ABSTRACT
Small bispecific antibodies (bsAbs) are important therapeutic molecules and represent the first bsAb format approved by the United States Food and Drug Administration. Diabody (Db), a small bsAb format, has four possible domain orders; we previously reported the differences in the expression levels and cancer growth inhibition effects upon rearranging the domain order of this format. However, there have been no comprehensive reports on domain rearrangements of bispecific single-chain Db (scDb) and tandem single-chain Fv (taFv), which are widely used bsAb formats. In this study, we designed all possible domain orders for scDb and taFv (each with eight variants) with identical Fv pairs and individually expressed all 16 variants using Escherichia coli, Pichia pastoris, and Brevibacillus choshinensis. Comprehensive investigations showed that the intrinsic functions of the variants were similar to each other, regardless of the expression host system, but expression levels varied depending on the format as well as on the host cell. Among the 16 variants, we found a promising candidate that exhibited high activity and productivity. Furthermore, we determined that B. choshinensis is an attractive expression host because of its secretory production of recombinant proteins.
Introduction
Conventional monoclonal therapeutic antibodies have been widely used to treat a variety of diseases that are difficult to cureCitation1,Citation2; however, production costs for those produced in mammalian expression systems can be high. In addition, especially in cancer treatments, adverse clinical outcomes and data from animal studies have highlighted important limitations in their modes of action and therapeutic efficacy.Citation3 Therefore, many strategies to improve the functions of antibodies have been explored; one such strategy is the design of non-natural antibody formats, particularly bispecific antibodies (bsAbs). These antibodies are characterized by their ability to simultaneously bind two targets, which, for example, allows effective redirection of diverse effectors such as cytotoxic T cells toward cancer cells. Blinatumomab (Blincyto®) was the first, and so far only, bispecific T-cell redirecting therapeutic antibody drug approved by the United States Food and Drug Administration.Citation3
Advances in recombinant technology have made it feasible to generate small recombinant bsAb constructed from two different variable antibody fragments, such as variable fragments (Fvs), single-chain Fvs (scFvs), and variable domains of the heavy chain of heavy-chain antibodies (VHH). These recombinant bsAbs include diabodies (Dbs),Citation4 single-chain diabodies (scDbs),Citation5 tandem scFvs (taFvs),Citation6 minibodies (dimeric scDb-CH3 fusion proteins),Citation7 and tandem VHH.Citation8 In contrast with classic bsAbs prepared through chemical conjugation or quadroma production,Citation9–Citation11 small bsAbs may allow greater tissue penetration and high target retention, and they are can be produced using cost-efficient microbial expression systems.Citation12,Citation13 Hosts such as Escherichia coliCitation14 and Pichia pastorisCitation15 have been used to produce small bsAbs; however, clinical-grade production using these hosts is still challenging. In fact, blinatumomab was produced in Chinese hamster ovary cells, i.e., a mammalian expression system.
Brevibacillus choshinensis was originally isolated from soil and found to secrete large quantities of protein with low extracellular protease activity.Citation16 This nonpathogenic Gram-positive bacterium can secrete recombinant proteins in culture directly without concern for contaminating endotoxins, facilitating the purification process.Citation17,Citation18 B. choshinensis has been used as a host for the production of industrial proteins as well as for biological products. Production of human epidermal growth factor (EGF) was reported in reclassified strain B. choshinensis HPD31;Citation19 however, there have been no reports of the production of bsAbs using this microorganism.
Several reports have suggested that the order of the variable domain affected the expression level and the function of small bsAb fragments such as bispecific taFv and Db.Citation20,Citation21 We previously described the construction of a functional humanized bispecific Db (bsDb) that targeted human EGF receptor (EGFR) and CD3 (hEx3-Db).Citation22 The bsDb format has four possible domain orders, and we reported the differences in the expression levels and cancer growth inhibition effects by rearranging the domain order.Citation23 Although the bsDb format has a potential concern regarding the formation of inactive homodimers concurrent with the active heterodimers, construction of scDb by connecting an additional middle linker can solve this problem. However, bispecific scDb and taFv formats have eight possible domain orders, and the effects of domain order on the expression levels and the function of these formats have not been investigated yet.
