ABSTRACT
A central dogma in immunology is that an antibody's in vivo functionality is mediated by 2 independent events: antigen binding by the variable (V) region, followed by effector activation by the constant (C) region. However, this view has recently been challenged by reports suggesting allostery exists between the 2 regions, triggered by conformational changes or configurational differences. The possibility of allosteric signals propagating through the IgG domains complicates our understanding of the antibody structure-function relationship, and challenges the current subclass selection process in therapeutic antibody design. Here we review the types of cooperativity in IgG molecules by examining evidence for and against allosteric cooperativity in both Fab and Fc domains and the characteristics of associative cooperativity in effector system activation. We investigate the origin and the mechanism of allostery with an emphasis on the C-region-mediated effects on both V and C region interactions, and discuss its implications in biological functions. While available research does not support the existence of antigen-induced conformational allosteric cooperativity in IgGs, there is substantial evidence for configurational allostery due to glycosylation and sequence variations.
Introduction
The ability of antibodies to target diverse antigens with high specificity and affinity has led to many successful antibody-based therapies for various diseases.Citation1-4 Immunoglobulin G (IgG) monoclonal antibodies (mAbs) have emerged as the largest class of biopharmaceuticals whose approval for therapeutic applications and use in clinical developments is occurring on a regular basis.Citation5,6 Because this class of biopharmaceutical products closely resembles natural human IgG molecules, they have therapeutic and economic benefits, including 1) infrequent dosing requirement due to their potency and long half-lives, 2) a favorable safety profile because of the absence of off-target binding, and 3) a wide range of therapeutic applications owing to their ability to target diverse antigens with various modes of action.
Functionally, IgG exhibits cooperativity in both antigen binding and effector function. Each IgG molecule consists of 2 separable regions with distinct functions: a variable (V) region responsible for specific antigen binding, and a constant (C) region whose binding sites determine which effector functions will occur, such as complement activation or specific Fc- receptor (FcR) binding ().Citation7-9 The combined functionality of target-antigen recognition and effector ligand binding by distinct regions of the IgG molecule, together with the modulatory role of Fc glycosylation, is critical for triggering a variety of immune responses to eradicate foreign pathogens.Citation10,11 For almost half a century, it was assumed that these 2 functional regions do not influence each other's activity. However, this classical view has been challenged by increasing evidence for allostery (synonymous with intramolecular signaling), between the V and C regions.Citation12 Although V-C allostery was explored in the 1970s as a mechanism for effector activation triggered by antigen-mediated conformational changes, it failed to gain wide support. Instead, an associative model was developed, in which the antigen-mediated crosslinking of V regions results in increased avidity for FcγR and C1q through the resulting increased proximity of C regions.Citation13,14 While both concepts could describe the experimentally observed functional cooperativity, one invoked intramolecular structural changes as the source of cooperativity, whereas the other used intermolecular interactions as the source. On the other hand, the finding that different IgG subclasses with identical V regions exhibit different target-binding affinities and specificities was evidence for allostery.Citation15,16 These observations led to the hypothesis that changes in the C region may cause conformational changes in the V region that re-shape the antigen-binding site, challenging the classical view of V-C independence.
Figure 1. IgG structure and function. (A) The crystal structure of a human IgG1 molecule is used to illustrate domain assignments (PDB ID: 1HZH). An individual IgG is composed of 2 identical heavy chains (blue) and 2 identical light chains (orange), linked together by inter-chain disulfide bonds. Each heavy chain consists of a variable (VH) domain and 3 constant (CH1, CH2, and CH3) domains, while the light chain is composed of a variable (VL) and a constant (CL) domain. The light chain pairs with the VH and CH1 domains to form the Fab, which interacts to form the antigen-binding region, also known as the complementarity-determining region (CDR, green). The CH2 and CH3 domains dimerize to form the Fc, which is connected to the upper Fab region via a flexible hinge containing several disulfide bridges that covalently link the CH1 and CH2 chains together. The interactions between the Fc and the Fc-gamma receptors (FcγR) expressed by effector cells or the complement component 1q (C1q), are vital to the clearance of target antigens through antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis (ADCP), or complement-dependent cytotoxicity (CDC), respectively. The highly conserved N-linked glycosylation site (gray) located in the CH2 domains is responsible for the overall structural integrity of the IgG molecule to mediate effector functions. In addition, the Fc can bind the neonatal Fc receptor (FcRn) in a strictly pH-dependent manner, an interaction that contributes to the long serum half-lives of human IgGs. (B) Schematic diagrams of N-linked glycoforms commonly found in human IgGs or industrial mAbs. The N-linked glycoforms attached to asparagine (Asn) 297 are categorized into 2 groups: fucosylated (top panels) and non-fucosylated (bottom panels). A core heptasaccharide structure (G0) is composed of 2 N-acetylglucosamine (GlcNAc), 3 mannose, and 2 GlcNAc residues that are β-1,2 linked to the mannose from the α-1,6 and α-1,3 arms, forming 2 antennae. In addition to fucose, galactose, bisecting GlcNAc, and sialic acid may be added to the core. Figure reproduced fromCitation18 with permission from Elsevier.
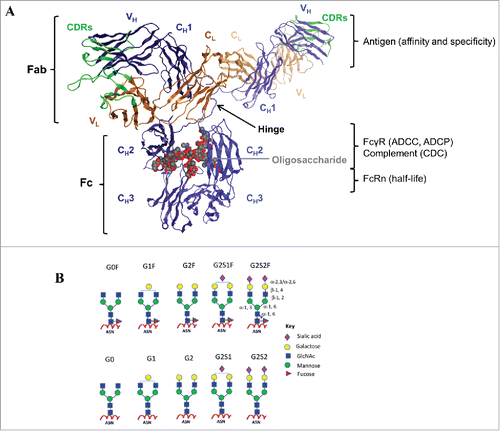
The functions of both the V and C regions are essential for the clinical efficacy of IgG therapeutics.Citation17,18 There are 4 subclasses of IgGs in humans, IgG1, IgG2, IgG3, and IgG4, which differ in their C regions and have distinct biological characteristics ().Citation19-21 Since V-C functional independence is widely accepted, the current design strategies for therapeutic mAbs involve 2 separate efforts: (1) the generation of V-genes with desirable affinity, specificity, and in vitro pharmacological properties for the target, and (2) the selection and optimization of C-region subclass to elicit effector functions associated with a suitable in vivo efficacy and half-life.Citation22-24 Currently, the majority of therapeutic mAbs available on the market are predominantly derived from the human IgG1 subclass, with a few in the IgG2 and IgG4 subclass;Citation25 the use of IgG3 has been largely excluded.Citation26 IgG1 is generally selected for the eradication of cancer cells, whereas, due to their reduced effector functions, IgG2 and IgG4 may be used as “benign blockers” for the neutralization of soluble antigens.Citation23 However, if the V- or C-region functions are influenced by allostery, or if the extent of cooperativity differs for different subclasses, then the selection process for the IgG subclass in therapeutic mAb discovery and development may be affected.
Table 1. Biological characteristics of human IgG subclasses.
In this review, we seek to address whether allosteric cooperativity is a common feature for antibody function, and how allostery can affect the respective V and C region functions. In the first section, the various forms of cooperativity occurring in IgGs are identified, focusing on the structure-function relationship in which the antigen-binding fragment (Fab) and crystallizable fragment (Fc) may exert cooperative influence on each other's function or within its own functions. The second section centers on IgG subclass differences and how C-region configurational allostery may manifest changes in V-region interactions, structure, and function. By examining the source of allostery and the mechanistic models concerning the existence and extent of V-C cooperativity, we seek to clarify the rules governing the relationship between the V and C regions, and improve our understanding of IgG cooperativity.
Cooperative mechanisms in antibody function
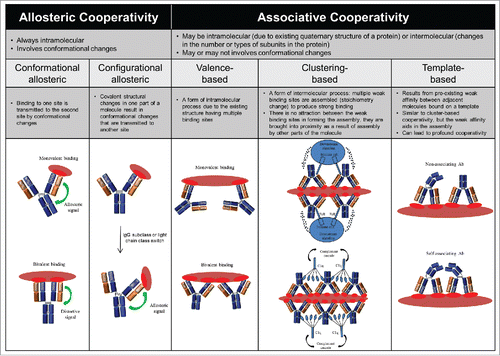
Cooperative interactions are a hallmark of biological processes. In a cooperative system, structural changes at one site affects binding at a second site, either through strengthening it (positive cooperativity) or weakening it (negative cooperativity). There are many types of cooperativity, and several mechanisms for achieving cooperative binding. Given the quaternary structure of IgG, in which the V and C regions are arranged to form 2 separate fragments, 2 Fabs and one Fc, cooperative binding could potentially occur between: 1) the 2 Fabs, 2) either Fab or the Fc, or 3) different sites within a given Fab or Fc domain. Two major types of cooperativity in IgGs have been reported: 1) allosteric cooperativity, which always involves intramolecular conformational changes; and 2) associative cooperativity, which can occur either intermolecularly or intramolecularly and involves conformational changes only in certain cases ( and Appendix I). Clearly, multiple cooperative mechanisms may be present simultaneously. Here, we examine historical and recent evidence for and against allostery in the different IgG regions and its implications for biological functions. We also compare and contrast the associative models concerning effector cell and complement activation and discuss the role of cooperativity in these models.
Figure 2. Classification of the potential forms of cooperativity in IgG and their nomenclature. Please refer to the Appendix I for additional information.
Historical development of Fab-Fc independence
How the initial antigen recognition in the Fab is communicated to the Fc to activate the effector system has been a central debate since the 1970s. Two mechanisms were proposed, with one involving Fab-Fc intramolecular cooperativity (i.e., ‘conformational allosteric’ cooperativity), and the other involving independent Fab-Fab and Fc-Fc intermolecular cooperativity (i.e., ‘clustering-based associative’ cooperativity) ().Citation27 Evidence for the allosteric mechanism arose from the detection of antigen-induced conformational transitions from spectroscopic measurements, where spectral differences observed upon antigen binding in intact antibodies and their respective Fab and Fc indicated an interaction between the Fab and Fc in the IgG molecule.Citation28,29 However, subsequent attempts to correlate these antigen-induced conformational changes with complement activation were negative.Citation30-33 Instead, the results indicated that complement activation requires binding of 2 IgG Fab arms to the antigen and Fc clustering through higher order antibody-antigen complex formation. These observations are consistent with antigen performing an associative (or templating) role by bringing IgGs into close proximity with each other instead of acting as an allosteric trigger (reviewed inCitation13). Similarly, effector cell activation through crosslinking of IgGs with both antigens and FcγRs expressed on immune cells was an attractive hypothesis. This was confirmed through observations that FcγR aggregation at the cell surface by antibodies and multivalent antigens, rather than antigen binding, was critical to generate a signal (reviewed inCitation34). These early studies indicate that functional avidity and effector functions attained through enhanced intermolecular cooperativity do not require intramolecular signaling between the Fab and the Fc.
Progress in the elucidation of the various intact IgG crystal structures has provided further insights into the interrelations of the Fab arms responsible for antigen binding and the Fc-mediated effector functions.Citation35,36 Support for the allosteric model was claimed from the X-ray crystal structure of the intact IgG molecules Mcg and Dob, in which close contact between a Fab and the Fc was observed.Citation37,38 However, following sequencing it was revealed that these molecules lacked a hinge region. The hinge-deletion compromised both complement C1q binding and monocyte FcR binding as a result of steric obstruction of Fc binding sites.Citation39,40 By contrast, when a full length IgG (Kol) was crystallized, only the Fab regions yielded interpretable diffractions, suggesting that either the Fc was mobile within the crystal or occupied multiple orientations.Citation41 These structures were obtained under extreme conditions and under physiologic conditions each Fab and the Fc exhibit independent mobility.
With the discovery of complement and effector molecule binding sites on antibodies, an IgG “dislocation” model has been proposed whereby hinge flexibility is exploited to enable independent mobility of Fab and Fc to bind respective ligands simultaneously.Citation10,42 However, given the bivalent nature of IgG, crosslinking of 2 epitopes by the 2 Fab arms may result in the Fabs assuming a bent conformation that allows Fab-Fc contacts. Huber et al. proposed such an allosteric liganded antibody model, describing the Kol molecule transitions from a Y-shape to a rigid T-shape in the presence of ligand, a process where the hinge undergoes extensive local structural change to facilitate the new contact formation between the CH1 and CH2 domains.Citation43 This model was disputed by an experiment showing the susceptibility of the hinge region to attack by thiols and proteolytic enzymes was unchanged regardless of whether antigen was present or absent, suggesting that no conformational change occurred in the hinge region upon antigen binding.Citation44 Furthermore, structural studies over the next decade also contradicted this allosteric model, as different crystal forms of the same antibody-antigen complex showed different hinge-region angles and no correlation between the observed angles for different Fabs and antigen binding.Citation45 Therefore, the observed structural differences are likely to result from the molecular flexibility inherent to the IgG, and are independent of antigen binding. The comparison of the complete X-ray crystal structures of IgG molecules revealed that the intact IgG1 b12 molecule with a full length hinge is highly flexible and asymmetric, with each Fab capable of adopting different positions relative to the Fc.Citation46 Together, these results confirm the crucial role of the hinge in providing the flexibility needed to permit Fab-Fc functional independence. This hinge-mediated Fab-Fc functional independence is recently supported by Kiyoshi et al., who showed that both the IgG1 and cleaved Fc bind to FcγRI at comparable kinetic rate constants and affinities.Citation47 The similarity in their binding characteristics demonstrates that the influence of the 2 Fab moieties of IgG is essentially negligible in Fc interactions.
