ABSTRACT
Increasing attention has been paid to developability assessment with the understanding that thorough evaluation of monoclonal antibody lead candidates at an early stage can avoid delays during late-stage development. The concept of developability is based on the knowledge gained from the successful development of approximately 80 marketed antibody and Fc-fusion protein drug products and from the lessons learned from many failed development programs over the last three decades. Here, we reviewed antibody quality attributes that are critical to development and traditional and state-of-the-art analytical methods to monitor those attributes. Based on our collective experiences, a practical workflow is proposed as a best practice for developability assessment including in silico evaluation, extended characterization and forced degradation using appropriate analytical methods that allow characterization with limited material consumption and fast turnaround time.
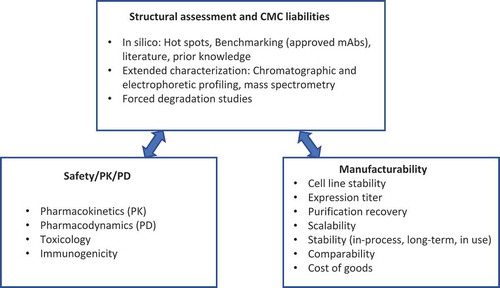
The discovery and development of monoclonal antibody (mAb) therapeutics is resource demanding and technically challenging. Specifically, challenges associated with Chemistry, Manufacturing, and Controls (CMC) development such as high aggregation, high viscosity and susceptibility to chemical degradation and insufficient product stability have been commonly recognized. Conventionally, only limited criteria such as antigen binding and in vivo properties including safety, pharmacokinetics (PK) and pharmacodynamics (PD) in animal models are used to select a mAb candidate from the early discovery to development stage. Without extensive characterization to understand the biochemical and biophysical properties of the selected candidate, issues can arise from unexpected modifications, stability or poor PK and PD, which can result in delayed project progress or even termination. The development risks are often associated with the intrinsic properties of the drug candidates. Therefore, it is critical to carry out a developability assessment before entering process development. Developability assessment is a process used to systematically evaluate drug candidates, including structural assessment and CMC liabilities, safety, PK and PD, as well as manufacturability (). Although, many interdependent factors contribute to the successful development of an mAb therapeutic, selection of a candidate with favorable biophysical and biochemical behavior help lay down a solid foundation. Thus, the primary goal of a developability assessment is to critically evaluate the biochemical and biophysical properties of mAb lead candidates and select the molecules with the lowest risks for development.
Numerous studies have shown the importance of developability assessment of mAb lead candidates. For example, poor biophysical properties resulted in mAbs with lower expression, instability or shorter in vivo half-life.Citation1,Citation2 Continuous asparagine (Asn) deamidation in the complementarity-determining region (CDR) has also caused loss of potency of a mAb.Citation3 Given the limitations of timelines and resources at the early stage of development, a thorough developability evaluation may not eliminate all risks that could occur later, but it does allow the selection of lead candidates with fewer development risks. Meanwhile, the knowledge gained through thorough evaluation also provides a strong foundation that in turn allows for a quality by design (QbD) approach for process and formulation development to mitigate any remaining identified risks. When risks are deemed critical and cannot be mitigated, an early decision to re-engineer the molecule is much more preferable than a later decision because it allows companies to save resources and avoid excessive delays of the timeline.
The biochemical and biophysical properties of mAb candidates are evaluated based on in silico and experimental evaluation, and according to: 1) the general properties of the approved mAbs; 2) scientific literature regarding the general properties of mAb molecules including posttranslational modifications (PTMs), stability and degradation pathways; and 3) drug developers’ internal knowledge from development of similar molecules.
To date, approximately 80 mAb and Fc-fusion protein drug products have been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA),Citation4,Citation5 and more than 70 are in late-stage development.Citation6 Among them, some generally preferred drug properties for mAbs have begun to emerge (). It should be noted that some attributes such as high molecular weight (HMW) species are dependent on the sample shelf-life and handling history. MAbs with attributes within or better than these ranges are expected to have relatively lower development risks.
Table 1. Quality attributes of a panel of FDA/EMA approved and clinical stage mAb products.
Because of the highly conserved primary and similar high-order structures of mAbs, the commonly recognized degradation hot-spots can guide drug candidate evaluation to identify potential problematic features. For example, Asn and aspartate (Asp) residues in the flexible CDRs are susceptible to deamidation and isomerization,Citation17 respectively, and thus need more careful examination. The correlation between aggregation or faster clearance and exposed hydrophobicCitation18-Citation22 or charged patchesCitation23,Citation24 in the variable domains can also be applied to mAb candidate evaluation. These general principles allow identification of potential risks based on the amino acid sequences of mAb candidates.
Drug developers’ internal knowledge from process and formulation development also plays a significant role in developability evaluation. Limitations of stable cell line, cell culture media components, and drug substance process steps are common elements that may exist across the pipeline and should be part of the developability assessment consideration. Therefore, we are proposing an overall developability assessment (), focusing on evaluation of biochemical and biophysical properties at early stage.
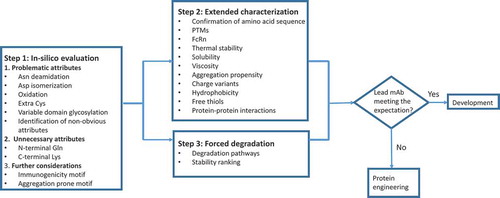
Below is a three-step workflow for the assessment of structural and CMC liabilities (), taking into consideration previous proposals.Citation3,Citation25-Citation29 Step 1 is in silico evaluation, including the use of computational methods, based on amino acid sequence to identify the known degradation hot spots and problematic motifs. Step 2 is to perform in-depth characterization to experimentally evaluate key biochemical and biophysical properties, such as structure, stability, charge profiles, PTMs, solubility, and hydrophobicity. Step 3 is to carry out limited forced degradation studies to, first, further confirm hot-spots identified from steps 1 and 2, and second, reveal candidate-specific degradation hot-spots that were not identified from Step 1. Forced degradation studies also provide additional stability information to rank the candidates. Step 2 and Step 3 can be carried out simultaneously to allow a thorough evaluation of mAb candidates within a short timeframe.
In silico evaluation
We propose to categorize mAb structural features into three groups (), namely, Problematic, Unnecessary and Further Considerations for in silico evaluation, based on the general properties of mAbs, including PTMs and degradation pathways.Citation30,Citation31 Problematic attributes have been associated with known safety or efficacy concerns. Unnecessary attributes have not been linked to any biological functionalities. Attributes for further considerations have not been considered traditionally for mAb lead selection, but may be considered for next-generation molecules with improved properties, such as minimal immunogenicity, enhanced stability and extended half-life.
Problematic attributes
Problematic attributes of mAb candidates mainly encompass PTMs in CDRs that can negatively impact potency, immunogenicity and stability. MAbs are subject to PTMs and degradation during cell culture, purification, storage and even after administration. Exposure to various stress conditions during manufacturing, such as elevated temperature (e.g., cell culture, process hold steps), extreme pH (e.g., low pH protein A chromatography elution, or virus inactivation), agitation (e.g., cell culture, pumping, mixing, filtration, or shipping), shear forces (e.g.,UF/DF) and ambient light can accelerate degradation.
Asn deamidation is one of the most commonly encountered degradation pathways in mAbs, especially for Asn residues in CDRs. Citation17 Asn followed by the small and flexible glycine (Gly) residue (NG motif) is highly susceptible to deamidation.Citation3,Citation17,Citation32-Citation37 Additionally, protein structures can have a substantial impact on Asn deamidation, and this has been demonstrated in cases where Asn not in NG motif are susceptible to deamidation,Citation17,Citation37 whereas, cases where Asn in NG motif are resistant to deamidation.Citation34,Citation37 Asn deamidation in CDRs may cause a decrease in antigen binding affinity Citation33,Citation35-Citation37; therefore, deamidation in the CDRs continues to occur in circulation after administrated to humans,Citation32,Citation33 resulting in loss of potency.Citation3 For this reason, Asn followed by Gly, and to a lesser degree, Asn followed by serine (Ser), threonine (Thr) in the CDRs, should be highlighted during in silico assessment and evaluated during extended characterization and forced degradation to confirm deamidation liability. IgG also contains susceptible deamidation sites in the so-called “PENNY” loop peptide in the Fc.Citation38,Citation39 Deamidation at this site continues to occur with mAbs in humans and to endogenous IgG.Citation40 Because this region is conserved and not associated with negative impact, deamidation at this site should not be a concern for developability assessment.
Asp isomerization is another common degradation pathway for mAbs. Similar to deamidation, Asp residues in CDRs are generally prone to isomerization,Citation17 especially when followed by a Gly residue.Citation17,Citation37,Citation41-Citation46 Isomerization of Asp followed by HisCitation42 or SerCitation47 have also been reported, suggesting the involvement of other factors such as residue flexibility, size and structure.Citation17,Citation26 Isomerization of Asp in CDRs may also cause a decrease in antigen binding affinity.Citation37,Citation41,Citation42,Citation46,Citation48 Asp isomerization is favored around pH 5,Citation47,Citation49 making it challenging to formulate mAb in liquid formulation around this commonly used pH range. To reduce development risks, Asp followed by Gly, to a lesser degree, followed by Asp or His, should be highlighted during in silico evaluation and further evaluated during extended characterization and forced degradation.
Oxidation occurs frequently with methionine (Met) and tryptophan (Trp) residues. Studies demonstrated that oxidation of a Met in the heavy chain CDR2,Citation46 as well as one in the framework region,Citation50 did not impact antigen binding. However, a negative impact may be expected with either higher levels of oxidation or at locations that are more critical to antigen binding. Two conserved Met residues close to the CH2-CH3 domain interface and part of the neonatal Fc receptor (FcRn), Protein A, and Protein G binding sites have been shown to be susceptible to oxidation.Citation50-Citation52 Oxidation of these Met residues results in decreased thermal stability,Citation50-Citation53 increased aggregation,Citation51,Citation53 decreased complement-dependent cytotoxicity (CDC),Citation50 decreased binding affinity to FcRnCitation50,Citation54,Citation55 and shorter in vivo half-life.Citation56 Oxidation of Trp residues in the CDRs has been reported, which can lead to reduced potency, decreased thermal stability and increased aggregation propensity.Citation57-Citation59 Trp oxidation has also been shown to cause a yellow coloration to the mAb solution,Citation60 due to the formation of kynurenine. Because higher Trp oxidation correlates with higher solvent exposure,Citation26 Trp residues in CDRs are expected to be more susceptible to oxidation. Overall, Met and Trp in CDRs should be carefully evaluated to determine their susceptibility, and implement an appropriate control strategy during processing and formulation, if necessary.
Though rare, mAbs may have an unpaired cysteine (Cys) residue in CDRs. The unpaired Cys can be readily modified by free cysteine in cell culture medium,Citation61,Citation62 which has been shown to decrease antigen binding affinity.Citation61 Candidates with unpaired Cys in the CDRs or other regions should be eliminated due to the highly reactive nature of the Cys side chain. However, the formation of a disulfide bond from two Cys residues in the heavy chain CDRs offers some unique epitope recognition properties, though no impact on stability and solubility has been observed.Citation63
It is not uncommon for mAbs to have a consensus sequence for N-glycosylation (NXS/T, X cannot be P) in variable domains, in addition to the conserved N-glycosylation site in the Fc region. The variable domain glycosylation showed variable effects on antigen binding,Citation64-Citation69 but no impact for in vivo half-life.Citation67,Citation70 Variable domain glycosylation adds another level of uncertainty with regard to potency and comparability later in development. The higher level of terminal galactose of Fab glycosylation increases the likelihood for further galactosylation by α1,3-galactose and sialylation. Fab-associated oligosaccharides with the addition of α1,3-galactose (Gal) have been shown to cause immunogenicity.Citation71 Sialylation could also add the immunogenic moiety of N-glycolylneuraminic acid (NGNA).Citation12,Citation72 It is worth mentioning that the addition of α-1,3 Gal and NGNA is highly dependent on cell line,Citation12,Citation13,Citation72 and their levels should be evaluated using material from the intended stable cell line.
In silico evaluation, especially with the use of computational methods, may also assist in identifying less apparent problematic attributes. The low expression and low stability of the mAb candidate was caused by uncommon amino acids identified by statistical sequence analysis.Citation73 Hydrophobic amino acids in CDRs were responsible for precipitation and absorption of mAb to filters during manufacturing and shorter in vivo half-life.Citation2 Yet, another study demonstrated that asymmetric charge distribution, and to a lesser degree hydrophobicity, contributes significantly to the observed high viscosity.Citation26 These hydrophobic or charged patches may not be obvious at the primary sequence level, but visible through proper sequence analysis and structural simulation using various computational methods. Extended characterization and forced degradation can confirm these non-obvious problematic attributes.
