ABSTRACT
Bispecific antibodies are a growing class of therapeutic molecules. Many of the current bispecific formats require DNA engineering to convert the parental monoclonal antibodies into the final bispecific molecules. We describe here a method to generate bispecific molecules from hybridoma IgGs in 3–4 d using chemical conjugation of antigen-binding fragments (Fabs) (bisFabs). Proteolytic digestion conditions for each IgG isotype were analyzed to optimize the yield and quality of the final conjugates. The resulting bisFabs showed no significant amounts of homodimers or aggregates. The predictive value of murine bisFabs was tested by comparing the T-cell redirected cytotoxic activity of a panel of antibodies in either the bisFab or full-length IgG formats. A variety of antigens with different structures and expression levels was used to extend the comparison to a wide range of binding geometries and antigen densities. The activity observed for different murine bisFabs correlated with those observed for the full-length IgG format across multiple different antigen targets, supporting the use of bisFabs as a screening tool. Our method may also be used for the screening of bispecific antibodies with other mechanisms of action, allowing for a more rapid selection of lead therapeutic candidates.
Introduction
Although the concept of bispecific antibodies and their potential benefits over monoclonal antibodies (mAbs) were described many years ago, manufacturing processes that allow multiple molecules to progress into clinical trials have only recently been developed.Citation1 The success of bispecific antibodies is due in part to the fact that, in addition to targeting 2 antigens by a single molecule, bispecific antibodies enable novel mechanism of actions not accessible to monospecific antibodies.Citation2,3 Examples include the recruitment of effector cells such as T cells, natural killer (NK) cells, macrophages; enabling transcytosis across the blood-brain barrier; the biomimetic replacement of one component of a protein complex (Factor VIII) and the improvement of the uptake of antibody-drug conjugates by increasing the internalization of the target protein.Citation2 These novel mechanisms are unavailable to mAbs and result from the physical connection of 2 different binding specificities in the same molecule.
As with any other antibody-based therapeutic, the development of bispecific antibodies requires careful selection of the lead clones, a process that involves screening multiple antibodies to select the ones better matching the desired properties. Unlike mAbs that can be screened directly after hybridoma production, each component of a bispecific antibody needs to be tested in the context of the bispecific molecule. Here, we describe a method to produce heterodimeric (Fab’)2 from any murine IgG isotype using chemical crosslinking. Using T-cell redirected cytolysis as a screening assay, we validated the use of the crosslinked F(ab’)2 as a suitable tool for predicting the activity of hybridoma clones in the final full-length human IgG bispecific format.
Results
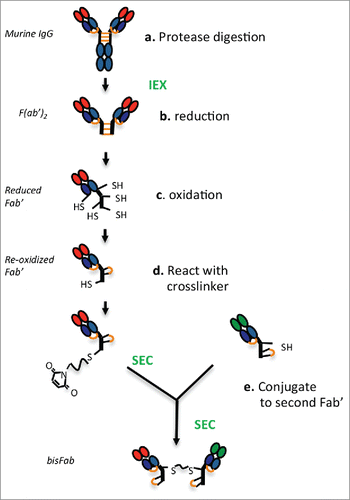
A general scheme of the strategy and the main steps involved in generating bispecific F(ab’)2 (bisFabs) from murine IgGs is shown in . First (a), antibodies are digested with proteolytic enzymes to generate F(ab’)2. After purification, (b) the F(ab’)2 are reduced to release the individual Fab’ molecules, and they are then (c) oxidized to reform the disulfide bond between the light and heavy chains. The resulting Fab’ molecules bearing a single reactive cysteine in the hinge are then (d) coupled to a bis-maleimide crosslinker, and finally (e) conjugated to another Fab’ with a different binding specificity.
Figure 1. Schematic diagram showing the main steps involved in producing bispecific bisFabs from murine IgGs. Purification steps are indicated in green font. The inter-chain disulfide bonds are indicated in orange. (a) The purified IgGs are digested with the proteases indicated in . to generate F(ab’)2. (b) Reduction with TCEP releases the monomeric Fab’ molecules and reduces the heavy-light chain disulfide bond. (c) Re-oxidation of the thiol groups re-form the heavy-light disulfide bond while cyclizing a pair of cysteines in the hinge, leaving a single reduced cysteine. (d) The reoxidized Fab’ is reacted with an excess of bismaleimide crosslinker. (e) the resulting modified Fab’ is conjugated to a second Fab’ with a different specificity.