Allosteric cooperativity in the fab domains
Although the Fab-Fc allostery hypothesis for effector functions was disregarded in favor of the associative mechanism, structural analyses on antibody-antigen bound complexes suggested there may be intramolecular signaling within the Fab domains.Citation48,49 While the 2 Fabs of an IgG are identical in sequence, differences may exist in the relative disposition of the VL/VH and CL/CH1 domains due to local flexibility provided by the “switch” residues in the VL/CL and VH/CH1 junction that form an “elbow angle.” This elbow angle is characteristic for a given Fab and can vary extensively between different Fabs.Citation50 In particular, a statistical survey of Fab structures by Stanfield et al. has shown that lambda (λ) light chain Fabs exhibit a larger elbow angle than kappa (κ) light chain Fabs, potentially due to an additional glycine in the switch region that increases the flexibility of the molecule.Citation51 Several studies have probed the influence of these structural differences on IgG-Fab structure and function, with conflicting reports. A study investigating the effect of light chain class switch on a catalytic IgG revealed the inverse finding to that of Stanfield et al., where a switch of Cκ to Cλ resulted in a decreased elbow angle that accounted for changes in peptide substrate binding affinity and catalytic efficiency of the antibody.Citation52 These results implied covalently-induced allostery triggered by configurational differences in sections of the light chain that are not part of the complementarity-determining regions (CDRs) can influence inter-domain cooperativity and antigen interaction. The CL class-mediated changes on inter-domain cooperativity were recently illustrated by Toughiri et al., who compared the thermal unfolding profiles of both κ- and λ-containing Fabs and found that, while the Cκ domain-containing Fab unfolds cooperatively as a single unit, separate unfolding events were observed in the Cλ domain-containing Fabs.Citation53 Despite these cooperative unfolding differences, there was no significant discrepancy in antigen binding among all the Fabs tested, nor were Fc-mediated functional differences demonstrated.
Studies on the influence of inter-domain allosteric signal transmission on respective V and C domain interactions have also resulted in conflicting reports. The same concept but using different techniques was used to probe the existence of ligand-induced allostery by comparing the effects of antigen binding in the V domain on the binding of ligands in the C domains within the Fab or the Fc. If there is V-C allostery, ligand binding to any of the C domains may change when the V domain is occupied with antigen. Reciprocally, antigen binding would be altered when ligand is bound to the C domains. Wright and Jaton et al. in the late 1970s used spectroscopy to address this possibility, but generated negative findings: no mutual structural changes could be detected using protein A (CH2-CH3 specific) as the probe indicating no allosteric transmission to the Fc region.Citation32 On the other hand, almost 3 decades later a similar study by Oda et al. using label-free surface plasmon resonance (SPR) biosensor technique showed that the binding to either protein A or protein G (CH1 specific) in several mAbs was inhibited in the presence of increasing antigen concentrations.Citation54,55 These results are consistent with antigen-induced allostery in both the Fab and Fc. Perhaps advances in methodology enabled researchers to identify subtle changes that were undetectable in earlier studies. Alternatively, it is possible that antigen binding simply resulted in the steric hindrance of binding of the domain ligands. It was, however, unclear whether the observed antigen-induced allostery could also inhibit the binding of FcγR and C1q to implicate significance in antibody functions.
Sela-Culang et al. recently sought structural support for binding-associated allostery by comparing a large number of free and bound antibody crystal structures.Citation56 They detected conformational changes in the relative orientation of the H and L chains in both the V and C domains, in the V-C elbow angle, and most significantly in an CH1–1 loop far from the antigen-binding site.Citation56 The authors hypothesized an allosteric signaling pathway in which antigen binding in the V domains may be transmitted through the V-C interface (i.e., changes in elbow angle), into the CH1 domain, and possibly other Fc domains, and influence Fc function. Since the CH1–1 loop is part of the CH1-CL interface involved in complement binding, this hypothesis is plausible, though correlation experiments are needed to determine whether these allosteric transmissions are functionally relevant using the same antibodies as in the data set.
Allosteric cooperativity in the Fc region
Three different classes of human FcγRs have been identified: FcγRI (CD64), FcγRII (CD32) with A, B, and C isoforms, and FcγRIII (CD16) with 2 isoforms.Citation9 Monomeric IgG binds to each FcγR with affinity ranges from 108 M−1 for FcγRI to 104–107 M−1 for FcγRII and FcγRIII.Citation57 It is established that this monovalent interaction is nonfunctional; the crosslinking of FcγR membrane molecules by multivalent ligand forms (i.e., IgG immune complexes) is a prerequisite for immune cell activation.Citation14 Although antigen is involved in the formation of the IgG immune complexes, it is not required to reveal an occult binding site. Site-directed mutagenesis in combination with X-ray crystallographic analysis of IgG-Fc in complex with FcγR confirmed that the Fc-FcγR contacts sites involve the lower hinge and hinge proximal CH2 domain residues on the Fc.Citation58-60 Like FcγR, it is accepted that the key binding motif for C1q is also located at the lower hinge and the CH2 domain.Citation7,61,62
While the role of the CH2-CH3 interface for FcγR binding was shown to be nonessential from competition studies using ligands specific to this interface (i.e., FcRn and protein A),Citation63 mutational screening of amino acids in CH2 and CH3 domains by Shields et al. provided evidence that residues distant from the receptor-Fc binding sites, including those located at the “bottom” of the CH3 domain, can influence IgG-Fc affinity for FcγRs.Citation58 For example, single amino acid mutant K414A resulted in a 40% reduction in binding for FcγRIIA and FcγRIIB, whereas E430A in the CH2-CH3 interface showed a 20–30% improvement in binding for FcγRIIA, FcγRIIB, and FcγRIIIA.Citation58 More recently, Greys et al. reported that mutations intended for serum half-life regulation have affected both FcγR and C1q binding that correlated with changes in effector functions.Citation64 The IgG1-MN (i.e., M428L/N434S) mutant that improved IgG-FcRn binding and extended serum half-life had reduced ADCC, ADCP, and CDC.Citation64,65 In contrast, the IgG1-HN (i.e., H433K/N434F) mutant that reduced IgG-FcRn binding and shortened serum half-life displayed enhanced ADCC, ADCP, and CDC.Citation64,66 These findings therefore confirm allosteric cooperativity between the C domains, by which inter-domain allosteric signal can be triggered by amino acid interchanges distant from the effector binding sites to positively or negatively affect Fc effector mechanisms (i.e., ‘configurational allosteric’ cooperativity).
Besides the sequence mediated inter-domain allostery, Fc glycosylation at position Asn 297 can induce allostery to result in profound changes in antibody functions. Although the Fc oligosaccharides are not in direct contact with FcγR,Citation59 it is well described that a- or deglycosylated IgGs are almost completely devoid of all Fc-mediated immune effector functions as a result of significantly reduced binding to FcγRs or to C1q.Citation67-69 In addition to functionality, it has been reported that the thermal and colloidal stability of the IgGs is compromised by deglycosylation.Citation70,71 Modern technologies, including X-ray crystallography,Citation72 nuclear magnetic resonance (NMR) spectroscopy,Citation73 and hydrogen/deuterium exchange mass spectrometry (H/DX-MS),Citation74 have been used to assess the link between glycoform-dependent conformational alteration and IgG stability and function. Crystal structures of the stepwise truncated Fc-oligosaccharides investigated by Krapp et al. provided direct evidence for the incremental decrease interactions with FcγR due to increased conformational changes in the individual CH2 domain.Citation72 This observed allostery was shown by a mutual approach of both CH2 domains to form a “closed” Fc conformation, thereby hindering the binding to FcγR.Citation72 These results are consistent with conformational changes detected by NMR showing that the FcγR and C1q binding sites were disturbed upon deglycosylation,Citation73 and also by H/DX-MS displaying altered deuterium incorporation profiles on critical FcγR binding residues between the glycosylated and deglycosylated forms.Citation74 These findings led to the prevailing horseshoe-shaped Fc structural model that the Fc-oligosaccharide moieties are required for maintaining the structural integrity of the effector binding sites (i.e., positive ‘configurational allosteric’ effect).
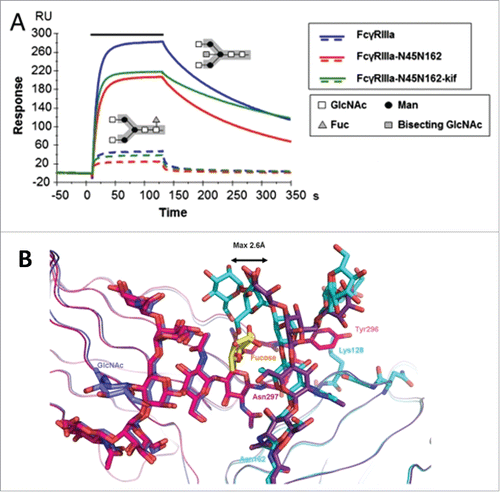
How heterogeneity in Fc glycostructures manifests configurational allosteric cooperativity is provided by additional lines of investigation. For example, depletion of fucose from the core oligosaccharides improved binding to FcγRIIIA independent of IgG subclasses, resulting in enhanced ADCC with no effect on CDC.Citation75,76 SPR and in vitro binding studies by Ferrara et al. revealed that the presence of carbohydrate at Asn 162 of FcγRIIIA is essential for the high affinity binding to the Fc and for discrimination between fucosylated and afucosylated IgG glycoforms ().Citation77 Subsequent structural analyses by independent laboratories presented evidence that the binding improvement to afucosylated IgG was attributed to the cooperative interactions between the carbohydrate moieties of both FcγRIIIA and Fc.Citation78,79 Comparison of the crystal structure of glycosylated FcγRIIIA in complex with afucosylated Fc as well as fucosylated Fc showed that the carbohydrate-mediated interactions are weakened when the fucose is linked to the Fc as a result of the displacement of oligosaccharides on Asn 162 ().Citation79 These results together confirm the core fucose as the key allosteric trigger negatively affecting the FcγR binding through steric hindrance.
Figure 3. Fucosylation-induced allostery on FcγR binding. (A) Comparison of the binding interactions between FcγRIIIA and human IgG1 glycovariants. Overlay of SPR sensorgrams for binding of 125 nM FcγRIIIA glycovariants to fucosylated (dotted line) and afucosylated (continuous line) IgG1s. The association phase is indicated by a solid bar above the curves. The afucosylated IgG significantly enhanced binding to all FcγRIIIA glycovariants with up to 100-fold increase in affinity as compared with the fucosylated version. The N-linked glycosylation is shown in the insert containing the core pentasaccharide (gray box) and the additional carbohydrate residues (legend box). (B) Overlay of the crystal interaction interface between glycosylated FcγRIIIA and Fc glycovariants. Chain A of the afucosylated (blue) bound to FcγRIIIA (cyan) and of the fucosylated (magenta) Fc bound to FcγRIIIA (dark violet) with core fucose (yellow). The oligosaccharide at Asn 162 is displaced by a maximum distance of 2.6 Å in comparison to its position in the structure with a fucosylated Fc. Figure reproduced from an open access article from.Citation79
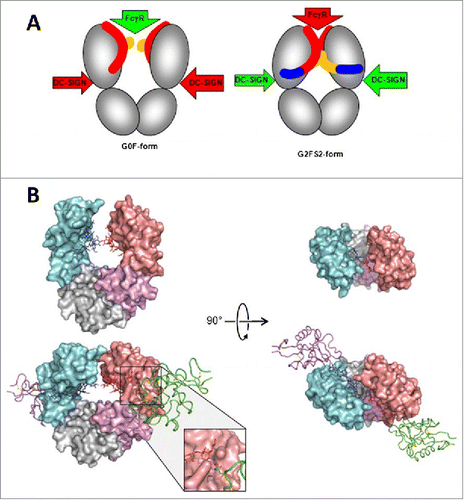
Similar to core fucose residues, increase in sialic acids was associated with decreased ADCC activity in human IgG1 mAbs, because of reduced binding affinity to FcγRIIIA.Citation80 In the same study, Scallon et al. also observed that while increased Fc sialylation did not affect the binding affinity to the soluble antigen in all 3 mAbs tested, reduced binding avidity to the cell surface antigen was observed as an indication for allostery.Citation80 Although no structural information of these sialylated mAbs was available to establish a correlation between the extent of sialylation and conformational change, Raju and colleagues hypothesized that since the sialic acid residue is a negatively charged bulky sugar, it can form ionic interactions along with imposing spatial constraints to cause conformational changes in the IgG molecule to affect both proximal (i.e., Fc-FcγR) and distal (i.e., Fab-antigen) interactions.Citation68,80,81 This hypothesis is supported by Sondermann et al., who recently illustrated significant structural alterations in the CH2 domain upon sialylation in a human IgG1-Fc. These results led them to propose a general allosteric model by which the regulation of antibody effector functions is achieved through shifting between conformational states in the Fc ().Citation82 While this model is also in agreement with the proposed Fc structure model by Krapp et al. (see earlier discussion on deglycosylation), it does not explain how these Fc conformational changes may transmit allosteric signals to the Fab to affect antigen binding. Modeling of an intact IgG in both the sialylated and asialylated forms and in complex with the cognate antigen would be needed to validate the distal allosteric effect.