Unnecessary attributes
Some sequence and structural features of mAbs cause mAb heterogeneity, but not linked to any biological functions, and do not raise safety or efficacy concerns. The presence of these attributes possesses unnecessary challenges for the manufacturing of product with consistent profiles, and complicates analytical method development.
Cyclization of N-terminal glutamine (Gln) to form pyroglutamate (pyroGlu) is a major source of heterogeneity of mAbs. This reaction occurs spontaneously during drug substance production process, storage in liquid formulation and continues under physiological conditionsCitation74 and during storage. This N-terminal modification has no impact on structure, stability, or biological functions of mAbs.Citation75 N-terminal Gln of human endogenous IgGs is almost completely converted to pyroGlu.Citation76 It has been proposed to substitute N-terminal Gln with other amino acids to eliminate this source of heterogeneity.
Incomplete processing of C-terminal lysine (Lys) is another common modification. C-terminal Lys has no impact on mAb potencyCitation77,Citation78 PK, PD or immunogenicity.Citation78 However, removal of C-terminal Lys showed optimal C1q binding and CDC.Citation79 C-terminal Lys is rapidly removed during circulation,Citation80 explaining its absence from endogenous IgGs.Citation80 Removal of the codon for C-terminal Lys can eliminate this heterogeneity.
Further considerations
MAbs for therapeutic purposes have evolved from murine origin, to chimeric, humanized and fully human molecules to reduce immunogenicity.Citation81 However, immunogenicity remains a concern even for mAbs with full human sequences.Citation81,Citation82 Various tools including in silico calculation, in vitro assays or in vivo animal models are designed to identify amino acid sequences causing immunogenicities.Citation81,Citation83,Citation84 Additional immunogenic components such as alpha 1,3 galactose can be eliminated with the removal of variable domain glycosylation sites.Citation81,Citation83
Aggregation of mAb therapeutics is another contributor to immunogenicity, as well as to processing difficulties.Citation85 Attempts have been made to identify regions in mAbs that mediate aggregation through computational algorithms,Citation18,Citation19,Citation86-Citation90 or experimental screening (e.g., phage display),Citation91 and then to improve mAb stability by mutagenesis.Citation2,Citation19,Citation83,Citation88,Citation91,Citation92
Host cell protein (HCP) in mAb therapeutics can also contribute to immunogenicity.Citation93,Citation94 The type and amount of HCP in drug substance can be dependent on the specific mAb sequence or manufacturing process conditions, such as cell line, cell culture and stringency of purification parameters. However, mAbs with more general stickiness due to exposed hydrophobic or charged patches are expected to have more HCP problems.
The potential for self-administration through subcutaneous injection requires mAbs to be formulated at high concentration (≥100 mg/mL) and delivered at small volumes (≤2mL). In terms of developability, high solubility and low viscosity are thus required. Since strong mAb self-association is often the cause of low solubility and high viscosity, protein engineering can be applied to reduce self-association by modifying the protein sequences and thus increase mAb solubilityCitation95 or decrease viscosity.Citation26,Citation96-Citation99 Aside from the previously discussed potential issues with variable domain glycosylation, the introduction of glycosylation sites near aggregation-prone regions (APRs) was demonstrated to improve mAb solubility.Citation95,Citation100-Citation103
Modulation of in vivo half-life has been explored by changing amino acid sequence around the FcRn binding siteCitation83,Citation104 or in CDRs by introduction of a pH switch using histidine (His)Citation105 or disruption of CDR positively charged patchesCitation106 Additionally, mAbs with rapid clearance due to off-target binding can be addressed by altering amino acid sequences to disrupt charged or hydrophobic patches.Citation2,Citation26 MAbs can be tailored to have either extended or shortened half-life to better fit their therapeutic purposes and to increase patient compliance, e.g., longer half-life reducing dosing frequency.
Experimental evaluation by extended characterization
Following in silico evaluation, lead candidates are usually evaluated experimentally through extended characterization. Quality attributes that are highly relevant to mAb developability assessment are summarized in . Usually, the initial material available for testing are produced by transient transfection (e.g., HEK293 cells). Properties that are independent of the expression hosts such as primary sequence, hydrophobicity, solubility, thermal stability, and antigen binding affinity, can be evaluated without any observable differences. On the other hand, most PTMs are highly dependent on cell line and cell culture conditions, such as pH, temperature, and cell culture duration. Those cell line-dependent attributes should be evaluated using materials produced from the stable cell lines that will be used for clinical and/or commercial manufacturing.
Table 2. Categories of mAb attributes.
Table 3. mAb quality attributes evaluated during developability assessment.
Table 4. Known PTMs of mAbs identified by LC-MS.
Table 5. Modifications that form either acidic or basic species.
Table 6. Modifications causing HIC retention time shift.
Table 7. Forced degradation conditions, degradation pathways, and recommendations for developability evaluation.
Primary structure confirmation and sequence variants
The confirmation of the intended amino acid sequence is a prerequisite for further analysis and development of a mAb lead candidate. Modern mass spectrometry (MS) has the capability of accurately measuring the molecular weight of an IgG at approximately 150 kDa with the accuracy of ≤2 Da. The ability to obtain and confirm the monoisotopic molecular weights of mAbs at the subunit level provides strong evidence for confirming the primary structure.Citation107 Ultimately, the full primary sequence can be confirmed by liquid chromatography (LC)-MS and MS/MS peptide mapping.
Several studies have shown the presence of low abundance sequence variants.Citation108-Citation112 Detection and identification of low levels of sequence variants are made possible by using LC-MS/MS in combination with database searches.Citation113-Citation115 The presence of very low abundance sequence variants is likely caused by the naturally occurring low frequency errors during transcription and translation. Selection of mAb candidates and clones with minimal sequence variants is made possible by extensive characterization.
Posttranslational modifications
LC-MS plays an essential role for developability assessment because of its high sensitivity, fast turnaround time and, most importantly, the ability to obtain an in-depth level of information. PTMs and degradation of mAb lead candidates can be obtained from LC-MS analysis at intact, subunit or peptide levels. LC-MS analysis at the intact level enables detection of modifications above its resolution and detection limit, such as glycoforms, N-terminal pyroGlu, C-terminal Lys, C-terminal amidation, and glycation. LC-MS analysis at the subunit level or after reduction into light and heavy chains localizes modifications to either the Fab, F(ab’)2, Fc regions, light chain or heavy chain.Citation8,Citation116,Citation117 In addition to the traditionally used papain, more specific digestion can be achieved using limited Lys-CCitation117,Citation118 or Ides enzyme digestion.Citation107,Citation119,Citation120 The combination of IdeS digestion and reduction decreases the molecular weight of each fragment to 23–25 kDa, allowing the measurement of monoisotopic molecular weight.Citation107 Ultimately, analysis at the peptide level can precisely localize modification sites detected at intact and subunit levels. More importantly, analysis at the peptide level can detect modifications that cannot be detected at intact and subunit levels, such as Asn deamidation, where the molecular weight difference is about 1 Da. Because of chromatographic separation, modifications without molecular weight differences, such as Asp isomerization,Citation41-Citation43 L-Cys to D-Cys,Citation121,Citation122 and Ser racemizationCitation123 can also be detected by LC-MS.
All the reported modifications (to our best knowledge) for mAbs detected by LC-MS are listed in . Focus should be on those modifications that correspond to the potentially problematic attributes. For those PTMs that are highly dependent on cell lines, re-evaluation using materials from the stable cell line throughout the development is necessary. Comparison of the different candidates is based on the nature of modifications and their relative percentages.
Modifications with safety or efficacy concerns
This group of modifications are known to be linked with safety and efficacy issues. These modifications correspond to Problematic attributes listed in , which includes deamidation, isomerization, Met and Trp oxidation, unpaired cysteine and additional glycosylation in the variable domains. This group of modifications should be carefully examined during extended characterization and forced degradation studies.
There are three types of oligosaccharides, α1,3-Gal, NGNA and high mannose, that should be evaluated carefully. As discussed previously, α1,3-Gal and NGNA are immunogenic. High mannose has been shown to cause shorter in vivo half-lifeCitation185-Citation191 and enhanced antibody-dependent cell-meditated cytotoxicity (ADCC) due to the lack of core-fucose.Citation187,Citation190,Citation191 Additionally, the afucosylation level must be monitored because of its correlation with enhanced ADCC,Citation141 which could be either beneficial or harmful depending on the mAb’s mechanism of action (MOA).Citation192 Higher levels of afucosylation are beneficial for mAbs targeting cell surface antigen and initiating cell killing, while it is harmful for mAbs that only block cell surface antigens. The levels of those oligosaccharides should be re-evaluated using material generated from the stable cell lines that will be used for clinical and commercial production.
Modifications corresponding to degradation
This group of modifications has not been reported to impact product safety or efficacy, but could potentially cause immunogenicity because these modifications are not present in either human endogenous IgG or degradation products. This group of modifications includes the presence of partial leader sequence, trisulfide bond, thioether, and glycation. To minimize this risk, mAb lead candidates with the lowest levels of these types of modification should be selected. Modifications in this category may also be highly dependent on cell lines and cell culture parameters such as temperature, pH, media composition and formulation.
Modifications causing heterogeneity
N-terminal pyroGlu formation and partial removal of C-terminal Lys are two of the well characterized modifications that cause molecule heterogeneity, but have no impact on safety or efficacy. Additionally, the levels of these modifications are highly dependent on cell line and cell culture conditions.
The level of terminal Gal associated with the glycosylation of Fc is also of note due to its sensitivity to process changes. The terminal Gal has no impact on structure,Citation179-Citation181,Citation193,Citation194 stability Citation184,Citation195 or clearance.Citation70,Citation185,Citation186,Citation189,Citation196,Citation197 Recent studies have demonstrated that the terminal galactose may have minimal impact on ADCC, but substantial influence on CDC.Citation139,Citation198 Therefore, the level of terminal galactose should be considered for mAb candidates with MOA involving CDC. Since the level of terminal galactosylation varies with different cell lines and cell culture conditions, it may have to be re-evaluated later in the development process.
FcRn affinity
FcRn binding is one of the most critical factors affecting mAb half-life.Citation104 The FcRn binding affinities of mAb candidates are usually measured by Biacore, but alternative assays using biolayer interferometry (BLI) or FcRn affinity chromatography have been established. In a study evaluating mAbs, it was found that delayed elution of mAbs from an FcRn affinity column at neutral pH correlated with poor pK.Citation24,Citation199 Compared to Biacore and affinity chromatography, much higher throughput can be obtained using BLI.Citation200 In general, mAbs with stronger FcRn binding at acidic pH, but fast dissociation at neutral pH, show longer in vivo half-life.Citation104
Thermal stability
Thermal stability is the ability of a protein to maintain its structural and functional integrity under different temperature environment, and is an intrinsic property of mAbs that can influence product stability, such as aggregation, during manufacturing and storage. High thermal stability of a mAb candidate indicates a well-packed structure that requires more energy to unfold. Therefore, higher thermal stability of a mAb generally correlates with a lower tendency towards partial unfolding and thus aggregation.Citation19,Citation73,Citation201-Citation206 Besides aggregation, mAbs with lower thermal stability have been shown to have lower expression.Citation73,Citation207
Thermal stability has been commonly measured by differential scanning calorimetry (DSC). Citation19,Citation73,Citation201-Citation206 An alternative method using differential scanning fluorimetry (DSF) allows high throughput thermal stability screening of 96 or 384 samples.Citation202,Citation206,Citation208-Citation210 Multiple candidates analyzed under the same conditions can be ranked based on the obtained thermodynamic parameters such as the midpoint temperature (Tm) of unfolding or the onset of unfolding temperature (Tonset).
Solubility
Solubility is an important developability parameter for mAbs,Citation211,Citation212 especially in consideration of the industry trend towards higher concentration formulations (100 mg/mL and above).Citation213 MAbs must remain soluble throughout processing, storage and administration.Citation97,Citation211 Low solubility can lead to issues during purification, sterile filtration, fill and finish, shipping, storageCitation214 and more importantly, can adversely affect activity, bioavailability, and immunogenicity.Citation212,Citation215 From the process perspective, a minimum solubility (e.g., 20–30 mg/mL) in the buffers used for bioprocessing is necessary for all the chromatographic steps. During the final ultrafiltration/diafiltration (UF/DF) step, mAbs are buffer exchanged into formulation buffers at concentrations above the targeted drug product, thus requiring much higher solubility.
