ABSTRACT
Preclinical efficacy studies of antibodies targeting a tumor-associated antigen are only justified when the expression of the relevant antigen has been demonstrated. Conventionally, antigen expression level is examined by immunohistochemistry of formalin-fixed paraffin-embedded tumor tissue section. This method represents the diagnostic “gold standard” for tumor target evaluation, but is affected by a number of factors, such as epitope masking and insufficient antigen retrieval. As a consequence, variances and discrepancies in histological staining results can occur, which may influence decision-making and therapeutic outcome. To overcome these problems, we have used different fluorescence-labeled therapeutic antibodies targeting human epidermal growth factor receptor (HER) family members and insulin-like growth factor-1 receptor (IGF1R) in combination with fluorescence imaging modalities to determine tumor antigen expression, drug-target interaction, and biodistribution and tumor saturation kinetics in non-small cell lung cancer xenografts. For this, whole-body fluorescence intensities of labeled antibodies, applied as a single compound or antibody mixture, were measured in Calu-1 and Calu-3 tumor-bearing mice, then ex vivo multispectral tumor tissue analysis at microscopic resolution was performed. With the aid of this simple and fast imaging method, we were able to analyze the tumor cell receptor status of HER1–3 and IGF1R, monitor the antibody-target interaction and evaluate the receptor binding sites of anti-HER2-targeting antibodies. Based on this, the most suitable tumor model, best therapeutic antibody, and optimal treatment dosage and application schedule was selected. Predictions drawn from obtained imaging data were in excellent concordance with outcome of conducted preclinical efficacy studies. Our results clearly demonstrate the great potential of combined in vivo and ex vivo fluorescence imaging for the preclinical development and characterization of monoclonal antibodies.
Abbreviation
| TAA | = | tumor-associated antigen |
| mAb | = | monoclonal antibody |
| IHC | = | immunohistochemistry |
| FISH | = | fluorescence in situ hybridization |
| FFPE | = | formalin-fixed paraffin-embedded |
| MSFI | = | multispectral fluorescence imaging |
| NSCLC | = | non-small cell lung cancer |
| PK | = | pharmacokinetics |
| PD | = | pharmacodynamics |
| TV | = | tumor volume |
Introduction
Targeting a tumor-associated antigen (TAA) by a monoclonal antibody (mAb) is a successful approach for the treatment of different malignancies.Citation1-4 Before therapeutic intervention with a given antibody is initiated, expression of the relevant cancer antigen must be confirmed in needle core or excisional biopsies. Immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) are considered “gold standards” for the evaluation of TAAs in formalin-fixed paraffin-embedded (FFPE) tumor tissue section, and subsequently for the stratification of patients. These analytical methods enable morphological and molecular biological differentiation between healthy and diseased tissue. Furthermore, specific antigen staining can be used to determine the expression level of various tumor cell antigens. In combination with other technologies, such as blood analysis, IHC can support the availability of predictive biomarkers allowing the selection of an individualized and targeted therapy, and monitor resulting therapeutic treatment effects. This approach should increase drug efficacy and decrease toxicity, thus leading to a more personalized and improved cancer treatment.Citation5-8
Although FFPE-IHC has achieved worldwide acceptance and is used as a standard procedure in morphology-based sciences and pharmacological drug-related research, the exact molecular mechanism of this procedure is still not well understood. The quality, reproducibility and validity of immunostains depend substantially on 3 major factors: 1) tissue fixation and processing; 2) unmasking of epitopes, which is also termed antigen retrieval (AR); and 3) the affinity and specificity of the primary detection antibody to the relevant epitope.Citation9-14 In particular, the loss of immunoreactivity of TAAs after fixation is a critical factor in FFPE tissue section. The chemical fixation process can mask and modify antibody binding sites, resulting in staining variations that may lead to an inaccurate determination of the antigen expression level. Over the past decades, different antigen retrieval methods were developed to reverse the formalin-fixation effect and restore the antigenicity of proteins.Citation15,16 Furthermore, approaches that minimize discrepancies in IHC, e.g., fixation mediaCitation14,17,18 and fixation time using ultrasound,Citation19,20 and help with the selection of the right antibodyCitation21,22 to examine humanCitation12 and rodentCitation23 tissue are being investigated.
Besides establishing the most efficient and appropriate parameters for tissue preparations, the primary detection antibody has to fulfill certain criteria (e.g., paraffin permeability, affinity and specificity to the formalin-fixed antigen) to provide optimal staining results. Since most therapeutic antibodies are not paraffin permeable, a large number of primary non-therapeutic detection antibodies from different species (e.g., goat, rabbit, sheep, monkey) are commercially available and routinely used in FFPE-IHC. However, their binding characteristics may differ from the therapeutic antibody under evaluation. Recent work has demonstrated that primary antibodies targeting the same tumor antigen can differ significantly in their binding affinity and specificity, and therefore lead to different staining results.Citation10,12,24 Thus, rigorous research and comprehensive guidelines on specimen fixation, processing, storage and selection of the most appropriate detection antibody are needed in order to improve the preclinical and clinical antibody development process.
An excellent alternative to classical histology for preclinical evaluation of TAAs is multispectral fluorescence imaging (MSFI), which also offers different advantages in whole-body imaging and anatomic pathology.Citation25-29 Over the past decades, such optical imaging modalities have undergone rapid development and been used to monitor primary tumor growth and metastases,Citation30,31 tumor cell and enzyme activity,Citation32-34 tumor cell surface receptors,Citation35-37 apoptosis,Citation38 anti-tumor treatment effectsCitation39 and biodistribution and accumulation of fluorescence-labeled molecules, such as therapeutic antibodies.Citation40-43 The majority of this macroscopic in vivo research was not correlated with microscopic ex vivo fluorescence histology analysis. From our point of view, this is a basic requirement to validate the accuracy of in vivo results on a cellular level and also receive deeper insight and understanding of investigated biological processes. Furthermore, conclusions and decisions derived from obtained imaging results have only been marginally correlated with efficacy data to confirm its validity and significance for preclinical drug development. As a consequence, the real potential and relevance of fluorescence imaging techniques for tumor target evaluation and drug development needs more in-depth analysis.
Here, we address this topic and illustrate the excellent possibilities of in vivo and ex vivo fluorescence imaging, in direct comparison to conventional IHC, for analyzing tumor-associated cell surface antigen expression in non-small cell lung cancer (NSCLC) xenografts and determine important pharmacokinetic (PK) and pharmacodynamic (PD) antibody parameters. Utilizing this combination of fluorescence imaging techniques, we successfully examined the distribution and tumor binding behavior of different fluorescence-labeled therapeutic antibodies targeting human epidermal growth factor receptor (HER) family members and insulin-like growth factor-1 receptor (IGF1R) in tumor-bearing mice. Furthermore, we performed epitope mapping studies of 2 anti-HER2 antibodies and selected the most suitable xenograft model, optimal treatment dosage and application schedule for antibody therapy. The imaging results were verified with preclinical efficacy data. An IHC study also investigated the problems associated with antigen retrieval buffer, and affinity and specificity variations of anti-HER2 non-therapeutic detection antibodies.
Results
Problems of tumor cell receptor evaluation using conventional immunohistochemistry
Variation of binding affinity and specificity of different anti-HER2 detection antibodies in Calu-3 tumor xenograft
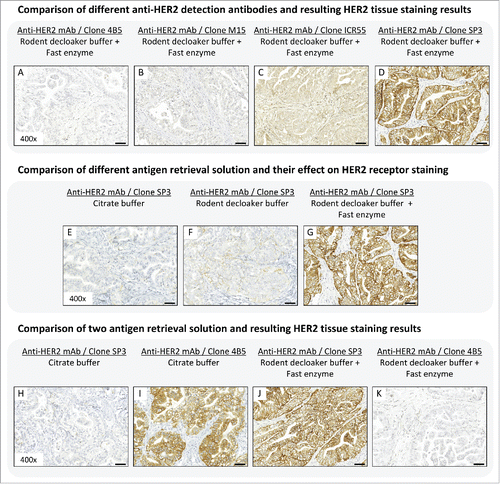
In order to demonstrate the high variations and difficulties in tumor cell-associated antigen evaluation arising from IHC analysis, we compared 4 commercially available anti-HER2 antibody clones (4B5, M15, ICR55, SP3) regarding target affinity, specificity, and resulting HER2 staining. HER2 staining, conducted under equivalent conditions, showed high variation in antibody binding affinity and specificity (). Staining with the anti-HER2 antibody clone 4B5 () and clone M15 () demonstrated no specific staining (IHC score: 0+), whereas the anti-HER2 antibody clone ICR55 showed moderate binding specificity to the tumor cells receptor (IHC score: 1–2+), as well as cross reactivity with murine stroma tissue (). Only the anti-HER2 antibody clone SP3 provided a suitable staining result, with strong HER2 signal intensity (IHC score: 3+) and no unspecific cross reactivity (). The latter is thus the most appropriate antibody reagent for this use because it possesses the ability to display the HER2 status in Calu-3 tissue correctly. Interestingly, the mAb clone 4B5, which showed no HER2 staining, is the primary and US Food and Drug Administration-approved HER2 detection antibody of Ventana Medical Systems Inc.. for breast cancer diagnostics.