In this study, we constructed expression vectors for all possible domain orders of scDb and taFv formats of hEx3. We prepared 16 hEx3 variants using E. coli, P. pastoris, and B. choshinensis expression systems. Finally, we evaluated and compared their expression levels and function. To our knowledge, this is the first report on the preparation and evaluation of all configurations of bispecific scDb and taFv comprising identical Fv pairs, as well as of the production of bsAb in B. choshinensis. Our results showed that the intrinsic functions of hEx3 in each format were similar regardless of host cells used, but the expression levels varied in each format in each host cell. Furthermore, we found that B. choshinensis is a good candidate expression host for the secretory production of small bsAb.
Results
Design and construction of expression vectors for hEx3-scDbs and -taFvs with different domain orders
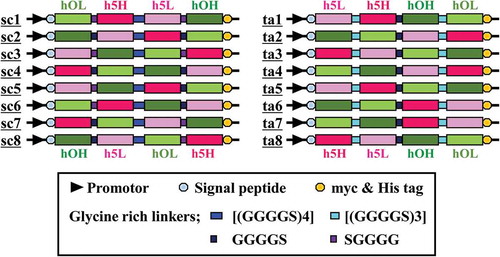
To comprehensively investigate the effects of rearranging the domain order on the functions of small bsAbs, we designed all possible domain orders for the single-chain bsAb format of hEx3, resulting in eight types of hEx3-scDb and eight types of hEx3-taFv. We also constructed expression vectors for these variants. Schematic diagrams of the gene constructs of all 16 types of hEx3 bsAbs are summarized in . Because of the similarity in the codon used by E. coli and B. choshinensis, whole genes optimized for E. coli codons were synthesized and used for the construction of the expression vectors for both E. coli and B. choshinensis. In contrast, genes optimized for P. pastoris codons were used for the construction of vectors for the yeast expression system. We effectively constructed 48 whole-gene expression vectors accounting for 16 hEx3 variants for each of the three host cells.
Evaluation of bsAbs expressed in E. coli
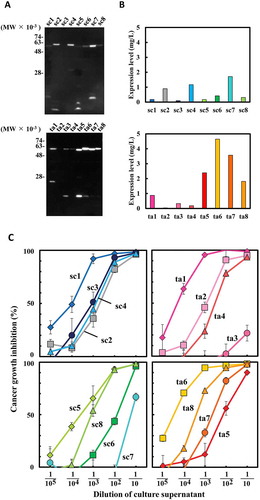
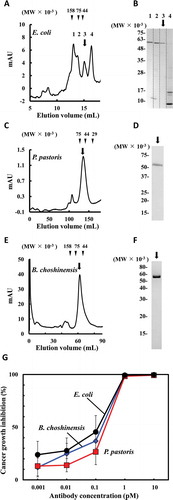
To evaluate the influence of the domain order of hEx3 bsAb on the inhibition of human carcinoma cell growth, we prepared 16 hEx3 variants using E. coli as the expression system and conducted analysis using 3-(4,5-dimethylthiazole-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). Western blot analysis of the culture supernatant showed that there were differences in the expression levels in each variant (,). We also directly used each culture supernatant to evaluate cancer growth inhibition effects.Citation24 In brief, we used culture supernatant diluted more than 10-fold, which showed no background cytotoxicity. Optimally, biologicals should be both highly effective and easily produced in large quantities. The scDb formats sc1, sc3, sc5, and sc8 were promising candidates as they showed high activity despite low expression levels. The taFv formats ta1, ta2, ta6, and ta8 were also promising candidates, with ta1 and ta2 showing high activity but low expression levels, while ta6 and ta8 showed both high activity and expression levels (,).
Figure 2. Expression of 16 hEx3 variants in Escherichia coli and their growth inhibition effects against epidermal growth factor receptor (EGFR)-positive TFK-1 cells. Expression levels were estimated by western blotting analysis of culture supernatant (a, b). Diluted culture supernatants of E. coli transformants and T-LAK cells were added to TFK-1 cells (T-LAK:TFK-1 ratio, 5:1) (c).