Selection of digestion enzymes
Because the first step in the process is to generate F(ab’)2 from the intact hybridoma antibodies, we started optimizing the method for each murine isotype by screening and selecting enzymes capable of generating F(ab’)2 with the desired structural properties. The factors taken into consideration to choose between different enzymes were: 1) complete, specific digestion to maximize the yield of Fab’ molecules, 2) limited heterogeneity in the cleavage site to facilitate the final quality control by mass spectrometry, and 3) an odd number of cysteine residues in the hinge of the resulting Fab’ to facilitate the downstream steps.
For mouse IgG1, methods for generating Fab’ molecules using pepsin or ficin have been described.Citation4,5 However, since having an even or odd number of cysteines in the hinge region affects the methods used downstream and the resulting final yields, we wanted to characterize in detail the cleavage site(s). We digested a series of mIgG1s with both ficin and pepsin and then analyzed the cleaved products by mass spectrometry and SDS-PAGE. The results indicated that ficin and pepsin cleave mIgG1 primarily at the positions indicated in , with both enzymes producing F(ab’)2 with 3 cysteine residues in the hinge region. Digestion with ficin led to a slightly lower digestion efficiency (, Fig. S1) than digestion with pepsin (, Fig. S2). Extending the digestion time with ficin led to an increase of the proportion of IgG converted into F(ab’)2, but at the expense of the species containing 3 cysteines in the hinge (data not shown). Because of the more consistent results obtained with pepsin, we used it as the primary enzyme for generating F(ab’) molecules from mIgG1. Ficin served as a second option for clones that did not digest well with pepsin.
Mouse IgG2a and mouse IgG3 can be digested with IdeS, an enzyme with exquisite selectivity without the risk of over-digestion.Citation6 Digestion of mIgG2a (Fig. S3) and mIgG3 (Fig. S5) with IdeS produces Fab’ molecules with 3 and 1 cysteine residues in the hinge, respectively (), facilitating the subsequent conjugation steps.
Table 1. Enzymes used to generate F(ab’)2s from the different murine isotypes.
Antibodies of the IgG2b isotype can be cleaved in the hinge by Lys-C digestion. Two Lys-C digestion sites are present in the hinge region of mIgG2b such that digestion of one site or the other creates Fab’ molecules with 2 or 3 cysteines.Citation7 At enzyme concentration of 1:200, most of the antibody was digested, but the resulting F(ab’)2 contained mainly Fab species with 2 cysteine residues in the hinge, whereas, when Lys-C was used at 1:1,000, the most abundant (80–90%) Fab’ species contained 3 cysteines, but the digestion was not complete (∼60% digestion) (Fig. S4, ). Because the method using a lower concentration of Lys-C led to a final yield of bisFab that was greater than the method using a higher concentration of Lys-C, we adopted the former condition for the generation of bisFabs from mIgG2b.
Reduction and re-oxidization
After digestion, F(ab’)2 were purified from enzymes and unwanted IgG fragments by ion exchange chromatography. The next step in the process was to generate Fab’ molecules with a single reactive cysteine to make sure not more than one crosslinker molecule was incorporated () to reduce the possibility of creating crosslinked Fab multimers. For Fab’ species bearing an odd number of cysteines in the hinge (mIgG1, mIgG2a, mIgG2b digested with Lys-C 1:1,000, mIgG3) the strategy to obtain a single reactive thiol group was to first reduce all inter-chain disulfide bonds with tris(2-carboxyethyl) phosphine (TCEP) followed by re-oxidation with dehydroascorbic acid. In addition to reforming the heavy-light chain disulfide, the re-oxidation treatment of Fab’ species with 3 hinge cysteines (mIgG1 and mIGg2a) also led to the formation of an intra-hinge disulfide bond, leaving a single cysteine in the reduced form (data not shown). Re-oxidation of F(ab’)2 from mIgG3, which contains a single cysteine in the hinge, also produced Fab’ molecules with a single cysteine in the reduced form. For all isotypes, oxidation was performed at 0.5 mg/ml to minimize the formation of Fab-Fab dimers. We did observe the formation of small amounts of dimers (< 10%, Fig. S6) that were removed by a purification step before the conjugation (see below). Although reoxidation of the inter-chain disulfide was almost complete for all isotypes, a small fraction (< 12%) of the Fab's did not reform the covalent bond as evidenced by SDS-PAGE analysis (Fig. S6). Further investigation of the number of thiol groups reacting with N-ethylmaleimide (NEM) showed incorporation of one NEM molecule in the light chain and none in the heavy chain (data not shown), suggesting the 3 cysteines in the hinge and the cysteine normally involved in pairing with the light chain cyclized into 2 intra-chain disulfides, leaving the cysteine in the light chain unpaired.