Figure 1. (A-D) Binding affinity and specificity of different anti-HER2 antibody clones in Calu-3 tumor xenograft. Antigen retrieval was performed with rodent decloaker buffer and fast enzyme solution. (E-G) Influence of different antigen retrieval buffer on HER2 tissue staining results in Calu-3 tumor xenograft. The anti-HER2 antibody clone SP3 was use for target detection. (H-K) Comparison of 2 antigen retrieval solution and resulting HER2 tissue staining results in Calu-3 tissue slices. Antigen retrieval using citrate buffer followed by HER2 staining with anti-HER2 mAb clone SP3 (H) and anti-HER2 mAb clone 4B5 (I). Antigen retrieval using rodent decloaker plus fast enzyme followed by HER2 staining with anti-HER2 mAb clone SP3 (J) and anti-HER2 mAb clone 4B5 (K) showed contradictory staining results. Magnification of the tissue slices: x400. Scale bar: 50 µm.
Influence of antigen retrieval buffer to antibody binding affinity
In addition to analyzing specificity variations of different anti-HER2 detection antibodies, we investigated the influence of the antigen retrieval buffer on tissue staining results. The following 3 antigen retrieval buffer solutions were tested on de-paraffinized Calu-3 tumor tissue slices: 1) citrate buffer; 2) rodent decloaker buffer; and 3) rodent decloaker buffer in combination with fast enzyme solution. After antigen retrieval was performed, binding specificity of anti-HER2 antibody clone SP3 to its corresponding tumor cell receptor epitope was investigated.
Our results clearly demonstrated the influence of the selected antigen retrieval buffer on HER2 staining (). Tumor tissue slices pre-treated with citrate buffer showed no HER2 staining (IHC score: 0+; ), whereas the pre-incubation with rodent decloaker buffer lead to weak staining of HER2 (IHC score: 1+; ). Only the combination of rodent decloaker and fast enzyme solution was able to perform a complete antigen retrieval of HER2 and showed a strong staining result (IHC score: 3+) when anti-HER2 antibody clone SP3 was used (). Therefore, tissue slice pre-treatment with rodent decloaker and fast enzyme would be the antigen retrieval buffer of choice for this specific IHC application.
We also compared the IHC staining results of the 2 anti-HER2 antibody clones SP3 and 4B5 when using citrate buffer or rodent decloaker buffer plus fast enzyme solution for antigen retrieval of Calu-3 tissue slices (). Performing the retrieval step with citrate buffer resulted in negative HER2 staining of mAb clone SP3 (IHC score: 0+), whereas mAb clone 4B5 showed strong HER2 staining intensity (IHC score: 3+). Opposite HER2 staining results were obtained when using the same anti-HER2 detection antibodies in combination with the antigen retrieval buffer rodent decloaker plus fast enzyme. This is a clear proof that tumor cell receptor staining results are highly dependent on the selected primary detection antibody and utilized antigen-retrieval solution.
Evaluation of different tumor cell receptors in Calu-3 NSCLC xenograft
Tumor cell receptor evaluation using conventional IHC
In order to prioritize the most suitable xenograft model for a preclinical efficacy study, tumor cell receptor status must be evaluated. In the following experiment, we used established and standardized Ventana and Roche in-house IHC staining protocols (Supplementary Methods) to determine the expression level of 4 tumor cell receptors, HER1, HER2, HER3, and IGF1R, in Calu-3 tumor tissue slices ().
Figure 2. (A-D) Tumor cell receptor evaluation in Calu-3 xenograft using immunohistochemistry. All IHC staining protocols are available in Supplementary Methods. (E-J) Tumor cell receptor evaluation in Calu-3 xenograft using in vivo and ex vivo fluorescence imaging of single labeled therapeutic mAbs. Diagram illustrates in vivo fluorescence signal intensities in Calu-3 tumors 24h after i.v. injection. Animals per group: n = 5. Values are given as mean ± s.d.. ** P < 0.01, t-test. (K-Q) Tumor cell receptor evaluation in Calu-3 xenograft using a mixture of therapeutic mAbs labeled with different fluorophores. Diagram illustrates spectral library for unmixing the different Alexa fluorophores. Magnification of the tissue slices: x400. Scale bar: 50 µm.
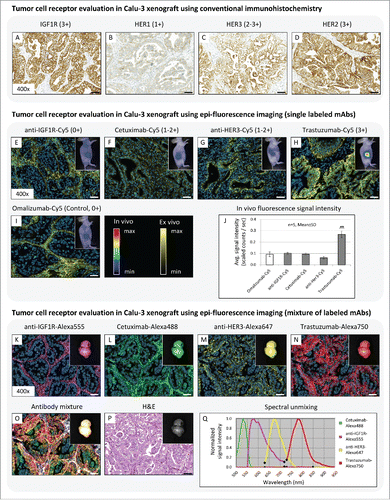
Results showed an inhomogeneous receptor staining between the different tumor cell receptors. Only weak staining intensity was visible for the HER1 receptor (1+), whereas HER3 showed an intense expression level (2+-3+) (). In contrast, strong overexpression of the IGF1R and HER2 (3+) could be detected (). Due to their high receptor overexpression, all investigated tumor cell receptors, excluding HER1, are potential targets for tumor therapy, and therefore can be prioritized as suitable epitopes for subsequent preclinical efficacy studies.
Tumor cell receptor evaluation using in vivo and ex vivo epi-fluorescence imaging
In the previous section, we evaluated the expression level of 4 tumor cell receptors in Calu-3 tissue slices using conventional immunohistochemistry and prioritized 3 of them (HER2, HER3, and IGF1R) as potential targets for tumor therapy. Although clearly identified by IHC, the current receptor status cannot provide any information about the penetration, distribution, and binding properties of the therapeutic antibody in vivo, and therefore may not be indicative for preclinical efficacy studies. To overcome this problem, we determined the Calu-3 receptor status using fluorescent-labeled therapeutic antibodies (cetuximab-Cy5 (anti-HER1), trastuzumab-Cy5 (anti-HER2), anti-HER3-Cy5, anti-IGF1R-Cy5) in combination with in vivo and ex vivo fluorescence imaging modalities. The IgE specific antibody omalizumab-Cy5 possesses no in vivo activity, and therefore served as a negative control.
The in vivo fluorescence imaging results showed no signal intensity differences in the tumor region between the control antibody omalizumab-Cy5, which circulates as a consequence of non-tumor binding, cetuximab-Cy5, anti-HER3-Cy5, and anti-IGF1R-Cy5 mAbs (). Only the trastuzumab-Cy5 signal was significantly increased in tumor region (). The subsequent ex vivo tumor tissue analysis clarified the distribution, penetration, and binding behavior of the individual antibodies in more detail at cellular resolution. Ex vivo fluorescence imaging results demonstrated an exclusive localization of omalizumab-Cy5 and anti-IGF1R-Cy5 in stroma tissue, but both antibodies showed no specific tumor cell binding at all (IHC score: 0+). In contrast to this, cetuximab-Cy5 and anti-HER3-Cy5 mAb were located in tumor stroma tissue and weak binding to the corresponding tumor cell receptor (IHC score: 1+-2+) was observed. Furthermore, cetuximab-Cy5 was located on the tumor cell surface, whereas the anti-HER3-Cy5 mAb was internalized into the Calu-3 cells (). Only trastuzumab-Cy5 possesses a very strong binding affinity and specificity to HER2 (IHC score: 3+) ().
Consequently, the in vivo and ex vivo evaluation of TAAs in Calu-3 xenograft using fluorescent-labeled therapeutic antibodies prioritized HER2 as the most suitable target for a subsequent preclinical efficacy study. Nevertheless, HER1 and HER3, which demonstrated weak antibody-target interaction, could also be potential targets for tumor therapy and might possess treatment efficacy in other xenografts.