Evaluation of bsAbs expressed in P. pastoris
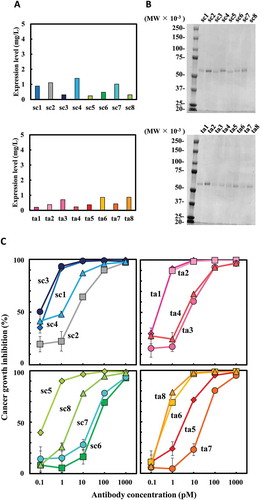
To confirm the validity of our variant selection in E. coli, we purified bsAbs expressed in P. pastoris, which was expected to produce high-purity proteins in the supernatant. The expression levels estimated by western blot analysis of the culture supernatant revealed differences in the pattern of expression levels in P. pastoris compared to E. coli. In P. pastoris, sc1 and ta6 showed moderate expression levels (). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis verified the single bands of each purified His-tagged bsAbs prepared from culture supernatants (). Growth inhibition assays using the samples showed high activities for sc1, sc3, sc5, ta1, ta2, ta6, and ta8, all promising candidates from E. coli expression (). These results indicate the applicability of using unpurified culture supernatant for rapid and easy prescreening.
Figure 3. Expression of 16 hEx3 variants in Pichia pastoris and their growth inhibition effects against epidermal growth factor receptor (EGFR)-positive TFK-1 cells. Expression levels were estimated by western blotting analysis of culture supernatant (a). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses were performed after His-tag-purification of hEx3s (b). Each purified sample was added along with T-LAK cells to TFK-1 cells (T-LAK:TFK-1 ratio, 5:1) (c). The value of ta2 at 0.1 pM was not evaluated correctly due to technical error.
Evaluation of bsAbs expressed in B. choshinensis
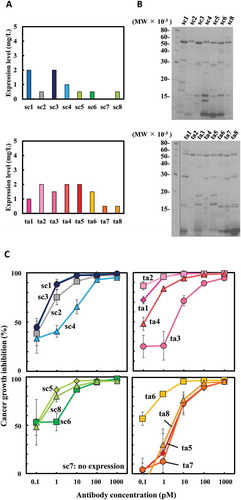
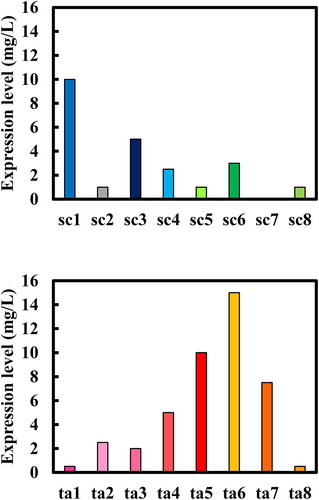
Unlike E. coli, B. choshinensis is a highly safe Gram-positive bacterium that can secrete soluble recombinant proteins directly into culture. Therefore, we used B. choshinensis as an expression host and evaluated the function of bsAbs prepared from this expression system. All the bsAbs, except sc7, were successfully expressed and secreted in the culture supernatant. The levels estimated by western blot analysis of the culture supernatant also revealed differences in the pattern of expression levels in B. choshinensis compared to E. coli. In B. choshinensis, sc1, sc3, and ta2, ta4, ta5 showed relatively high expression levels (). SDS-PAGE analysis also showed that purified His-tagged bsAbs prepared from B. choshinensis culture supernatants still contained a considerable amount of impurities (). A growth inhibition assay was performed using the bsAbs concentration calculated from band intensity. To confirm whether impurities from host cells affected growth, we first performed an MTS assay using culture supernatant directly. The diluted supernatants did not profoundly enhance the activities of T-LAK cells, indicating that the impurities in the bsAb solutions prepared from B. choshinensis had limited effects on cytotoxicity (Supplementary Figure 1). High growth inhibitory effects were observed in sc1, sc3, sc5, sc8, ta1, ta2, and ta6, similar to those expressed in P. pastoris. This indicates that B. choshinensis is a potentially viable production host for next-generation therapeutic bsAbs ().
Figure 4. Expression of 16 hEx3 variants in Brevibacillus choshinensis and their growth inhibition effects against epidermal growth factor receptor (EGFR)-positive TFK-1 cells. Expression levels were estimated by western blotting analysis of culture supernatant (a). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses were performed after His-tag-purification of hEx3s (b). Each His-tag-purified sample was added along with T-LAK cells to TFK-1 cells (T-LAK:TFK-1 ratio, 5:1) (c).