Lower solubility is usually caused by strong mAb self- association with exposed hydrophobic or charged patches. Naturally, the bivalent nature of mAbs amplifies their self-association tendencies.Citation216 Colloidal instability caused by conformational changes or chemical modifications can also contribute to the poor solubility of a mAb. Additionally, mAb solubility is often influenced by solution properties, such as buffer composition, ionic strength, pH, and temperature.Citation214,Citation217-Citation219
It is challenging to predict solubility of mAb candidates based on the amino acid sequences, therefore solubility of mAbs should be studied experimentally. However, studying mAb solubility directly requires large amounts of protein, typically several hundred milligrams, and it is often not practical to produce all candidates at such large quantities for solubility study. Given the limited sample quantities available for developability assessment studies, indirect measurement methods are commonly used. For example, addition of polyethylene glycol (PEG) to mAb solutions causes precipitation at much lower concentrations, and thus can be used to determine the apparent solubility of mAbs through extrapolation to zero PEG concentration.Citation212,Citation220-Citation222 This approach can be implemented in a high-throughput manner for mAb candidate selection. However, PEG-induced precipitation may not truly reflect the mechanisms of the poor solubility of mAbs,Citation218,Citation223 and thus orthogonal methods or direct evaluation of solubility at high concentration should be considered to confirm the predicted solubility or validate the rank order.
Prediction of the high concentration behavior of a mAb using low concentration sample may also be done through the measurement of the osmotic second virial coefficient B22, a thermodynamic parameter related to intermolecular interactions. Positive and negative B22 values indicate repulsive or attractive forces, respectively. Parameters affecting B22 include electrostatic interactions, van der Waals force, excluded volumes, hydration forces, and hydrophobic effects.Citation224,Citation225 Among many different ways to obtain B22 values, such as self-interaction chromatography (SIC),Citation226 membrane osmometry (MO)Citation227 and analytical ultracentrifugation (AUC),Citation228 the most common method is through static light scattering (SLS).Citation225,Citation229,Citation230 Recently this value was used to determine a universal solubility line, the “liquidus” line, as part of a phase diagram for a mAb.Citation231,Citation232
Cross-interaction chromatography (CIC) assay, introduced by Jacob et al.Citation233 takes advantage of the accumulative effect on a column to capture weak binding between testing mAb and a large quantity (30 mgs) of immobilized human serum IgGs. MAbs with late elution by CIC assay correlate with poor solubility, due to exposed sticky (hydrophobic or charge) surfaces. The other method worth mentioning is self-interaction nanoparticle spectroscopy, which uses gold nanoparticles to concentrate mAb molecule to a high local concentration to amplify weak self-interaction.Citation216,Citation234 This method can also be applied to high throughput screening of mAb candidates.Citation234
Viscosity
High concentration drug products administrated via subcutaneous (SC) injection require a formulation with manageable viscosity, making it another critical factor for evaluation at an early stage to ease developability concerns.Citation235 High viscosity can pose challenges to the final UF/DF step Citation28,Citation236,Citation237 and fill/finish operation.Citation238,Citation239 Viscous drug product can lead to difficulties in delivery causing low patient compliance.Citation240 Viscous samples can also pose sampling challenges for analytical method development and instrumentation.Citation241
High viscosity has been shown to be caused by strong mAb self-association through electrostatic Citation26,Citation98,Citation242-Citation246 or hydrophobic interactions,Citation26,Citation242 or the combination of both.Citation26,Citation242,Citation247 Although, a number of formulation parameters, including pH, salts, sugars, and various small molecule excipients and detergents can be explored to lower viscocity,Citation97,Citation248-Citation251 selection of mAb lead candidates with minimal inherent problems such as exposure of hydrophobic, or charged patches is one of the most efficient means to minimize high viscosity risk.Citation242,Citation252
A variety of methods have been used to measure viscosity, including Cannon-Fenske Routine viscometer, Taylor Cone plate method, and various rheometers. Most of the conventional techniques for measuring viscosity require a large amount of materials. To overcome this challenge, in particular for developability assessment, a high throughput DLS method has been developed based on measurement of apparent polystyrene bead radii in high concentration mAb solutions to back calculate the viscosity of a mAb solution.Citation253 This method can only be used for mAbs without interaction with the beads, otherwise the apparent bead radii cannot be reliably measured. High throughput diffusion interaction parameters derived from DLS measurement have also been shown to correlate with viscosity.Citation254 In recent years, the instruments allowing viscosity measurement using ≤100 µL and with automated sample handling have become commercially available, and these are suitable for measuring viscosity during developability assessment.
Because of the significance of viscosity for process and product development, having a carefully defined viscosity target for developability assessment is important. At minimum, the formulation viscosity should be low enough to allow the drug formulation to be delivered with manual injection. The viscosity target is typically developed based on each company’s internal development experience, in particular for developing SC product with prefilled syringes and auto-injector devices. For example, it was proposed that the viscosity can be grouped into 3 categories: 1) “preferred” viscosity of 10 cP or lower; 2) “acceptable” viscosity between 10 to 20 cP; and 3) “unacceptable’ viscosity of > 20 cP. This can be used as a starting point for defining the viscosity target with additional considerations of the internal experience of process and product development and product knowledge of delivery devices, such as autoinjector.
Aggregation propensity
Aggregates are the most commonly observed product-related impurities, requiring close monitoring due to immunogenicity concerns.Citation85,Citation255 Therefore, it is a critical component of developability assessment. In addition to utilizing predictive tools, aggregation propensity can be directly measured during extended characterization and forced degradation studies. Though typically only low concentration data is available due to material limitation, it is important to evaluate the colloidal stability and aggregation propensity of a mAb at medium to high (50–100 mg/mL) concentration ranges.
Routinely, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or capillary electrophoresis (CE-SDS) are used to determine mAb monomer, fragments and covalent aggregates under denaturing conditions with or without reduction. A variety of other methods are available to measure mAb soluble aggregates such as dimer, oligomer or subvisible particles under native conditions.Citation255-Citation258 Size-exclusion chromatography (SEC) is the most commonly used method to determine mAb HMW species (e.g., dimer, trimer or oligomers) and LMW species. SEC is typically included for product release and often available as a generic or platform method, therefore it is suitable for evaluation of aggregates during extended characterization and forced degradation. It is worth mentioning that SEC can sometimes reveal properties other than the percentage of monomer, aggregates and fragments. Abnormal SEC behaviors of a mAb, such as peak tailing, could indicate non-ideal biophysical properties.Citation1 Longer retention time and asymmetric peak shape can suggest nonspecific interactions between a mAb and the SEC column.Citation2 Studies showed that SEC can even separate mAb variants containing succinimide intermediate from those with Asn deamidation (17Da) or Asp isomerization (18Da).Citation167,Citation259 SEC has also shown that a mAb variant containing oxidized Trp eluting earlier than the main peak.Citation158 These examples suggest that interpretation of SEC data should be done cautiously because earlier and later peaks may not always represent HMW or LMW species.
For large aggregates, light scattering can be used to characterize particles in the range of <1 nm to 1–10 µm. The DLS method can be used to determine the hydrodynamic diameters of mAbsCitation242 and interaction parameters, such as KD,Citation260,Citation261 which can be run in high throughput mode with low sample consumption. Methods that measure turbidity, such as optical density at visible wavelength and nephelometry, can also be considered for detection of submicron/sub visible particles. These techniques may be developed with high throughout and low volume consumption, and thus can be used for developability assessment. Light obscuration (e.g., HIAC) and flow imaging methods (e.g., micro-flow imaging (MFI)) and FlowCam) can be used for sub-visible particle characterization and quantification for sizes greater than 2 µm. A visual inspection method is used for detecting the protein particles in the visible range, typically > 70 to 100 µm.
In addition to directly measuring the level of aggregates, aggregation propensity can be ranked based on hydrophobicity or protein interactions as they are the main driving forces for aggregation. Fluorescence dyes such as 1-anilino-naphthalenesulfonate (ANS) and thioflavin can be used to probe exposed hydrophobic patches in a high throughput manner with minimal sample requirement.Citation262 Affinity capture self-interaction nanoparticle spectroscopy (AC-SINS), which provides coarse-grained information about interactions and aggregation propensity in different solution conditions, is useful to leverage during developability evaluations.Citation234
Charge variants
Charge variation of mAbs reflects the sum of various PTMs. Variants of mAbs need to be closely monitored throughout the development process to ensure consistent peak profiles. Because of its sensitivity to process changes, charge variation is one of the quality attributes that could be challenging for demonstrating comparability, when process changes are introduced.
A typical mAb charge variant profile characterized by charged-based methods such as ion exchange chromatography and isoelectric focusing usually contains one major peak and several smaller acidic and basic peaks. Reported modifications resulting in the formation of acidic or basic species are shown in . It is worth mentioning that several modifications may impact chromatographic separations of mAb variants by modulating mAb structures. For examples, mAbs with smaller oligosaccharides can contribute to the formation of basic species,Citation263 while the oxidized Met may contribute to the formation of either acidicCitation172,Citation264 or basicCitation265,Citation266 species. Similarly, the presence of the incompletely formed disulfide bond in the heavy chain variable domain can either contribute to acidicCitation163 or basicCitation162 species.
Isoelectric focusing gel electrophoresis (IEF) was traditionally used to analyze mAb charge variants.Citation37,Citation267,Citation268,Citation276 This semi-quantitative, labor-intensive method relies on dye staining for detection. It also suffers from low throughput, lack of automation and poor reproducibility. Capillary IEF (cIEF) overcame most of the IEF limitations and offered additional advantages, including high sensitivity, automation, and low sample consumption.Citation276-Citation278 Moreover, imaged cIEF (icIEF) has gained popularity for the analysis of mAb charge variantsCitation279-Citation281 because the whole capillary imaging eliminates the troublesome mobilization step used by cIEF.Citation282
Capillary zone electrophoresis (CZE) separates mAb charge variants based on both charge and hydrodynamic radius. This method can be readily platformed with relatively high throughput compared to cIEF.Citation277,Citation278,Citation283-Citation286 CZE can also be coupled on-line with a mass spectrometer. CZE-MS has been used to profile N-linked glycans from tryptic peptidesCitation16 and analyze the sites of deamidation and isomerization.Citation287 A single CE-MS run has been shown to confirm 100% of the primary structure and reveal several PTMs, including glycosylation, N-terminal Gln cyclization, deamidation and isomerization.Citation288
Ion exchange chromatography (IEX), including cation exchangeCitation36,Citation37,Citation75,Citation166,Citation264,Citation267,Citation268,Citation271,Citation272 and anion exchange,Citation116,Citation155,Citation276 has been widely used to monitor mAb charge variants. IEX allows fraction collection for further characterization. Multiple mAbs can be analyzed when a pH gradient is used,Citation289 implying the potential for establishment of a platform method. Strong cation exchange (SCX) chromatography allows a relatively higher throughput compared to weak cation exchange chromatography.Citation290,Citation291 When comparing the overall charge profiles, IEF usually shows comparable results with either cationCitation37or anionCitation276 exchange chromatography. However, different profiles have been observed due to differences in the separation mechanisms.Citation267,Citation268
Hydrophobicity and related heterogeneity
Hydrophobicity can impact mAb aggregation, solubility and viscosity.Citation292,Citation293 Higher hydrophobicity correlates with higher propensity towards aggregationCitation18,Citation19,Citation21,Citation22 and precipitation.Citation293,Citation294 Hydrophobic patches in CDRs can lead to a higher degree of inter-molecule interaction, higher viscosity and shorter in vivo half-life.Citation2,Citation26
Hydrophobic interaction chromatography (HIC) has been used to measure the relative hydrophobicity of different mAbsCitation7,Citation25,Citation292 or separate variants of the same mAb caused by PTMs or degradation.Citation37,Citation41,Citation43,Citation161 Reported modifications causing HIC retention time shift, as compared to the main peak, are listed in . Some modifications such as Asp isomerization and deamidation can shift HIC retention times both ways, suggesting the involvement of other factors impacting chromatographic behavior.
Alternative methods for measuring mAb relative hydrophobicity of mAbs have also been reported, such as the use of gold nanoparticle via salt gradient screening.Citation295 In this method, the testing mAb is loaded onto gold nanoparticles, followed by salt gradient stress to strip water molecules from hydrophobic patches on the surface of a mAb molecule. The results demonstrated a good correlation with HIC retention times for tested mAbs.
Free thiols
The presence of significant levels of free Cys negatively impacts mAb stability and potency. The level of free Cys and free Cys-related modifications and degradations are highly dependent on mAb sequence, as well as environmental factors during cell culture and purification.