We extended our imaging studies by using 4 antibodies, each labeled with a different fluorochrome (cetuximab-Alexa488, trastuzumab-Alexa750, anti-HER3-Alexa647, anti-IGF1R-Alexa555) (). Due to the application of fluorochromes from the visible wavelength range (Alexa-488 and Alexa-555), where animal skin cause strong light absorption and scattering and consequently reduce depth penetration, in vivo fluorescence imaging could not be conducted. Therefore, only the explanted Calu-3 tumor was measured and spectrally unmixed using the Maestro imaging system. Although direct comparison of signal intensities between the individual fluorochromes were not possible due to the different extinction coefficient and nonlinear energy content of the light source, spectral unmixing results clearly indicated the presence of all applied therapeutic antibodies in Calu-3 tumor tissue (). Subsequent histological tumor tissue analysis demonstrated the same distribution and binding behavior of each individual therapeutic antibody already shown in the single mAb experiment, where the combination of HER2 and trastuzumab was predicted as the most suitable candidate for high therapeutic efficacy.
Comparison of IHC and epi-fluorescence imaging results with preclinical efficacy data
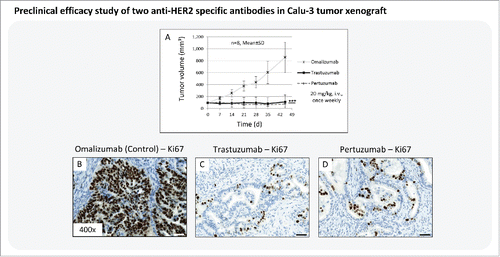
To verify obtained immunohistochemistry and epi-fluorescence imaging results regarding their significance in tumor cell receptor evaluation and response prediction, a preclinical efficacy study in Calu-3 xenograft was conducted with the 4 therapeutic antibodies, cetuximab, trastuzumab, anti-HER3 and anti-IGF1R, in comparison to the control antibody omalizumab ().
Figure 3. Preclinical efficacy study of different therapeutic antibodies in Calu-3 xenograft. (A) Tumor growth kinetics of the Calu-3 xenograft model treated with anti-IGF1R mAb, anti-HER3 mAb, cetuximab (anti-HER1 mAb), and trastuzumab (anti-HER2 mAb). The antibody omalizumab was used as a negative control. Animals per control group: n = 8. Values are given as mean ± s.d.. ** P < 0.01, *** P < 0.001, t-test. (B-F) Tumor tissue slices of the control and treatment groups were stained for Ki67 to monitor the tumor cell proliferation rate. Magnification of the tissue slices: x400. Scale bar: 50 µm.
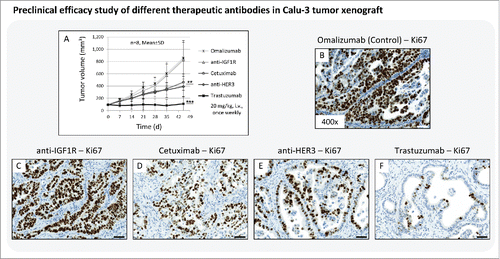
The results showed no treatment effect with anti-IGF1R on tumor growth, and the resultant growth curve was nearly congruent to the control group. Tumors treated with cetuximab and anti-HER3 mAb showed significant growth inhibition in comparison to control, but nevertheless tumor volume increased over time. Only treatment with trastuzumab exerted tumor growth inhibition over the complete treatment period (). Subsequent Ki67 staining of Calu-3 tumor tissue slices confirmed the in vivo results of the preclinical efficacy study. The tumor cell proliferation rate was nearly identical between control and the anti-IGF1R-treated group (). The treatment with cetuximab and anti-HER3 mAb decreased tumor cell proliferation by ∼50% (), whereas trastuzumab reduced cell division rate almost entirely ().
The preclinical efficacy study was able to confirm the epi-fluorescence imaging results. The efficacy data could perfectly reproduce the previous response predictions obtained by fluorescence imaging.
Receptor binding site evaluation of 2 anti-HER2 therapeutic antibodies in Calu-3 NSCLC xenograft
Receptor binding site evaluation using in vivo and ex vivo epi-fluorescence imaging
In addition to evaluating the receptor status and binding behavior of fluorescent-labeled therapeutic antibodies in tumor xenografts, the combination of in vivo and ex vivo fluorescence imaging can be used to determine the binding site and specificity of different antibody formats directed against the same tumor cell receptor. The evaluation of such characteristics is of great value in understanding their mode of action in more detail, and it may open the possibility of selecting potential combination strategies for more efficient tumor therapy.
To evaluate the application of fluorescence imaging for this purpose, we performed “blocking experiments” and analyzed the binding behavior of the fluorescent-labeled anti-HER2 antibodies trastuzumab and pertuzumab (). Both molecules are excellently suited for this application because binding characteristics were already described in the literatureCitation44 and their combination therapy is successfully used in clinical practice.Citation45-47
Figure 4. Application of fluorescence imaging for epitope mapping of 2 anti-HER2 specific mAbs. (A-C) Basic principle of performed imaging experiment. (D-K) Binding site evaluation of fluorescent labeled trastuzumab-Cy5 (T-Cy5) and Pertuzumab-Cy5 (P-Cy5) in Calu-3 tumor xenografts. Pre-treatment was performed with unlabeled therapeutic anti-HER2 mAbs (20 mg/kg, i.v., 24h), followed by injection of fluorescence labeled compound (2 mg/kg, i.v., 24h) and in vivo and ex vivo fluorescence imaging. (L,M) Diagrams illustrate in vivo fluorescence imaging results. T = trastuzumab, P = pertuzumab. Animals per group: n = 3. Values are given as mean ± s.d.. ** P < 0.01, t-test. All plots were compared to the in vivo signal intensity value of the control antibody Omalizumab-Cy5 (red dotted line; 2 mg/kg, i.v., 24h; 0.1 scaled counts / sec). Magnification of the tissue slices: x400. Scale bar: 50 µm.
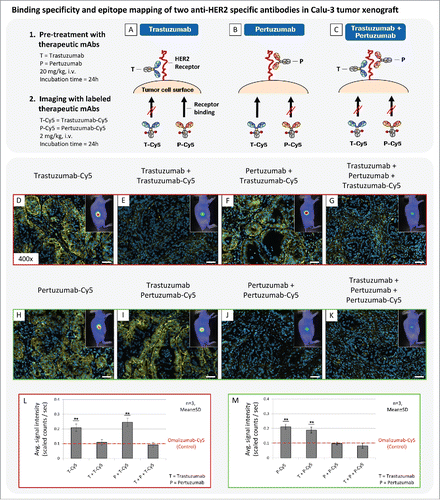
The in vivo imaging results demonstrated that fluorescent-labeled trastuzumab and pertuzumab showed identical penetration and accumulation behavior in Calu-3 tumor xenograft after 24 h of incubation (). In the subsequent ex vivo analysis, identical binding specificity to HER2 and fluorescence signal intensities were visible for both antibodies. In contrast, pre-treatment of Calu-3 tumors with trastuzumab or pertuzumab cover corresponding HER2 epitopes and lead to significant reduction of fluorescence signal intensity for the labeled respective anti-HER2 antibodies (). Resultant in vivo signal intensities of uniform treatment schedules, such as trastuzumab followed by trastuzumab-Cy5, were comparable to the control mAb, omalizumab-Cy5. Only the combination of different antibodies, such as trastuzumab followed by pertuzumab-Cy5, showed strong in vivo signal intensities and specific receptor binding at cellular resolution (). Furthermore, combined in vivo injection of trastuzumab and pertuzumab, each labeled with a different fluorescence label (Alexa488 and Alexa647), revealed simultaneous binding of both antibodies to HER2 (Fig. S1) and thus confirmed previous imaging results.
These experiments clearly demonstrate the potential of in vivo and ex vivo fluorescence imaging to determine the binding behavior of the 2 anti-HER2 antibodies regarding receptor specificity and binding site. Both antibodies recognize different epitopes of the extracellular HER2 domain, but no significant differences in binding affinity and specificity were visible. As a result, comparable therapeutic treatment effects should be achieved with both antibodies in the Calu-3 xenograft model.
Comparison of obtained epi-fluorescence imaging results with preclinical efficacy data
In order to demonstrate the relevance of imaging data for subsequent efficacy studies, the therapeutic treatment effect of trastuzumab and pertuzumab was monitored in Calu-3 xenograft. Preclinical efficacy data confirm the findings of our imaging experiments. In comparison to the control group, both anti-HER2 antibodies induced significant tumor growth inhibition over the complete treatment period (). The following histological ex vivo Ki67 analysis of tumor tissue slices showed almost entire reduction of Calu-3 cell proliferation ().