Evaluation of highly purified bsAbs expressed in three different host cells
To confirm whether the same variants expressed from E. coli, P. pastoris, and B. choshinensis had comparable activities, we purified and evaluated bsAbs from the three different expression systems. The multimeric form of small recombinant antibodies often results in higher activity due to avidity.Citation25–Citation27 There was a substantial amount of impurities in the purified His-tagged bsAbs from B. choshinensis (). For further investigation, we chose ta6 because it showed high growth inhibitory activity and relatively high expression levels in all the expression systems. While several peaks caused by multimeric structures and impurities were found in ta6 from E. coli (,), single peaks corresponding to the monomers were mainly observed in ta6 from P. pastoris (,) and B. choshinensis (,). The final yields for monomers of ta6 from E. coli, P. pastoris, and B. choshinensis were approximately 10, 50, and 100 μg/L culture, respectively. The monomer fractions following His-tag purification were used for cancer growth inhibition assay, and no major differences were observed among the monomers of ta6 prepared using the different expression hosts (). These results show that expression levels vary depending on domain rearrangements and host species, but growth inhibitory effects of bsAbs are independent of the expression host used, at least as far as the representative microorganisms (E. coli, P. pastoris, and B. choshinensis) we tested in this study are concerned.
Figure 5. Comparison of growth inhibition effects against epidermal growth factor receptor (EGFR)-positive TFK-1 cells using the ta6 monomers prepared from three different host cells. A chromatograph and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis for each peak after gel filtration of ta6 expressed in Escherichia coli, Pichia pastoris, and Brevibacillus choshinensis are shown (a, b, c, d, e, f). Each ta6 monomer indicated in arrow was added along with T-LAK cells to TFK-1 cells (T-LAK:TFK-1 ratio, 5:1) (g).
Discussion
A variety of expression systems for the production of recombinant antibodies such as bacteria, yeasts, filamentous fungi, insect cells, and plant cells have been explored. Although eukaryotic and prokaryotic expression systems have concerns regarding the immunogenicity of non-human glycosylation patterns and functional depression due to a lack of glycosylation,Citation28 both systems can be applied for small recombinant antibodies because many of them lack the Fc region that contains conserved functional glycosylation sites. B. choshinensis is a Gram-positive bacterium that secretes proteins directly into culture, has low extracellular protease activity, produces no endotoxins, and is nonpathogenic.Citation16–Citation18 It is an attractive protein expression host, but it has never been used as a production host for small recombinant bsAbs.
Among small recombinant bsAbs, the Dbs and taFvs formats are the most widely used, and their features and structural differences have been reviewed.Citation11,Citation29,Citation30 The bsDb format has four possible configurations with different domain orders, and we previously reported that domain rearrangement affected the expression levels and cancer growth inhibition effects of hEx3-Db.Citation23 In contrast, both single-chain formats, scDb and taFv, have eight possible configurations, and effects of rearranging domain orders on either expression level or function have not been reported. Thus, in this study, we constructed all 16 configurations of small bsAbs based on hEx3 and determined their optimal host cells considering both expression levels and function.
Our comprehensive investigations on hEx3 formats using optimized whole genes showed that the expression levels varied depending on the bsAb formats and microbial hosts (,, , ), while variation in cancer growth inhibition effects was solely format-dependent (, , ). When we used highly purified samples, it was confirmed that the intrinsic functions of hEx3 were similar to each other regardless of host cells (). In bsDb formats, we previously reported that LH type, in which both components are in the VL-VH order, exerted the strongest anti-tumor activity in vitro and in vivo among the four possible configurations.Citation23,Citation31 We considered that the structure of LH type could avoid steric hindrance with cell surface molecules, and this feature might contribute to the intense cytotoxicity. In this study, sc1 and sc3 reflect the LH type single-chain formats. Among eight possible configurations for bispecific scDb, these LH type configurations also showed relatively high inhibitory effects, and we could not find other configurations with obvious significant effects other than them ().