In the case of mIgG2b digested with Lys-C 1:200 where most of the Fab’ molecules contained 2 hinge cysteines, the strategy to generate Fab’ molecules with only one reactive cysteine was to partially block one of the 2 cysteines with NEM.Citation8 After completely reducing the F(ab’)2, the heavy-light chain disulfide was re-oxidized in conditions that preferentially oxidized the heavy-light disulfide, keeping the hinge cysteines reduced. The subsequent reaction with one molar equivalent of NEM led to ∼30–40% of the material carrying just one reduced cysteine in the hinge.
Conjugation reactions
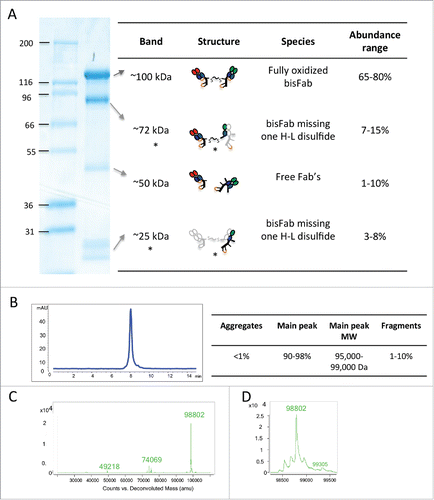
Re-oxidized Fab’ molecules were incubated with an excess of bismaleimide crosslinker, and then purified by size exclusion chromatography (SEC) or ion exchange chromatography (IEX) to remove the excess of crosslinker and small amounts of dimers formed during the oxidation reaction. The derivatized Fab's were then reacted with a similarly purified Fab’ with a different target specificity. The resulting bispecific bisFabs were purified from non-reacted Fab’ molecules by SEC and characterized by SDS-PAGE, mass spectrometry and analytical SEC. Purified bisFabs typically showed a main band of ∼100 kDa by SDS-PAGE with minor bands around ∼72 kDa, 50 kDa and ∼30 kDa (). Mass spectrometry analysis indicated that, while the ∼100 kDa band corresponded to the fully oxidized bisFabs, the ∼72 kDa and ∼30 kDa bands corresponded to bisFabs where one of Fab's did not reform the heavy-light disulfide during the reoxidation step. The ∼50 kDa band corresponded to unconjugated Fab molecules. Thus, most of the heterogeneity observed by SDS-PAGE was due to heterogeneous disulfide bonding in the bisFabs and the only non-bispecific species were the residual Fab's. Although we observed some variability in the relative abundance of the different bands (), the abundance of unconjugated Fab's was always below 10%. Consistent with our method including gel filtration as the final purification step, analytical SEC analysis of the purified bisFAbs showed very low levels of aggregates (< 1%) and unconjugated Fab's (< 10%)(). As mentioned above, reoxidation at low protein concentration minimized the formation of homodimers, and the subsequent SEC step removed any small amounts that may have formed during the reoxidation reaction. Mass spectrometry analysis confirmed the absence or presence of very low levels (< 2%) of homodimers in all bisFabs preparations (). Analysis of thermal and colloidal stability of 2 different bisFabs showed no major differences with their corresponding F(ab’)2 counterparts (Fig. S7) indicating the different reaction steps did not affect basic biophysical properties of the Fabs.
Figure 2. Biochemical characterization of a representative purified bisFab. (A) Non-reducing SDS-PAGE. The table indicates the structures of the corresponding bands as deduced based on the mass spectrometry analysis. The species indicated with asterisks are likely bisFabs missing one S-S bond between a heavy and light chain that dissociates under denaturing conditions. The light gray chain in the structure indicates the dissociated chain. The relative abundance of each band was determined by densitometry of 30 independent bisFabs and shown is the range observed for each band. (B) Analytical size-exclusion chromatography. The Table shows the observed range of aggregation and low molecular weight fragments obtained for the panel of bisFabs and the molar masses calculated by multi-angle laser light scattering. (C) Mass spectrometry analysis showing mass deconvolution between 20 kDa and 120 kDa, and (D), a zoom-in of the mass spectrometry analysis in the 98 kDa area. Homodimers were absent or below 2%.
Although considerable variability was observed for different clones within a given isotype, in general, murine IgG2a and IgG3 isoforms produced the highest yield, typically 200 μg purified bisFabs per 1 mg of starting hybridoma antibody. In contrast, mIgG1 and mIgG2b isoforms yielded ∼150 and 100 μg of final bisFab per mg of starting hybridoma antibody, respectively ().