Figure 5. Preclinical efficacy study of 2 anti-HER2 specific antibodies in Calu-3 xenograft. (A) Tumor growth kinetics of Calu-3 xenograft treated with trastuzumab and pertuzumab (20 mg/kg, i.v., once weekly). The therapeutic antibody omalizumab was used as negative control. Animals per group: n = 8. Values are given as mean ± s.d.. *** P < 0.001, t-test. (B-D) Tumor tissue slices of the control and treatment groups were stained for Ki67 to monitor tumor cell proliferation. Magnification of the tissue slice: x400. Scale bar: 50 µm.
This example proves again that fluorescence imaging possesses the ability to address and answer relevant questions that arise during preclinical development, and may be helpful in the design of clinical studies.
Evaluation of tumor saturation to determine optimal treatment dosages and schedule
Analysis of tumor saturation using in vivo and ex vivo epi-fluorescence imaging
Another very important aspect in drug development is the determination of the optimal treatment dosage and schedule for preclinical efficacy studies. Conventionally, the therapeutic treatment response of escalating antibody dosages are investigated in different treatment groups. Tumor growth inhibition and performed blood analysis are used to determine the PK and PD behavior of the agent, and select the optimal treatment dosage for further efficacy studies. This procedure is very labor-intensive and time-consuming, requires large numbers of experimental animals, which conflicts with the “3R” concept of reducing the use of animals, and, most importantly, provides no direct insight into the drug-target interaction.
A straightforward alternative is the use of in vivo and ex vivo fluorescence imaging in studies of tumor saturation kinetics, the results of which can aid with the selection of the optimal treatment dosage for preclinical efficacy studies. To evaluate fluorescence imaging for this purpose, we performed “blocking experiments,” similar to those described above, by pretreating Calu-3-bearing mice for 24 h with escalating trastuzumab dosages of 1, 3, 10, 20 mg/kg, and subsequently analyzed the binding behavior of fluorescent-labeled trastuzumab to HER2.
The in vivo imaging results showed decreasing fluorescence signal intensities in tumor xenografts depending on the trastuzumab concentration (). Pretreatment with 1, 3 and 10 mg/kg trastuzumab constantly reduced signal intensity in Calu-3 tumors (). Nevertheless, only the antibody concentration of 20 mg/kg trastuzumab lead to complete coverage of all available HER2 receptors (), and showed no significant differences in signal intensity compared to circulating control antibody omalizumab-Cy5 (). Subsequent ex vivo tumor tissue analysis confirmed the in vivo imaging results ().
Figure 6. (A-I) The application of in vivo and ex vivo fluorescence imaging to determine optimal treatment dosage. (A,B) Basic principle of performed imaging experiment. (C-H) In vivo and ex vivo fluorescence imaging was used to visualize the binding specificity of trastuzumab-Cy5 (2 mg/kg, i.v., 24h) in Calu-3 tumors pretreated with different concentration of trastuzumab (0, 1, 3, 10, 20 mg/kg, i.v., 24h). (I) Diagram illustrate in vivo fluorescence imaging results. O = omalizumab, T = trastuzumab. Animals per group: n = 10. Values are given as mean ± s.d.. ** P < 0.01, *** P < 0.001, t-test. All plots were compared to the in vivo signal intensity value of the control mAb Omalizumab-Cy5 (red dotted line; 2 mg/kg, i.v., 24h). (J-K) Preclinical efficacy study of different trastuzumab concentration in Calu-3 xenograft. (J) Tumor growth kinetics of Calu-3 xenograft treated with escalating trastuzumab dosages (0, 1, 3, 10, 20 mg/kg, i.v., once weekly). The therapeutic mAb omalizumab was used as negative control (20 mg/kg, i.v., once weekly). Animals per group: n = 8. Values are given as mean ± s.d.. ** P < 0.01, *** P < 0.001, t-test. (M-K) Tumor tissue slices of the control and treatment groups were stained for Ki67 to monitor tumor cell proliferation. Magnification of the tissue slice: x400. Scale bar: 50 µm.
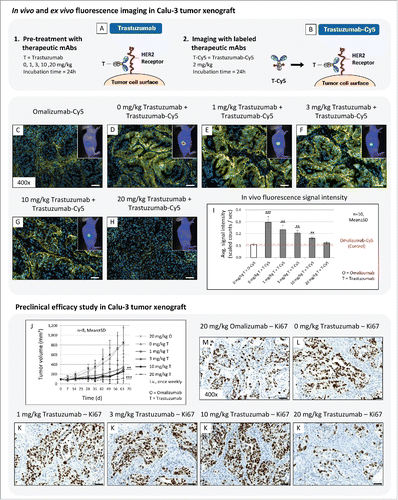
Based on the in vivo and ex vivo fluorescence imaging results, we hypothesized that an antibody concentration of 20 mg/kg trastuzumab is the optimal treatment dosage for anti-HER2 tumor therapy in the Calu-3 xenograft model, and thus may provide the most efficient tumor growth inhibition over time. In contrast, when lower treatment dosages of trastuzumab are used for tumor therapy, the HER2 receptors are incompletely covered and, as a result, signaling of tumor cell proliferation is still active or only insufficiently inhibited.
In addition to determining treatment dosages, we investigated the optimal application schedule. For this, we injected different concentrations of trastuzumab-Cy5 (1, 3, 10, 20, 30 mg/kg) intravenously (i.v.) to investigate the dose and time-dependent penetration, distribution, and clearing process in Calu-3 xenografts. These imaging data were then correlated with corresponding tumor growth kinetics (Fig. S3). We found a clear relationship between fluorescence signal intensity in tumor tissue and tumor growth inhibition. The results indicate that a trastuzumab treatment schedule of once weekly to be optimal for dosages of 20 mg/kg antibody. A detailed description of how the dose selection was performed is included in Fig. S3.
Comparison of epi-fluorescence imaging results with preclinical efficacy data
In order to prove our hypothesis regarding the selecting of optimal treatment dosage for Calu-3 tumor therapy, we performed a preclinical efficacy study. The results confirmed our assumption and demonstrated superiority of the selected treatment dosage compared to lower trastuzumab concentration (). The selected treatment dosage of 20 mg/kg trastuzumab induced a nearly complete tumor stasis in Calu-3 xenograft. In contrast, tumor therapy with antibody concentrations of 1, 3 and 10 mg/kg trastuzumab lead to significant growth inhibition compared to the control and untreated group, but tumors started regrowth at day 28 for 1 mg/kg and day 42 for 3 and 10 mg/kg trastuzumab treatment. Subsequent Ki67 staining of tumor tissue slices demonstrated high proliferation rates in all Calu-3 tissue samples, excluding tumors treated with 20 mg/kg trastuzumab. The latter showed marked reduction in tumor cell proliferation.
Discussion
Demonstration of the expression of a TAA is a prerequisite for a subsequent efficacy study with the relevant antibody targeting this antigen. Such antigen detection studies are conventionally performed with tissue slides prepared from explanted tumor tissue. Commercially available and paraffin-permeable detection antibodies are used for IHC analysis to assess tumor antigen expression. Considering the variation in staining intensities when antibodies from different vendors were used and the problems associated with optimizing fixation and antigen-retrieval procedures, this approach may not be optimal for tumor cell receptor evaluation. Furthermore, an enhanced staining intensity recorded by optimization of the different parameters may not reflect the real situation because epitope masking can still be relevant. The tissue fixation and staining problems that occur with in situ hybridization and IHC of human tissue samples were addressed in previous work.Citation9,10,12,48-50
We confirmed the IHC results in a preclinical NSCLC tumor xenograft model, illustrating high HER2 staining variability for different anti-HER2 detection antibodies, with staining intensities ranging from 0+ to 3+, and also the substantial effect of the selected antigen retrieval buffer on receptor staining results. In addition to these fixation and antigen retrieval problems, the fact that TAAs can be modulated during the growth of tumor cells must be taken into consideration.Citation51,52 In this context, it was shown that extracellular receptor domains can be shedCitation53,54 and found in serumCitation55-58 or internalized into tumor cells.Citation59 Furthermore, different disintegrin and metalloproteinases (ADAMs), which are considered to be responsible for shedding,Citation60,61 can cleave antibodies, thereby compromising anti-tumoral efficacy.Citation62,63 Thus, TAA expression should be verified in vivo at the initiation of antibody therapy to allow better predictions for preclinical studies.