Figure 4. A proposed allosteric model for Fc effector function regulation through sialylation. (A) Schematic of the blockade of FcγR binding as a result of sialylation-induced conformational changes within the Fc. The G0F-Fc maintains an “open” conformation (left) that allows FcγR binding, whereas the fully sialylated G2FS2 (blue) interacts with the CH2 domain to induce a “closed” conformation that prevents FcγR binding while revealing the binding site for DC-SIGN. DC-SIGN is an alternative cellular receptor responsible for anti-inflammatory responses (please refer to the article for details). (B) The “open” crystal front (upper left) and top (upper right) view of the G0F-Fc and the “closed” crystal front (lower left) and top (lower right) view of the G2FS2-Fc with DC-SIGN bound. Figure reproduced fromCitation82 with permission from PNAS.
Regarding the influence of galactose residues on IgG-Fc structure and function, it has been reported that changes in Fc galactosylation have resulted in noticeable alternations in C1q-mediated activity on a few marketed IgG1 mAbs.Citation83 Removal of the terminal galactose residues reduced the CDC activity in CD52-targeting Campath-1H while retaining ADCC activity.Citation84 Similarly, the ability of CD20-targeting rituximab to kill tumor cells by CDC improved with increasing terminal galactose content as a result of increased binding to C1q.Citation68,85 No galactose-mediated effect on ADCC was detected also confirmed by observations from HER2-targeting trastuzumab.Citation68 A most recent study by Peschke et al. on 4 human IgG subclasses constructed with identical V-region from rituximab also revealed that while the addition of galactose further increased the binding affinity for C1q and enhanced CDC in IgG1 and IgG3 mAbs, no change on the affinity for FcγRIIIA and for the antigen was observed in all the mAbs tested.Citation86 Together with earlier studies showing minimal differences in crystal structure and thermal stability between the galactosylated and agalactosylated forms,Citation70,87,72 these findings imply that although the key binding motif on IgG for C1q is similar to that of FcγR, the galactose-dependent CDC activity may not involve Fc conformational changes as required for FcγR-mediated activity. Instead it has been suggested that galactose exerts an “associative” role by enhancing Fc-Fc cooperative interactions (see Section 2.5).Citation86
Associative cooperativity for effector cell activation
The interactions between IgGs and FcγRs that lead to downstream signaling are not limited to monomeric interactions, but can be influenced by multimeric interactions between antigen-bound IgGs and FcγRs. The low-affinity FcγRIII in complex with Fc revealed a 1:1 receptor: Fc stoichiometry in which the formation of asymmetric contacts between the single FcγR molecule and the Fc preclude the binding of a second FcγR molecule to the same Fc.Citation59,60 This explains why the crosslinking of FcγR and cellular activation is dependent on the binding of IgGs present only in immune complexes, and is not elicited by monomeric IgG binding. The 1:1 stoichiometry prevents continuous activation of inflammation cascades by circulating IgGs in vivo that would be enabled by a 2:1 receptor: Fc stoichiometry. Additionally, the 1:1 stoichiometry also highlights the importance of antigen valency and epitope density in crosslinking the FcγRs through increased proximity and avidity of the binding interactions (i.e., ‘clustering-based associative’ cooperativity).
Multiple associative models of FcγR-mediated effector cell activation have been described. Radaev and Sun proposed a simple avidity model and an ordered receptor aggregation model ( and ).Citation88 The avidity model assumes that the increased avidity and proximity of FcγR on effector cell membrane by multivalent IgG and antigen interactions are sufficient for activation. The ordered receptor aggregation model, which can also be referred to as the clustering model, assumes that the formation of an antigen-bound FcγR aggregation complex leads to activation as a result of added stabilization. Several examples, including imaging studies on T cells and natural killer (NK) cell receptor activation processes, and crystallographic studies on a NK cell receptor in complex with its ligand, were presented as evidence for the clustering model.Citation89-91 Furthermore, the FcγRIIA noncovalent homodimers observed in the crystals of glycosylated receptor are consistent with the clustering model, by which the assembly of a dimeric activation complex composed of 2 antigen-bound IgGs in association with the FcγR dimer offers a platform for optimal signal transduction.Citation92,93
Figure 5. Proposed associative models of FcγR crosslinking and activation. (A) The simple avidity model and (B) the ordered receptor aggregation model by Radaev and Sun. Figure reproduced fromCitation88 with permission from Elsevier. The IgG dislocation models proposed by Woof and Burton: (C) Fc array formation from adjacent antigen-bound IgGs facilitates FcγR binding and (D) FcγR binding to distant antigen-bound IgGs coupled with membrane rearrangement facilitates Fc array formation. Figure reproduced fromCitation8 with permission from Macmillan Publishers Ltd.
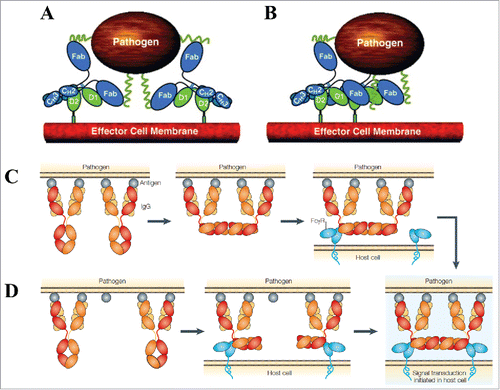
Woof and Burton described 2, non-exclusive types of associative models of FcγR crosslinking by IgG immune complexes ( and ).Citation8 These models were proposed with the knowledge from IgG crystal structure that the Fab and Fc are “dislocated” with respect to each other, allowing simultaneous interaction with antigen and effector molecule (see earlier discussion). The 2 models differ in the order of Fc array formation, but both rely on the effector cell membrane rearrangement to position FcγR binding. In the first model, the FcγR molecules are crosslinked through random movement within the effector cell membrane, eventually binding to a pre-existing antigen-bound Fc array (formed through perpendicular rotation of nearby IgG Fcs), and resulting in signal transduction. In the second model, where 2 IgG immune complex molecules initially are distributed further apart on the antigen surface, each Fc binds an individual FcγR molecule, and membrane rearrangements then occur to facilitate the formation of an FcγR-bound Fc array, again leading to signal transduction. Since FcγRs are known to relocate to specialized sphingolipid-cholesterol-rich compartments in the plasma membrane rich in signaling molecules after crosslinking by immune complexes, the array formation would be beneficial for relocation into lipid rafts.Citation8
While there is no experimental evidence to distinguish between the different models, they all encompass the valence-based, clustering-based, and template-based cooperative features ( and Appendix I). The major difference lies in that Woof and Burton's models require conformational changes in the IgG molecules to assume appropriate positions to initiate signal transduction, whereas those of Radaev and Sun emphasize the extent of intermolecular additivity for activation. It is possible that the efficacy of effector cell function depends on a synergy of these various events, and the variation within Fc allosteric cooperativity provides a tenable explanation for why different models exist. If an IgG exhibits configurational glycosylation-mediated allostery (e.g., addition of fucose or sialic acid), the activation likely will involve conformational changes, whereas if an IgG does not exhibit configurational glycosylation-mediated allostery (e.g., a controlled glycoform), the activation will simply be avidity based.
Associative cooperativity for complement activation
Early support for the associative cooperativity responsible for complement activation was provided by multiple biophysical and biochemical studies. First, hexameric C1q exhibited significantly stronger binding to IgG immune complexes than did monomeric C1q. The reverse was also true, i.e., aggregated IgG exhibited stronger interactions with C1q than monomeric IgG.Citation94,95 Second, the extent of complement fixation increased in proportion to the oligomeric state of the IgG.Citation96,97 Although monomeric IgG could bind C1q, only aggregated IgG or large IgG immune complexes could activate the complement system in plasma.Citation98 Third, the observed increase in C1q binding and complement activation was independent of whether aggregation was achieved by bivalent antigen (i.e., immune complex) binding, by heat, or by chemical crosslinking (i.e., non-immune complex).Citation96,99 And last, the C1q binding avidity and complement activation to chemically crosslinked IgG aggregates or IgG immune complexes was unaffected by antigen binding, nor by the extent of antigen binding site occupancy.Citation33,100,101
The mechanism of association for complement activity has been clarified with visual support by Diebolder et al., who illustrated that IgG hexamerization through specific mutations in the CH2-CH3 interface that enhanced Fc-Fc non-covalent interactions increased the ability of the IgG to activate complement ().Citation102 Contrary to the standard associative model, in which functional cooperativity is mediated by the IgG Fab through multivalent interactions, the authors observed that monovalent Fab binding achieved stronger complement activity than bivalent Fab binding. They reasoned that the monovalent binding of IgG molecules is less structurally constrained by antigen epitope geometry, thereby allowing efficient Fc-Fc cooperative assembly for optimal C1q recruitment. Their observations of ordered clustering into hexamers through increased Fc-Fc interactions is reminiscent of the ordered receptor aggregation model by Radaev and Sun, highlighting the need to optimize intermolecular cooperativity for maximal activation.
Figure 6. Proposed associative models of IgG hexamerization and complement activation. (A) Structure-function relationship of a triple mutant IgG1 mAb RGY (E345R/E430G/S440Y) in solution: 1) enhanced CDC activity relative to wild-type IgG1–005 and IgG1-E345R; 2) an overview electron tomography (ET) image showing a monomer (small circle) and a hexamer (large circle); 3) a representative hexamer with colored Fab pairs; and 4) ET average of 200 subtomograms at a resolution of 2.9 nm. Figure reproduced fromCitation102 with permission from AAAS. (B) Native mass spectrometry analysis of reconstructed C1, C1:IgG, and C1:IgG:Ag complexes. The assembly C1q:C1r:C1s stoichiometry of 1:2:2 is consistent with the reported composition of natural C1.Citation103 The C1 exhibits the same IgG binding stoichiometry as C1q. In the presence of excess soluble antigen, the assembly C1:IgG:Ag stoichiometry of 1:6:12 is the predominant species. (C) Model summarizing the molecular determinants for IgG-mediated activation of the classical component pathway: 1) availability of antigen and epitope distribution; 2) ability of antigen to cluster IgG at the cell surface or in solution; 3) Fc-Fc associative cooperativity required for hexamerization; 4) avidity binding sites for hexavalent C1q; 5) composition of Fc oligosaccharide; 6) Fab-Fab intermolecular cooperativity; and 7) antigen-induced conformational allostery to affect downstream Fc-mediated complement activation. Figures reproduced fromCitation103 with permission from Elsevier.
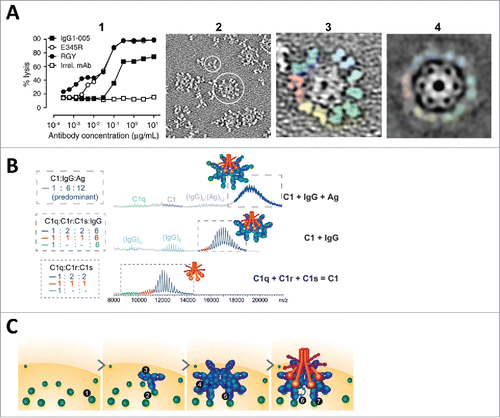
Most recently, Wang et al. within the same group used native mass spectrometry to further characterize the IgG hexamerization process in solution, with a focus on the influence of Fc glycosylation, Fab valency, and antigen binding on C1q binding and complement activation.Citation103 They not only provided detailed analysis of the formation of IgG hexamers and the assembly of hexameric immune complex in solution (), but also illustrated the role of glycosylation in enabling Fc-Fc interactions for IgG hexamerization, rather than directly affecting the C1q-Fc binding.Citation103 These results provided an allostery-independent explanation for both the detrimental effect of deglycosylation and the beneficial effect of galactosylation on complement activity observed in previous studies, through which the antibody function could be modulated by Fc-Fc intermolecular associative cooperativity independent of Fc conformational changes.
On the other hand, findings regarding the influence of Fab valency and antigen binding on IgG hexamerization and complement activity are inconsistent. While the deletion of one or both Fab arms resulted in a decrease in the abundance of IgG hexamers by ∼30% without affecting C1q binding, an unexpected increase in complement activation was observed.Citation103 Similarly, although antigen binding did not affect the C1q-binding avidity of IgG hexamers formed in solution, the complement activity was augmented in the presence of cognate antigens for 2 different mAbs. These findings are therefore in contradiction with earlier studies in the 1980s as well as that of Diebolder et al., which implied a dispensable role of Fab- and antigen- affiliated cooperativity for complement activation. Instead, these data gave rise to the abandoned antigen-induced allosteric model, leading the authors to propose that the transmission of an allosteric signal from the antigen-bound Fab into the Fc (i.e., ‘conformational allosteric’ cooperativity) was an essential component for complement activation (). Although evidence is lacking in support of the conformational changes for the observed functional consequence, the proposal to revisit the allosteric role of antigen should warrant further studies given the uncertainty in our current understanding of the molecular determinants for complement activation.
C-Region subclass effects on V-region-identical antibodies
By the 1990s, it was generally accepted that antibodies consist of 2 independent non-interacting V and C regions with distinct functions. However, many studies from the early 1990s to the present have shown that different IgG C-region subclasses can influence V-region properties despite conservation of the V region sequence. Although the amino acid sequences of the C regions of the human IgG subclasses exhibit greater than 95% similarity, major structural differences are found in the hinge region with respect to the number of residues and interchain disulfide bonds ().Citation104,105 In contrast to V→C allostery, which involves conformational changes instigated by antigen binding (i.e., conformational allostery), C→V allostery results from inherent C-region sequence differences (i.e., configurational allostery) between the subclasses (Appendix II).Citation106-113 The influence of the C region on sequence-identical V regions has been reported for at least 12 different antigen-antibody systems, as summarized in .Citation114-141 The listed studies are grouped by antigen target and presented in roughly chronological order of publication. The observations included C-region-mediated changes in V-region interactions, structure, and function. The antibody origin, subclass variant, and analytical technique used to detect the changes are also included, highlighting the extent of the observations and the diversity of antigen-antibody systems. This section reviews a representative subset of this published work, and discusses the contributing factors, molecular origin, and proposed mechanisms by which the C region may exert allosteric influence on the V region through configuration-based cooperativity.