Validation of the bisFab format as surrogate of full-length IgG bispecific antibodies
Because we intended to use the murine bisFabs to determine the biologic activity of different hybridoma clones as bispecifics in the full-length format, we next compared the activity of different bispecific antibodies in both bisFab and full-length IgG formats. Production and characterization of full-length bispecific antibodies was done as described elsewhere.Citation9,10,11 To explore potential differences between the 2 formats in the context of different binding geometries and antigen densities, we used 10 different antibodies directed against 5 different antigens: human epidermal growth factor receptor 2 (HER2); B-cell antigens CD20, CD79, and antigen A; and melanoma antigen B. A description of the isotypes, enzymes used and yields obtained for those 10 clones is shown in Table S1.
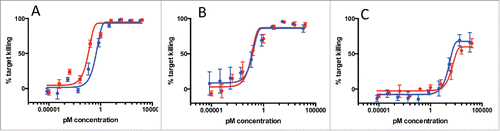
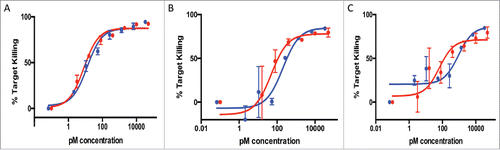
Antibodies against 3 different epitopes of human HER2 (4D5,Citation12 7C2,Citation13 and 2C414) were paired with an anti-CD3 antibody in either the bisFab or full-length IgG format. Clone 4D5 binds to an epitope very proximal to the plasma membrane in domain IV of the molecule, whereas clones 2C4 and 7C2 bind to more membrane-distal regions in domains II and I, respectively.Citation15 The activity of the 2 formats was compared in cell killing assays using SKBR3 as the target cells (∼2,000,000 copies of HER2 per cell) and human peripheral blood mononuclear cells (PBMC) as effector cells. The results show very similar dose-response curves between the 2 formats for all 3 antibodies clones (). A comparison of the 3 clones in the full-length format showed that, while clones 4D5 and 2C4 showed comparable potencies, clone 7C2 had a pronounced lower potency and a lower extent of maximum killing. Although we did not perform a formal kinetic analysis of killing mediated with the clone 7C2, it has been previously shown that maximal cytotoxicity of HER2-CD3 bispecific antibody is enhanced until 48 h,Citation10 suggesting that the incomplete killing observed with clone 7C2 may reflect a kinetic difference between molecules in their ability to mediate T-cell activation and cytolysis rather than a true plateau. Both the EC50 and the maximum percentages of cell killing between the 2 formats were very close for the 3 antibodies, and the potency ranking was the same in both formats.
Figure 3. Comparison of T-cell recruiting antibodies using 3 separate anti-HER2 antibody clones (A: 4D5, B: 2C4, C: 7C2) in either full-length IgG (blue line) or bisFab format (red line). Shown is the percentage of target cell (SKBR-3) killing in the presence of human PBMCs and different concentrations of the antibodies. Values are the mean ± standard deviation of 3 replicate experiments. The calculated EC50 and extent of maximum killing are: (A) IgG: 0.24 pM, 95%; bisFab: 0.07 pM, 95%; (B) IgG: 0.14 pM, 90%; bisFab: 0.06 pM, 90%; (C) IgG: 46 pM, 65%; bisFab: 129 pM, 62%.
To expand the diversity of targets used for the comparison between the 2 different formats, we next analyzed antibodies against targets expressed in cells from the hematologic lineage. First, we tested a clone against CD20 and 2 clones against CD79. BJAB cells, which express 160,000 copies of CD20 per cell and 120,000 copies of CD79, were used in an in vitro cell-killing assay. While for the most potent antibody (against CD20) the 2 formats showed very similar potencies, there was a slightly higher potency of the bisFab over the full-length format for the 2 other clones (against CD79, ). Nevertheless, the ranking of the potencies for the 3 different antibodies was the same in both formats. A set of 3 additional antibodies directed against a different protein also expressed in B cells (antigen A), and one antibody against a target for melanoma cells (antigen B) was used on cell lines expressing 600–2,000 molecules of the target protein with comparable observed potencies and extent of killing maxima between the 2 formats (data not shown).
Figure 4. T-cell killing activity of 3 antibodies against B-cell targets (A: anti-CD20, B: anti-CD79 clone A, C: anti-CD79 clone B). Antibodies in the full-length IgG format (blue line) or bisFabs (red line) were tested against BJAB cells in an in vitro cell killing assay using human PBMCs. Values are the average of duplicate experiments ± standard deviation. The calculated EC50 and extent of maximum killing are: (A) IgG: 1.9 pM, 85%; bisFab: 0.8 pM, 85%; (B) IgG: 200 pM, 80%; bisFab: 49 pM, 75%; (C) IgG: 920 pM, 78%; bisFab: 65 pM, 67%.