Compared to conventional histology, visualization and analysis of TAAs in vivo has many advantages. Most importantly, the tumor antigens can be considered to be in its natural state, and thus fixation problems and insufficient antigen retrieval are not relevant. Numerous studies with radiolabeled antibodies or fragments thereof have been conducted to determine their PK behavior and TAA expression using non-invasive positron emission tomography (PET) imaging or ex vivo tumor tissue autoradiography.Citation64-68 Although both imaging modalities are widely used for preclinical and clinical applications, they possess clear limitations for TAA analysis, such as long acquisition time, restricted half-life of radioactive isotopes, which limit long-term investigations, and the missing multispectral ability of in vivo PET imaging. In addition, the ex vivo autoradiography analysis possesses insufficient spatial resolution to detect drug-target interaction on a cellular level. However, the demonstration of specific binding to tumor cells is a fundamental aspect and prerequisite for successful evaluation of TAAs and selection of the right tumor model for preclinical efficacy studies needed to achieve optimal treatment results.
In contrast, our results clearly demonstrate that in vivo application of fluorescence-labeled therapeutic antibodies in combination with in vivo and ex vivo fluorescence imaging modalities is a valuable tool for tumor-target evaluation, and to determine the PK and PD properties of therapeutic monoclonal antibodies in preclinical settings. Non-invasive longitudinal in vivo imaging studies of fluorescence-labeled therapeutic antibodies for up to several weeks provide detailed information regarding drug behavior, especially penetration, accumulation and retention time in tumor tissue. Furthermore, specific accumulation of the labeled antibody in different mouse organs, such as liver, spleen or kidney, might give an indication regarding antibody cross reactivity and potential side effects. Nevertheless, macroscopic in vivo fluorescence imaging studies should always be confirmed by ex vivo multispectral fluorescence histology. Such microscopic analysis of explanted tumor tissue delivers valuable high-resolution information of morphological and molecular nature, and allows detection of unspecific mAb binding to murine tissue components, accumulation in central necrosis and, most importantly, verifies antibody-target interaction. The latter is a prerequisite for anti-tumor efficacy.
By using this synergistic in vivo and ex vivo fluorescence imaging strategy, we were able to monitor the interaction of 4 fluorescence labeled therapeutic antibodies with their relevant TAAs as single antibodies (same label) as well as antibody mixtures (different label). The latter approach is of great importance when analyzing the in vivo interaction of different therapeutic compounds to understand their mode of action for combination therapy. The predictions made from the imaging results regarding tumor treatment response correlated perfectly with tumor growth inhibition observed in the preclinical efficacy study. The labeled anti-HER2 antibody trastuzumab showed the highest TAA affinity, specificity, and signal intensity and demonstrated complete tumor growth inhibition, whereas labeled anti-IGF1R showed no specific binding and no treatment efficacy. In contrast to the imaging results, conventional immunohistochemical evaluation of TAAs identified 3 tumor cell receptors (HER2, HER3 and IGF1R) as potential targets for tumor therapy. This result was not verified by our preclinical efficacy data, and provides a clear confirmation that in vivo evaluation of TAAs with fluorescence-labeled therapeutic antibodies is more indicative regarding treatment response, and thus might also possess better prediction accuracy. This aspect is relevant for preclinical research, and may also influence tumor target evaluation in patients. We believe that clinical translation of the presented methodology would improve existing diagnostic and theranostic approaches and support personalized medicine strategies.Citation69 The application of fluorescence-labeled therapeutic antibodies at microdosing for in vivo evaluation of TAAs followed by microscopic fluorescence analysis of needle biopsy material could complement the existing histological “gold-standards” (IHC and FISH) and improve decision making for appropriate treatment strategies.
In addition to tumor penetration/distribution analysis and evaluation of TAAs, we used the combined application of in vivo and ex vivo fluorescence imaging for binding site evaluation of anti-HER2 antibodies (trastuzumab and pertuzumab) targeting the same tumor cell receptor, selected the optimal tumor xenograft model for preclinical efficacy studies and performed tumor saturation kinetics with different mAb concentrations to determine the optimal treatment dosage and application schedule for effective antibody therapy. All the imaging data demonstrated excellent correlation with preclinical efficacy outcome, which confirms the high significance and the great potential of fluorescence imaging for the preclinical development and evaluation of new monoclonal antibodies.
Another important aspect that should be considered is the possibility of combining the introduced method with other fluorescence or bioluminescence imaging techniques. For example, the PK behavior of antibodies or fragments can be monitored non-invasively in the blood by measuring fluorescence signal intensities in the eye of mice.Citation70 Combination of fluorescence and bioluminescence can be measured simultaneously by using luciferase-transfected tumor cellsCitation71 or tumor cells equipped with split-luciferase.Citation72,73 The latter allows monitoring of apoptosis induction non-invasively. In addition, in the context with tumor morphology and vasculature, tumor penetration of labeled antibodies can be also assessed in its 3-dimensionality using light-sheet fluorescence microscopy in combination with optical tissue clearing methods.Citation74 Furthermore, ex vivo visualization of drug-target interaction in FFPE tumor tissue slices can be also combined with FISH to quantitatively determine HER2 gene amplification (Fig. S4). Such purposeful combination of macroscopic/microscopic in vivo and ex vivo fluorescence imaging methods open up new avenues for deep and holistic analysis of the complex interaction between therapeutic antibodies, tumor cells, tumor microenvironment and resulting treatment effects.
In summary, the in vivo application of fluorescence-labeled therapeutic antibodies in combination with in vivo and ex vivo fluorescence imaging modalities is an excellent and straightforward method to support the preclinical development and evaluation of monoclonal antibodies. Basic biological questions and relevant preclinical parameters, such as expression level of TAAs, drug-target interaction, selection of the most suitable xenograft model and most promising therapeutic antibody for tumor therapy, as well as dose and schedule optimization, can be perfectly addressed with the “in vivo histology” method described herein.
Materials and methods
Compounds and labeling
Trastuzumab (Herceptin®, anti-HER2; Roche), pertuzumab (Perjeta®, anti-HER2; Roche), cetuximab (Erbitux®, anti-HER1; Merck Serono), and omalizumab (Xolair®, anti-human IgE; Novartis) were used for imaging experiments and efficacy studies. The latter served as a negative control. The non-commercial therapeutic monoclonal IgG1 antibodies against HER3 (anti-HER3; Roche) and IGF1R (anti-IGF1R; Roche) were also tested. Antibodies were labeled in-house with the different Cyanine and Alexa fluorophores (Invitrogen, Darmstadt, Germany). Labels were attached via mono-reactive N-hydroxysuccinimide (NHS) ester to lysine residues with a labeling ratio of 3 fluorophores per molecule. The labeled antibodies were purified by dialysis and size-exclusion chromatography. Surface plasmon resonance analysis was performed to measure binding characteristics. Results indicated no significant differences in the kon and koff constants and the resulting binding affinity between labeled and non-labeled antibodies (data not shown).
Cell lines and culture
Calu-3 NSCLC tumor cells were cultured in RPMI-1640 medium and Calu-1 NSCLC tumor (10% FCS and 2 mM L-glutamine) and incubated at 37°C and 5% CO2. The viability of the Calu-3 cells was assessed using a Vi-Cell viability analyzer (Beckman Coulter, Krefeld, Germany) and reached 95%.
Tumor xenograft
Xenografts were generated by subcutaneous injection of Calu-3 and Calu-1 tumor cells (5 × 106 cells / 100 µl in phosphate-buffered saline) into the right flank of anesthetized female SCID hairless outbred (SHO) mice (age: 6–8 weeks, weight: 20–25 g; Charles River, Sulzfeld, Germany). The implanted tumors grew until they reached a tumor volume (TV) of 100 mm3. Tumor bearing animals were then randomized and divided in individual groups. All experimental study protocols were reviewed and approved by local government. The animals were handled according to committed guidelines of the Society of Laboratory Animals and Federation for Laboratory Animal Science Associations. The animal facility has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
In vivo epi-fluorescence imaging
In vivo fluorescence imaging experiments were performed with a 2-dimensional planar-reflectance imaging system (Maestro®; PerkinElmer, Rodgau, Germany). Fluorescent-labeled antibodies (2 mg/kg) were injected intravenously (i.v.) into Calu-3 tumor-bearing mice (TV = 80–150 mm3, n = 3–10 SHO mice/group). Then, fluorescence signal intensities were measured non-invasively 24 h after injection. For “blocking experiments,” which were utilized for epitope mapping and tumor saturation kinetics, animals were pre-treated for about 24 h with single therapeutic antibodies (for epitope mapping: 20 mg/kg trastuzumab or pertuzumab, i.v.; for tumor saturation kinetics: 1, 3, 10, 20 mg/kg trastuzumab, i.v.) or a mixture of both mAbs (for epitope mapping: 20 mg/kg trastuzumab and pertuzumab each, i.v.) followed by fluorescence-labeled trastuzumab or pertuzumab. After 24 h of incubation, fluorescence imaging was performed as described before. We further utilized different concentration of trastuzumab-Cy5 (1, 3, 10, 20, 30 mg/kg, i.v.) to monitor the penetration and retention time in Calu-3 at several time points (7, 14, 21, 28 days).