MAbs contain low levels of free thiols at each Cys residue.Citation298-Citation300 Free thiols have been shown to lower thermal stabilityCitation298 and increase formation of reducible covalent aggregates.Citation301,Citation302 These mAb-associated free cysteines can react with free cysteine present in cell culture media to form cysteinylated or other covalent adducts.Citation61,Citation62,Citation128,Citation149,Citation150 In a few cases, relatively higher levels of free cysteine were detected, mainly due to incomplete formation of heavy chain variable domain disulfide bonds,Citation161-Citation163 which could result in reduced potency.Citation161
Protein-protein interactions
Protein-protein interaction has drawn increasing attention during developability evaluation due to its impact on solubility, viscosity, and aggregation propensityCitation96,Citation254,Citation294,Citation303-Citation305 In addition, non-specific off-target binding in vivo resulted in fast clearance and poor PKCitation23,Citation306
A variety of techniques have been developed to study protein-protein interactions for mAbs, including self-interactions and non-specific interactions with other molecules. Among these techniques, Biacore,Citation307 bio-layer interferometry (BLI),Citation308 and self-interaction nanoparticle spectroscopy (SINS).Citation216,Citation234,Citation309,Citation310 have been used to study self-interaction. On the other hand, cross-interaction chromatography (CIC) can be used to study non-specific interaction when different proteins were immobilized.Citation233,Citation293,Citation311 Positive correlation between delayed retention between CIC and HIC suggests that hydrophobic interaction being a major contributing factor to the general stickiness (non-specific interaction) of these mAbs.Citation293 Other assays including the polyspecificity reagent binding assay,Citation312 and binding to heparin,Citation313 HEK293 cells,Citation106 baculovirus particles,Citation314 chaperone proteins,Citation315 and yeastCitation316 have also been used to study non-specific interactions.
Experimental evaluation by forced degradation
Forced degradation studies are playing an ever-increasing role providing critical information to support mAb drug development.Citation31 Forced degradation studies can be predictive of in vivo degradationCitation3,Citation33,Citation35,Citation40 and can also be used to validate liable spots identified from in silico evaluation, reveal degradation hot-spots that are not obvious from in-silico and provide a ranking order of mAb candidates based on stability. The commonly used forced degradation conditions and major degradation pathways, and recommendations to support developability are shown in .
Forced degradation studies are important for confirmationCitation3 or rejection Citation34 of the predicted degradation hot-spots from in silico assessment. More importantly, forced degradation studies can reveal the hidden degradation hot-spots that are not generally recognized or specific to individual mAb lead candidates. The relatively harsh conditions used in forced degradation studies can increase the detectability of degradation products that are normally present at low levels during extended characterization. For example, susceptibility of Asp to isomerization and peptide bond hydrolysis in an Asp-Asp motif in the CDRCitation315 or susceptibility of a Ser-Ser motif in the CDR to peptide bond hydrolysis Citation316 can only be detected during forced degradation.
Forced degradation studies are valuable for defining process parameters and for prediction of long-term stability.Citation317 Low pH is a common stress during protein A chromatography elution and virus inactivation steps used for a typical mAb purification process. MAb candidates with poor low pH stability will require extensive efforts using alternative purification and virus inactivation processes. MAbs could also be transiently exposed to high pH conditions during anion exchange chromatography elution and pH neutralization after low pH exposure. MAbs that are sensitive to agitation, light, freeze/thaw cycle can be a challenge to the establishment of a robust process due to limited design space.
Forced degradation results for any given mAb are highly dependent on the selected conditions. It is well known that the first and second Asn residues in the “PENNY” peptide are highly susceptible to deamidation.Citation38,Citation39 However, the Asn residue within the amino acid sequence VSNK was found to have an even higher level of deamidation compared to the “PENNY” peptides when stored at pH 5.2.Citation169 Specific guidance regarding photostability is provided in ICH Q1B, but the recommended conditions are different from room light conditions.Citation349 From the developability assessment perspective, forced degradation conditions may be best selected based on their relevance to process, stability and in vivo conditions, while taking into consideration the intrinsic properties of the mAb candidates.
Computational tools
Computational approaches are attractive for mAb developability assessment as the amino acid sequence is the only necessary input. Thus, they can be applied to in-silico evaluation for in-depth evaluation, in addition to identifying the obvious degradation hot-spots. Potential problematic attributes from known motifs or the presence of less frequently observed amino acid at certain positions can be easily highlighted.Citation73,Citation84,Citation90 Several computational tools, based on machine learning, are available to calculate the solvent-accessible surface area (SASA).Citation350 SASA showed good predictability of the chemical liability, such as oxidation of MetCitation8 and TrpCitation26 or Asp isomerization,Citation26 as well as HIC retention times.Citation350
Computational tools are also available to rank viscosity of mAb lead candidates based on the known amino acid sequences. The variable domain net charge, asymmetric charge distribution and, to a lesser degree hydrophobicity, were found to contribute to higher viscosity.Citation26 The spatial charge map was established based on amino acid sequence and homology modeling to predict viscosity.Citation351 Calculation of biochemical and biophysical properties based on homology modeling of variable regions of low and high viscosity mAbs revealed that net negative charge, zeta-potential and variable domain isoelectric point are the critical parameters impacting viscosity.Citation245 Homology modeling has identified surface negatively charged patches causing high viscosity.Citation99 Interestingly, homology modeling also identified positively charged patches in the variable domain of a mAb responsible for its shorter half-life, which was related to decreased dissociation from FcRn at neutral pH.Citation24 Coarse-grained modeling has also been used to understand inter-molecule interactions, and has revealed that domain-level electrostatic interactions play an important role.Citation349,Citation352,Citation353
Various computational tools have also been developed to identify structural features that could trigger aggregation.Citation86,Citation87 Spatial-aggregation-propensity (SAP), based on molecular simulation, can be used to identify aggregation-prone motifs, due to formation of hydrophobic patches in the tertiary structure.Citation18,Citation19,Citation89,Citation294 Mutation of the identified hydrophobic residues to hydrophilic ones has been shown to increase stability.Citation18,Citation19,Citation294 SAP identified APRs in the constant domains,Citation18 as well as in variable domains,Citation90 highlighting the need to balance the affinity and aggregation propensity. Lauer et al proposed the concept of a developability index, to rank mAbs based on aggregation propensity calculated from the net charge and SAP of the CDRs.Citation27 Based on experimental data from over 500 mAbs, a model was built using statistical modeling and machine learning to categorize mAbs to high or low risk towards aggregation.Citation354
Computational tools have also been developed to identify unwanted in vivo behaviors such as poor PKCitation29 and immunogeneicity.Citation355 Studies have shown that faster mAb clearance correlated with exposed hydrophobic or charged patches in the variable domains.Citation26,Citation356 T-cell epitopes can be identified by computational tools,Citation355 which can be used to rank mAb candidates to lower the potential immunogenicity risk.
Emerging analytical methods
Emerging analytical methods may have the potential to gather information faster with less material consumption, and thus can be applied to mAb developability assessment. Hydrophilic interaction liquid chromatography (HILIC) has been applied to mAb characterization.Citation357,Citation358 With a polar stationary phase and an organic mobile phase, HILIC is fully compatible with MS and offers a complementary retention mechanism compared to reversed-phase high-performance liquid chromatography (RP-HPLC). This chromatographic method was mostly used for released glycan profiling and glycopeptide separations. It can also be used as an orthogonal method to RP-HPLC at the subunit level following IdeS digestion.
Two-dimensional LC (2D-LC) with MS and other detection methods (e.g., UV) clearly facilitate a deep structural understanding of mAbs.Citation359-Citation361 The additional chromatographic selectivity and resolution of 2D-LC compared to the conventional 1D-LC methods enables the direct and efficient identification of different variants present in these materials. 2D-LC with various combinations, such as SEC×RP–MS, CEX×RP–MS, and HIC×RP–MS, have the potential to provide extensive characterization of mAbs with automation and low material consumption to support developability evaluation.
Recommendations
Acceleration of development activities to bring mAb drug candidates into first-in-human clinical studies, and then to market is the ultimate goal of drug developers. There is a fine balance between minimizing the risks at early stages and accelerating the later stages of development to provide the essential therapies to patients. It is not practical, nor necessary, to apply all of the discussed methods and studies for a developability evaluation.
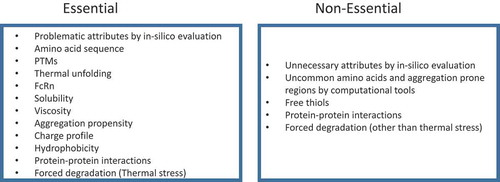
We propose a workflow as outlined in , which categorizes studies and testing as “Essential”, which must be included, or “Non-essential”, which are optional. The goal of this workflow is to gather scientific information and experimental data within a reasonable timeframe for candidate ranking and selection.
It is worth mentioning that ex vivo and in vivo serum stability has gained popularity because of the relevance to physiological conditions. Those studies can be considered under specific occasions in order to ease the remaining concerns of the selected mAb candidates. For example, in the case of incomplete heavy chain variable domain disulfide bond formation, additional studies demonstrated that they can be formed rapidly under ex vivo and in vivo conditions, thus eliminating the concern of reduced potency.Citation166 In contrast, in the case of CDR region deamidation, although the rate of deamidation may be controlled by appropriate process controls and formulation, the risk of continuous deamidation and loss of potency in vivo means that this risk cannot be mitigated.Citation3
Conclusions
Developability assessment has been recognized as a critical step that should occur early in the process of selecting a drug candidate for development. The candidate is selected based on a thorough evaluation using in silico assessment with the aid of computational approaches and experimental data from extended characterization and forced degradation. Candidates with the most favorable biochemical and biophysical properties, and thus lower inherent risks, are selected for further development. Knowledge gained from developability assessments will also help guide process and product development to reduce timelines and resource consumption, thus providing affordable and high-quality mAb therapeutics to patients.
Abbreviations
| AC-SINS | = | Affinity capture self-interaction nanoparticle spectroscopy |
| ADCC | = | Antibody-dependent cell-mediated cytotoxicity |
| ANS | = | 1-anilino-naphthale-nesulfonate |
| Asn | = | Asparagine |
| Asp | = | Aspartate |
| AUC | = | Analytical ultracentrifugation |
| BLI | = | Biolayer interferometry |
| C1q | = | Component of complement |
| CDC | = | Complement-dependent cytotoxicity |
| CDR | = | Complementarity-determining region |
| CE | = | Capillary electrophoresis |
| CIC | = | Cross-interaction chromatography |
| cIEF | = | Capillary isoelectric focusing |
| CMC | = | Chemistry, Manufacturing, and Controls |
| Cys | = | Cysteine |
| CZE | = | Capillary zone electrophoresis |
| DLS | = | Dynamic light scattering |
| DSC | = | Differential scanning calorimetry |
| DSF | = | Differential scanning fluorimetry |
| EMA | = | European Medicines Agency |
| FDA | = | US Food and Drug Administration |
| Gal | = | Galactose |
| Gln | = | Glutamine |
| Glu | = | Glutamate |
| Gly | = | Glycine |
| HCP | = | Host cell protein |
| HIC | = | Hydrophobic interaction chromatography |
| HILIC | = | Hydrophilic interaction liquid chromatography |
| HMW | = | High molecular weight |
| kDa | = | Kilodalton |
| LC-MS | = | Liquid chromatography-mass spectrometry |
| LMW | = | Low molecular weight |
| IEX | = | Ion exchange chromatography |
| Lys | = | Lysine |
| mAb | = | Monoclonal antibody |
| Met | = | Methionine |
| MFI | = | Micro-flow imaging |
| MO | = | Membrane osmometry |
| MOA | = | Mechanism of action |
| NGNA | = | N-glycolylneuraminic acid |
| PD | = | Pharmacodynamics |
| PD | = | Pharmacokinetics |
| PEG | = | Polyethylene glycol |
| PTMs | = | Post-translational modifications |
| QbD | = | Quality by design |
| SAP | = | Spatial-aggregation propensity |
| SC | = | Subcutaneous |
| SCX | = | Strong cation exchange |
| SDS-PAGE | = | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEC | = | Size exclusion chromatography |
| SIC | = | Self-interaction chromatography |
| SLS | = | Static light scattering |
| Trp | = | Tryptophan |
| Tyr | = | Tyrosine |
| UF/DF | = | Ultrafiltration/Diafiltration |
| WCX | = | Weak cation exchange |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lavoisier A, Schlaeppi JM. Early developability screen of therapeutic antibody candidates using Taylor dispersion analysis and UV area imaging detection. MAbs. 2015;7:77–83. doi:10.4161/19420862.2014.985544.
- Dobson CL, Devine PW, Phillips JJ, Higazi DR, Lloyd C, Popovic B, Arnold J, Buchanan A, Lewis A, Goodman J, et al. Engineering the surface properties of a human monoclonal antibody prevents self-association and rapid clearance in vivo. Sci Rep. 2016;6:38644. doi:10.1038/srep38644.
- Yang X, Xu W, Dukleska S, Benchaar S, Mengisen S, Antochshuk V, Cheung J, Mann L, Babadjanova Z, Rowand J, et al. Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies. MAbs. 2013;5:787–794. doi:10.4161/mabs.25269.
- Strohl WR. Current progress in innovative engineered antibodies. Protein Cell. 2018;9:86–120. doi:10.1007/s13238-017-0457-8.
- Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat Rev Drug Discov. 2018;17:197–223. doi:10.1038/nrd.2017.227.
- Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10:183–203. doi:10.1080/19420862.2018.1415671.