Figure 7. Interchain disulfide linkage characteristics and structural isoforms of human IgG subclasses. (A) Schematic comparison of disulfide linkages and hinge amino acid sequences between the subclasses. The core hinge region sequences are displayed under each schematic. (B) Structural isoforms of IgG2 resulting from inter-chain disulfide shuffling: IgG2-A is the known classical form, IgG2-B is created by a symmetric disulfide linkage of both Fab regions to the hinge, and IgG2-A/B is an intermediate form with an asymmetric disulfide linkage of one Fab arm to the hinge. (C) Formation of a bispecific monovalent IgG4 molecule resulting from Fab arm exchange between 2 different monospecific bivalent IgG4 molecules. The non-covalently linked half molecule is created by the formation of intra-chain disulfide bonds as depicted in the insert. The C regions are shown in solid colors and the V regions are patterned.
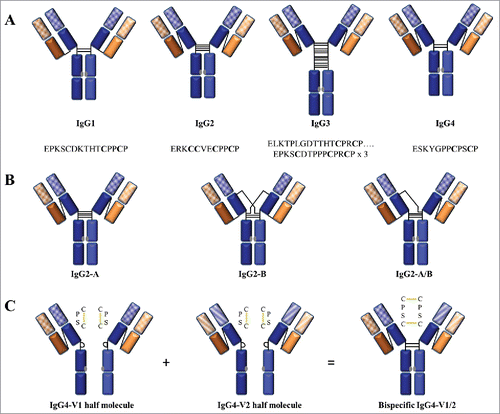
Table 2. Observations of differential effects of Ig subclass on identical V regions.
Modifications in antigen-binding characteristics
In 1988, Persson et al. extracted each of the 4 human IgG subclasses by subclass-specific affinity purification from sera obtained from 285 hepatitis B-vaccinated and 74 naturally infected individuals.Citation114 They then compared the antigen binding of the IgG subclass antibodies and found that the binding affinities to either a hepatitis B surface protein antigen or a monovalent peptide derivative differed among the subclasses in the order IgG1 > IgG2 > IgG3 > IgG4, in sera from both vaccinated and naturally infected individuals. The relative avidity (also referred to as functional affinity) for a multivalent pneumococcal polysaccharide antigen had the reverse pattern, with IgG2 exhibiting 2-fold higher avidity than IgG1.Citation114 Another comparison study was performed in 1991 by Kato et al., who used NMR to detect the effect of antigen binding on anti-dansyl murine IgG1, IgG2a, and IgG2b class-switched variants.Citation115 They found that despite sharing identical VL, CL, and VH sequences, significant differences in chemical shifts upon antigen binding were observed between the subclasses. These differences were located in the H chain CDR3, which was identified as the paratope (also called the antigen-binding site), as well as in other residues apart from the paratope throughout the Fab.Citation115 These 2 studies provided the initial evidence for subclass-associated binding variations in an immune response that are independent of antibody origins and antigen types. Although the mechanism underlying the binding differences was unknown, the binding potency of an individual IgG subclass appeared to vary depending on the valence of the antigen and on the site of the antigen (i.e., epitope) and antibody (i.e., paratope) interaction.
Around the same time, a series of studies conducted by Cooper et al. using a variety of analytical techniques further confirmed the role of IgG subclass-specific C-region determinants in modulating the interaction with a multivalent group A polysaccharide antigen.Citation116-118 The antibody system consisted of a panel of V-region-identical murine mAbs of the IgG1, IgG2b, and IgG3 subclasses, confirmed by cDNA sequencing. The results from multiple techniques consistently showed that the IgG3 mAb bound more strongly and effectively than the IgG1 or IgG2a mAbs or IgG3-derived F(ab’)Citation2 fragments.Citation116 SPR biosensor analysis revealed that the greater affinity of the IgG3 mAb was associated with both faster on-rates and slower off-rates compared with the IgG1 and IgG2b mAbs.Citation118 Consistent with this finding, Schreiber et al. reported that another murine IgG3 mAb had stronger binding avidity than the V-domain-identical IgG1 mAb to a different multivalent polysaccharide antigen in both purified and whole bacterial form.Citation119 In addition to the avidity differences between IgG subclasses, Cooper et al. revealed that the binding varied for target bacterial strains expressing different epitope densities on their surface: while the IgG3 mAb bound best to the strain with intermediate epitope density, the IgG1 and IgG2b mAbs bound best to the strain with the highest epitope density.Citation117 These observations indicated that antigen surface properties also can affect the multivalent interactions with IgGs. Thus, in interactions between IgGs and multivalent antigens, C-region subclass differences can influence both the avidity of the interactions, and the ability of the IgGs to bind targets of varying epitope density.
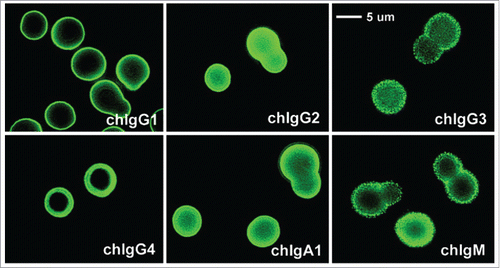
Throughout the first decade of the 2000s, Casadevall's group performed extensive IgG subclass comparison studies using a family of V-region-identical mAbs specific to the Cryptococcus neoformans capsular polysaccharide antigen, with murine and chimeric IgG forms.Citation124-128 In addition to evaluating the binding avidity for the multivalent antigen with repeating epitopes, binding affinity and thermodynamic characterizations were performed for monovalent peptide antigen derivatives. Unlike previous studies that addressed only a few parameters, Casadevall's group applied several sensitive techniques to obtain strong and comprehensive evidence that C-region differences, regardless of the origin being mouse or human, can significantly affect V-region interactions. For example, McLean et al. showed differences between various chimeric IgG mAbs in the immunofluorescence pattern and intensity on the bacterial capsule (), the binding potency for both the multivalent and monovalent antigen forms, and the reactivity with an anti-idiotypic mAb.Citation124 The authors used the term “fine specificity” to describe the nature of these binding characteristics, which are manifested by IgG complexes formed as a result of differences in the folding and exposure of antigenic epitopes of the V region.Citation124 Further SPR biosensor studies by Torres et al. found no significant difference in antigen binding between the parent murine mAb and its chimeric IgG1 form, but found altered binding affinity and specificity between the heterologous mouse and human C regions.Citation127 These findings were consistent with a study by Torres et al. on a different family of murine IgG subclass mAbs, which found significant variations in the affinity rank order among the 4 murine IgG subclasses for 3 different peptide derivations.Citation125 Differences in the localization of the fluorescence signal also were observed; while IgG2b produced a thicker outer ring and fluorescence throughout the cell compared with IgG1 and IgG2b, IgG3 produced an even stronger intensity in the outer ring but no fluorescence in the body of the cell.Citation125 Isothermal titration calorimetry (ITC) studies on a 12-mer peptide derivative revealed a 2:1 peptide to mAb binding stoichiometry and different association constants in the order IgG3 > IgG1 > IgG2b > IgG2a.Citation128 These studies collectively demonstrated that C-region differences between the IgG subclasses can alter the V-region binding characteristics with an antigen, for either multivalent (i.e., avidity) or monovalent (i.e., affinity) interactions.
Figure 8. Immunofluorescence patterns of V-region identical chimeric mAbs binding to encapsulated Cryptococcus neoformans cells. Differences in the fluorescence pattern and intensity throughout the capsule are observed: IgG1, IgG2, IgG4, and IgA1 produced an annular pattern, whereas IgG3 and IgM revealed a punctate pattern. IgG4 gave a thick annular pattern that is different from the other subclasses. Figure reproduced fromCitation124 with permission from Copyright 2002. The American Association of Immunologists, Inc.
Modifications in in vivo antibody function
In 2013, Hovenden et al. performed a series of in vitro and in vivo studies comparing the murine IgG3 mAb to its class-switched IgG1, IgG2a, and IgG2b variants with identical V-region sequences specific to the capsule of Bacillus anthracis.Citation133 Compared to the original murine IgG3 mAb, the 3 class-switched variants showed a 2–3-times lower binding avidity for the multivalent antigen by SPR, and an 18–120 times lower intrinsic affinity for a 5-mer peptide derivative by fluorescence detection.Citation133 These in vitro results were consistent with observations obtained using a pulmonary anthrax in vivo murine model, in which the protective efficacy diminished when IgG3 was switched to the other 3 subclass variants (). Although Hovenden et al. demonstrated a clear correlation between in vitro loss-of-binding and in vivo loss-of-function, the biological outcome could have resulted from factors besides the antigen-binding avidity/affinity, such as the differential ability of the murine IgG subclasses to activate the effector system. However, the authors contend that the difference in effector function alone could not explain the greater protective activity observed with the mouse IgG3 mAb versus the other subclasses, because both mouse IgG2a and IgG2b interact with Fc receptors more strongly than does mouse IgG3.Citation142
Figure 9. In vivo protective efficacy and capsule reactivity of V-region identical murine IgG subclass mAbs against Bacillus anthracis. (A) Overall survival of mAb-treated mice after receiving lethal dose of Bacillus anthracis spores. Only IgG3 mAbs were protective in a dose-dependent manner; treatment with IgG1, IgG2a, or IgG2b mAbs did not significantly increase the overall survival percentage at any given dose. (B) Incubation of Bacillus anthracis cells with each mAb variant and evaluation by differential interference contrast microscopy. The IgG3 mAbs produced dual-capsule binding reactions at both the outer edge (red arrow) and inner layer near the cell wall (blue arrow); the other subclass variants produced a “puffy” type of reaction where no reactivity to either region was observed. Figure reproduced from an open access article from.Citation133
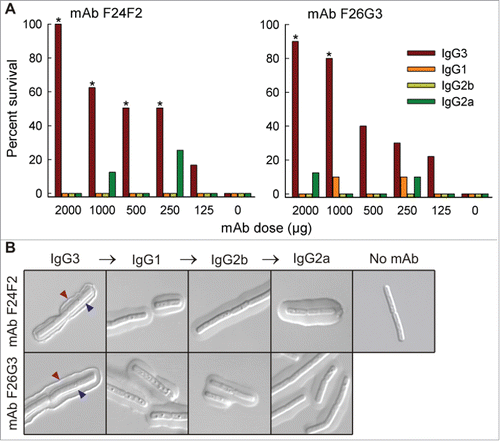
A study by Hubbard et al. using the same family of V-region sequences on each of the 4 human IgG subclasses, also showed differential binding characteristics to the capsule of Bacillus anthracis cells.Citation134 SPR measurements of each chimeric mAb binding to a 25-mer peptide derivative found 9- to 20-fold lower avidity compared with the original mouse IgG3 mAb. Among the 4 chimeric mAbs, the binding potency was decreased in the order IgG2 > IgG4 > IgG1 > IgG3.Citation134 Although the authors did not analyze the protective efficacy of these chimeric mAbs in an in vivo disease model, the microbial binding patterns revealed by microscopy were very similar to those of inactive mouse non-IgG3 mAbs. Therefore, they postulated that the chimeric IgG mAbs also lost their protective activity in a manner influenced by C-region-dependent effects.
Similarly, another series of in vitro and in vivo studies was performed by Xia et al. to examine the C-region influence on a renal pathogenicity that was correlated with altered antigen-binding characteristics.Citation135 Anti-DNA murine mAbs of the IgM, IgG1, IgG2a, and IgG2b subclasses were first compared with the parental IgG3 mAb in terms of their binding affinity for nuclear and renal antigens. The results showed differential binding to both DNA and chromatin with decreasing affinity in the order IgG3 > IgG2a > IgG1 > IgG2b > IgM by enzyme-linked immunosorbent assay (ELISA) and SPR analyses. On the other hand, IgG2a showed a higher affinity than IgG3 for a variety of renal antigens while IgG1, IgG2b, and IgM displayed weak or no binding to these antigens.Citation135 Since the binding for nuclear antigens and cross-reactivity with renal antigens have implications for systemic lupus erythematosus (SLE), the authors explored the functional consequences of these antibodies in a murine disease model. They found increased renal IgG deposition and kidney damage in vivo that were consistent with the higher binding potency of IgG2a and IgG3 mAbs in vitro, and were also correlated with shorter survival compared with the non-reactive IgM mAb.Citation135 The authors concluded that the mouse IgG2a subclass conferred greater effector function and complement activation against lupus disease compared with the other subclasses. They also suggested that an increased functional affinity through Fc-Fc interactions in the mouse IgG3 mAb was responsible for its greater pathogenic potential.