Animals received inhalation anesthesia (2 Vol% isoflurane; Delta Select, Pfullingen, Germany) and were placed on a heating plate (37°C) inside the imaging system. Subsequently, a bright field image of the animal and a multispectral image cube, consisting of a series of single 12-bit fluorescence images at different wavelengths, were created. Following this, the penetration, distribution, and binding specificity of labeled therapeutic antibodies to the relevant TAA were analyzed using ex vivo fluorescence histology.
Overall performance of the in vivo imaging system allows a very simple, fast and high-throughput measurement of multispectral image cubes. Obtained imaging data were spectrally unmixed and fluorescence signals from different fluorophores separated. Unmixed grayscale component images were used to quantify the fluorescence signal intensity in selected tumor region. The grayscale values were normalized, converted into pseudo-color, and overlaid with the monochrome bright field image of the animal. The software ImageJ and GIMP (both open source) were used for image fusion and preparation of the individual images.
Preclinical efficacy studies
To evaluate the significance of the in vivo and ex vivo fluorescence imaging results regarding predication of therapeutic responses, we performed preclinical efficacy studies in Calu-1 and Calu-3 tumor xenograft (TV = 80–150 mm3, n = 8–10 SHO mice/group) using unlabeled therapeutic antibodies [cetuximab (anti-HER1), trastuzumab (anti-HER2), pertuzumab (anti-HER2), anti-HER3, and anti-IGF1R]. The antibody omalizumab was used as a negative control. All animals were treated i.v. once weekly with an appropriate antibody concentration of 20 mg/kg. Animal conditions were checked on a daily basis and tumor growth was measured once weekly via caliper.
Histological tissue analysis
Upon completion of in vivo imaging experiments or efficacy studies, mice were sacrificed and explanted tumor tissue was fixed in 10% buffered formalin (VWR International, Ismaning, Germany) for about 12 hours at 4°C in the dark, and then dehydrated in a graded ethanol and xylene series (3 × 70%, 2 × 95%, 2 × 100% ethanol and 2 × 100% xylene for 1h each). Afterwards, tumor specimens were incubated (4 × paraffin for 30 min each) and embedded in paraffin. All incubation steps were carried out using the Tissue Tek® VIP Vacuum Infiltration Processor (Sakura Finetek, Heppenheim, Germany). The embedded tumor samples were cut into serial tissue slices and further prepared for conventional immunohistochemistry or ex vivo fluorescence imaging.
Immunohistochemistry
Serial tissue were de-paraffinized in a declining alcoholic row of xylene and ethanol (3 × 100% xylene; 2 × 100%, 2 × 95%, 2 × 80%, 2 × 70% ethanol for 2 min each; and deionized water for 20 sec). Afterwards, antigen retrieval, blocking of free peroxidases, and a protein block were conducted. Various primary detection antibodies were used for IHC staining of different tumor antigens (HER1, 2 and 3, IGF1R) and proliferation marker (Ki-67). The primary antibody was captured by a secondary antibody-chromogene detection system. Additionally, counterstaining of the nucleus via hematoxylin was conducted. The stained IHC slices were dehydrated in a graded alcoholic row of ethanol and xylene (2 × 70%, 2 × 80%, 2 × 95%, 2 × 100% ethanol and 3 × 100% xylene for 2 min each), covered with eukitt mounting medium and eventually locked air bubble-free with a cover slip. The details of the individual IHC protocols are included in the supplementary materials.
Ex vivo fluorescence imaging
For the analysis of fluorescent-labeled tissue section, the slices were de-paraffinized in a declining alcoholic row of xylene and ethanol (as described in the previous section). Afterwards, the slices were covered with a DAPI-containing mounting medium (Fluoro-Gel II, Electron Microscopy Sciences, Hatfield, USA) and locked with a cover slide. All bright filed and fluorescence tissue slides were scanned with the Pannoramic 250 Flash scanner (3D Histech, Budapest, Hungary). Image visualization and analysis were accomplished with the corresponding slide scanner software (Pannoramic viewer).
Disclosure of potential conflicts of interest
The authors M.D., U.H. and W.S. are employees of Roche Diagnostics GmbH.
Supplemental_Data.docx
Download MS Word (1.3 MB)Acknowledgments
We are thankful to A. Wessner and R. Vogel for fluorescence labeling, U. Jucknischke for performing the Biacore studies, H. Sade and A. Zabarella for histological support and F. Osl for cell culturing. We are indebted to G. Niederfellner, K. Bosslet, F. Herting and C. Rommel for constant support and A. Rehemtulla for excellent cooperation.
References
- Shuptrine CW, Surana R, Weiner LM. Monoclonal antibodies for the treatment of cancer. Semin Cancer Biol 2012; 22:3-13; PMID:22245472; http://dx.doi.org/10.1016/j.semcancer.2011.12.009
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/10.1038/nrc3236
- Reichert JM. Antibodies to watch in 2015. Mabs 2015; 7:1-8; PMID:25484055; http://dx.doi.org/10.4161/19420862.2015.988944
- Reichert JM. Antibodies to watch in 2016. Mabs 2016; 8:197-204; PMID:26651519; http://dx.doi.org/10.1080/19420862.2015.1125583
- Diamandis M, White NMA, Yousef GM. Personalized Medicine: Marking a New Epoch in Cancer Patient Management. Mol Cancer Res 2010; 8:1175-87; PMID:20693306; http://dx.doi.org/10.1158/1541-7786.MCR-10-0264
- Duffy MJ, Crown J. Companion biomarkers: paving the pathway to personalized treatment for Cancer. Clin Chem 2013; 59:1447-56; PMID:23656699; http://dx.doi.org/10.1373/clinchem.2012.200477
- Kalia M. Personalized oncology: Recent advances and future challenges. Metab-Clin Exp 2013; 62:S11-S4; PMID:22999010; http://dx.doi.org/10.1016/j.metabol.2012.08.016
- Kalia M. Biomarkers for personalized oncology: recent advances and future challenges. Metab-Clin Exp 2015; 64:S16-S21; PMID:25468140; http://dx.doi.org/10.1016/j.metabol.2014.10.027
- Bussolati G, Leonardo E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J Clin Pathol 2008; 61:1184-92; PMID:18326011; http://dx.doi.org/10.1136/jcp.2007.047720
- Anagnostou VK, Welsh AW, Giltnane JM, Siddiqui S, Liceaga C, Gustavson M, Syrigos KN, Reiter JL, Rimm DL. Analytic variability in immunohistochemistry biomarker studies. Cancer Epidemiol Biomarkers Prev 2010; 19:982-91; PMID:20332259; http://dx.doi.org/10.1158/1055-9965.EPI-10-0097
- Paavilainen L, Edvinsson A, Asplund A, Hober S, Kampf C, Ponten F, Wester K. The Impact of Tissue Fixatives on Morphology and Antibody-based Protein Profiling in Tissues and Cells. J Histochem Cytochem 2010; 58:237-46; PMID:19901271; http://dx.doi.org/10.1369/jhc.2009.954321
- Engel KB, Moore HM. Effects of Preanalytical Variables on the Detection of Proteins by Immunohistochemistry in Formalin-Fixed, Paraffin-Embedded Tissue. Arch Pathol Lab Med 2011; 135:537-43; PMID:21526952; http://dx.doi.org/10.1043/2010-0702-RAIR.1
- Fairley JA, Gilmour K, Walsh K. Making the Most of Pathological Specimens: Molecular Diagnosis in Formalin-Fixed, Paraffin Embedded Tissue. Current Drug Targets 2012; 13:1475-87; PMID:22974391; http://dx.doi.org/10.2174/138945012803530125