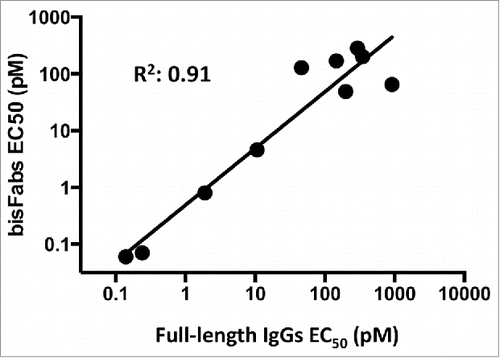
Analysis of the data for all different antibodies indicated a good correlation (r = 0.91) between the EC50 values of the bisFab and full-length antibody formats (), suggesting that bisFabs are a useful tool to assess the activity of different antibody clones as bispecifics across a diverse range of antigen targets and range of expression levels.
Discussion
This study describes the development of a method that allows generation of bispecific molecules from hybridoma IgGs in 3–4 days, enabling rapid screening of antibody clones for their activity in bispecific format prior subcloning. Although methods to produce bispecific F(ab’)2 from recombinant Fab’ molecules or from human and murine full-length IgG1 have been described previously,Citation16,8,17-19 no comprehensive method to produce bispecific antibodies directly from all murine IgG isotype antibodies has been published. We described previously a method to produce bispecific F(ab)’2 from human IgG1 using a bismaleimide crosslinker.Citation8 Here, because murine isotypes differ from human IgG1 in their susceptibility to protease digestion and in the number of cysteines in the hinge region, careful selection of the most appropriate digestion enzymes was performed as the first step. We found conditions to efficiently digest mIgG1, mIgG2a and mIgG3 into Fab’ containing an odd number of cysteines in the hinge, which leads to almost complete conversion into Fab’ with a single reactive maleimide. The digestion conditions used for mIgG2b led to ∼30–40% of the digested species being converted into bispecific F(ab’)2s, a yield sufficient to support many cell-based screening assays. While those isotypes capable of generating F(ab’)2 by IDES digestion (mIgG2a and mIgG3) consistently yielded complete digestion, isotypes that required digestion with other enzymes (mIG1, mIgG2b) showed variation in the yields from clone to clone. Although it was possible to efficiently generate F(ab’)2 for all mIgG1 clones tested, maximizing the yield required exploring different digestion times. In the case of mIgG2b clones, some variability was observed in the relative abundance of Fab's with 2 or 3 hinge cysteine. These results are reflective of the exquisite specificity of IDES in contrast to other proteases like pepsin or Lys-C.
BisFabs produced by the procedure described here did not contain significant amounts of homodimers (< 2%) or aggregates (< 2%), similarly to knob-into-hole bispecific antibodies.Citation9,10,11 Unlike full-length bispecific Abs, bisFabs showed a minor population (< 12%) with incomplete heavy-light chain disulfide formation, originating from disulfide scrambling in the heavy chain during reoxidation of the Fab's. While the presence of a fraction of bisFabs with scrambled disulfide did not affect the potency of bisFabs in T-cell redirected cell killing, the effect on other mechanisms of actions remains to be studied.
The intended use of bisFabs in this work was to screen hybridoma clones, retaining only those clones showing good in vitro activity. For bisFabs to be useful for determining the activity of antibody clones in the final full-length IgG bispecific format, their activities should correlate, and so the potency ranking of the clones should be similar in the 2 formats. It is widely accepted that, for T-cell redirecting applications, the format of the bispecific molecule may significantly influence the potency. In spite of a similar general architecture between bisFab and full-length antibodies, bisFabs likely have greater flexibility around the linker than Fabs in an IgG. The difference in flexibility may affect their efficiencies in bridging effector to target cells. In fact, there are examples in the field of T-cell engaging antibodies of molecules with small structural differences but significantly different potencies. For example, the BiTE (Bispecific T-cell Engagers) and DART (Dual Affinity Re-Targeting) formats are both based on different arrangements of single-chain variable fragments (scFvs). An engineered disulfide bond in DARTs stabilizes the molecules, but also restricts the degree of rotation and flexibility of the 2 binding domains. A direct comparison between a BiTE and a DART using the same clones showed a pronounced higher (4–60 fold, depending on the target cell line) potency of the DART,Citation20 presumably due to a geometry or flexibility more conducive to the simultaneous binding of the 2 targets. In addition to flexibility, the size of the 2 different formats could also affect potencies. The relationship between the immune synapse gap and the efficiency of T-cell activation and cytoIysis is well documented.Citation21 Consistent with these observations, it has been reported that the size of the extracellular domain of antigens targeted by BiTEs influences the efficacy of the cell killing, such that molecules with large extracellular domains do not lead to efficient redirected cell lysis.Citation22 In a similar way, it could be hypothesized that the size of the bispecific molecules could affect the potency of the cell killing, with larger molecules being less efficient than smaller molecules.