- Goyon A, D’Atri V, Colas O, Fekete S, Beck A, Guillarme D. Characterization of 30 therapeutic antibodies and related products by size exclusion chromatography: feasibility assessment for future mass spectrometry hyphenation. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1065–1066:35–43. doi:10.1016/j.jchromb.2017.09.027.
- Yang R, Jain T, Lynaugh H, Nobrega RP, Lu X, Boland T, Burnina I, Sun T, Caffry I, Brown M, et al. Rapid assessment of oxidation via middle-down LCMS correlates with methionine side-chain solvent-accessible surface area for 121 clinical stage monoclonal antibodies. MAbs. 2017;9:646–653. doi:10.1080/19420862.2017.1290753.
- Goyon A, Excoffier M, Janin-Bussat MC, Bobaly B, Fekete S, Guillarme D, Beck A. Determination of isoelectric points and relative charge variants of 23 therapeutic monoclonal antibodies. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1065–1066:119–128. doi:10.1016/j.jchromb.2017.09.033.
- Raju TS, Jordan RE. Galactosylation variations in marketed therapeutic antibodies. MAbs. 2012;4:385–391. doi:10.4161/mabs.19868.
- Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 2011;29:310–312. doi:10.1038/nbt.1839.
- Maeda E, Kita S, Kinoshita M, Urakami K, Hayakawa T, Kakehi K. Analysis of nonhuman N-glycans as the minor constituents in recombinant monoclonal antibody pharmaceuticals. Anal Chem. 2012;84:2373–2379. doi:10.1021/ac300234a.
- Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8:2858–2871. doi:10.1002/pmic.200700968.
- Kamoda S, Ishikawa R, Kakehi K. Capillary electrophoresis with laser-induced fluorescence detection for detailed studies on N-linked oligosaccharide profile of therapeutic recombinant monoclonal antibodies. J Chromatogr A. 2006;1133:332–339. doi:10.1016/j.chroma.2006.08.028.
- Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18. doi:10.1016/j.ab.2007.01.023.
- Giorgetti J, D’Atri V, Canonge J, Lechner A, Guillarme D, Colas O, Wagner-Rousset E, Beck A, Leize-Wagner E, François Y-N. Monoclonal antibody N-glycosylation profiling using capillary electrophoresis - Mass spectrometry: assessment and method validation. Talanta. 2018;178:530–537. doi:10.1016/j.talanta.2017.09.083.
- Sydow JF, Lipsmeier F, Larraillet V, Hilger M, Mautz B, Mølhøj M, Kuentzer J, Klostermann S, Schoch J, Voelger HR, et al. Structure-based prediction of asparagine and aspartate degradation sites in antibody variable regions. PLoS One. 2014;9:e100736. doi:10.1371/journal.pone.0100736.
- Chennamsetty N, Helk B, Voynov V, Kayser V, Trout BL. Aggregation-prone motifs in human immunoglobulin G. J Mol Biol. 2009;391:404–413. doi:10.1016/j.jmb.2009.06.028.
- Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci U S A. 2009;106:11937–11942. doi:10.1073/pnas.0904191106.
- Grebenau RC, Goldenberg DM, Chang CH, Koch GA, Gold DV, Kunz A, Hansen HJ. Microheterogeneity of a purified IgG1 due to asymmetric Fab glycosylation. Mol Immunol. 1992;29:751–758.
- Lee CC, Perchiacca JM, Tessier PM. Toward aggregation-resistant antibodies by design. Trends Biotechnol. 2013;31:612–620. doi:10.1016/j.tibtech.2013.07.002.
- Perchiacca JM, Tessier PM. Engineering aggregation-resistant antibodies. Annu Rev Chem Biomol Eng. 2012;3:263–286. doi:10.1146/annurev-chembioeng-062011-081052.
- Kelly RL, Yu Y, Sun T, Caffry I, Lynaugh H, Brown M, Jain T, Xu Y, Wittrup KD. Target-independent variable region mediated effects on antibody clearance can be FcRn independent. MAbs. 2016;8:1269–1275. doi:10.1080/19420862.2016.1208330.
- Schoch A, Kettenberger H, Mundigl O, Winter G, Engert J, Heinrich J, Emrich T. Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proc Natl Acad Sci U S A. 2015;112:5997–6002. doi:10.1073/pnas.1408766112.
- Jarasch A, Koll H, Regula JT, Bader M, Papadimitriou A, Kettenberger H. Developability assessment during the selection of novel therapeutic antibodies. J Pharm Sci. 2015;104:1885–1898. doi:10.1002/jps.24430.
- Sharma VK, Patapoff TW, Kabakoff B, Pai S, Hilario E, Zhang B, Li C, Borisov O, Kelley RF, Chorny I, et al. In silico selection of therapeutic antibodies for development: viscosity, clearance, and chemical stability. Proc Natl Acad Sci U S A. 2014;111:18601–18606. doi:10.1073/pnas.1421779112.
- Lauer TM, Agrawal NJ, Chennamsetty N, Egodage K, Helk B, Trout BL. Developability index: a rapid in silico tool for the screening of antibody aggregation propensity. J Pharm Sci. 2012;101:102–115. doi:10.1002/jps.22758.
- Yang Y, Velayudhan A, Thornhill NF, Farid SS. Multi-criteria manufacturability indices for ranking high-concentration monoclonal antibody formulations. Biotechnol Bioeng. 2017;114:2043–2056. doi:10.1002/bit.26329.
- Dostalek M, Prueksaritanont T, Kelley RF. Pharmacokinetic de-risking tools for selection of monoclonal antibody lead candidates. MAbs. 2017;9:756–766. doi:10.1080/19420862.2017.1323160.
- Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–352. doi:10.1038/nri2747.
- Nowak C, Cheung K, Dellatore J,M, Katiyar A, Bhat R, Sun J, Ponniah G, Neill A, Mason B, Beck A, et al. Forced degradation of recombinant monoclonal antibodies: A practical guide. MAbs. 2017;9:1217–1230. doi:10.1080/19420862.2017.1368602.
- Bults P, Bischoff R, Bakker H, Gietema JA, van de Merbel NC. LC-MS/MS-based monitoring of in vivo protein biotransformation: quantitative determination of trastuzumab and its deamidation products in human plasma. Anal Chem. 2016;88:1871–1877. doi:10.1021/acs.analchem.5b04276.
- Huang L, Lu J, Wroblewski VJ, Beals JM, Riggin RM. In vivo deamidation characterization of monoclonal antibody by LC/MS/MS. Anal Chem. 2005;77:1432–1439. doi:10.1021/ac0494174.
- Phillips JJ, Buchanan A, Andrews J, Chodorge M, Sridharan S, Mitchell L, Burmeister N, Kippen AD, Vaughan TJ, Higazi DR, et al. Rate of asparagine deamidation in a monoclonal antibody correlating with hydrogen exchange rate at adjacent downstream residues. Anal Chem. 2017;89:2361–2368. doi:10.1021/acs.analchem.6b04158.
- Tran JC, Tran D, Hilderbrand A, Andersen N, Huang T, Reif K, Hotzel I, Stefanich EG, Liu Y, Wang J. Automated affinity capture and on-tip digestion to accurately quantitate in vivo deamidation of therapeutic antibodies. Anal Chem. 2016;88:11521–11526. doi:10.1021/acs.analchem.6b02766.
- Vlasak J, Bussat MC, Wang S, Wagner-Rousset E, Schaefer M, Klinguer-Hamour C, Kirchmeier M, Corvaïa N, Ionescu R, Beck A. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal Biochem. 2009;392:145–154. doi:10.1016/j.ab.2009.05.043.
- Harris RJ, Kabakoff B, Macchi FD, Shen FJ, Kwong M, Andya JD, Shire SJ, Bjork N, Totpal K, Chen AB. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J Chromatogr B Biomed Sci Appl. 2001;752:233–245.
- Chelius D, Rehder DS, Bondarenko PV. Identification and characterization of deamidation sites in the conserved regions of human immunoglobulin gamma antibodies. Anal Chem. 2005;77:6004–6011. doi:10.1021/ac050672d.
- Sinha S, Zhang L, Duan S, Williams TD, Vlasak J, Ionescu R, Topp EM. Effect of protein structure on deamidation rate in the Fc fragment of an IgG1 monoclonal antibody. Protein Sci. 2009;18:1573–1584. doi:10.1002/pro.173.
- Liu YD, van Enk JZ, Flynn GC. Human antibody Fc deamidation in vivo. Biologicals. 2009;37:313–322. doi:10.1016/j.biologicals.2009.06.001.
- Cacia J, Keck R, Presta LG, Frenz J. Isomerization of an aspartic acid residue in the complementarity-determining regions of a recombinant antibody to human IgE: identification and effect on binding affinity. Biochemistry. 1996;35:1897–1903. doi:10.1021/bi951526c.
- Rehder DS, Chelius D, McAuley A, Dillon TM, Xiao G, Crouse-Zeineddini J, Vardanyan L, Perico N, Mukku V, Brems DN, et al. Isomerization of a single aspartyl residue of anti-epidermal growth factor receptor immunoglobulin gamma2 antibody highlights the role avidity plays in antibody activity. Biochemistry. 2008;47:2518–2530. doi:10.1021/bi7018223.
- Sreedhara A, Cordoba A, Zhu Q, Kwong J, Liu J. Characterization of the isomerization products of aspartate residues at two different sites in a monoclonal antibody. Pharm Res. 2012;29:187–197. doi:10.1007/s11095-011-0534-2.
- Wakankar AA, Borchardt RT, Eigenbrot C, Shia S, Wang YJ, Shire SJ, Liu JL. Aspartate isomerization in the complementarity-determining regions of two closely related monoclonal antibodies. Biochemistry. 2007;46:1534–1544. doi:10.1021/bi061500t.
- Wakankar AA, Liu J, Vandervelde D, Wang YJ, Shire SJ, Borchardt RT. The effect of cosolutes on the isomerization of aspartic acid residues and conformational stability in a monoclonal antibody. J Pharm Sci. 2007;96:1708–1718. doi:10.1002/jps.20823.
- Yan Y, Wei H, Fu Y, Jusuf S, Zeng M, Ludwig R, Krystek SR, Chen G, Tao L, Das TK. Isomerization and oxidation in the complementarity-determining regions of a monoclonal antibody: A study of the modification-structure-function correlations by hydrogen-deuterium exchange mass spectrometry. Anal Chem. 2016;88:2041–2050. doi:10.1021/acs.analchem.5b02800.
- Chu GC, Chelius D, Xiao G, Khor HK, Coulibaly S, Bondarenko PV. Accumulation of succinimide in a recombinant monoclonal antibody in mildly acidic buffers under elevated temperatures. Pharm Res. 2007;24:1145–1156. doi:10.1007/s11095-007-9241-4.
- Valliere-Douglass J, Jones L, Shpektor D, Kodama P, Wallace A, Balland A, Bailey R, Zhang Y. Separation and characterization of an IgG2 antibody containing a cyclic imide in CDR1 of light chain by hydrophobic interaction chromatography and mass spectrometry. Anal Chem. 2008;80:3168–3174. doi:10.1021/ac702245c.
- Yan B, Steen S, Hambly D, Valliere-Douglass J, Vanden Bos T, Smallwood S, Yates Z, Arroll T, Han Y, Gadgil H, et al. Succinimide formation at Asn 55 in the complementarity determining region of a recombinant monoclonal antibody IgG1 heavy chain. J Pharm Sci. 2009;98:3509–3521. doi:10.1002/jps.21655.
- Mo J, Yan Q, So CK, Soden T, Lewis MJ, Hu P. Understanding the impact of methionine oxidation on the biological functions of IgG1 antibodies using hydrogen/deuterium exchange mass spectrometry. Anal Chem. 2016;88:9495–9502. doi:10.1021/acs.analchem.6b01958.
- Liu D, Ren D, Huang H, Dankberg J, Rosenfeld R, Cocco MJ, Li L, Brems DN, Remmele RL. Structure and stability changes of human IgG1 Fc as a consequence of methionine oxidation. Biochemistry. 2008;47:5088–5100. doi:10.1021/bi702238b.
- Liu H, Gaza-Bulseco G, Xiang T, Chumsae C. Structural effect of deglycosylation and methionine oxidation on a recombinant monoclonal antibody. Mol Immunol. 2008;45:701–708. doi:10.1016/j.molimm.2007.07.012.
- Zhang A, Hu P, MacGregor P, Xue Y, Fan H, Suchecki P, Olszewski L, Liu A. Understanding the conformational impact of chemical modifications on monoclonal antibodies with diverse sequence variation using hydrogen/deuterium exchange mass spectrometry and structural modeling. Anal Chem. 2014;86:3468–3475. doi:10.1021/ac404130a.
- Bertolotti-Ciarlet A, Wang W, Lownes R, Pristatsky P, Fang Y, McKelvey T, Li Y, Li Y, Drummond J, Prueksaritanont T, et al. Impact of methionine oxidation on the binding of human IgG1 to Fc Rn and Fc gamma receptors. Mol Immunol. 2009;46:1878–1882. doi:10.1016/j.molimm.2009.02.002.
- Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci. 2009;18:424–433. doi:10.1002/pro.45.
- Wang W, Vlasak J, Li Y, Pristatsky P, Fang Y, Pittman T, Roman J, Wang Y, Prueksaritanont T, Ionescu R. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol Immunol. 2011;48:860–866. doi:10.1016/j.molimm.2010.12.009.
- Dashivets T, Stracke J, Dengl S, Knaupp A, Pollmann J, Buchner J, Schlothauer T. Oxidation in the complementarity-determining regions differentially influences the properties of therapeutic antibodies. MAbs. 2016;8:1525–1535. doi:10.1080/19420862.2016.1231277.
- Qi P, Volkin DB, Zhao H, Nedved ML, Hughes R, Bass R, Yi SC, Panek ME, Wang D, Dalmonte P, et al. Characterization of the photodegradation of a human IgG1 monoclonal antibody formulated as a high-concentration liquid dosage form. J Pharm Sci. 2009;98:3117–3130. doi:10.1002/jps.21617.
- Wei Z, Feng J, Lin H-Y, Mullapudi S, Bishop E, Tous GI, Casas-Finet J, Hakki F, Strouse R, Schenerman MA. Identification of a single tryptophan residue as critical for binding activity in a humanized monoclonal antibody against respiratory syncytial virus. Anal Chem. 2007;79:2797–2805. doi:10.1021/ac062311j.
- Li Y, Polozova A, Gruia F, Feng J. Characterization of the degradation products of a color-changed monoclonal antibody: tryptophan-derived chromophores. Anal Chem. 2014;86:6850–6857. doi:10.1021/ac404218t.
- Banks DD, Gadgil HS, Pipes GD, Bondarenko PV, Hobbs V, Scavezze JL, Kim J, Jiang X-R, Mukku V, Dillon TM. Removal of cysteinylation from an unpaired sulfhydryl in the variable region of a recombinant monoclonal IgG1 antibody improves homogeneity, stability, and biological activity. J Pharm Sci. 2008;97:775–790. doi:10.1002/jps.21014.
- Gadgil HS, Bondarenko PV, Pipes GD, Dillon TM, Banks D, Abel J, Kleemann GR, Treuheit MJ. Identification of cysteinylation of a free cysteine in the Fab region of a recombinant monoclonal IgG1 antibody using Lys-C limited proteolysis coupled with LC/MS analysis. Anal Biochem. 2006;355:165–174. doi:10.1016/j.ab.2006.05.037.
- Almagro JC, Raghunathan G, Beil E, Janecki DJ, Chen Q, Dinh T, LaCombe A, Connor J, Ware M, Kim PH, et al. Characterization of a high-affinity human antibody with a disulfide bridge in the third complementarity-determining region of the heavy chain. J Mol Recognit. 2012;25:125–135. doi:10.1002/jmr.1168.
- Leung SO, Dion AS, Pellegrini MC, Losman MJ, Grebenau RC, Goldenberg DM, Hansen HJ. Effect of VK framework-1 glycosylation on the binding affinity of lymphoma-specific murine and chimeric LL2 antibodies and its potential use as a novel conjugation site. Int J Cancer. 1995;60:534–538.
- Co MS, Scheinberg DA, Avdalovic NM, McGraw K, Vasquez M, Caron PC, Queen C. Genetically engineered deglycosylation of the variable domain increases the affinity of an anti-CD33 monoclonal antibody. Mol Immunol. 1993;30:1361–1367.
- Wright A, Tao MH, Kabat EA, Morrison SL. Antibody variable region glycosylation: position effects on antigen binding and carbohydrate structure. Embo J. 1991;10:2717–2723.
- Coloma MJ, Trinh RK, Martinez AR, Morrison SL. Position effects of variable region carbohydrate on the affinity and in vivo behavior of an anti-(1–>6) dextran antibody. J Immunol. 1999;162:2162–2170.
- Leibiger H, Wustner D, Stigler RD, Marx U. Variable domain-linked oligosaccharides of a human monoclonal IgG: structure and influence on antigen binding. Biochem J. 1999;338(Pt 2):529–538.
- Wallick SC, Kabat EA, Morrison SL. Glycosylation of a VH residue of a monoclonal antibody against alpha (1—-6) dextran increases its affinity for antigen. J Exp Med. 1988;168:1099–1109.
- Huang L, Biolsi S, Bales KR, Kuchibhotla U. Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization. Anal Biochem. 2006;349:197–207. doi:10.1016/j.ab.2005.11.012.
- Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, James H, van Berkel PHC, van de Winkel JGJ, Platts-Mills TAE, et al. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29:574–576. doi:10.1038/nbt.1912.
- Raju TS, Briggs JB, Borge SM, Jones AJ. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology. 2000;10:477–486.
- Seeliger D, Schulz P, Litzenburger T, Spitz J, Hoerer S, Blech M, Enenkel B, Studts JM, Garidel P, Karow AR. Boosting antibody developability through rational sequence optimization. MAbs. 2015;7:505–515. doi:10.1080/19420862.2015.1017695.
- Dick LW Jr., Kim C, Qiu D, Cheng KC. Determination of the origin of the N-terminal pyro-glutamate variation in monoclonal antibodies using model peptides. Biotechnol Bioeng. 2007;97:544–553. doi:10.1002/bit.21260.
- Lyubarskaya Y, Houde D, Woodard J, Murphy D, Mhatre R. Analysis of recombinant monoclonal antibody isoforms by electrospray ionization mass spectrometry as a strategy for streamlining characterization of recombinant monoclonal antibody charge heterogeneity. Anal Biochem. 2006;348:24–39. doi:10.1016/j.ab.2005.10.003.
- Liu YD, Goetze AM, Bass RB, Flynn GC. N-terminal glutamate to pyroglutamate conversion in vivo for human IgG2 antibodies. J Biol Chem. 2011;286:11211–11217. doi:10.1074/jbc.M110.185041.
- Antes B, Amon S, Rizzi A, Wiederkum S, Kainer M, Szolar O, Fido M, Kircheis R, Nechansky A. Analysis of lysine clipping of a humanized Lewis-Y specific IgG antibody and its relation to Fc-mediated effector function. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:250–256. doi:10.1016/j.jchromb.2007.01.024.
- Jiang G, Yu C, Yadav DB, Hu Z, Amurao A, Duenas E, Wong M, Iverson M, Zheng K, Lam X, et al. Evaluation of Heavy-Chain C-Terminal Deletion on Product Quality and Pharmacokinetics of Monoclonal Antibodies. J Pharm Sci. 2016;105:2066–2072. doi:10.1016/j.xphs.2016.04.027.
- van Den Bremer ET, Beurskens FJ, Voorhorst M, Engelberts PJ, de Jong RN, van der Boom BG, Cook EM, Lindorfer MA, Taylor RP, van Berkel PH, et al. Human IgG is produced in a pro-form that requires clipping of C-terminal lysines for maximal complement activation. MAbs. 2015;7:672–680. doi:10.1080/19420862.2015.1046665.
- Cai B, Pan H, Flynn GC. C-terminal lysine processing of human immunoglobulin G2 heavy chain in vivo. Biotechnol Bioeng. 2011;108:404–412. doi:10.1002/bit.22933.
- Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2:256–265.
- West RL, Zelinkova Z, Wolbink GJ, Kuipers EJ, Stokkers PC, van der Woude CJ. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2008;28:1122–1126. doi:10.1111/j.1365-2036.2008.03828.x.
- Ducancel F, Muller BH. Molecular engineering of antibodies for therapeutic and diagnostic purposes. MAbs. 2012;4:445–457. doi:10.4161/mabs.20776.
- Seeliger D. Development of scoring functions for antibody sequence assessment and optimization. PLoS One. 2013;8:e76909. doi:10.1371/journal.pone.0076909.
- Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:E501–7. doi:10.1208/aapsj080359.
- Agrawal NJ, Kumar S, Wang X, Helk B, Singh SK, Trout BL. Aggregation in protein-based biotherapeutics: computational studies and tools to identify aggregation-prone regions. J Pharm Sci. 2011;100:5081–5095. doi:10.1002/jps.22705.
- Buck PM, Kumar S, Wang X, Agrawal NJ, Trout BL, Singh SK. Computational methods to predict therapeutic protein aggregation. Methods Mol Biol. 2012;899:425–451. doi:10.1007/978-1-61779-921-1_26.
- Courtois F, Schneider CP, Agrawal NJ, Trout BL. Rational design of biobetters with enhanced stability. J Pharm Sci. 2015;104:2433–2440. doi:10.1002/jps.24520.
- Voynov V, Chennamsetty N, Kayser V, Helk B, Trout BL. Predictive tools for stabilization of therapeutic proteins. MAbs. 2009;1:580–582.
- Wang X, Singh SK, Kumar S. Potential aggregation-prone regions in complementarity-determining regions of antibodies and their contribution towards antigen recognition: a computational analysis. Pharm Res. 2010;27:1512–1529. doi:10.1007/s11095-010-0143-5.
- Dudgeon K, Rouet R, Kokmeijer I, Schofield P, Stolp J, Langley D, Stock D, Christ D. General strategy for the generation of human antibody variable domains with increased aggregation resistance. Proc Natl Acad Sci U S A. 2012;109:10879–10884. doi:10.1073/pnas.1202866109.
- Courtois F, Agrawal NJ, Lauer TM, Trout BL. Rational design of therapeutic mAbs against aggregation through protein engineering and incorporation of glycosylation motifs applied to bevacizumab. MAbs. 2016;8:99–112. doi:10.1080/19420862.2015.1112477.
- Abiri N, Pang J, Ou J, Shi B, Wang X, Zhang S, Sun Y, Yang D, Wang Z. Assessment of the immunogenicity of residual host cell protein impurities of OsrHSA. PLoS One. 2018;13:e0193339. doi:10.1371/journal.pone.0193339.
- Jawa V, Joubert MK, Zhang Q, Deshpande M, Hapuarachchi S, Hall MP, Flynn GC. Evaluating immunogenicity risk due to host cell protein impurities in antibody-based biotherapeutics. AAPS J. 2016;18:1439–1452. doi:10.1208/s12248-016-9948-4.
- Wu SJ, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, Baker A, Huang M, Tang QM, Raju TS, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel. 2010;23:643–651. doi:10.1093/protein/gzq037.
- Nichols P, Li L, Kumar S, Buck PM, Singh SK, Goswami S, Balthazor B, Conley TR, Sek D, Allen MJ. Rational design of viscosity reducing mutants of a monoclonal antibody: hydrophobic versus electrostatic inter-molecular interactions. MAbs. 2015;7:212–230. doi:10.4161/19420862.2014.985504.
- Pindrus M, Shire SJ, Kelley RF, Demeule B, Wong R, Xu Y, Yadav S. Solubility challenges in high concentration monoclonal antibody formulations: relationship with amino acid sequence and intermolecular interactions. Mol Pharm. 2015;12:3896–3907. doi:10.1021/acs.molpharmaceut.5b00336.
- Yadav S, Sreedhara A, Kanai S, Liu J, Lien S, Lowman H, Kalonia DS, Shire SJ. Establishing a link between amino acid sequences and self-associating and viscoelastic behavior of two closely related monoclonal antibodies. Pharm Res. 2011;28:1750–1764. doi:10.1007/s11095-011-0410-0.
- Yadav S, Laue TM, Kalonia DS, Singh SN, Shire SJ. The influence of charge distribution on self-association and viscosity behavior of monoclonal antibody solutions. Mol Pharm. 2012;9:791–802. doi:10.1021/mp200566k.
- Werner RG, Kopp K, Schlueter M. Glycosylation of therapeutic proteins in different production systems. Acta Paediatr. 2007;96:17–22. doi:10.1111/j.1651-2227.2007.00199.x.
- Tiller KE, Tessier PM. Advances in antibody design. Annu Rev Biomed Eng. 2015;17:191–216. doi:10.1146/annurev-bioeng-071114-040733.
- Kuhn AB, Kube S, Karow-Zwick AR, Seeliger D, Garidel P, Blech M, Schäfer LV. Improved solution-state properties of monoclonal antibodies by targeted mutations. J Phys Chem B. 2017;121:10818–10827. doi:10.1021/acs.jpcb.7b09126.
- Casaz P, Boucher E, Wollacott R, Pierce BG, Rivera R, Sedic M, Ozturk S, Thomas WD, Wang Y. Resolving self-association of a therapeutic antibody by formulation optimization and molecular approaches. MAbs. 2014;6:1533–1539. doi:10.4161/19420862.2014.975658.
- Liu L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell. 2018;9:15–32. doi:10.1007/s13238-017-0408-4.
- C S, Günther R, Rhiel L, Becker S, Toleikis L, Doerner A, Becker J, Schönemann A, Nasu D, Neuteboom B, et al. A generic approach to engineer antibody pH-switches using combinatorial histidine scanning libraries and yeast display. MAbs. 2015;7:13.