Molecular origin of C-region subclass-mediated influence on V region
Several investigators have suggested that changes in the CH1 domain are the molecular origin of functional changes in the V region. In 1996, Pritsch et al., studying human anti-tubulin mAb clones derived from a lymphoma patient, found that despite sharing identical VH and VL sequences, the IgG1 mAb exhibited less than 10% of the affinity of the IgA mAb.Citation120,121 To determine which C region domain was responsible for the difference, they prepared Fab and F(ab’)Citation2 fragments. Their results revealed that the binding difference was found at the Fab level, suggesting that the CH1 domain is involved in causing conformational changes in the antigen-binding site to influence antigen-binding kinetics.Citation120 To support this claim, the authors constructed a model using crystallographic data from proteins with homologous V domains. They found that one of the CH1 loops involved in the VH-CH1 contacts had a different conformation for each subclass.Citation121 Based on this structural evidence, they concluded that specific differences in the CH1 domain can impose structural or kinetic constraints on the paratope through contacts at the VH-CH1 interface. The authors also related these findings to the roles of clonal selection and maturation in generating an effective immune response. They postulated that the C-region modulation of antigen-binding affinity may present an alternative mechanism for the affinity maturation of V regions, in which maturation is achieved through C-region class switching in the absence of further somatic mutations.Citation121
Further evidence for the role of CH1 in modulating antigen binding was obtained from Torres et al.'s work on different mouse IgG subclasses and related Fabs targeting Cryptococcus neoformans with identical V regions. Examination of the kinetic and equilibrium rate constants revealed that all 4 intact mAbs and Fabs derived from the IgG2a and IgG3 subclass mAbs exhibited different kinetic and thermodynamic properties upon monovalent peptide binding.Citation126 While the IgG3 Fab had the lowest binding affinity among the 4 molcules, the IgG2a Fab had higher affinity than the intact IgG2a or the parental intact IgG3 mAb. Further homology modeling analysis of these IgG subclass mAbs identified 3 regions in the CH1 domain with structural differences.Citation126 The authors postulated that these differences in the CH1 domain could translate to structural isomers that alter the antibody-antigen complex formation kinetics. Furthermore, since one or more amino acids located in these regions can participate in the formation of electrostatic and hydrophobic interactions, the CH1 domain may influence antigen binding through changes in the microenvironment of the paratope site.Citation126
In addition to the CH1 domain, the CH2 and CH3 domains have also been suggested to play a role in modulating V-region binding in antibody-antigen systems. McCloskey et al. compared the binding kinetics of intact chimeric IgGs and their respective F(ab’)Citation2 fragments to a multivalent glycoprotein from tumors by SPR. They found that the apparent binding avidity among the intact IgGs differed in the order: IgG4 > IgG3 > IgG2 > IgG1.Citation123 On the other hand, identical kinetic parameters were observed for all of the derived F(ab’)Citation2 fragments, supporting the notion that the binding differences were mediated by the CH2-CH3 domains in the C region (). Additional evidence for CH2-CH3 domain involvement was provided by domain-swapping studies by Hovenden et al. using a mouse antibody system.Citation133 The authors demonstrated different IgG subclass functions in vivo, and then constructed hybrid mAbs by substituting each C domain from the (non-responsive) IgG2b into the (functionally active) IgG3 mAb.Citation133 They found that while both the CH2 and CH3 domains from IgG2b compromised the mAb's protective activity and affinity (more so with the CH2 domain), the CH1 domain had no influence.
Figure 10. SPR sensorgrams of V-region identical (A) intact human IgG subclass mAbs and (B) respective F(ab’)Citation2 domains binding to mucin immobilized onto the sensor surface. Each set was analyzed using the same sample concentration. IgG1 and IgG2 exhibited faster dissociation than IgG3 and IgG4. IgG4 achieved the highest maximal binding response at the end of the association phase. In contrast, insignificant binding pattern difference was observed between the F(ab’)Citation2 fragments. IgG4 F(ab’)Citation2 showeda slightly higher binding response than the others. Figure reproduced from Citation123 with permission from Wiley.
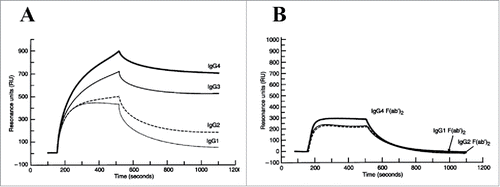
Besides the 3 C domains, the difference in C region hinge flexibility has also been postulated to affect multivalent interactions. Using a competitive ELISA technique, Morelock et al. showed that the binding of a chimeric IgG followed the order, IgG1 > IgG4 > IgG2, with respect to its ability to compete with the V-identical mouse IgG1 mAb for intracellular adhesion molecule-1.Citation122 They also examined the binding of Fabs generated from the chimeric IgG4 and mouse IgG1 mAbs, and found that while the binding constants were equivalent between the 2 Fabs, they were 70- and 300-times weaker than their intact IgG counterparts. The authors attributed these differences to the cooperative bivalent binding involving 2 Fab arms on multivalent antigen surfaces, for which the avidity is determined by hinge region flexibility, as opposed to monovalent binding involving each unhinged Fab arm. The competition rank order of these intact chimeric mAbs was consistent with that of the Fab-Fc flexibility established for human IgG subclasses. Therefore, the authors suggested that the binding differences could be explained by hinge flexibility, where the more flexible IgG1 permitted bivalent binding better than the less flexible IgG2.Citation122
Mechanisms for C-region subclass-mediated influence on V region
Several mechanisms have been proposed to explain the influence of C-region changes on V-region properties. First, Greenspan and Cooper hypothesized that the C region can enhance the avidity for an antigen with repeating epitopes through non-covalent intermolecular Fc-Fc interactions between 2 antigen-bound IgG3 molecules in close proximity.Citation143 This model, which is consistent with the ‘Template-based’ cooperativity concept ( and Appendix I), was proposed to explain the observation that mouse IgG3 mAbs can bind cooperatively to multivalent antigens, whereas V-region-identical IgG1 and IgG2b mAbs do not exhibit cooperative binding.Citation116,144,145 Mouse IgG3 is the preferential subclass in humoral immune responses to bacterial polysaccharides.Citation146 The authors thus reasoned that since antibodies against bacterial polysaccharides generally exhibit low intrinsic affinities, the Fc-Fc intermolecular cooperative binding in the mouse IgG3 subclass may be an adaptation by nature to stabilize the antibody-antigen bound complex to bacterial surfaces to attain more efficient bacterial clearance. The ability to participate in such intermolecular Fc-Fc association is subclass-specific, and may depend on the inherent C-region configurational characteristics.Citation143 The authors postulated that IgG segmental flexibility plays a role in the binding differences observed at varying epitope densities.Citation117 Since mouse IgG3 has a rigid structure, bacterial surfaces with high epitope density facilitate the intermolecular Fc-Fc association between closely spaced IgG3 molecules. Conversely, because IgG2b is the most flexible mouse IgG subclass, it is plausible that under low epitope density the IgG2b mAb binds more effectively than the other subclasses, owing to the greater probability of bivalent interaction by each individual IgG molecule.Citation117
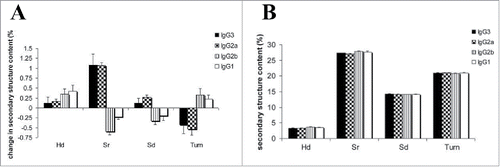
Second, configurational differences between the C regions can impose distinct allosteric effects on V-region conformations to alter antigen binding. Using the V-identical murine IgG set against the Cryptococcus neoformans capsular polysaccharide antigen, Janda and Casadevall performed circular dichroism (CD) studies comparing the spectra of the 4 murine IgG subclass mAbs, with and without bound antigen.Citation129 Their results showed that in the absence of antigen, the IgG1 and IgG2b mAbs exhibited similar CD spectra to each other, while the CD spectra of the IgG2a and IgG3 mAbs were also highly comparable to each other. The CD spectra were interpreted as showing that IgG1 and IgG2b contained greater α helical content, whereas IgG2s and IgG3 had greater β sheet content.Citation129 In the presence of antigen, all 4 mAbs exhibited shifts in their CD spectra, indicative of secondary structural changes (). Furthermore, each mAb pair with similar native spectra (e.g., IgG1/IgG2b) exhibited similar spectral changes, with a magnitude of change in the order IgG2a > IgG3 > IgG2b > IgG1 at antigen saturation.Citation129 The differences in secondary structure observed between the subclasses upon antigen binding provide evidence for allostery between the C and V regions. The conformations of the final antigen-antibody complexes differ depending on the subclass. These results, together with previous binding studies, support the notion that the C-region configuration can allosterically impose structural constraints on the V region, resulting in conformational changes that are manifested as differences in V-region functionality.
Figure 11. Circular dichroism analysis of V-region identical murine IgG subclass mAbs (A) with and (B) without bound antigen. The antigen-bound subclass pairs IgG1/IgG2b and IgG2a/IgG3 shared similar patterns of changes in secondary structure with difference in magnitude, whereas the secondary structure content was similar between all the subclasses in the absence of antigen. H(d) represents α helix (disordered) secondary structure; S(r) represents β sheet (regular) secondary structure; S(d) represents β sheet (disordered) secondary structure. Figure reproduced fromCitation129 with permission from Elsevier.
Third, local chemical and electrostatic differences in the C-region microenvironment can restrain V-region function through direct manipulation of the paratope. To provide direct evidence for C-region-induced alterations in antibody paratope, Janda et al. used tryptophan (Trp) fluorescence and NMR spectroscopy to explore changes in the V region upon binding to the Cryptococcus neoformans polysaccharide antigen in both the multivalent and monovalent forms.Citation130 The mAbs exhibited a shift in Trp fluorescence after binding the polysaccharide complex. Binding to the IgG3 mAb induced the greatest shift, whereas binding to IgG1 induced the smallest shift. The 4 mAbs shared the same V-region sequence, which contained 4 Trp residues, one of which appeared to be directly involved in the antigen interactions. Therefore, the observed differences implied that changes in the binding pocket were mediated by the C region.Citation130 NMR studies were performed with an isotype-labeled 12-mer peptide and mAbs that could bind and cleave it. Negligible chemical shifts were observed for each mAb upon binding the peptide. However, differences in the chemical shift perturbation upon cleavage of the peptide were observed among the mAb subclasses. The IgG2b mAb cleaved the peptide at both 25°C and 37°C, the IgG1 and IgG2a mAbs only exhibited proteolytic activity at 37°C, and the IgG3 mAb showed no proteolytic activity at either temperature.Citation130 These results indicated that different C regions attached to the same V region could confer different properties by influencing the chemical and electronic environment of the antibody paratope, profoundly affecting antigen interactions.
The notion of V-region conformation or paratope landscape changes as manifestations of C-region allosteric influence on the V region was further explored by Xia et al. using a set of murine anti-DNA IgG subclasses with an identical V-region sequence.Citation136,137 The catalysis of nucleic acid or peptide cleavage is an intrinsic property of certain antibodies with implications in homeostasis, autoimmune disease, and protection against infection.Citation147,148 Such anti-DNA mAbs are a serological hallmark of SLE. Similar to the findings of Janda and Casadevall, CD and Trp fluorescence spectroscopic analyses showed differences in antibody secondary structure between the subclasses after antigen binding. The IgG3 mAb showed the greatest fluorescence shift after binding to double-stranded DNA, single-stranded DNA, and histone 2A, while the IgG1 mAb exhibited the most prominent shift for histone 2B.Citation136 These results indicated that variations in binding between the IgG subclasses were not only related to C-region differences, but also to the properties of the antigen. The authors related the differences in emission intensity to the different ways the mAbs interacted with their cognate antigens, for which the mAbs used different binding paratopes despite sharing the same V region. In an NMR study, the authors observed differences between the IgG subclasses with respect to their catalytic potential to cleave DNA, as well as the proteolytic activity toward a peptide mimic of the antigen.Citation137 While the 4 mAbs had the same primary cleavage sites for the peptide antigen, the catalytic efficiency differed among the subclasses. There was no correlation between the catalytic rates and the binding affinities to the peptide antigens.Citation137 These studies provided further evidence that C regions can exert allosteric influence on the V region by altering the local electronic environment of the V-region paratopes, as well as their secondary structures.
Summary and perspectives
A central concept in antibody structure and function is that the V region determines antigen specificity, whereas the C region determines the class type, effector functions, and pharmacological characteristics of Ig molecules. This view of V-C functional independence was accepted in light of negative evidence for antigen-induced conformational allosteric (or intramolecular) cooperativity in antibody effector functions. Meanwhile, considerable direct experimental evidence favored the associative hypothesis whereby tenable mechanistic models, coupled with insights from crystallographic studies, indicated that antibody function is achieved mainly through clustering-based (or intermolecular) cooperativity in the form of increased avidity by crosslinking. In particular, the hinge between the Fab and Fc regions provides antibody flexibility that allows simultaneous interactions with antigen and effector molecules. Such flexible structural configuration argues against the transmission of secondary and tertiary structural changes between the Fab and Fc regions, thus contributing to the abandonment of the allosteric hypothesis.
However, polymorphisms in the Fc receptor molecules and the differential influence of IgG glycoforms have changed our understanding of allostery in IgG molecules. In contrast to the scarcity of literature on allosteric effects due to antigen binding-dependent conformational changes in the antibody, there is substantial evidence for glycosylation-induced configurational allostery in the C region. Many studies have established that the heterogeneity of N-linked glycoforms can display positive or negative cooperativity in the binding of IgGs to FcγRs and C1q through changes in the Fc structure. There is also substantial support for inter-domain configurational allostery triggered by amino acid changes distant from the effectors' binding sites. Together these findings implicate that regardless of V-region antigen specificity, the C-region interactions with effector molecules are dictated by configurational allosteric cooperativity through localized structural modifications in the Fc to directly impact downstream Fc-mediated effector functions.
Furthermore, a compelling body of work by multiple independent groups using a variety of antigens and antibodies of mouse, chimeric, or human origin has provided additional evidence for configurational allosteric cooperativity, in which covalent changes in the C region may affect V region conformation, and consequently, antigen binding. These observations have challenged our traditional view of V-C functional independence and led to the expeditions for the allosteric mechanisms and the molecular origins through which these effects may be occurring. Through fragmentation studies using a wide variety of biophysical analytical methods, it is revealed that no single IgG region appears responsible for the functional changes. Instead, particular domains and regional differences spanning from the Fab to the Fc, including CL, CH1, CH2-CH3, hinge flexibility, or V-C elbow, can all play central roles in the antibody paratope structure, and thus impact its antigen affinity and specificity.