- Howat WJ, Wilson BA. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods 2014; 70:12-9; PMID:24561827; http://dx.doi.org/10.1016/j.ymeth.2014.01.022
- D'Amico F, Skarmoutsou E, Stivala F. State of the art in antigen retrieval for immunohistochemistry. J Immunol Methods 2009; 341:1-18; PMID:19063895; http://dx.doi.org/10.1016/j.jim.2008.11.007
- Shi S-R, Shi Y, Taylor CR. Antigen Retrieval Immunohistochemistry: Review and Future Prospects in Research and Diagnosis over Two Decades. J Histochem Cytochem 2011; 59:13-32; PMID:21339172; http://dx.doi.org/10.1369/jhc.2010.957191
- Burns JA, Li Y, Cheney CA, Ou Y, Franlin-Pfeifer LL, Kuklin N, Zhang ZQ. Choice of Fixative Is Crucial to Successful Immunohistochemical Detection of Phosphoproteins in Paraffin-embedded Tumor Tissues. J Histochem Cytochem 2009; 57:257-64; PMID:19001637; http://dx.doi.org/10.1369/jhc.2008.952911
- Gruchy JR, Barnes PJ, Hache KAD. CytoLyt (R) Fixation and Decalcification Pretreatments Alter Antigenicity in Normal Tissues Compared With Standard Formalin Fixation. Appl Immunohistochem Mol Morphol 2015; 23:297-302; PMID:25265430; http://dx.doi.org/10.1097/PAI.0000000000000082
- Chu WS, Furusato B, Wong K, Sesterhenn IA, Mostofi FK, Wei MQ, Zhu Z, Abbondanzo SL, Liang Q. Ultrasound-accelerated formalin fixation of tissue improves morphology, antigen and mRNA preservation. Modern Pathol 2005; 18:850-63; PMID:15605077; http://dx.doi.org/10.1038/modpathol.3800354
- Nietner T, Jarutat T, Mertens A. Systematic comparison of tissue fixation with alternative fixatives to conventional tissue fixation with buffered formalin in a xenograft-based model. Virchows Arch 2012; 461:259-69; PMID:22814649; http://dx.doi.org/10.1007/s00428-012-1248-5
- Bordeaux J, Welsh AW, Agarwal S, Killiam E, Baquero MT, Hanna JA, Anagnostou V, Rimm D. Antibody validation. Biotechniques 2010; 48:197-209; PMID:20359301; http://dx.doi.org/10.2144/000113382
- Pauly D, Hanack K. How to avoid pitfalls in antibody use. F1000Research 2015; 4:691; PMID:26834988; http://dx.doi.org/10.12688/f1000research.6894.1
- Ward JM, Rehg JE. Rodent Immunohistochemistry: Pitfalls and Troubleshooting. Vet Pathol 2014; 51:88-101; PMID:24078006; http://dx.doi.org/10.1177/0300985813503571
- Hashizume K, Hatanaka Y, Kamihara Y, Kato T, Hata S, Akashi S, Kato T, Koyatsu J, Tani Y, Tsujimoto M, et al. Interlaboratory comparison in HercepTest assessment of HER2 protein status in invasive breast carcinoma fixed with various formalin-based fixatives. Appl Immunohistochem Mol Morphol 2003; 11:339-44; PMID:14663361; http://dx.doi.org/10.1097/00129039-200312000-00011
- Ballou B, Ernst LA, Waggoner AS. Fluorescence imaging of tumors in vivo. Curr Med Chem 2005; 12:795-805; PMID:15853712; http://dx.doi.org/10.2174/0929867053507324
- Levenson RM, Mansfield JR. Multispectral imaging in biology and medicine: Slices of life. Cytometry Part A 2006; 69A:748-58; PMID:16969820; http://dx.doi.org/10.1002/cyto.a.20319
- Zhou L, El-Deiry WS. Multispectral Fluorescence Imaging. J Nucl Med 2009; 50:1563-6; PMID:19759119; http://dx.doi.org/10.2967/jnumed.109.063925
- Mansfield JR. Multispectral imaging: a review of its technical aspects and applications in anatomic pathology. Vet Pathol 2014; 51:185-210; PMID:24129898; http://dx.doi.org/10.1177/0300985813506918
- Leblond F, Davis SC, Valdes PA, Pogue BW. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J Photochem Photobiol B-Biol 2010; 98:77-94; PMID:20031443; http://dx.doi.org/10.1016/j.jphotobiol.2009.11.007
- Koenig A, Herve L, Josserand V, Berger M, Boutet J, Da Silva A, Dinten JM, Peltié P, Coll JL, Rizo P. In vivo mice lung tumor follow-up with fluorescence diffuse optical tomography. J Biomed Opt 2008; 13; PMID:18315357; http://dx.doi.org/10.1117/1.2884505
- Yang M, Baranov E, Jiang P, Sun FX, Li XM, Li LN, Hasegawa S, Bouvet M, Al-Tuwaijri M, Chishima T, et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci U S A 2000; 97:1206-11; PMID:10655509; http://dx.doi.org/10.1073/pnas.97.3.1206
- Ntziachristos V, Tung C-H, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med 2002; 8:757-60; PMID:12091907; http://dx.doi.org/10.1038/nm729
- Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 1999; 17:375-8; PMID:10207887; http://dx.doi.org/10.1038/7933
- Mahmood U, Tung CH, Bogdanov A, Weissleder R. Near-infrared optical imaging of protease activity for tumor detection. Radiology 1999; 213:866-70; PMID:10580968; http://dx.doi.org/10.1148/radiology.213.3.r99dc14866
- von Wallbrunn A, Hoeltke C, Zuehlsdorf M, Heindel W, Schaefers M, Bremer C. In vivo imaging of integrin alpha nu beta(3) expression using fluorescence-mediated tomography. Eur J Nucl Med Mol Imaging 2007; 34:745-54; PMID:17131149; http://dx.doi.org/10.1007/s00259-006-0269-1
- Ke S, Wen XX, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, Li C. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res 2003; 63:7870-5; PMID:14633715; http://dx.doi.org/10.1371/journal.pone.0046255
- Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grötzinger C. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat Biotechnol 2001; 19:327-31; PMID:11283589; http://dx.doi.org/10.1038/86707
- Bullok KE, Maxwell D, Kesarwala AH, Gammon S, Prior JL, Snow M, Stanley S, Piwnica-Worms D. Biochemical and in vivo characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. Biochemistry 2007; 46:4055-65; PMID:17348687; http://dx.doi.org/10.1021/bi061959n
- Ntziachristos V, Schellenberger EA, Ripoll J, Yessayan D, Graves E, Bogdanov A, Josephson L, Weissleder R. Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc Natl Acad Sci U S A 2004; 101:12294-9; PMID:15304657; http://dx.doi.org/10.1073/pnas.0401137101
- Oliveira S, Cohen R, Stigter-van Walsum M, van Dongen GAMS, Elias SG, van Diest PJ, Mali W, van Bergen En Henegouwen PM. A novel method to quantify IRDye800CW fluorescent antibody probes ex vivo in tissue distribution studies. Ejnmmi Res 2012; 2(1):50,1-9; PMID:23009555; http://dx.doi.org/10.1186/2191-219X-2-50
- Suzuki T, Miyazaki C, Ishii-Watabe A, Tada M, Sakai-Kato K, Kawanishi T, Kawasaki N. A fluorescent imaging method for analyzing the biodistribution of therapeutic monoclonal antibodies that can distinguish intact antibodies from their breakdown products. Mabs 2015; 7:759-69; PMID:25891896; http://dx.doi.org/10.1080/19420862.2015.1038683
- Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009; 69:9330-6; PMID:19934333; http://dx.doi.org/10.1158/0008-5472.CAN-08-4597
- Peterson NC, Wilson GG, Huang Q, Dimasi N, Sachsenmeier KF. Biodistribution analyses of a near-infrared, fluorescently labeled, bispecific monoclonal antibody using optical imaging. Comparative Med 2016; 66:90-9; PMID:27053562
- Fuentes G, Scaltriti M, Baselga J, Verma CS. Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res 2011; 13(3):R54,1-9; PMID:21600050; http://dx.doi.org/10.1186/bcr2888
- Maly JJ, Macrae ER. Pertuzumab in Combination with Trastuzumab and Chemotherapy in the Treatment of HER2-Positive Metastatic Breast Cancer: Safety, Efficacy, and Progression Free Survival. Breast cancer 2014; 8:81-8; PMID:24855372; http://dx.doi.org/10.4137/BCBCR.S9032