- Datta-Mannan A, Thangaraju A, Leung D, Tang Y, Witcher DR, Lu J, Wroblewski VJ. Balancing charge in the complementarity-determining regions of humanized mAbs without affecting pI reduces non-specific binding and improves the pharmacokinetics. MAbs. 2015;7:483–493. doi:10.1080/19420862.2015.1016696.
- Ayoub D, Jabs W, Resemann A, Evers W, Evans C, Main L, Baessmann C, Wagner-Rousset E, Suckau D, Beck A. Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. MAbs. 2013;5:699–710. doi:10.4161/mabs.25423.
- Guo D, Gao A, Michels DA, Feeney L, Eng M, Chan B, Laird MW, Zhang B, Yu XC, Joly J, et al. Mechanisms of unintended amino acid sequence changes in recombinant monoclonal antibodies expressed in Chinese Hamster Ovary (CHO) cells. Biotechnol Bioeng. 2010;107:163–171. doi:10.1002/bit.22780.
- Harris RJ, Murnane AA, Utter SL, Wagner KL, Cox ET, Polastri GD, Helder JC, Sliwkowski MB. Assessing genetic heterogeneity in production cell lines: detection by peptide mapping of a low level Tyr to Gln sequence variant in a recombinant antibody. Biotechnology (N Y). 1993;11:1293–1297.
- Khetan A, Huang Y-M, Dolnikova J, Pederson NE, Wen D, Yusuf-Makagiansar H, Chen P, Ryll T. Control of misincorporation of serine for asparagine during antibody production using CHO cells. Biotechnol Bioeng. 2010;107:116–123. doi:10.1002/bit.22771.
- Wen D, Vecchi MM, Gu S, Su L, Dolnikova J, Huang Y-M, Foley SF, Garber E, Pederson N, Meier W. Discovery and investigation of misincorporation of serine at asparagine positions in recombinant proteins expressed in Chinese hamster ovary cells. J Biol Chem. 2009;284:32686–32694. doi:10.1074/jbc.M109.059360.
- Yu XC, Borisov OV, Alvarez M, Michels DA, Wang YJ, Ling V. Identification of codon-specific serine to asparagine mistranslation in recombinant monoclonal antibodies by high-resolution mass spectrometry. Anal Chem. 2009;81:9282–9290. doi:10.1021/ac901541h.
- Que HZ, Yang Y, Zhang J, Derfus G, Amanullah A. Sequence variant analysis using peptide mapping by LC–MS/MS. Bioprocess Int. 2010;8:52–60.
- Yang Y, Strahan A, Li C, Shen A, Liu H, Ouyang J, Katta V, Francissen K, Zhang B. Detecting low level sequence variants in recombinant monoclonal antibodies. MAbs. 2010;2:285–298.
- Zeck A, Regula JT, Larraillet V, Mautz B, Popp O, Göpfert U, Wiegeshoff F, Vollertsen UEE, Gorr IH, Koll H, et al. Low level sequence variant analysis of recombinant proteins: an optimized approach. PLoS One. 2012;7:e40328. doi:10.1371/journal.pone.0040328.
- Neill A, Nowak C, Patel R, Ponniah G, Gonzalez N, Miano D, Liu H. Characterization of recombinant monoclonal antibody charge variants using OFFGEL fractionation, weak anion exchange chromatography, and mass spectrometry. Anal Chem. 2015;87:6204–6211. doi:10.1021/acs.analchem.5b01452.
- Sinha S, Pipes G, Topp EM, Bondarenko PV, Treuheit MJ, Gadgil HS. Comparison of LC and LC/MS methods for quantifying N-glycosylation in recombinant IgGs. J Am Soc Mass Spectrom. 2008;19:1643–1654. doi:10.1016/j.jasms.2008.07.004.
- Kleemann GR, Beierle J, Nichols AC, Dillon TM, Pipes GD, Bondarenko PV. Characterization of IgG1 immunoglobulins and peptide-Fc fusion proteins by limited proteolysis in conjunction with LC-MS. Anal Chem. 2008;80:2001–2009. doi:10.1021/ac701629v.
- An Y, Zhang Y, Mueller HM, Shameem M, Chen X. A new tool for monoclonal antibody analysis: application of IdeS proteolysis in IgG domain-specific characterization. MAbs. 2014;6:879–893. doi:10.4161/mabs.28762.
- Fornelli L, Ayoub D, Aizikov K, Beck A, Tsybin YO. Middle-down analysis of monoclonal antibodies with electron transfer dissociation orbitrap fourier transform mass spectrometry. Anal Chem. 2014;86:3005–3012. doi:10.1021/ac4036857.
- Amano M, Hasegawa J, Kobayashi N, Kishi N, Nakazawa T, Uchiyama S, Fukui K. Specific racemization of heavy-chain cysteine-220 in the hinge region of immunoglobulin gamma 1 as a possible cause of degradation during storage. Anal Chem. 2011;83:3857–3864. doi:10.1021/ac200321v.
- Zhang Q, Flynn GC. Cysteine racemization on IgG heavy and light chains. J Biol Chem. 2013;288:34325–34335. doi:10.1074/jbc.M113.506915.
- Huang L, Lu X, Gough PC, De Felippis MR. Identification of racemization sites using deuterium labeling and tandem mass spectrometry. Anal Chem. 2010;82:6363–6369. doi:10.1021/ac101348w.
- Kaschak T, Boyd D, Lu F, Derfus G, Kluck B, Nogal B, Emery C, Summers C, Zheng K, Bayer R, et al. Characterization of the basic charge variants of a human IgG1: effect of copper concentration in cell culture media. MAbs. 2011;3:577–583. doi:10.4161/mabs.3.6.17959.
- Kotia RB, Raghani AR. Analysis of monoclonal antibody product heterogeneity resulting from alternate cleavage sites of signal peptide. Anal Biochem. 2010;399:190–195. doi:10.1016/j.ab.2010.01.008.
- Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, Yao Z, Sreedhara A, Cano T, Tesar D, et al. Charge variants in IgG1: isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. 2010;2:613–624. doi:10.4161/mabs.2.6.13333.
- Meert CD, Brady LJ, Guo A, Balland A. Characterization of antibody charge heterogeneity resolved by preparative immobilized pH gradients. Anal Chem. 2010;82:3510–3518. doi:10.1021/ac902408r.
- Buchanan A, Clementel V, Woods R, Harn N, Bowen MA, Mo W, Popovic B, Bishop SM, Dall’Acqua W, Minter R, et al. Engineering a therapeutic IgG molecule to address cysteinylation, aggregation and enhance thermal stability and expression. MAbs. 2013;5:255–262. doi:10.4161/mabs.23392.
- Chumsae C, Zhou LL, Shen Y, Wohlgemuth J, Fung E, Burton R, Radziejewski C, Zhou ZS. Discovery of a chemical modification by citric acid in a recombinant monoclonal antibody. Anal Chem. 2014;86:8932–8936. doi:10.1021/ac502179m.
- Quan C, Alcala E, Petkovska I, Matthews D, Canova-Davis E, Taticek R, Ma S. A study in glycation of a therapeutic recombinant humanized monoclonal antibody: where it is, how it got there, and how it affects charge-based behavior. Anal Biochem. 2008;373:179–191. doi:10.1016/j.ab.2007.09.027.
- Zhang B, Yang Y, Yuk I, Pai R, McKay P, Eigenbrot C, Dennis M, Katta V, Francissen KC. Unveiling a glycation hot spot in a recombinant humanized monoclonal antibody. Anal Chem. 2008;80:2379–2390. doi:10.1021/ac701810q.
- Banks DD, Hambly DM, Scavezze JL, Siska CC, Stackhouse NL, Gadgil HS. The effect of sucrose hydrolysis on the stability of protein therapeutics during accelerated formulation studies. J Pharm Sci. 2009;98:4501–4510. doi:10.1002/jps.21749.
- Fischer S, Hoernschemeyer J, Mahler HC. Glycation during storage and administration of monoclonal antibody formulations. Eur J Pharm Biopharm. 2008;70:42–50. doi:10.1016/j.ejpb.2008.04.021.
- Gadgil HS, Bondarenko PV, Pipes G, Rehder D, McAuley A, Perico N, Dillon T, Ricci M, Treuheit M. The LC/MS analysis of glycation of IgG molecules in sucrose containing formulations. J Pharm Sci. 2007;96:2607–2621. doi:10.1002/jps.20966.
- Goetze AM, Liu YD, Arroll T, Chu L, Flynn GC. Rates and impact of human antibody glycation in vivo. Glycobiology. 2012;22:221–234. doi:10.1093/glycob/cwr141.
- Miller AK, Hambly DM, Kerwin BA, Treuheit MJ, Gadgil HS. Characterization of site-specific glycation during process development of a human therapeutic monoclonal antibody. J Pharm Sci. 2011;100:2543–2550. doi:10.1002/jps.22504.
- Mo J, Jin R, Yan Q, Sokolowska I, Lewis MJ, Hu P. Quantitative analysis of glycation and its impact on antigen binding. MAbs. 2018;10:406–415. doi:10.1080/19420862.2018.1438796.
- Butko M, Pallat H, Cordoba A, Yu XC. Recombinant antibody color resulting from advanced glycation end product modifications. Anal Chem. 2014;86:9816–9823. doi:10.1021/ac5024099.
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–478. doi:10.1016/j.coi.2008.06.007.
- Chumsae C, Hossler P, Raharimampionona H, Zhou Y, McDermott S, Racicot C, Radziejewski C, Zhou ZS. When good intentions go awry: modification of a recombinant monoclonal antibody in chemically defined cell culture by xylosone, an oxidative product of ascorbic acid. Anal Chem. 2015;87:7529–7534. doi:10.1021/acs.analchem.5b00801.
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SHA, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi:10.1074/jbc.M202069200.
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi:10.1074/jbc.M210665200.
- Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, Crocker L, Pabonan O, Baginski T, Meng G, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–4489. doi:10.1158/0008-5472.CAN-09-3704.
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622. doi:10.1002/bit.20151.
- Valliere-Douglass JF, Brady LJ, Farnsworth C, Pace D, Balland A, Wallace A, Wang W, Treuheit MJ, Yan B. O-fucosylation of an antibody light chain: characterization of a modification occurring on an IgG1 molecule. Glycobiology. 2009;19:144–152. doi:10.1093/glycob/cwn116.
- Santora LC, Stanley K, Krull IS, Grant K. Characterization of maleuric acid derivatives on transgenic human monoclonal antibody due to post-secretional modifications in goat milk. Biomed Chromatogr. 2006;20:843–856. doi:10.1002/bmc.603.
- Dick LW Jr., Qiu D, Mahon D, Adamo M, Cheng KC. C-terminal lysine variants in fully human monoclonal antibodies: investigation of test methods and possible causes. Biotechnol Bioeng. 2008;100:1132–1143. doi:10.1002/bit.21855.
- Harris RJ. Processing of C-terminal lysine and arginine residues of proteins isolated from mammalian cell culture. J Chromatogr A. 1995;705:129–134.
- Kita A, Ponniah G, Nowak C, Liu H. Characterization of cysteinylation and trisulfide bonds in a recombinant monoclonal antibody. Anal Chem. 2016;88:5430–5437. doi:10.1021/acs.analchem.6b00822.
- McSherry T, McSherry J, Ozaeta P, Longenecker K, Ramsay C, Fishpaugh J, Allen S. Cysteinylation of a monoclonal antibody leads to its inactivation. MAbs. 2016;8:718–725. doi:10.1080/19420862.2016.1160179.
- Zhao J, Saunders J, Schussler SD, Rios S, Insaidoo FK, Fridman AL, Li H, Liu Y-H. Characterization of a novel modification of a CHO-produced mAb: evidence for the presence of tyrosine sulfation. MAbs. 2017;9:985–995. doi:10.1080/19420862.2017.1332552.
- Chumsae C, Gifford K, Lian W, Liu H, Radziejewski CH, Zhou ZS. Arginine modifications by methylglyoxal: discovery in a recombinant monoclonal antibody and contribution to acidic species. Anal Chem. 2013;85:11401–11409. doi:10.1021/ac402384y.
- Valliere-Douglass JF, Connell-Crowley L, Jensen R, Schnier PD, Trilisky E, Leith M, Follstad BD, Kerr J, Lewis N, Vunnum S, et al. Photochemical degradation of citrate buffers leads to covalent acetonation of recombinant protein therapeutics. Protein Sci. 2010;19:2152–2163. doi:10.1002/pro.495.
- Gu S, Wen D, Weinreb PH, Sun Y, Zhang L, Foley SF, Kshirsagar R, Evans D, Mi S, Meier W, et al. Characterization of trisulfide modification in antibodies. Anal Biochem. 2010;400:89–98. doi:10.1016/j.ab.2010.01.019.
