposomal doxorubicin in patients with platinum-sensitive epithelial ovarian cancer. Gynecol Oncol 2016; 140:210-4; PMID:26644263; http://dx.doi.org/10.1016/j.ygyno.2015.11.031
- Armstrong DK, White AJ, Weil SC, Phillips M, Coleman RL. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol Oncol 2013; 129:452-8; PMID:23474348; http://dx.doi.org/10.1016/j.ygyno.2013.03.002
- Vergote I, Armstrong D, Scambia G, Teneriello M, Sehouli J, Schweizer C, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Study to Assess Efficacy and Safety of Weekly Farletuzumab in Combination With Carboplatin and Taxane in Patients With Ovarian Cancer in First Platinum-Sensitive Relapse. J Clin Oncol 2016; 34:2271–8. PMID: 27001568 doi: 10.1200/JCO.2015.63.2596
- Marchetti C, Palaia I, Giorgini M, De Medici C, Iadarola R, Vertechy L, Domenici L, Di Donato V, Tomao F, Muzii L, et al. Targeted drug delivery via folate receptors in recurrent ovarian cancer: a review. Onco Targets Ther 2014; 7:1223-36; PMID:25031539; http://dx.doi.org/10.2147/OTT.S40947
- Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, Chao Q, Jacob S, Turchin H, Gibbs L, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun 2007; 7:20; PMID:18088084
- Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010; 16:6132-8; PMID:21037025; http://dx.doi.org/10.1158/1078-0432.CCR-10-2275
- Patel D, Lahiji A, Patel S, Franklin M, Jimenez X, Hicklin DJ, Kang X. Monoclonal antibody cetuximab binds to and down-regulates constitutively activated epidermal growth factor receptor vIII on the cell surface. Anticancer Res 2007; 27:3355-66; PMID:17970081
- Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 2003; 21:2787-99; PMID:12860957; http://dx.doi.org/10.1200/JCO.2003.01.504
- Schilder RJ, Pathak HB, Lokshin AE, Holloway RW, Alvarez RD, Aghajanian C, Min H, Devarajan K, Ross E, Drescher CW, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol Oncol 2009; 113:21-7; PMID:19162309; http://dx.doi.org/10.1016/j.ygyno.2008.12.003
- Konner J, Schilder RJ, DeRosa FA, Gerst SR, Tew WP, Sabbatini PJ, Hensley ML, Spriggs DR, Aghajanian CA. A phase II study of cetuximab/paclitaxel/carboplatin for the initial treatment of advanced-stage ovarian, primary peritoneal, or fallopian tube cancer. Gynecol Oncol 2008; 110:140-5; PMID:18554700; http://dx.doi.org/10.1016/j.ygyno.2008.04.018
- Secord AA, Blessing JA, Armstrong DK, Rodgers WH, Miner Z, Barnes MN, Lewandowski G, Mannel RS. Phase II trial of cetuximab and carboplatin in relapsed platinum-sensitive ovarian cancer and evaluation of epidermal growth factor receptor expression: a Gynecologic Oncology Group study. Gynecol Oncol 2008; 108:493-9; PMID:18191993; http://dx.doi.org/10.1016/j.ygyno.2007.11.029
- Wu M, Rivkin A, Pham T. Panitumumab: human monoclonal antibody against epidermal growth factor receptors for the treatment of metastatic colorectal cancer. Clin Ther 2008; 30:14-30; PMID:18343240; http://dx.doi.org/10.1016/j.clinthera.2008.01.014
- Vlahovic G, Meadows KL, Uronis HE, Morse MA, Blobe GC, Riedel RF, Zafar SY, Alvarez-Secord A, Gockerman J, Starodub AN, et al. A phase I study of bevacizumab, everolimus and panitumumab in advanced solid tumors. Cancer Chemother Pharmacol 2012; 70:95-102; PMID:22638798; http://dx.doi.org/10.1007/s00280-012-1889-8
- Steffensen KD, Waldstrom M, Pallisgard N, Lund B, Bergfeldt K, Wihl J, Keldsen N, Marth C, Vergote I, Jakobsen A. Panitumumab and pegylated liposomal doxorubicin in platinum-resistant epithelial ovarian cancer with KRAS wild-type: the PaLiDo study, a phase II nonrandomized multicenter study. Int J Gynecol Cancer 2013; 23:73-80; PMID:23211422; http://dx.doi.org/10.1097/IGC.0b013e3182775fae
- Seiden MV, Burris HA, Matulonis U, Hall JB, Armstrong DK, Speyer J, Weber JD, Muggia F. A phase II trial of EMD72000 (matuzumab), a humanized anti-EGFR monoclonal antibody, in patients with platinum-resistant ovarian and primary peritoneal malignancies. Gynecol Oncol 2007; 104:727-31; PMID:17126894; http://dx.doi.org/10.1016/j.ygyno.2006.10.019
- Vanhoefer U, Tewes M, Rojo F, Dirsch O, Schleucher N, Rosen O, Tillner J, Kovar A, Braun AH, Trarbach T, et al. Phase I study of the humanized antiepidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumors that express the epidermal growth factor receptor. J Clin Oncol 2004; 22:175-84; PMID:14701780; http://dx.doi.org/10.1200/JCO.2004.05.114
- Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol 2003; 21:283-90; PMID:12525520; http://dx.doi.org/10.1200/JCO.2003.10.104
- Wuerkenbieke D, Wang J, Li Y, Ma C. miRNA-150 downregulation promotes pertuzumab resistance in ovarian cancer cells via AKT activation. Arch Gynecol Obstet 2015; 292:1109-16; PMID:25986891; http://dx.doi.org/10.1007/s00404-015-3742-x
- Mullen P, Cameron DA, Hasmann M, Smyth JF, Langdon SP. Sensitivity to pertuzumab (2C4) in ovarian cancer models: cross-talk with estrogen receptor signaling. Mol Cancer Ther 2007; 6:93-100; PMID:17237269; http://dx.doi.org/10.1158/1535-7163.MCT-06-0401
- Takai N, Jain A, Kawamata N, Popoviciu LM, Said JW, Whittaker S, Miyakawa I, Agus DB, Koeffler HP. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer 2005; 104:2701-8; PMID:16265675; http://dx.doi.org/10.1002/cncr.21533
- Makhija S, Amler LC, Glenn D, Ueland FR, Gold MA, Dizon DS, Paton V, Lin CY, Januario T, Ng K, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol 2010; 28:1215-23; PMID:19901115; http://dx.doi.org/10.1200/JCO.2009.22.3354
- Nagumo Y, Faratian D, Mullen P, Harrison DJ, Hasmann M, Langdon SP. Modulation of HER3 is a marker of dynamic cell signaling in ovarian cancer: implications for pertuzumab sensitivity. Mol Cancer Res 2009; 7:1563-71; PMID:19737968; http://dx.doi.org/10.1158/1541-7786.MCR-09-0101
- Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol 2005; 23:2534-43; PMID:15699478; http://dx.doi.org/10.1200/JCO.2005.03.184
- Gordon MS, Matei D, Aghajanian C, Matulonis UA, Brewer M, Fleming GF, Hainsworth JD, Garcia AA, Pegram MD, Schilder RJ, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. J Clin Oncol 2006; 24:4324-32; PMID:16896006; http://dx.doi.org/10.1200/JCO.2005.05.4221
- Kaye SB, Poole CJ, Bidzinski M, Gianni L, Gorbunova V, Novikova E, Strauss A, McNally VA, Ross G, Vergote I. A randomised phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab (P) versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. J Clin Oncol 2008; 26:15s 5520; http://dx.doi.org/10.1200/JCO.2008.16.2123
- Huang J, Wang S, Lyu H, Cai B, Yang X, Wang J, Liu B. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer 2013; 12:134; PMID:24215614; http://dx.doi.org/10.1186/1476-4598-12-134
- Liu J, Ray-Coquard IL, Selle F, Poveda A, Cibula D, Hirte HW, Raspagliesi F, Gladieff L, Harter P, Schiavetto I, et al. A phase II randomized open-label study of MM-121, a fully human monoclonal antibody targeting ErbB3, in combination with weekly paclitaxel versus weekly paclitaxel in patients with platinum-resistant/refractory ovarian cancers. J Clin Oncol 2014; 32:5s 5519; http://dx.doi.org/10.1200/JCO.2013.49.4757
- Chiorean EG, Sweeney C, Youssoufian H, Qin A, Dontabhaktuni A, Loizos N, Nippgen J, Amato R. A phase I study of olaratumab, an anti-platelet-derived growth factor receptor alpha (PDGFRalpha) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol 2014; 73:595-604; PMID:24452395; http://dx.doi.org/10.1007/s00280-014-2389-9
- Matsuo K, Nishimura M, Komurov K, Shahzad MMK, Ali-Fehmi R, Roh JW, Lu CH, Cody DD, Ram PT, Loizos N, et al. Platelet-derived growth factor receptor alpha (PDGFR alpha) targeting and relevant biomarkers in ovarian carcinoma. Gynecol Oncol 2014; 132:166-75; PMID:24183729; http://dx.doi.org/10.1016/j.ygyno.2013.10.027
- Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, Yancopoulos GD, Jaffe RB. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res 2003; 9:5721-8; PMID:14654557
- Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007; 25:5165-71; PMID:18024863; http://dx.doi.org/10.1200/JCO.2007.11.5345
- Burger RA. Role of vascular endothelial growth factor inhibitors in the treatment of gynecologic malignancies. J Clin Oncol 2010; 21:3-11; PMID: 20379441; http://dx.doi.org/10.3802/jgo.2010.21.1.3
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365:2484-96; PMID:22204725; http://dx.doi.org/10.1056/NEJMoa1103799
- Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014; 32:1302-8; PMID:24637997; http://dx.doi.org/10.1200/JCO.2013.51.4489
- Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res 2008; 68:2329-39; PMID:18381440; http://dx.doi.org/10.1158/0008-5472.CAN-07-5167
- Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, Weiss G, Yazji S, Ng C, Wilding G. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res 2008; 14:7924-9; PMID:19047123; http://dx.doi.org/10.1158/1078-0432.CCR-08-0378
- Bell-McGuinn KM, Matthews CM, Ho SN, Barve M, Gilbert L, Penson RT, Lengyel E, Palaparthy R, Gilder K, Vassos A, et al. A phase II, single-arm study of the anti-alpha5beta1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol Oncol 2011; 121:273-9; PMID:21276608; http://dx.doi.org/10.1016/j.ygyno.2010.12.362
- Vergote I, Colombo N, Kutarska E, del Campo J, Pippitt C, Casado A, Lengyel E, Gilder K, Ho S, Schilder RJ. Phase II study comparing volociximab (an antiangiogenic antibody) and pegylated liposomal doxorubicin (PLD) with PLD alone in recurrent ovarian or primary peritoneal cancer. J Clin Oncol 2009; 27:15s 5560; http://dx.doi.org/10.1200/JCO.2009.23.9822
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 2001; 19:3312-22; PMID:11454878
- Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S, Joly F, et al. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2014; 20:2192-204; PMID:24563479; http://dx.doi.org/10.1158/1078-0432.CCR-13-2200
- Mahalingam D, Patel RM, Sachdev JC, Hart LL, Halama N, Ramanathan RK, Sarantopoulos J, Liu X, Yazji S, Jaeger D, et al. First-in-human, phase I study assessing imalumab (Bax69), a first-in-class anti-oxidized macrophage migration inhibitory factor (oxMIF) antibody in advanced solid tumors. J Clin Oncol 2015; 33:2518.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/10.1056/NEJMoa1200690
- Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol 2015; 33:4015-22; PMID:26351349; http://dx.doi.org/10.1200/JCO.2015.62.3397
- Varga A, Piha-Paul SA, Ott PA. Antitumour activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: interim results from a phase Ib study. J Clin Oncol 2015; 33:5510.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/10.1056/NEJMoa1200694
- Disis ML, Patel MR, Pant S, Infante JR, Lockhart AC, Kelly K, Beck JT, Gordon MS, Weiss GJ, Ejadi S, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with previously treated, recurrent or refractory ovarian cancer: A phase Ib, open-label expansion trial. J Clin Oncol 2015; 33:15s 5509.
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 2003; 100:4712-7; PMID:12682289; http://dx.doi.org/10.1073/pnas.0830997100
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008; 105:3005-10; PMID:18287062; http://dx.doi.org/10.1073/pnas.0712237105
- Callahan MK, Ott PA, Odunsi K, Bertolini SV, Pan LS, Venhaus RR, Karakunnel JJ, Hodi FS, Wolchok JD. A phase 1 study to evaluate the safety and tolerability of MEDI4736, an anti–PD-L1 antibody, in combination with tremelimumab in patients with advanced solid tumors. J Clin Oncol 2014; 32:5s TPS3120; http://dx.doi.org/10.1200/JCO.2013.49.4757
- Seymour L, Tinker A, Hirte H, Wagtmann N, Dodion P. Phase I and dose ranging, phase II studies with IPH2201, a humanized monoclonal antibody targeting HLA-E receptor CD94/NKG2A. Ann Oncol 2015; 26 (suppl 2): ii3; http://dx.doi.org/10.1093/annonc/mdv081.2
- Smith DC, Eisenberg PD, Manikhas G, Chugh R, Gubens MA, Stagg RJ, Kapoun AM, Xu L, Dupont J, Sikic B. A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors. Clin Cancer Res 2014; 20:6295-303; PMID:25324140; http://dx.doi.org/10.1158/1078-0432.CCR-14-1373
- Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov 2013; 12:329-32; PMID:23629491; http://dx.doi.org/10.1038/nrd4009
- Ab O, Whiteman KR, Bartle LM, Sun X, Singh R, Tavares D, LaBelle A, Payne G, Lutz RJ, Pinkas J, et al. IMGN853, a Folate Receptor-alpha (FRalpha)-Targeting Antibody-Drug Conjugate, Exhibits Potent Targeted Antitumor Activity against FRalpha-Expressing Tumors. Mol Cancer Ther 2015; 14:1605-13; PMID:25904506; http://dx.doi.org/10.1158/1535-7163.MCT-14-1095
- Borghael H, O'Malley D, Seward SM, Bauer TM, Perez RP, Oza AM, Jeong W, Michenzie MF, Kirby MW, Chandorkar G, et al. Phase 1 study of IMGN853, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC) in patients (Pts) with epithelial ovarian cancer (EOC) and other FRA-positive solid tumors. J Clin Oncol 2015; 33:15s 5558.