Since structural differences between formats can lead to different potencies, it was important to validate the use of bisFabs by comparing their activities with their corresponding counterparts in the full-length IgG format. We used a panel of 10 antibodies targeting different antigens or different epitopes in a given antigen to compare the 2 formats in the context of different binding geometries that may cause steric hindrance preferentially to one format. Our analysis showed a good correlation between the 2 formats across a range of typical antigen expression levels. These results suggest that the presence of the Fc does not reduce the potency of full-length IgGs, and support the use of bisFabs as a tool for predicting their activity in the IgG format. This good correlation is likely due to the close similarity between the Fab-Fab architecture in bisFabs and full-length IgGs. It is interesting to note, however, that while very potent full-length antibodies (EC50 of single digit pM) showed comparable potencies to the bisFabs, the bisFabs tended to be more potent than the full-length IgG format for antibodies with EC50 in the range of hundreds pM. This should not be a concern for the intended application of bisFabs because the potency ranking was maintained even for less potent antibodies. In addition, it would be preferable to have a few false positives rather than false negatives. While false positives could be identified and removed in subsequent assays using the full-length format, false negatives would represent the loss of valuable clones.
Besides their use in vitro, bisFabs may also be used for the initial exploration of the in vivo activity of a given bispecific antibody, but the short half-life of F(ab’)2s limits this application.Citation23 Although F(ab’)2 conjugated with maleimide crosslinkers are more stable than disulfide-bonded F(ab’)2 and they show longer half lives in circulation,Citation24 the half-life is still shorter than a full-length IgG. Nevertheless, it is possible to dose laboratory animals daily with bispecific F(ab’)2 to maintain exposure and assess their in vivo efficacy. This is in particular feasible for molecules recruiting effector cells, which are normally very potent and require minute amounts of reagents.Citation25
The methods described here apply to murine IgGs, but we have easily adapted them for use with IgGs from rat and hamster. Although we have validated the use of bisFabs in the context of bispecific antibodies for redirecting the killing activity of T-cells, they may also be used for the in vitro screening of bispecific antibodies using other mechanisms of actions. The use of bisFabs as a strategy for obtaining a quick assessment of the activity of a given antibody combination in a full-length IgG format could thus be extended to antibodies from other species and other biologic systems.
Materials and methods
Proteolytic digestions
Purified mIgG1 clones were digested as described previously.Citation26 Briefly, antibodies were diluted in 25 mM sodium bicarbonate pH 3.5, and treated with pepsin (P6887, Lot# 091M7020V, Sigma) at a 1:50 (weight : weight) at 37°C. A time course (0–4 hr) analysis of the reaction by SDS-PAGE was conducted to find the optimal reaction time for each mIgG1 clone. Incubation times in the 2–4 hr range were optimal for most clones, but occasionally some clones required shorter digestion reaction (1 hr) to avoid overdigestion. A scale-up digestion was performed using the optimized conditions for each clone. mIgG2a or mIgG3 were digested with IdeS (A0-FR8–050, Genovis, Sweden) according to the instructions of the supplier: 5 units of enzyme per μg of IgG at 37°C, overnight reaction in phosphate-buffered saline. mIgG2b was digested with lysyl endoproteinase C (Lys-C) (129–02541, Wako) at variable concentrations at pH 8 in an overnight reaction at 37°C.
| • | After digestion, all clones were diluted into 20 mM sodium acetate, pH5 and loaded onto a cation exchange column, (HiTrap SP HP, 17115101, GE Healthcare Life Sciences) and the F(ab’)2 were purified from the enzymes and other diges-tion fragments by elution with a linear gradient (0-to 50%) 1 M sodium chloride in 20 mM sodium acetate, pH 5. | ||||
Reduction and reoxidation
After purification, F(ab’)2 molcules were treated with 2 mM TCEP (C4706, Sigma). The reducing agent was directly added into the eluate from the cation exchange column and allowed to react overnight at room temperature. Completion of the reduction was assessed by SDS-PAGE or mass spectrometry. To reform the disulfide bond between the heavy and light chains, as well as to cyclize 2 cysteines in the hinge in species containing 3 hinge cysteines, the Fab’ molecules were treated with 5 mM dehydroascorbic acid (DHAA, 261556, Sigma) at pH 5 at room temperature at a concentration of approximately 0.5 mg/ml to minimize the formation of dimers. The following day, samples were analyzed by mass spectrometry for completion of the reoxidation. While formation of the heavy-light interchain disulfide was assessed by the molecular weight of the detected species, reoxidation of the hinge cysteines was analyzed by probing the Cys residues with NEM. Fab’ molecules with 3 or one cysteine residues in the hinge typically showed the incorporation of only one NEM molecules (+125 Da shift) after the reoxidation treatment.