No comprehensive investigations on the effects of configuration of bispecific taFv formats on antibody function have previously been reported. In our study, taFvs prepared using P. pastoris showed relatively high purities and high activities for ta1, ta2, ta6, and ta8, almost consistent with the results observed for other expression systems (). An important structural feature of the taFv format is its high flexibility because of the two binding sites that can rotate freely and allow it to facilitate the simultaneous binding of two antigen epitopes.Citation11 Our results showed that, despite the high structural flexibility of the taFv format, the cytotoxic activity of the antibody varied depending on the domain order. This suggests that each taFv variant also has different ranges of mobility and permissive angles for crosslinking two target cells, influencing cytotoxic properties.
B. choshinensis is an attractive candidate as a host cell for the production of next-generation biologicals, including small bsAbs. We optimized the culture conditions for expressing 16 hEx3 bsAb variants in B. choshinensis. As shown in , yields greater than 10 mg/L were observed in several variants upon addition of 0.05–0.2 M L-arginine for each variant, with ta6 expressing one of the highest yields (15 mg/L), as well as one of the highest cytotoxic effects among the 16 variants (). Similar expression levels were also confirmed in three different ta6-expressing B. choshinensis clones by western blotting (Supplementary Figure 2). Several examples of recombinant proteins, including antibody fragments, being produced in the g/L range in B. choshinensis have been reported.Citation32–Citation34 A host-vector system using genetically engineered B. choshinensis HPD31-SP3 strain that cannot form spores nor secrete proteases is commercially available. Furthermore, in this system, three promoters with different expression activities can be combined with four signal sequences to optimize recombinant protein production.Citation32 We are currently working on the development and optimization of the fed-batch culture for promising variants such as ta6, considering vector constructions. Since the final yields (i.e., purification efficiencies) are still low, we are also developing an efficient downstream process.
Figure 6. Optimization of expression by investigating the culture conditions of Brevibacillus choshinensis. Expression levels were estimated by western blotting analysis of culture supernatant.
In this study, we report the first evaluation of all configurations of bispecific scDb and taFv comprising identical Fv pairs. Comprehensive investigations showed that the intrinsic functions of hEx3 in each format were similar to each other, regardless of host cell, but expression levels varied depending on the format and host cell. Among the 16 hEx3 bsAb variants, we found a promising candidate that exhibited high activity and productivity. Moreover, B. choshinensis is an attractive microbial host cell for recombinant protein expression because of its secretory production of recombinant proteins.
Materials and methods
Construction of expression vectors for bsAbs with different domain orders
As in our previous report,Citation22 we designated here the VH and VL regions of the humanized anti-EGFR antibody 528 as h5H and h5L, and those of the humanized anti-CD3 antibody OKT3 as hOH and hOL. The high affinity mutant HY52W, in which Tyr52 was replaced by Trp, was used as h5H. We previously isolated this mutant using a phage display system.Citation35 We first designed all possible domain orders for single-chain bsAb format of hEx3, including eight types of hEx3-scDb and eight types of hEx3-taFv, as shown in . To reduce linker-inherent effects on the function of bsAbs, we chose the standard simple glycine-rich linkers. Genes of variable regions optimized for E. coli codons and for P. pastoris codons were synthesized and used to construct expression vectors of 16 kinds of hEx3 bsAbs for each of the expression host species. The pRA vector, which is a previously constructed T7 promoter-based expression vector with N-terminus pel-B signal peptide and C-terminus Myc and His tags,Citation36 was used for E. coli expression system. The gene cassettes with the N-terminus α-factor secretion signal and C-terminus Myc and His tags were amplified using polymerase chain reaction (PCR) and were inserted into the pJ902-15 vector for expression in P. pastoris.Citation37 Because of the similarity in codon usage of E. coli and B. choshinensis, genes optimized for E. coli codons were inserted into the pROXb3 vector with N-terminus R2L6 signal peptide and C-terminus Myc and His tags for expression in B. choshinensis.