- Lindegren S, Andrade LN, Back T, Machado CM, Horta BB, Buchpiguel C, Moro AM, Okamoto OK, Jacobsson L, Cederkrantz E, et al. Binding Affinity, Specificity and Comparative Biodistribution of the Parental Murine Monoclonal Antibody MX35 (Anti-NaPi2b) and Its Humanized Version Rebmab200. PLoS One 2015; 10:e0126298; PMID:25970341; http://dx.doi.org/10.1371/journal.pone.0126298
- Burris HA, Gordon MS, Gerber DE, Spigel DR, Mendelson DS, Schiller JH, Wang Y, Choi Y, Kahn RS, Wood K, et al. A phase I study of DNIB0600A, an antibody-drug conjugate (ADC) targeting NaPi2b, in patients (pts) with non-small cell lung cancer (NSCLC) or platinum-resistant ovarian cancer (OC). J Clin Oncol 2014; 32:5s 2504; http://dx.doi.org/10.1200/JCO.2013.49.4757
- Hong DS, Garrido-Laguna I, Krop IE, Subbiah V, Werner TW, Cotter CM, Hamilton EP, Velastegul K, Xuan D, Bugarini R, et al. First-in-human dose escalation, safety, and PK study of a novel EFNA4-ADC in patients with advanced solid tumors. J Clin Oncol 2015; 33:15s 2520; http://dx.doi.org/10.1200/JCO.2015.60.8554
- NCI. NCI Thesaurus. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C124133. 2016.
- Sahin U, Jaeger D, Marme F, Mavratzas A, Krauss J, De Greve J, Vergote I, Tureci O. First-in-human phase I/II dose-escalation study of IMAB027 in patients with recurrent advanced ovarian cancer (OVAR): Preliminary data of phase I part. J Clin Oncol 2015; 33:5537.
- Gould HJ, Mackay GA, Karagiannis SN, O'Toole CM, Marsh PJ, Daniel BE, Coney LR, Zurawski VR, Jr., Joseph M, Capron M, et al. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol 1999; 29:3527-37; PMID:10556807; http://dx.doi.org/10.1002/(SICI)1521-4141(199911)29:11%3c3527::AID-IMMU3527%3e3.0.CO;2-5
- Josephs DH, Spicer JF, Karagiannis P, Gould HJ, Karagiannis SN. IgE immunotherapy: a novel concept with promise for the treatment of cancer. MAbs 2014; 6:54-72; PMID:24423620; http://dx.doi.org/10.4161/mabs.27029
- Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG, Thompson RG, Durham SR, Schwartz LB, Balkwill FR, et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol 2003; 33:1030-40; PMID:12672069; http://dx.doi.org/10.1002/eji.200323185
- Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, Fear DJ, Thompson RG, East N, Burke F, et al. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol 2007; 179:2832-43; PMID:17709497; http://dx.doi.org/10.4049/jimmunol.179.5.2832
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 2009; 8:226-34; PMID:19247305; http://dx.doi.org/10.1038/nrd2804
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/10.1038/nrc3236
- Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, Waldmann H, Neuberger MS. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med 1987; 166:1351-61; PMID:3500259; http://dx.doi.org/10.1084/jem.166.5.1351
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991-8; PMID:12407406; http://dx.doi.org/10.1038/ni1102-991
- Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013; 73:3591-603; PMID:23633484; http://dx.doi.org/10.1158/0008-5472.CAN-12-4100
- Kandalaft LE, Powell DJ, Jr., Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology 2013; 2:e22664; PMID:23482679; http://dx.doi.org/10.4161/onci.22664