It is noteworthy that not all subclass changes result in V region changes;Citation149 the functional differences are mainly attributed to subclass-specific variations in FcγR interactions.Citation23,26,150,151 Therefore, it is plausible that the susceptibility of the V region to modifications in the C region may be more a function of subtle differences in V-region structure that either facilitate or inhibit the signaling between the C and V region, rather than being a direct function of the C-region structure. This explains the inconsistent results reported in the literature, in which allosteric changes are facilitated by a variety of mechanisms and molecular origins, or in contrast are only observed in some V-C combinations but not others. Given that the cooperativity exhibited by each antibody-antigen system involves different sets of variables, including antigen valency and epitope density, antibody intrinsic affinity or avidity for the antigen, structural flexibility with respect to Fab-Fab and Fab-Fc orientations, and Fc glycosylation, etc., we suggest that V-C allosteric cooperativity is not an inherent property of either region; rather, it is a system property that depends on a synergy of the factors that determine the characteristics of cooperative interactions between the antibody and the antigen.
Nevertheless, the observation that some antibodies are permissive to V-C allostery while others are not add a layer of unpredictability in antibody function that deviates from the central dogma of immunology. This unpredictability can have profound implications in therapeutic antibody development because it precludes the ability to predict the clinical outcome of antibody-antigen interaction at a given time. Our current understanding of antibody functional cooperativity comes from in vitro data, and our lack of knowledge about the architecture of the immune complexes formed in vivo adds uncertainly to our understanding of allostery.Citation152,153 This uncertainty may explain why some models of the effector cell and complement activation assume that conformational changes and/or transmission of allosteric signal are at work while others models do not make this assumption. The observations that antibodies specific for the same target (e.g., several approved anti-CD20 mAbs)Citation154 exhibit significant variation in effector functions and clinical outcomes illustrate the complexity in extrapolating from activities demonstrated in vitro to in vivo IgG cooperative mechanisms, and highlights the need for further study.
In summary, the successful optimization of mAbs for therapeutic purposes requires a thorough understanding of how each antibody domain interacts and behaves to execute all of the antibody functions. Clearly, tremendous amounts of recent work are alerting us of the need to expand our current knowledge of the antibody structure and function relationship beyond the central dogma. Given that the biological activities of an IgG molecule depend on diverse inter- and intra- molecular cooperative interactions that can be influenced by a variety of C-region configurational allostery, rather than assuming nature's knowledge on the native function of V and C regions, there should be increased attention on the impact of allosteric cooperativity in developing antibody therapeutics, and a greater focus placed on gaining further insights into the rules governing the intramolecular signaling through the IgG domains particularly under physiologically relevant settings.
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301-16. doi:10.1038/nri2761. PMID:20414204
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192-8. doi:10.1126/science.1241145. PMID:24031011
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. doi:10.1126/science.aaa8172. PMID:25838373
- Melis JPM, Strumane K, Ruuls SR, Beurskens FJ, Schuurman J, Parren PWHI. Complement in therapy and disease: Regulating the complement system with antibody-based therapeutics. Mol Immunol. 2015;67:117-30. doi:10.1016/j.molimm.2015.01.028. PMID:25697848
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2015;7:9-14. doi:10.4161/19420862.2015.989042. PMID:25529996
- Reichert JM. Antibodies to watch in 2017. mAbs. 2017;9:167-81. doi:10.1080/19420862.2016.1269580
- Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738-40. doi:10.1038/332738a0. PMID:3258649
- Woof JM, Burton DR. Human antibody–Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol. 2004;4:89-99. doi:10.1038/nri1266. PMID:15040582
- Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34-47. doi:10.1038/nri2206. PMID:18064051
- Burton DR. Antibody: the flexible adaptor molecule. Trends Biochem Sci. 1990;15:64-9. doi:10.1016/0968-0004(90)90178-E. PMID:2186517
- Jefferis R, Lund J, Pound JD. IgG-Fc-mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol Rev. 1998;163:59-76. doi:10.1111/j.1600-065X.1998.tb01188.x. PMID:9700502
- Bowen A, Casadevall A. Revisiting the Immunoglobulin Intramolecular Signaling Hypothesis. Trends Immunol. 2016;37:721-3. doi:10.1016/j.it.2016.08.014. PMID:27639628
- Metzger H. The effect of antigen on antibodies: recent studies. Contemp Top Mol Immunol. 1978;7:119-52. doi:10.1007/978-1-4757-0779-3_4. PMID:365443
- Metzger H. Transmembrane signaling: the joy of aggregation. J Immunol. 1992;149:1477-87. PMID:1324276
- Torres M, Casadevall A. The immunoglobulin constant region contributes to affinity and specificity. Trends Immunol. 2008;29:91-7. doi:10.1016/j.it.2007.11.004. PMID:18191616
- Casadevall A, Janda A. Immunoglobulin isotype influences affinity and specificity. Proc Natl Acad Sci. 2012;109:12272-3. doi:10.1073/pnas.1209750109. PMID:22826242
- Jefferis R. The antibody paradigm: present and future development as a scaffold for biopharmaceutical drugs. Biotechnol Genet Eng Rev. 2010;26:1-42. doi:10.5661/bger-26-1. PMID:21415874
- Liu L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-Fusion proteins. J Pharm Sci. 2015;104:1866-84. doi:10.1002/jps.24444. PMID:25872915
- Hamilton RG. The Human IgG Subclasses. Calbiochem. 1989. http://wolfson.huji.ac.il/purification/PDF/affinity/CALBIOCHEM_HumanIgG_Subclasses.pdf
- Correia IR. Stability of IgG isotypes in serum. mAbs. 2010;2:221-32. doi:10.4161/mabs.2.3.11788. PMID:20404539
- Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi:10.3389/fimmu.2014.00520. PMID:25368619
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343-57. doi:10.1038/nri1837. PMID:16622479
- Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol. 2007;25:1369-72. doi:10.1038/nbt1207-1369. PMID:18066027
- Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526:159-66. doi:10.1016/j.abb.2012.03.021. PMID:22465822
- The Antibody Society. Therapeutic monoclonal antibodies approved or in review in the European Union or the United States. 2017 [cited 2017 May 22]; Available from: http://www.antibodysociety.org/wordpress/wp-content/uploads/2017/05/EU_US-approved-mAbs-May-6_2017.pdf
- Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401-13. doi:10.1517/14712598.7.9.1401. PMID:17727329
- Metzger H. Effect of antigen binding on the properties of antibody. Adv Immunol. 1974;18:169-207. doi:10.1016/S0065-2776(08)60310-7. PMID:4133409
- Schlessinger J, Steinberg IZ, Givol D, Hochman J, Pecht I. Antigen-induced conformational changes in antibodies and their Fab fragments studied by circular polarization of fluorescence. Proc Natl Acad Sci U S A. 1975;72:2775-9. doi:10.1073/pnas.72.7.2775. PMID:1058492
- Jaton JC, Huser H, Braun DG, Givol D, Pecht I, Schlessinger J. Conformational changes induced in a homogeneous antitype III pneumococcal antibody by oligosaccharides of increasing size. Biochemistry (Mosc). 1975;14:5312-5. doi:10.1021/bi00695a014
- Pecht I, Ehrenberg B, Calef E, Arnon R. Conformational changes and complement activation induced upon antigen binding to antibodies. Biochem Biophys Res Commun. 1977;74:1302-10. doi:10.1016/0006-291X(77)90584-8. PMID:557325
- Jaton JC, Huser H, Riesen WF, Schlessinger J, Givol D. The binding of complement by complexes formed between a rabbit antibody and oligosaccharides of increasing size. J Immunol Baltim Md. 1976;116:1363-6
- Wright JK, Engel J, Brandt DC, Jaton J-C. Independence of the binding of domain-specific ligands to Fab and Fc suggests that antigen-induced effects in IgG antibodies are not allosteric. FEBS Lett. 1978;90:79-83. doi:10.1016/0014-5793(78)80302-0. PMID:658444
- Tschopp J, Schulthess T, Engel J, Jaton JC. Antigen-independent activation of the first component of complement C1 by chemically crosslinked rabbit IgG-oligomers. FEBS Lett. 1980;112:152-4. doi:10.1016/0014-5793(80)80168-2. PMID:7371851
- Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203-34. doi:10.1146/annurev.immunol.15.1.203. PMID:9143687
- Davies DR, Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87-117. doi:10.1146/annurev.iy.01.040183.000511. PMID:6399980
- Burton DR. Immunoglobulin G: functional sites. Mol Immunol. 1985;22:161-206. doi:10.1016/0161-5890(85)90151-8. PMID:3889592
- Steiner LA, Lopes AD. The crystallizable human myeloma protein Dob has a hinge-region deletion. Biochemistry (Mosc). 1979;18:4054-67. doi:10.1021/bi00586a002
- Guddat LW, Herron JN, Edmundson AB. Three-dimensional structure of a human immunoglobulin with a hinge deletion. Proc Natl Acad Sci U S A. 1993;90:4271-5. doi:10.1073/pnas.90.9.4271. PMID:8483943
- Klein M, Haeffner-Cavaillon N, Isenman DE, Rivat C, Navia MA, Davies DR, Dorrington KJ. Expression of biological effector functions by immunoglobulin G molecules lacking the hinge region. Proc Natl Acad Sci U S A. 1981;78:524-8. doi:10.1073/pnas.78.1.524. PMID:6787591
- Woof JM, Nik Jaafar MI, Jefferis R, Burton DR. The monocyte binding domain(s) on human immunoglobulin G. Mol Immunol. 1984;21:523-7. doi:10.1016/0161-5890(84)90068-3. PMID:6235444
- Marquart M, Deisenhofer J, Huber R, Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980;141:369-91. doi:10.1016/0022-2836(80)90252-1. PMID:7441755
- Burton DR, Woof JM. Human antibody effector function. Adv Immunol. 1992;51:1-84. doi:10.1016/S0065-2776(08)60486-1. PMID:1502974
- Huber R, Deisenhofer J, Colman PM, Matsushima M, Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976;264:415-20. doi:10.1038/264415a0. PMID:1004567
- Wright JK, Engel J, Jaton J-C. Selective reduction and proteolysis in the hinge region of liganded and unliganded antibodies. Identical kinetics suggest lack of major conformational change in the hinge region. Eur J Immunol. 1978;8:309-14. doi:10.1002/eji.1830080505. PMID:28953
- Sheriff S, Silverton EW, Padlan EA, Cohen GH, Smith-Gill SJ, Finzel BC, Davies DR. Three-dimensional structure of an antibody-antigen complex. Proc Natl Acad Sci U S A. 1987;84:8075-9. doi:10.1073/pnas.84.22.8075. PMID:2446316
- Saphire EO, Stanfield RL, Crispin MDM, Parren PWHI, Rudd PM, Dwek RA, Burton DR, Wilson IA. Contrasting IgG structures reveal extreme asymmetry and flexibility. J Mol Biol. 2002;319:9-18. doi:10.1016/S0022-2836(02)00244-9. PMID:12051932
- Kiyoshi M, Caaveiro JMM, Kawai T, Tashiro S, Ide T, Asaoka Y, Hatayama K, Tsumoto K. Structural basis for binding of human IgG1 to its high-affinity human receptor FcγRI. Nat Commun. 2015;6:7866. doi:10.1038/ncomms7866. PMID:26198319
- Wilson IA, Stanfield RL. Antibody-antigen interactions: new structures and new conformational changes. Curr Opin Struct Biol. 1994;4:857-67. doi:10.1016/0959-440X(94)90267-4. PMID:7536111
- Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169-217. doi:10.1016/0161-5890(94)90001-9. PMID:8114766
- Davies DR, Chacko S. Antibody structure. Acc Chem Res. 1993;26:421-7. doi:10.1021/ar00032a005
- Stanfield RL, Zemla A, Wilson IA, Rupp B. Antibody elbow angles are influenced by their light chain class. J Mol Biol. 2006;357:1566-74. doi:10.1016/j.jmb.2006.01.023. PMID:16497332
- Ponomarenko N, Chatziefthimiou SD, Kurkova I, Mokrushina Y, Mokrushina Y, Stepanova A, Smirnov I, Avakyan M, Bobik T, Mamedov A, et al. Role of κ→λ light-chain constant-domain switch in the structure and functionality of A17 reactibody. Acta Crystallogr D Biol Crystallogr. 2014;70:708-19. doi:10.1107/S1399004713032446. PMID:24598740
- Toughiri R, Wu X, Ruiz D, Huang F, Crissman JW, Dickey M, Froning K, Conner EM, Cujec TP, Demarest SJ. Comparing domain interactions within antibody Fabs with kappa and lambda light chains. mAbs. 2016;8:1276-85. doi:10.1080/19420862.2016.1214785. PMID:27454112
- Oda M, Kozono H, Morii H, Azuma T. Evidence of allosteric conformational changes in the antibody constant region upon antigen binding. Int Immunol. 2003;15:417-26. doi:10.1093/intimm/dxg036. PMID:12618486
- Sagawa T, Oda M, Morii H, Takizawa H, Kozono H, Azuma T. Conformational changes in the antibody constant domains upon hapten-binding. Mol Immunol. 2005;42:9-18. doi:10.1016/j.molimm.2004.07.004. PMID:15488939
- Sela-Culang I, Alon S, Ofran Y. A systematic comparison of free and bound antibodies reveals binding-related conformational changes. J Immunol Baltim Md. 2012;189:4890-9
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716-25. doi:10.1182/blood-2008-09-179754. PMID:19018092
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276:6591-604. doi:10.1074/jbc.M009483200. PMID:11096108
- Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267-73. doi:10.1038/35018508. PMID:10917521
- Radaev S, Motyka S, Fridman W-H, Sautes-Fridman C, Sun PD. The structure of a human Type III Fcγ receptor in complex with Fc. J Biol Chem. 2001;276:16469-77. doi:10.1074/jbc.M100350200. PMID:11297532
- Morgan A, Jones ND, Nesbitt AM, Chaplin L, Bodmer MW, Emtage JS. The N-terminal end of the CH2 domain of chimeric human IgG1 anti-HLA-DR is necessary for C1q, Fc gamma RI and Fc gamma RIII binding. Immunology. 1995;86:319-24. PMID:7490135
- Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol Baltim Md. 2000;164:4178-84
- Wines BD, Powell MS, Parren PWHI, Barnes N, Hogarth PM. The IgG Fc Contains Distinct Fc Receptor (FcR) binding sites: the leukocyte receptors FcγRI and FcγRIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and Protein A. J Immunol. 2000;164:5313-8. doi:10.4049/jimmunol.164.10.5313. PMID:10799893
- Grevys A, Bern M, Foss S, Bratlie DB, Moen A, Gunnarsen KS, Aase A, Michaelsen TE, Sandlie I, Andersen JT. Fc engineering of human IgG1 for altered binding to the neonatal Fc receptor affects Fc effector functions. J Immunol Baltim Md. 2015;194:5497-508
- Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 2006;281:23514-24. doi:10.1074/jbc.M604292200. PMID:16793771
- Vaccaro C, Bawdon R, Wanjie S, Ober RJ, Ward ES. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc Natl Acad Sci. 2006;103:18709-14. doi:10.1073/pnas.0606304103. PMID:17116867
- Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: current models. Immunol Lett. 2002;82:57-65. doi:10.1016/S0165-2478(02)00019-6. PMID:12008035
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471-8. doi:10.1016/j.coi.2008.06.007. PMID:18606225
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226-34. doi:10.1038/nrd2804. PMID:19247305
- Mimura Y, Church S, Ghirlando R, Ashton PR, Dong S, Goodall M, Lund J, Jefferis R. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol Immunol. 2000;37:697-706. doi:10.1016/S0161-5890(00)00105-X. PMID:11275255
- Zheng K, Bantog C, Bayer R. The impact of glycosylation on monoclonal antibody conformation and stability. mAbs. 2011;3:568-76. doi:10.4161/mabs.3.6.17922. PMID:22123061
- Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979-89. doi:10.1016/S0022-2836(02)01250-0. PMID:12527303
- Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta. 2006;1760:693-700. doi:10.1016/j.bbagen.2005.10.002. PMID:16343775
- Houde D, Arndt J, Domeier W, Berkowitz S, Engen JR. Characterization of IgG1 conformation and conformational dynamics by hydrogen/deuterium exchange mass spectrometry. Anal Chem. 2009;81:2644-51. doi:10.1021/ac802575y. PMID:19265386
- Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, Tsumoto K, Kumagai I, Shitara K. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcγRIIIa. J Mol Biol. 2004;336:1239-49. doi:10.1016/j.jmb.2004.01.007. PMID:15037082
- Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306:151-60. doi:10.1016/j.jim.2005.08.009. PMID:16219319
- Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032-6. doi:10.1074/jbc.M510171200. PMID:16330541
- Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells Devoted Mol Cell Mech. 2011;16:1071-80. doi:10.1111/j.1365-2443.2011.01552.x
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669-74. doi:10.1073/pnas.1108455108. PMID:21768335
- Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524-34. doi:10.1016/j.molimm.2006.09.005. PMID:17045339
- Raju TS, Lang SE. Diversity in structure and functions of antibody sialylation in the Fc. Curr Opin Biotechnol. 2014;30:147-52. doi:10.1016/j.copbio.2014.06.014. PMID:25032906
- Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci. 2013;110:9868-72. doi:10.1073/pnas.1307864110. PMID:23697368
- Raju TS, Jordan R. Galactosylation variations in marketed therapeutic antibodies. mAbs. 2012;4:385-91. doi:10.4161/mabs.19868. PMID:22531450
- Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol Immunol. 1995;32:1311-8. doi:10.1016/0161-5890(95)00118-2. PMID:8643100
- Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog. 2005;21:1644-52. doi:10.1021/bp050228w. PMID:16321047
- Peschke B, Keller CW, Weber P, Quast I, Lünemann JD. Fc-Galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol. 2017;8:646. doi:10.3389/fimmu.2017.00646. PMID:28634480
- Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, Jefferis R. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276:45539-47. doi:10.1074/jbc.M107478200. PMID:11567028
- Radaev S, Sun P. Recognition of immunoglobulins by Fcγ receptors. Mol Immunol. 2002;38:1073-83. doi:10.1016/S0161-5890(02)00036-6. PMID:11955599
- Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82-6. doi:10.1038/25764. PMID:9738502
- Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A. 1999;96:15062-7. doi:10.1073/pnas.96.26.15062. PMID:10611338
- Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature. 2000;405:537-43. doi:10.1038/35014520. PMID:10850706
- Powell MS, Barnes NC, Bradford TM, Musgrave IF, Wines BD, Cambier JC, Hogarth PM. Alteration of the FcγRIIa dimer interface affects receptor signaling but not ligand binding. J Immunol. 2006;176:7489-94. doi:10.4049/jimmunol.176.12.7489. PMID:16751395
- Ramsland PA, Farrugia W, Bradford TM, Sardjono CT, Esparon S, Trist HM, Powell MS, Tan PS, Cendron AC, Wines BD, et al. Structural basis for Fc gammaRIIa recognition of human IgG and formation of inflammatory signaling complexes. J Immunol Baltim Md. 2011;187:3208-17
- Hughes-Jones NC. Functional affinity constants of the reaction between 125I-labelled C1q and C1q binders and their use in the measurement of plasma C1q concentrations. Immunology. 1977;32:191-8. PMID:844894
- Hughes-Jones NC, Gardner B. Reaction between the isolated globular sub-units of the complement component C1q and IgG-complexes. Mol Immunol. 1979;16:697-701. doi:10.1016/0161-5890(79)90010-5. PMID:119162
- Wright JK, Tschopp J, Jaton JC, Engel J. Dimeric, trimeric and tetrameric complexes of immunoglobulin G fix complement. Biochem J. 1980;187:775-80. doi:10.1042/bj1870775. PMID:6985362
- Doekes G, Vanes LA, Daha MR. Influence of aggregate size on the binding and activation of the first component of human complement by soluble IgG aggregates. Immunology. 1982;45:705-13. PMID:7068172
- Brown PB, Nardella FA, Mannik M. Human complement activation by self-associated IgG rheumatoid factors. Arthritis Rheum. 1982;25:1101-7. doi:10.1002/art.1780250911. PMID:7126294
- Liberti PA, Baillie RD, Milligan KF, Bausch DM. On the interaction of rabbit C1q with sheep and rabbit immune complexes. J Immunol Baltim Md. 1979;123:2212-9
- Tschopp J, Villiger W, Lustig A, Jaton J-C, Engel J. Antigen-independent binding of IgG dimers to C1 q as studied by sedimentation equilibrium, complement fixation and electron microscopy. Eur J Immunol. 1980;10:529-35. doi:10.1002/eji.1830100709. PMID:7408940
- Liberti PA, Bausch DM, Schoenberg LM. On the mechanism of Clq binding to antibody-I. aggregation and/or distortion of IgG vs combining site-transmitted effects. Mol Immunol. 1982;19:143-9. doi:10.1016/0161-5890(82)90256-5. PMID:6176855
- Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJR, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260-3. doi:10.1126/science.1248943. PMID:24626930
- Wang G, de Jong RN, van den Bremer ETJ, Beurskens FJ, Labrijn AF, Ugurlar D, Gros P, Schuurman J, Parren PWHI, Heck AJR. Molecular basis of assembly and activation of complement component C1 in complex with immunoglobulin G1 and antigen. Mol Cell. 2016;63:135-45. doi:10.1016/j.molcel.2016.05.016. PMID:27320199
- Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol Baltim Md. 1997;159:3372-82
- Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. mAbs. 2012;4:17-23. doi:10.4161/mabs.4.1.18347. PMID:22327427
- Yoo EM, Wims LA, Chan LA, Morrison SL. Human IgG2 can form covalent dimers. j Immunol. 2003;170:3134-8. doi:10.4049/jimmunol.170.6.3134. PMID:12626570
- Liu YD, Chen X, Enk JZ, Plant M, Dillon TM, Flynn GC. Human IgG2 antibody disulfide rearrangement in Vivo. J Biol Chem. 2008;283:29266-72. doi:10.1074/jbc.M804787200. PMID:18713741
- Dillon TM, Ricci MS, Vezina C, Flynn GC, Liu YD, Rehder DS, Plant M, Henkle B, Li Y, Deechongkit S, et al. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J Biol Chem. 2008;283:16206-15. doi:10.1074/jbc.M709988200. PMID:18339626
- Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9-19. doi:10.1046/j.0019-2805.2001.01341.x. PMID:11849310
- van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554-7. doi:10.1126/science.1144603. PMID:17872445
- Schuurman J, Perdok GJ, Gorter AD, Aalberse RC. The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol Immunol. 2001;38:1-8. doi:10.1016/S0161-5890(01)00050-5. PMID:11483205
- Michaelsen TE, Natvig JB. Unusual molecular properties of human IgG3 proteins due to an extended hinge region. J Biol Chem. 1974;249:2778-85. PMID:4208142
- Bindon CI, Hale G, Brüggemann M, Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988;168:127-42. doi:10.1084/jem.168.1.127. PMID:3260935
- Persson MA, Brown SE, Steward MW, Hammarström L, Smith CI, Howard CR, Wahl M, Rynnel-Dagöö B, Lefranc G, Carbonara AO. IgG subclass-associated affinity differences of specific antibodies in humans. J Immunol Baltim Md. 1988;140:3875-9
- Kato K, Matsunaga C, Odaka A, Yamato S, Takaha W, Shimada I, Arata Y. Carbon-13 NMR study of switch variant anti-dansyl antibodies: antigen binding and domain-domain interactions. Biochemistry (Mosc). 1991;30:6604-10. doi:10.1021/bi00240a033
- Cooper LJ, Schimenti JC, Glass DD, Greenspan NS. H chain C domains influence the strength of binding of IgG for streptococcal group A carbohydrate. J Immunol Baltim Md. 1991;146:2659-63
- Cooper LJ, Shikhman AR, Glass DD, Kangisser D, Cunningham MW, Greenspan NS. Role of heavy chain constant domains in antibody-antigen interaction. Apparent specificity differences among streptococcal IgG antibodies expressing identical variable domains. J Immunol Baltim Md. 1993;150:2231-42
- Cooper LJ, Robertson D, Granzow R, Greenspan NS. Variable domain-identical antibodies exhibit IgG subclass-related differences in affinity and kinetic constants as determined by surface plasmon resonance. Mol Immunol. 1994;31:577-84. doi:10.1016/0161-5890(94)90165-1. PMID:7515151
- Schreiber JR, Cooper LJ, Diehn S, Dahlhauser PA, Tosi MF, Glass DD, Patawaran M, Greenspan NS. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J Infect Dis. 1993;167:221-6. doi:10.1093/infdis/167.1.221. PMID:8418172
- Pritsch O, Hudry-Clergeon G, Buckle M, Petillot Y, Bouvet JP, Gagnon J, Dighiero G. Can immunoglobulin C(H)1 constant region domain modulate antigen binding affinity of antibodies? J Clin Invest. 1996;98:2235-43. doi:10.1172/JCI119033. PMID:8941639
- Pritsch O, Magnac C, Dumas G, Bouvet J-P, Alzari P, Dighiero G. Can isotype switch modulate antigen-binding affinity and influence clonal selection? Eur J Immunol. 2000;30:3387-95. doi:10.1002/1521-4141(2000012)30:12%3c3387::AID-IMMU3387%3e3.0.CO;2-K. PMID:11093156
- Morelock MM, Rothlein R, Bright SM, Robinson MK, Graham ET, Sabo JP, Owens R, King DJ, Norris SH, Scher DS. Isotype choice for chimeric antibodies affects binding properties. J Biol Chem. 1994;269:13048-55. PMID:7909805
- McCloskey N, Turner MW, Steffner P, Owens R, Goldblatt D. Human constant regions influence the antibody binding characteristics of mouse-human chimeric IgG subclasses. Immunology. 1996;88:169-73. doi:10.1111/j.1365-2567.1996.tb00001.x. PMID:8690447
- McLean GR, Torres M, Elguezabal N, Nakouzi A, Casadevall A. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J Immunol Baltim Md. 2002;169:1379-86
- Torres M, May R, Scharff MD, Casadevall A. Variable-region-identical antibodies differing in isotype demonstrate differences in fine specificity and idiotype. J Immunol. 2005;174:2132-42. doi:10.4049/jimmunol.174.4.2132. PMID:15699144
- Torres M, Fernández-Fuentes N, Fiser A, Casadevall A. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J Biol Chem. 2007;282:13917-27. doi:10.1074/jbc.M700661200. PMID:17353196
- Torres M, Fernandez-Fuentes N, Fiser A, Casadevall A. Exchanging murine and human immunoglobulin constant chains affects the kinetics and thermodynamics of antigen binding and chimeric antibody autoreactivity. PloS One. 2007;2:e1310. doi:10.1371/journal.pone.0001310. PMID:18074033
- Dam TK, Torres M, Brewer CF, Casadevall A. Isothermal titration calorimetry reveals differential binding thermodynamics of variable region-identical antibodies differing in constant region for a univalent ligand. J Biol Chem. 2008;283:31366-70. doi:10.1074/jbc.M806473200. PMID:18806257
- Janda A, Casadevall A. Circular Dichroism reveals evidence of coupling between immunoglobulin constant and variable region secondary structure. Mol Immunol. 2010;47:1421-5. doi:10.1016/j.molimm.2010.02.018. PMID:20299100
- Janda A, Eryilmaz E, Nakouzi A, Cowburn D, Casadevall A. Variable region identical immunoglobulins differing in isotype express different paratopes. J Biol Chem. 2012;287:35409-17. doi:10.1074/jbc.M112.404483. PMID:22930758
- Janda A, Eryilmaz E, Nakouzi A, Pohl MA, Bowen A, Casadevall A. Variable region identical IgA and IgE to cryptococcus neoformans capsular polysaccharide manifest specificity differences. J Biol Chem. 2015;290:12090-100. doi:10.1074/jbc.M114.618975. PMID:25778397