Expression of bsAbs using three host cells
Three host cells (E. coli, P. pastoris, and B. choshinensis) were used for the expression of 16 hEx3 variants. E. coli strain BL21 Star (DE3) (Life Technologies) was transformed with the pRA vectors and cultured according to the methodology described in a previous report.Citation38 P. pastoris strain PPS-9010 (ATUM) was transformed with the pJ902-15 vectors via electroporation after linearizing with SacI and cultured in buffered minimal glycerol-complex (BMGY) medium (pH 6; 2% peptone, 1% yeast extract, 1% glycerol, 1.34% yeast nitrogen base with ammonium sulfate but without amino acids, and 0.00004% biotin in 100 mM potassium phosphate buffer) at 30°C with shaking at 200 rpm until the optical density at 600 nm (OD600) reached 20–50. To induce protein expression, the cells were resuspended in BMMY (same as BMGY, except 0.5% methanol is replaced with glycerol) to an OD600 of 10 and were cultured at 25°C for 48 h. The medium was re-supplemented with methanol every 12 h. B. choshinensis HPD31 strainCitation16 was transformed by electroporation and cultured at 30°C for 48 h in the medium with 4% PhytoneTM peptone (BD Biosciences), 0.5% yeast extract, 0.001% FeSO47H2O, 0.0001% ZnSO47H2O, 0.001% MnSO45H2O, and 2% glucose.Citation39 Each supernatant was used for western blotting using horseradish peroxidase (HRP)-conjugated anti-His tag antibody to estimate the expression levels using Multitope (Denatured, recombinant, Solution, FUJIFILM Wako Pure Chemical Corporation) as a calibration marker protein.
Purification of bsAbs expressed in three host cells
E. coli transformants were harvested and sonicated. The intracellular soluble fraction was loaded onto a HisTrap HP column (GE Healthcare Bio-Science), and the eluted fraction was loaded onto a Superdex 200 GL column (10/300; GE Healthcare) to fractionate the monomers. Culture supernatants of P. pastoris transformants were loaded onto a TALON spin column (Takara Bio USA, Inc.) and a HiLoad Superdex 200 pg column (26/600; GE Healthcare) to fractionate the monomers. In the case of the B. choshinensis expression system, purification and fractionation of the monomers for bsAb were performed using a HisTrap HP column and a HiLoad Superdex 200 pg column (26/600), respectively.
Cell lines
Human bile duct carcinoma (TFK-1) was used in this study. The TFK-1 cell line was established by our group.Citation40 The TFK-1 cell line was cultured in Roswell Park Memorial Institute 1640 (RPMI 1640) medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin.
In vitro killing assay
Lymphokine-activated killer cells with the T-cell phenotype (T-LAK) were induced as described in literature.Citation41 Peripheral blood mononuclear cells were cultured for 48 h at a density of 1 × 106 cells/mL in medium supplemented with 100 IU/mL recombinant human interleukin 2 (IL-2) (Shionogi Pharmaceutical Co.) in a culture flask (A/S Nunc) that was pre-coated with anti-CD3 monoclonal antibody (10 μg/mL). The in vitro growth inhibition of cancer cells was assayed using an MTS assay kit (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay; Promega), as reported in literature.Citation41
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download PDF (136 KB)Acknowledgments
We thank Ms. Mika Ohta, Ms. Sayuri Murasaki, and Maiko Ueki for their excellent technical assistance.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10:183–203. doi:10.1080/19420862.2018.1415671.
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7:9–14. doi:10.4161/19420862.2015.989042.
- Nunez-Prado N, Compte M, Harwood S, Alvarez-Mendez A, Lykkemark S, Sanz L, Álvarez-Vallina L. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20:588–594. doi:10.1016/j.drudis.2015.02.013.
- Holliger P, Winter G. Diabodies: small bispecific antibody fragments. Cancer Immunol Immunother. 1997;45:128–130. doi:10.1007/s002620050414.
- Stork R, Campigna E, Robert B, Muller D, Kontermann RE. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J Biol Chem. 2009;284:25612–25619. doi:10.1074/jbc.M109.027078.
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi:10.1126/science.1158545.
- Shahied LS, Tang Y, Alpaugh RK, Somer R, Greenspon D, Weiner LM. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J Biol Chem. 2004;279:53907–53914. doi:10.1074/jbc.M407888200.
- Els Conrath K, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem. 2001;276:7346–7350. doi:10.1074/jbc.M007734200.
- Raso V, Griffin T. Hybrid antibodies with dual specificity for the delivery of ricin to immunoglobulin-bearing target cells. Cancer Res. 1981;41:2073–2078.
- Suresh MR, Cuello AC, Milstein C. Bispecific monoclonal antibodies from hybrid hybridomas. Methods Enzymol. 1986;121:210–228.