| • | Reaction with the bis-maleimide crosslinker. After reoxi-dation the Fab’ molecules were reacted with 10X molar excess of bismaleimide crosslinker (#10232, Quanta Biode-sign) overnight at room temperature. After the complete incorporation of the crosslinker into the Fabs was confirmed by mass spectrometry, the excess crosslinker was removed by SEC using a Superdex 200 10/30 GL column (17517501, GE). | ||||
Conjugation reactions
The Fab’ molecules activated with the crosslinker were then incubated with an anti-human CD3 Fab’ (mIgG1 or mIgG2a) that had been re-oxidized to result in a single reactive thiol group in the hinge region. The Fab’ molecules were mixed in equal amounts and concentrated to 5 mg/ml. After an overnight reaction at room temperature, the sample was loaded into a Superdex 200 10/30 GL (GE) and the reacted bispecific product was purified from small amounts of aggregates and unreacted Fab’ molecules.
Characterization
The purified bispecific fragments were characterized by SDS-PAGE, SEC-MALS using a Dawn Heleos instrument (Wyatt Technologies) and by mass spectrometry (6230 TOF LC/MS, Agilent).
Production of full-length bispecific antibodies
Human IgG1 full-length bispecific antibodies using the same clones as in the different bisFabs were produced as described elsewhere.Citation10,11
Cell killing reactions
In vitro cell killing assays were performed as described previously.Citation10,11 Briefly, PBMCs were isolated form whole blood of healthy donors by Ficoll separation. Target cells were incubated with PBMCs at 1:5 ratio for 24–48 hr. Cytotoxicity was analyzed by flow cytometry: Target cells were either pre-labeled with carboxyfluorescein succinimidyl ester or labeled with specific fluorescent antibodies and live and dead cells were distinguished by vital staining with propidium iodide. The results were plotted using Prism6 software and the reported EC50 and maximum killing values were obtained from the sigmoidal fitted dose-response curves.
Biophysical characterization of bisFabs
Two different bisFabs were generated as described above and their corresponding full-length IgG versions were produced by in vitro annealing. After HIC purification the full-length antibodies were digested with LysC at a 1:200 concentration (wt/wt) at pH 8 for 2 hr at 37°C and then the F(ab’)2 were purified by cation exchange column. The purified bisFabs and F(ab’)2s were formulated in 20 mM sodium acetate, pH5, 150 mM sodium chloride at 2 mg/ml and tested in a thermal ramp for unfolding and aggregation using an OPTIM 1000 instrument (Avacta).
Antigen density determination
The number of molecules per cell was determined by Scatchard analysis or using Quantum simply cellular beads (Cat# 816, Bangs Laboratory).Citation27
Disclosure of potential conflict of interests
No potential conflicts of interest were disclosed.
Supplemental_Figures_1-7___Supplemental_Table_1.pptx
Download MS Power Point (18 MB)Funding
This study was funded by Genentech Inc. SP, JL, PW, DS, JJ, SB, SP, LS, TJ, JMS and DAE were Genentech employees at the time the study was conducted.