- Kawajiri H, Takashima T, Kashiwagi S, Noda S, Onoda N, Hirakawa K. Pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer. Exp Rev Anticancer Ther 2015; 15:17-26; PMID:25494663; http://dx.doi.org/10.1586/14737140.2015.992418
- Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015; 372:724-34; PMID:25693012; http://dx.doi.org/10.1056/NEJMoa1413513
- Ezaki T. Antigen retrieval: Its significance and drawbacks in immunohistochemistry. Acta Anatomica Nipponica 1996; 71:615-28; PMID:9038004
- van den Broek LJCM, van de Vijver MJ. Assessment of problems in diagnostic and research immunohistochemistry associated with epitope instability in stored paraffin sections. Appl Immunohistochem Mol Morphol 2000; 8:316-21; PMID:11127924; http://dx.doi.org/10.1097/00129039-200012000-00009
- Riechers A, Bosserhoff AK. Pitfalls in immunohistochemistry - a recent example. Int J Clin Exp Patho 2012; 5:137-9; PMID:22400073
- Zatovicova M, Sedlakova O, Svastova E, Ohradanova A, Ciampor F, Arribas J, Pastorek J, Pastorekova S. Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17. Br J Cancer 2005; 93:1267-76; PMID:16278664; http://dx.doi.org/10.1038/sj.bjc.6602861
- Schirrmeister W, Gnad T, Wex T, Higashiyama S, Wolke C, Naumann M, Lendeckel U. Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp Cell Res 2009; 315:3500-8; PMID:19665015; http://dx.doi.org/10.1016/j.yexcr.2009.07.029
- Liu PCC, Liu X, Li Y, Covington M, Wynn R, Huber R, Hillman M, Yang G, Ellis D, Marando C, et al. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol Ther 2006; 5:657-64; PMID:16627989; http://dx.doi.org/10.4161/cbt.5.6.2708
- Higashiyama S, Nanba D, Nakayama H, Inoue H, Fukuda S. Ectodomain shedding and remnant peptide signalling of EGFRs and their ligands. J Biochem 2011; 150:15-22; PMID:21610047; http://dx.doi.org/10.1093/jb/mvr068
- Friess T, Scheuer W, Hasmann M. Combination treatment with erlotinib and pertuzumab against human tumor xenografts is superior to monotherapy. Clin Cancer Res 2005; 11:5300-9; PMID:16033849; http://dx.doi.org/10.1158/1078-0432.CCR-04-2642
- Tse C, Gauchez A-S, Jacot W, Lamy P-J. HER2 shedding and serum HER2 extracellular domain: Biology and clinical utility in breast cancer. Cancer Treatment Rev 2012; 38:133-42; PMID:21549508; http://dx.doi.org/10.1016/j.ctrv.2011.03.008
- Tas F, Bilgin E, Karabulut S, Duranyildiz D. Clinical significance of serum epidermal growth factor receptor (EGFR) levels in patients with breast cancer. Cytokine 2015; 71:66-70; PMID:25259789; http://dx.doi.org/10.1016/j.cyto.2014.09.001
- Wang Z, Lu P, Liang Z, Zhang Z, Shi W, Cai X, Chen C. Increased insulin-like growth factor 1 receptor (IGF1R) expression in small cell lung cancer and the effect of inhibition of IGF1R expression by RNAi on growth of human small cell lung cancer NCI-H446 cell. Growth Factors 2015; 33:337-46; PMID:26430715; http://dx.doi.org/10.3109/08977194.2015.1088533
- Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 2014; 24:26-34; PMID:24295852; http://dx.doi.org/10.1016/j.tcb.2013.11.002
- Reiss K, Saftig P. The “A Disintegrin And Metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Semin Cell Dev Biol 2009; 20:126-37; PMID:19049889; http://dx.doi.org/10.1016/j.semcdb.2008.11.002
- van Kilsdonk JWJ, van Kempen LCLT, van Muijen GNP, Ruiter DJ, Swart GWM. Soluble adhesion molecules in human cancers: Sources and fates. Eur J Cell Biol 2010; 89:415-27; PMID:20227133; http://dx.doi.org/10.1016/j.ejcb.2009.11.026
- Brezski RJ, Vafa O, Petrone D, Tam SH, Powers G, Ryan MH, Luongo JL, Oberholtzer A, Knight DM, Jordan RE. Tumor-associated and microbial proteases compromise host IgG effector functions by a single cleavage proximal to the hinge. Proc Natl Acad Sci U S A 2009; 106:17864-9; PMID:19815504; http://dx.doi.org/10.1073/pnas.0904174106
- Brezski RJ, Kinder M, Grugan KD, Soring KL, Carton J, Greenplate AR, Petley T, Capaldi D, Brosnan K, Emmell E, et al. A monoclonal antibody against hinge-cleaved IgG restores effector function to proteolytically-inactivated IgGs in vitro and in vivo. Mabs 2014; 6:1265-73; PMID:25517311; http://dx.doi.org/10.4161/mabs.29825
- Dijkers ECF, Kosterink JGW, Rademaker AP, Perk LR, van Dongen GAMS, Bart J, de Jong JR, de Vries EG, Lub-de Hooge MN. Development and characterization of clinical-grade Zr-89-Trastuzumab for HER2/neu immunopet imaging. J Nucl Med 2009; 50:974-81; PMID:19443585; http://dx.doi.org/10.2967/jnumed.108.060392
- Zahnd C, Kawe M, Stumpp MT, de Pasquale C, Tamaskovic R, Nagy-Davidescu G, Dreier B, Schibli R, Binz HK, Waibel R, et al. Efficient Tumor Targeting with High-Affinity Designed Ankyrin Repeat Proteins: Effects of Affinity and Molecular Size. Cancer Res 2010; 70:1595-605; PMID:20124480; http://dx.doi.org/10.1158/0008-5472.CAN-09-2724
- van Dijk LK, Hoeben BAW, Kaanders JHAM, Franssen GM, Boerman OC, Bussink J. Imaging of epidermal growth factor receptor expression in head and neck cancer with SPECT/CT and In-111-Labeled Cetuximab-F(ab ')(2). J Nucl Med 2013; 54:2118-24; PMID:24136932; http://dx.doi.org/10.2967/jnumed.113.123612
- Viola-Villegas NT, Sevak KK, Carlin SD, Doran MG, Evans HW, Bartlett DW, Wu AM, Lewis JS. Noninvasive Imaging of PSMA in Prostate Tumors with Zr-89-Labeled huJ591 Engineered Antibody Fragments: The Faster Alternatives. Mol Pharm 2014; 11:3965-73; PMID:24779727; http://dx.doi.org/10.1021/mp500164r
- Marquez BV, Ikotun OF, Zheleznyak A, Wright B, Hari-Raj A, Pierce RA, Lapi SE. Evaluation of Zr-89-pertuzumab in Breast Cancer Xenografts. Mol Pharm 2014; 11:3988-95; PMID:25058168; http://dx.doi.org/10.1021/mp500323d
- Scheuer W, van Dam GM, Dobosz M, Schwaiger M, Ntziachristos V. Drug-based optical agents: infiltrating clinics at lower risk (vol 4, 138er3, 2012). Sci Transl Med 2012; 4(134):134ps11,1-5; PMID:22593172; http://dx.doi.org/10.1126/scitranslmed.3003572
- Dobosz M, Strobel S, Stubenrauch K-G, Osl F, Scheuer W. Noninvasive measurement of pharmacokinetics by near-infrared fluorescence imaging in the eye of mice. J Biomed Opt 2014; 19(1):1-8; PMID:24474508; http://dx.doi.org/10.1117/1.JBO.19.1.016022
- Poeschinger T, Renner A, Weber T, Scheuer W. Bioluminescence imaging correlates with tumor serum marker, organ weights, histology, and human DNA levels during treatment of orthotopic tumor xenografts with antibodies. Mol Imaging Biol 2013; 15:28-39; PMID:22528864; http://dx.doi.org/10.1007/s11307-012-0559-x
- Weber TG, Poeschinger T, Galban S, Rehemtulla A, Scheuer W. Noninvasive monitoring of pharmacodynamics and kinetics of a death receptor 5 antibody and its enhanced apoptosis induction in sequential application with doxorubicin. Neoplasia 2013; 15:863-74; PMID:23908588; http://dx.doi.org/10.1593/neo.13932
- Weber TG, Osl F, Renner A, Poeschinger T, Galban S, Rehemtulla A, Scheuer W. Apoptosis imaging for monitoring DR5 antibody accumulation and pharmacodynamics in brain tumors noninvasively. Cancer Res 2014; 74:1913-23; PMID:24509903; http://dx.doi.org/10.1158/0008-5472.CAN-13-3001
- Dobosz M, Ntziachristos V, Scheuer W, Strobel S. Multispectral fluorescence ultramicroscopy: three-dimensional visualization and automatic quantification of tumor morphology, drug penetration, and antiangiogenic treatment response. Neoplasia 2014; 16:1-U24; PMID:24563615; http://dx.doi.org/10.1593/neo.131848