- Kriangkum J, Xu B, Nagata LP, Fulton RE, Suresh MR. Bispecific and bifunctional single chain recombinant antibodies. Biomol Eng. 2001;18:31–40. doi:10.1016/S1389-0344(01)00083-1.
- Robinson MK, Doss M, Shaller C, Narayanan D, Marks JD, Adler LP, González Trotter DE, Adams GP. Quantitative immuno-positron emission tomography imaging of HER2-positive tumor xenografts with an iodine-124 labeled anti-HER2 diabody. Cancer Res. 2005;65:1471–1478. doi:10.1158/0008-5472.CAN-04-4557.
- Sundaresan G, Yazaki PJ, Shively JE, Finn RD, Larson SM, Raubitschek AA, Williams LE, Chatziioannou AF, Gambhir SS, Wu AM. 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med. 2003;44:1962–1969.
- Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA. 1993;90:6444–6448. doi:10.1073/pnas.90.14.6444.
- Liu MY, Hu XP, Xie M, Jiang SJ, Li LJ, Liu DX, Yang XS. Secretory expression of a bispecific antibody targeting tumor necrosis factor and ED-B fibronectin in Pichia pastoris and its functional analysis. Biotechnol Lett. 2014;36:2425–2431. doi:10.1007/s10529-014-1630-2.
- Takagi H, Kadowaki K, Udaka S. Screening and characterization of protein-hyperproducing bacteria without detectable exoprotease activity. Agric Biol Chem. 1989;53:691–699.
- Ilk N, Schumi CT, Bohle B, Egelseer EM, Sleytr UB. Expression of an endotoxin-free S-layer/allergen fusion protein in gram-positive Bacillus subtilis 1012 for the potential application as vaccines for immunotherapy of atopic allergy. Microb Cell Fact. 2011;10. doi:10.1186/1475-2859-10-6.
- Zou C, Duan XG, Wu J. Efficient extracellular expression of Bacillus deramificans pullulanase in Brevibacillus choshinensis. J Ind Microbiol Biotechnol. 2016;43:495–504. doi:10.1007/s10295-016-1735-9.
- Miyauchi A, Ozawa M, Mizukami M, Yashiro K, Ebisu S, Tojo T, Fujii T, Takagi H. Structural conversion from non-native to native form of recombinant human epidermal growth factor by Brevibacillus choshinensis. Biosci Biotechnol Biochem. 1999;63:1965–1969. doi:10.1271/bbb.63.1965.
- Lu D, Jimenez X, Witte L, Zhu Z. The effect of variable domain orientation and arrangement on the antigen-binding activity of a recombinant human bispecific diabody. Biochem Biophys Res Commun. 2004;318:507–513. doi:10.1016/j.bbrc.2004.04.135.
- Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, Offner S, Locher M, Urbig T, Raum T. MT110: A novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi:10.1016/j.molimm.2005.07.034.
- Asano R, Sone Y, Makabe K, Tsumoto K, Hayashi H, Katayose Y, Unno M, Kudo T, Kumagai I. Humanization of the bispecific epidermal growth factor receptor x CD3 diabody and its efficacy as a potential clinical reagent. Clin Cancer Res. 2006;12:4036–4042. doi:10.1158/1078-0432.CCR-06-0059.
- Asano R, Kumagai T, Nagai K, Taki S, Shimomura I, Arai K, Ogata H, Okada M, Hayasaka F, Sanada H, et al. Domain order of a bispecific diabody dramatically enhances its antitumor activity beyond structural format conversion: the case of the hEx3 diabody. Protein Eng Des Sel. 2013;26:359–367. doi:10.1093/protein/gzt009.
- Sugiyama A, Umetsu M, Nakazawa H, Niide T, Onodera T, Hosokawa K, Hattori S, Asano R, Kumagai I. A semi high-throughput method for screening small bispecific antibodies with high cytotoxicity. Sci Rep-Uk. 2017;7:2862. doi:10.1038/s41598-017-03101-4.
- Le Gall F, Reusch U, Little M, Kipriyanov SM. Effect of linker sequences between the antibody variable domains on the formation, stability and biological activity of a bispecific tandem diabody. Protein Eng Des Sel. 2004;17:357–366. doi:10.1093/protein/gzh091.