References
- Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today 2015; 20:838-47; PMID:25728220; http://dx.doi.org/10.1016/j.drudis.2015.02.008
- Kontermann R, ed. Bispecific Antibodies: Springer, 2011; http://dx.doi.org/10.1007/978-3-642-20910-9
- Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol 2015; 67:95-106; PMID:25637431; http://dx.doi.org/10.1016/j.molimm.2015.01.003
- Jones RG, Landon J. Enhanced pepsin digestion: a novel process for purifying antibody F(ab')(2) fragments in high yield from serum. J Immunol Methods 2002; 263:57-74; PMID:12009204; http://dx.doi.org/10.1016/S0022-1759(02)00031-5
- Mariani M, Camagna M, Tarditi L, Seccamani E. A new enzymatic method to obtain high-yield F(ab)2 suitable for clinical use from mouse IgGl. Mol Immunol 1991; 28:69-77; PMID:2011130; http://dx.doi.org/10.1016/0161-5890(91)90088-2
- von Pawel-Rammingen U, Johansson BP, Bjorck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J 2002; 21:1607-15; PMID:11927545; http://dx.doi.org/10.1093/emboj/21.7.1607
- Yamaguchi Y, Kim H, Kato K, Masuda K, Shimada I, Arata Y. Proteolytic fragmentation with high specificity of mouse immunoglobulin G. Mapping of proteolytic cleavage sites in the hinge region. J Immunol Methods 1995; 181:259-67; PMID:7745255; http://dx.doi.org/10.1016/0022-1759(95)00010-8
- Scheer JM, Sandoval W, Elliott JM, Shao L, Luis E, Lewin-Koh SC, Schaefer G, Vandlen R. Reorienting the Fab domains of trastuzumab results in potent HER2 activators. PloS One 2012; 7:e51817; PMID:23284778; http://dx.doi.org/10.1371/journal.pone.0051817
- Spiess C, Merchant M, Huang A, Zheng Z, Yang NY, Peng J, Ellerman D, Shatz W, Reilly D, Yansura DG, et al. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol 2013; 31:753-8; PMID:23831709; http://dx.doi.org/10.1038/nbt.2621
- Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G, et al. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res 2014; 74:5561-71; PMID:25228655; http://dx.doi.org/10.1158/0008-5472.CAN-13-3622-T
- Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E, et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 2015; 7:287ra70; PMID:25972002; http://dx.doi.org/10.1126/scitranslmed.aaa4802
- Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, Shepard HM. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother 1993; 37:255-63; PMID:8102322; http://dx.doi.org/10.1007/BF01518520
- Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res 1990; 50:1550-8; PMID:1689212
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002; 2:127-37; PMID:12204533; http://dx.doi.org/10.1016/S1535-6108(02)00097-1
- Eigenbrot C, Ultsch M, Dubnovitsky A, Abrahmsen L, Hard T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc Natl Acad Sci U S A 2010; 107:15039-44; PMID:20696930; http://dx.doi.org/10.1073/pnas.1005025107
- Shalaby MR, Shepard HM, Presta L, Rodrigues ML, Beverley PC, Feldmann M, Carter P. Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. J Exp Med 1992; 175:217-25; PMID:1346155; http://dx.doi.org/10.1084/jem.175.1.217
- Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science 1985; 229:81-3; PMID:3925553; http://dx.doi.org/10.1126/science.3925553
- Glennie MJ, McBride HM, Worth AT, Stevenson GT. Preparation and performance of bispecific F(ab' gamma)2 antibody containing thioether-linked Fab' gamma fragments. J Immunol 1987; 139:2367-75; PMID:2958547
- Luo H, Hernandez R, Hong H, Graves SA, Yang Y, England CG, Theuer CP, Nickles RJ, Cai W. Noninvasive brain cancer imaging with a bispecific antibody fragment, generated via click chemistry. Proc Natl Acad Sci U S A 2015; 112:12806-11; PMID:26417085; http://dx.doi.org/10.1073/pnas.1509667112
- Moore PA, Zhang W, Rainey GJ, Burke S, Li H, Huang L, Gorlatov S, Veri MC, Aggarwal S, Yang Y, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 2011; 117:4542-51; PMID:21300981; http://dx.doi.org/10.1182/blood-2010-09-306449
- James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature 2012; 487:64-9; PMID:22763440; http://dx.doi.org/10.1038/nature11214
- Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, Kufer P. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother 2010; 59:1197-209; PMID:20309546; http://dx.doi.org/10.1007/s00262-010-0844-y
- Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab')2, and Fab' in mice. Cancer Res 1986; 46:3969-78; PMID:3731067
- Quadri SM, Lai J, Mohammadpour H, Vriesendorp HM, Williams JR. Assessment of radiolabeled stabilized F(ab')2 fragments of monoclonal antiferritin in nude mouse model. J Nucl Med 1993; 34:2152-9; PMID:8254403
- Honeychurch J, Tutt AL, Valerius T, Heijnen IA, Van De Winkel JG, Glennie MJ. Therapeutic efficacy of FcgammaRI/CD64-directed bispecific antibodies in B-cell lymphoma. Blood 2000; 96:3544-52; PMID:11071653
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol 1983; 131:2895-902; PMID:6358356
- Serke S, van Lessen A, Huhn D. Quantitative fluorescence flow cytometry: a comparison of the three techniques for direct and indirect immunofluorescence. Cytometry 1998; 33:179-87; PMID:9773878; http://dx.doi.org/10.1002/(SICI)1097-0320(19981001)33:2%3c179::AID-CYTO12%3e3.0.CO;2-R