- Eryilmaz E, Janda A, Kim J, Cordero RJB, Cowburn D, Casadevall A. Global structures of IgG isotypes expressing identical variable regions. Mol Immunol. 2013;56:588-98. doi:10.1016/j.molimm.2013.06.006. PMID:23911417
- Hovenden M, Hubbard MA, Aucoin DP, Thorkildson P, Reed DE, Welch WH, Lyons CR, Lovchik JA, Kozel TR. IgG subclass and heavy chain domains contribute to binding and protection by mAbs to the poly γ-D-glutamic acid capsular antigen of Bacillus anthracis. PLoS Pathog. 2013;9:e1003306. doi:10.1371/journal.ppat.1003306. PMID:23637599
- Hubbard MA, Thorkildson P, Kozel TR, AuCoin DP. Constant domains influence binding of mouse–human chimeric antibodies to the capsular polypeptide of Bacillus anthracis. Virulence. 2013;4:483-8. doi:10.4161/viru.25711. PMID:23863605
- Xia Y, Pawar RD, Nakouzi AS, Herlitz L, Broder A, Liu K, Goilav B, Fan M, Wang L, Li Q-Z, et al. The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti-DNA antibodies. J Autoimmun. 2012;39:398-411. doi:10.1016/j.jaut.2012.06.005. PMID:22841793
- Xia Y, Janda A, Eryilmaz E, Casadevall A, Putterman C. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol Immunol. 2013;56:28-37. doi:10.1016/j.molimm.2013.04.004. PMID:23665381
- Xia Y, Eryilmaz E, Zhang Q, Cowburn D, Putterman C. Anti-DNA antibody mediated catalysis is isotype dependent. Mol Immunol. 2016;69:33-43. doi:10.1016/j.molimm.2015.11.001. PMID:26655427
- Tudor D, Yu H, Maupetit J, Drillet A-S, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel M. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci U S A. 2012;109:12680-5. doi:10.1073/pnas.1200024109. PMID:22723360
- Crespillo S, Casares S, Mateo PL, Conejero-Lara F. Thermodynamic analysis of the binding of 2F5 (Fab and Immunoglobulin G Forms) to its gp41 epitope reveals a strong influence of the immunoglobulin Fc region on affinity. J Biol Chem. 2014;289:594-9. doi:10.1074/jbc.C113.524439. PMID:24302742
- Tomaras GD, Ferrari G, Shen X, Alam SM, Liao H-X, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110:9019-24. doi:10.1073/pnas.1301456110. PMID:23661056
- Dodev TS, Bowen H, Shamji MH, Bax HJ, Beavil AJ, McDonnell JM, Durham SR, Sutton BJ, Gould HJ, James LK. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015;70:720-4. doi:10.1111/all.12607. PMID:25758595
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin G subclass activity through selective fc receptor binding. Science. 2005;310:1510-2. doi:10.1126/science.1118948. PMID:16322460
- Greenspan NS, Cooper LJN. Cooperative binding by mouse IgG3 antibodies: implications for functional affinity, effector function, and isotype restriction. Springer Semin Immunopathol. 1993;15:275-91. doi:10.1007/BF00201107. PMID:8256202
- Greenspan NS, Monafo WJ, Davie JM. Interaction of IgG3 anti-streptococcal group A carbohydrate (GAC) antibody with streptococcal group A vaccine: enhancing and inhibiting effects of anti-GAC, anti-isotypic, and anti-idiotypic antibodies. J Immunol Baltim Md. 1987;138:285-92
- Greenspan NS, Dacek DA, Cooper LJ. Cooperative binding of two antibodies to independent antigens by an Fc-dependent mechanism. FASEB J Off Publ Fed Am Soc Exp Biol. 1989;3:2203-7
- Perlmutter RM, Hansburg D, Briles DE, Nicolotti RA, Davie JM. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol Baltim Md. 1978;121:566-72
- Nevinsky GA, Buneva VN. Natural catalytic antibodies in norm, autoimmune, viral, and bacterial diseases. Sci World J. 2010;10:1203-33. doi:10.1100/tsw.2010.98
- Paul S, Planque SA, Nishiyama Y, Hanson CV, Massey RJ. Nature and nurture of catalytic antibodies. Nat Occur Antibodies NAbs. 2012;750:56-75. doi:10.1007/978-1-4614-3461-0_5
- Abboud N, Chow S-K, Saylor C, Janda A, Ravetch JV, Scharff MD, Casadevall A. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207:2395-405. doi:10.1084/jem.20100995. PMID:20921285
- Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015;67:171-82. doi:10.1016/j.molimm.2015.03.255. PMID:25900877
- Beers SA, Glennie MJ, White AL. Influence of immunoglobulin isotype on therapeutic antibody function. Blood. 2016;127:1097-101. doi:10.1182/blood-2015-09-625343. PMID:26764357
- Nesspor TC, Raju TS, Chin C-N, Vafa O, Brezski RJ. Avidity confers FcγR binding and immune effector function to aglycosylated immunoglobulin G1. J Mol Recognit JMR. 2012;25:147-54. doi:10.1002/jmr.2155. PMID:22407978
- Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol Baltim Md. 2013;190:4315-23
- Lim SH, Beers SA, French RR, Johnson PWM, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica. 2010;95:135-43. doi:10.3324/haematol.2008.001628. PMID:19773256
- Wypych J, Li M, Guo A, Zhang Z, Martinez T, Allen MJ, Fodor S, Kelner DN, Flynn GC, Liu YD, et al. Human IgG2 antibodies display disulfide-mediated structural isoforms. J Biol Chem. 2008;283:16194-205. doi:10.1074/jbc.M709987200. PMID:18339624
Appendix I:
Cooperative mechanisms in IgG
In the context of IgG molecules, ‘conformational allosteric’ cooperativity refers to a process in which an antigen-induced conformational change, instigated by binding in the V region, and propagated by secondary and tertiary structural variations, reveals appropriate binding sites in other segments of the molecule, such as the Fc domain. There are 2 models that fall into this category: the ‘allosteric’ and the ‘distortive’ model, proposed by Metzger in the 1970s as potential mechanisms for the activation of effector systems against antigens.Citation27 In the allosteric model, the monovalent binding of antigen to the V region causes conformational changes that propagate to the Fc to promote the binding of FcγRs/C1q. In the distortive model, tension generated by multivalent antigen binding forces structural alteration in the Fc to expose an otherwise inaccessible docking site for FcγRs/C1q. A major distinction between these 2 models is that the distortive model is more dependent on the topology of the antigen surface. On the other hand, for IgGs the ‘configurational allosteric’ cooperativity occurring in both the heavy and light chain class switching results from inherent C region configurational differences (i.e., differences the amino acid sequence makes to the structure). The potential for configurational allosteric cooperativity is a concern in mAb-based biotherapeutic development as a configurational change between IgG subclasses (e.g., IgG2 → IgG1) or heterogeneity in Fc glycoforms (e.g., G2F vs. G0) may result in structural changes that affect antigen binding in the V region or effector ligand binding in the C region. Note that allosteric cooperativity always involves intramolecular interactions, in which the allosteric signals may propagate through both the V → C and C → V directions within the IgG molecule, and that the magnitude of the free energy changes are the same regardless of the direction of propagation.
There are at least 3 types of associative cooperativity in IgGs. In the first, a single protein may possess multiple binding sites by virtue of its quaternary structure. In this ‘valence-based’ cooperativity, bivalent binding to the antigen is enabled due to IgG having 2 identical Fab arms. While the binding strength at a single site is described by affinity (e.g., a free Fab binding to its epitope) and reported by the equilibrium dissociation constant (Kd), the bivalent binding is described by avidity (i.e., apparent affinity). In the simplest case, where the epitopes are arranged such that the 2 Fab regions on one IgG may bind 2 epitopes independently, the avidity Kd is the square of the affinity Kd. However, this situation is rare, and the bivalent antibody binding to a multivalent antigen usually exhibits cooperativity that depends not only on the affinity of the antibody for the epitope, but also on the density and structural arrangement of the epitopes. Therefore, avidity provides an overall measure of whether an antibody-antigen binding complex behaves “ideally” or with positive or negative cooperativity. Notably, valence-based cooperativity is purely an intramolecular process. This process can lead to another form of ‘clustering-based’ quaternary cooperativity when multiple IgGs participate in binding on a multivalent antigenic surface that subsequently drives Fc and FcγR/C1q binding. Clustering involves the geometrical arrangement of adjacent IgGs bound to an array of antigens, assuming there is no interaction between them. For such a cluster to occur, the antigen must be in the form of an array of sites such that the adjacent bound IgGs provide an array of Fc domains that are in close proximity. This newly formed Fc array may then exhibit valence-based cooperative binding that initiates an effector function more efficiently than a single Fc domain. In this case there is no affinity between adjacent IgGs, and the formation of the Fc array is purely a consequence of the antigen array. Another related form of clustering-based cooperativity results from a weak affinity between adjacent IgG molecules bound to a template, a situation referred to as ‘template-based’ cooperativity. The weak affinity may result from allosteric changes in the first bound molecule, or it may be intrinsic to the molecules (i.e., a weak association would be observed in solution). In either case, the binding of an IgG molecule to one site dramatically increases the avidity of adjacent IgG-binding sites. This cooperative model was proposed by Greenspan and Cooper in the 1990s to explain the predominance of murine IgG3 subclass response to bacterial polysaccharide antigens through non-covalent Fc-Fc interactions.Citation143 Note that the clustering-based and template-based processes are both intermolecular cooperative mechanisms that involve multiple IgG molecules brought in close proximity either through multivalent binding (cluster-based) or through weak non-covalent attractions between adjacent IgG molecules (template-based).
Appendix II:
IgG subclass structures and possible allosteric differences
To understand how allostery may vary for different IgG subclasses, it is helpful to outline their structural characteristics, which play important roles in their physiologic properties and biological functions. The hinge region links the 2 Fab arms to the Fc portion of the IgG molecule and provides flexibility to the molecule. Overall, the relative flexibility of the Fab arms with respect to the Fc is ranked as follows: IgG3 > IgG1 > IgG4 > IgG2.Citation104 Disulfide bond structural variants other than the classical structures shown in have been observed for IgG2 and IgG4, but not for IgG1 and IgG3.Citation105 It is possible that these C-region structural differences between the IgG subclasses result in functional changes in the V region.
Compared to IgG1, IgG2 has a shorter hinge with 2 additional disulfide bridges at the Fab base. In addition to the classical IgG2-A disulfide bond structure, IgG2-B and IgG2-A/B disulfide bond isoforms that result from disulfide shuffling in the upper hinge region () have been observed.Citation155 Furthermore, a disulfide-bond-linked covalent IgG2 dimer has been detected in both cell culture medium and human serum.Citation106,107 Thus, disulfide structural heterogeneity is a natural feature of the human IgG2 subclass. Different IgG2 inter-heavy chain isoforms have been shown to exhibit modified biological functions despite having the same V region.Citation108 It is possible that these changes in biological function are a consequence of configurational allostery.
A unique phenomenon in human IgG4 subclass antibodies is the in vivo exchange of disulfide bond isoforms, in the form of intra-chain disulfide-linked half molecules ().Citation109 The exchange of half molecules between different monospecific bivalent IgG4 antibodies leads to the formation of bispecific monovalent antibodies with 2 distinct Fab arms. Due to the monovalent Fab domain functionality, the bispecific IgG4 antibodies lose the ability to crosslink identical antigens to form immune complexes through the V region. This process has been postulated to modulate the immune response by decreasing the binding strength to the cognate antigen through a switch from avidity to affinity interaction in the V region.Citation110 There is a sequence difference between IgG1 and IgG4 in the core hinge region (CPPC in IgG1 and CPSC in IgG4). Stable inter-heavy chain disulfide bonds were achieved in IgG4 by engineering its core hinge to be identical to that of IgG1, i.e., CPPC.Citation111
IgG3 differs substantially from the other 3 subclasses because its unique extended hinge region forms an inflexible poly-proline double helix ().Citation112 Since the elongated hinge separates the Fab farther away from the Fc than in the other subclasses, greater upper hinge flexibility in the Fab arms is observed, while at the same time the hinge structure allows greater downward flexing of Fab arms toward the Fc than is observed in subclasses with a shorter hinge.Citation104 The unique structure of IgG3 argues against V-C conformational signal transmission: in spite of IgG3 having the longest hinge region and largest distance between the Fab and Fc domains, IgG3 exhibits the strongest complement-activating ability of all the subclasses.Citation113