- Asano R, Ikoma K, Sone Y, Kawaguchi H, Taki S, Hayashi H, Nakanishi T, Umetsu M, Katayose Y, Unno M, et al. Highly enhanced cytotoxicity of a dimeric bispecific diabody, the hEx3 tetrabody. J Biol Chem. 2010;285:20844–20849. doi:10.1074/jbc.M110.120444.
- Asano R, Ikoma K, Shimomura I, Taki S, Nakanishi T, Umetsu M, Kumagai I. Cytotoxic enhancement of a bispecific diabody by format conversion to tandem single-chain variable fragment (taFv): the case of the hEx3 diabody. J Biol Chem. 2011;286:1812–1818. doi:10.1074/jbc.M110.172957.
- Frenzel A, Hust M, Schirrmann T. Expression of recombinant antibodies. Front Immunol. 2013;4. doi:10.3389/fimmu.2013.00217.
- Kufer P, Lutterbuse R, Baeuerle PA. A revival of bispecific antibodies. Trends Biotechnol. 2004;22:238–244. doi:10.1016/j.tibtech.2004.03.006.
- Kontermann RE. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol Sin. 2005;26:1–9. doi:10.1111/j.1745-7254.2005.00008.x.
- Asano R, Nagai K, Makabe K, Takahashi K, Kumagai T, Kawaguchi H, Ogata H, Arai K, Umetsu M, Kumagai I. Structural considerations for functional anti-EGFR x anti-CD3 bispecific diabodies in light of domain order and binding affinity. Oncotarget. 2018;9:13884–13893. doi:10.18632/oncotarget.24490.
- Mizukami M, Tokunaga H, Onishi H, Ueno Y, Hanagata H, Miyazaki N, Kiyose N, Ito Y, Ishibashi M, Hagihara Y, et al. Highly efficient production of VHH antibody fragments in Brevibacillus choshinensis expression system. Protein Expr Purif. 2015;105:23–32. doi:10.1016/j.pep.2014.10.001.
- Mizukami M, Hanagata H, Miyauchi A. Brevibacillus expression system: host-vector system for efficient production of secretory proteins. Curr Pharm Biotechnol. 2010;11:251–258. doi:10.2174/138920110791112031.
- Ebisu S, Takagi H, Kadowaki K, Yamagata H, Udaka S. The efficient production of human epidermal growth factor by Bacillus brevis. Ann Ny Acad Sci. 1996;782:115–122. doi:10.1111/j.1749-6632.1996.tb40553.x.
- Nakanishi T, Maru T, Tahara K, Sanada H, Umetsu M, Asano R, Kumagai I. Development of an affinity-matured humanized anti-epidermal growth factor receptor antibody for cancer immunotherapy. Protein Eng Des Sel. 2013;26:113–122. doi:10.1093/protein/gzs088.
- Makabe K, Asano R, Ito T, Tsumoto K, Kudo T, Kumagai I. Tumor-directed lymphocyte-activating cytokines: refolding-based preparation of recombinant human interleukin-12 and an antibody variable domain-fused protein by additive-introduced stepwise dialysis. Biochem Biophys Res Commun. 2005;328:98–105. doi:10.1016/j.bbrc.2004.12.193.
- Shi XZ, Karkut T, Chamankhah M, Alting-Mees M, Hemmingsen SM, Hegedus D. Optimal conditions for the expression of a single-chain antibody (scFv) gene in Pichia pastoris. Protein Expr Purif. 2003;28:321–330. doi:10.1016/S1046-5928(02)00706-4.
- Studier FW. Protein production by auto-induction in high-density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi:10.1016/j.pep.2005.01.016.
- Takagi H, Kagiyama S, Kadowaki K, Tsukagoshi N, Udaka S. Genetic-transformation of bacillus-brevis with plasmid DNA by electroporation. Agric Biol Chem. 1989;53:3099–3100.
- Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, Fukuhara K, Suzuki T, Matsuno S. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71.
- Asano R, Watanabe Y, Kawaguchi H, Fukazawa H, Nakanishi T, Umetsu M, Hayashi H, Katayose Y, Unno M, Kudo T, et al. Highly effective recombinant format of a humanized IgG-like bispecific antibody for cancer immunotherapy with retargeting of lymphocytes to tumor cells. J Biol Chem. 2007;282:27659–27665. doi:10.1074/jbc.M704719200.