- Pristatsky P, Cohen SL, Krantz D, Acevedo J, Ionescu R, Vlasak J. Evidence for trisulfide bonds in a recombinant variant of a human IgG2 monoclonal antibody. Anal Chem. 2009;81:6148–6155. doi:10.1021/ac9006254.
- Lam XM, Yang JY, Cleland JL. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J Pharm Sci. 1997;86:1250–1255. doi:10.1021/js970143s.
- Xie Q, Moore B, Beardsley RL. Discovery and characterization of hydroxylysine in recombinant monoclonal antibodies. MAbs. 2016;8:371–378. doi:10.1080/19420862.2015.1122148.
- Yang J, Wang S, Liu J, Raghani A. Determination of tryptophan oxidation of monoclonal antibody by reversed phase high performance liquid chromatography. J Chromatogr A. 2007;1156:174–182. doi:10.1016/j.chroma.2007.01.140.
- Yang Y, Stella C, Wang W, Schoneich C, Gennaro L. Characterization of oxidative carbonylation on recombinant monoclonal antibodies. Anal Chem. 2014;86:4799–4806. doi:10.1021/ac4039866.
- Liu M, Zhang Z, Cheetham J, Ren D, Zhou ZS. Discovery and characterization of a photo-oxidative histidine-histidine cross-link in IgG1 antibody utilizing (1)(8)O-labeling and mass spectrometry. Anal Chem. 2014;86:4940–4948. doi:10.1021/ac500334k.
- Harris RJ. Heterogeneity of recombinant antibodies: linking structure to function. Dev Biol (Basel). 2005;122:117–127.
- Ouellette D, Alessandri L, Chin A, Grinnell C, Tarcsa E, Radziejewski C, Correia I. Studies in serum support rapid formation of disulfide bond between unpaired cysteine residues in the VH domain of an immunoglobulin G1 molecule. Anal Biochem. 2010;397:37–47. doi:10.1016/j.ab.2009.09.027.
- Zhang T, Zhang J, Hewitt D, Tran B, Gao X, Qiu ZJ, Tejada M, Gazzano-Santoro H, Kao Y-H. Identification and characterization of buried unpaired cysteines in a recombinant monoclonal IgG1 antibody. Anal Chem. 2012;84:7112–7123. doi:10.1021/ac301426h.
- Liu H, Gaza-Bulseco G, Sun J. Characterization of the stability of a fully human monoclonal IgG after prolonged incubation at elevated temperature. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;837:35–43. doi:10.1016/j.jchromb.2006.03.053.
- Wang L, Amphlett G, Lambert JM, Blättler W, Zhang W. Structural characterization of a recombinant monoclonal antibody by electrospray time-of-flight mass spectrometry. Pharm Res. 2005;22:1338–1349. doi:10.1007/s11095-005-5267-7.
- Moorhouse KG, Nashabeh W, Deveney J, Bjork NS, Mulkerrin MG, Ryskamp T. Validation of an HPLC method for the analysis of the charge heterogeneity of the recombinant monoclonal antibody IDEC-C2B8 after papain digestion. J Pharm Biomed Anal. 1997;16:593–603.
- Ouellette D, Chumsae C, Clabbers A, Radziejewski C, Correia I. Comparison of the in vitro and in vivo stability of a succinimide intermediate observed on a therapeutic IgG1 molecule. MAbs. 2013;5:432–444. doi:10.4161/mabs.24458.
- Yu L, Vizel A, Huff MB, Young M, Remmele RL Jr., He B. Investigation of N-terminal glutamate cyclization of recombinant monoclonal antibody in formulation development. J Pharm Biomed Anal. 2006;42:455–463. doi:10.1016/j.jpba.2006.05.008.
- Chelius D, Jing K, Lueras A, Rehder DS, Dillon TM, Vizel A, Rajan RS, Li T, Treuheit MJ, Bondarenko PV. Formation of pyroglutamic acid from N-terminal glutamic acid in immunoglobulin gamma antibodies. Anal Chem. 2006;78:2370–2376. doi:10.1021/ac051827k.
- Huang HZ, Nichols A, Liu D. Direct identification and quantification of aspartyl succinimide in an IgG2 mAb by RapiGest assisted digestion. Anal Chem. 2009;81:1686–1692. doi:10.1021/ac802708s.
- Yu XC, Joe K, Zhang Y, Adriano A, Wang Y, Gazzano-Santoro H, Keck RG, Deperalta G, Ling V. Accurate determination of succinimide degradation products using high fidelity trypsin digestion peptide map analysis. Anal Chem. 2011;83:5912–5919. doi:10.1021/ac200750u.
- Valliere-Douglass J, Wallace A, Balland A. Separation of populations of antibody variants by fine tuning of hydrophobic-interaction chromatography operating conditions. J Chromatogr A. 2008;1214:81–89. doi:10.1016/j.chroma.2008.10.078.
- Zhang Q, Schenauer MR, McCarter JD, Flynn GC. IgG1 thioether bond formation in vivo. J Biol Chem. 2013;288:16371–16382. doi:10.1074/jbc.M113.468397.
- Tous GI, Wei Z, Feng J, Bilbulian S, Bowen S, Smith J, Strouse R, McGeehan P, Casas-Finet J, Schenerman MA. Characterization of a novel modification to monoclonal antibodies: thioether cross-link of heavy and light chains. Anal Chem. 2005;77:2675–2682. doi:10.1021/ac0500582.
- Johnson KA, Paisley-Flango K, Tangarone BS, Porter TJ, Rouse JC. Cation exchange-HPLC and mass spectrometry reveal C-terminal amidation of an IgG1 heavy chain. Anal Biochem. 2007;360:75–83. doi:10.1016/j.ab.2006.10.012.
- Tsubaki M, Terashima I, Kamata K, Koga A. C-terminal modification of monoclonal antibody drugs: amidated species as a general product-related substance. Int J Biol Macromol. 2013;52:139–147. doi:10.1016/j.ijbiomac.2012.09.016.
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457.
- Flynn GC, Chen X, Liu YD, Shah B, Zhang Z. Naturally occurring glycan forms of human immunoglobulins G1 and G2. Mol Immunol. 2010;47:2074–2082. doi:10.1016/j.molimm.2010.04.006.
- Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta. 2006;1760:693–700. doi:10.1016/j.bbagen.2005.10.002.
- Mimura Y, Church S, Ghirlando R, Ashton PR, Dong S, Goodall M, Lund J, Jefferis R. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol Immunol. 2000;37:697–706.
- Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, Jefferis R. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276:45539–45547. doi:10.1074/jbc.M107478200.
- Fang J, Richardson J, Du Z, Zhang Z. Effect of Fc-Glycan structure on the conformational stability of IgG revealed by hydrogen/deuterium exchange and limited proteolysis. Biochemistry. 2016;55:860–868. doi:10.1021/acs.biochem.5b01323.
- Falck D, Jansen BC, Plomp R, Reusch D, Haberger M, Wuhrer M. Glycoforms of immunoglobulin G based biopharmaceuticals are differentially cleaved by trypsin due to the glycoform influence on higher-order structure. J Proteome Res. 2015;14:4019–4028. doi:10.1021/acs.jproteome.5b00573.
- Lu Y, Westland K, Ma YH, Gadgil H. Evaluation of effects of Fc domain high-mannose glycan on antibody stability. J Pharm Sci. 2012;101:4107–4117. doi:10.1002/jps.23284.
- Alessandri L, Ouellette D, Acquah A, Rieser M, Leblond D, Saltarelli M, Radziejewski C, Fujimori T, Correia I. Increased serum clearance of oligomannose species present on a human IgG1 molecule. MAbs. 2012;4:509–520. doi:10.4161/mabs.20450.
- Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, Flynn GC. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology. 2011;21:949–959. doi:10.1093/glycob/cwr027.
- Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17:104–118. doi:10.1093/glycob/cwl057.
- Wright A, Morrison SL. Effect of altered CH2-associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J Exp Med. 1994;180:1087–1096.
- Wright A, Morrison SL. Effect of C2-associated carbohydrate structure on Ig effector function: studies with chimeric mouse-human IgG1 antibodies in glycosylation mutants of Chinese hamster ovary cells. J Immunol. 1998;160:3393–3402.
- Yu M, Brown D, Reed C, Chung S, Lutman J, Stefanich E, Wong A, Stephan J-P, Bayer R. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. MAbs. 2012;4:475–487. doi:10.4161/mabs.20737.
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, Culm-Merdek K, Park A, Pan C, Edmunds T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng. 2008;99:652–665. doi:10.1002/bit.21598.
- Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10:101–111. doi:10.1038/nrd3365.
- Ghirlando R, Lund J, Goodall M, Jefferis R. Glycosylation of human IgG-Fc: influences on structure revealed by differential scanning micro-calorimetry. Immunol Lett. 1999;68:47–52.
- Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010;9:1716–1728. doi:10.1074/mcp.M900540-MCP200.
- Onitsuka M, Kawaguchi A, Asano R, Kumagai I, Honda K, Ohtake H, Omasa T. Glycosylation analysis of an aggregated antibody produced by Chinese hamster ovary cells in bioreactor culture. J Biosci Bioeng. 2014;117:639–644. doi:10.1016/j.jbiosc.2013.11.001.
- Millward TA, Heitzmann M, Bill K, Langle U, Schumacher P, Forrer K. Effect of constant and variable domain glycosylation on pharmacokinetics of therapeutic antibodies in mice. Biologicals. 2008;36:41–47. doi:10.1016/j.biologicals.2007.05.003.
- Chen X, Liu YD, Flynn GC. The effect of Fc glycan forms on human IgG2 antibody clearance in humans. Glycobiology. 2009;19:240–249. doi:10.1093/glycob/cwn120.
- Liu L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J Pharm Sci. 2015;104:1866–1884. doi:10.1002/jps.24444.
- Schlothauer T, Rueger P, Stracke JO, Hertenberger H, Fingas F, Kling L, Emrich T, Drabner G, Seeber S, Auer J, et al. Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. MAbs. 2013;5:576–586. doi:10.4161/mabs.24981.
- Souders CA, Nelson SC, Wang Y, Crowley AR, Klempner MS, Thomas W Jr. A novel in vitro assay to predict neonatal Fc receptor-mediated human IgG half-life. MAbs. 2015;7:912–921. doi:10.1080/19420862.2015.1054585.
- Kayser V, Chennamsetty N, Voynov V, Forrer K, Helk B, Trout BL. Glycosylation influences on the aggregation propensity of therapeutic monoclonal antibodies. Biotechnol J. 2011;6:38–44. doi:10.1002/biot.201000091.
- He F, Hogan S, Latypov RF, Narhi LO, Razinkov VI. High throughput thermostability screening of monoclonal antibody formulations. J Pharm Sci. 2010;99:1707–1720. doi:10.1002/jps.21955.
- Nemergut M, Zoldak G, Schaefer JV, Kast F, Miskovsky P, Pluckthun A, Sedlak E. Analysis of IgG kinetic stability by differential scanning calorimetry, probe fluorescence and light scattering. Protein Sci. 2017;26:2229–2239. doi:10.1002/pro.3278.
- Brader ML, Estey T, Bai S, Alston RW, Lucas KK, Lantz S, Landsman P, Maloney KM. Examination of thermal unfolding and aggregation profiles of a series of developable therapeutic monoclonal antibodies. Mol Pharm. 2015;12:1005–1017. doi:10.1021/mp400666b.
- Majumdar R, Esfandiary R, Bishop SM, Samra HS, Middaugh CR, Volkin DB, Weis DD. Correlations between changes in conformational dynamics and physical stability in a mutant IgG1 mAb engineered for extended serum half-life. MAbs. 2015;7:84–95. doi:10.4161/19420862.2014.985494.
- Shi S, Semple A, Cheung J, Shameem M. DSF method optimization and its application in predicting protein thermal aggregation kinetics. J Pharm Sci. 2013;102:2471–2483. doi:10.1002/jps.23633.
- Jain T, Sun T, Durand S, Hall A, Houston NR, Nett JH, Sharkey B, Bobrowicz B, Caffry I, Yu Y, et al. Biophysical properties of the clinical-stage antibody landscape. Proc Natl Acad Sci U S A. 2017;114:944–949. doi:10.1073/pnas.1616408114.
- Lavinder JJ, Hari SB, Sullivan BJ, Magliery TJ. High-throughput thermal scanning: a general, rapid dye-binding thermal shift screen for protein engineering. J Am Chem Soc. 2009;131:3794–3795. doi:10.1021/ja8049063.
- Huynh K, Partch CL. Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc Protein Sci. 2015;79:28–29. doi:10.1002/0471140864.ps2809s79.
- Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2:2212–2221. doi:10.1038/nprot.2007.321.
- Bye JW, Platts L, Falconer RJ. Biopharmaceutical liquid formulation: a review of the science of protein stability and solubility in aqueous environments. Biotechnol Lett. 2014;36:869–875. doi:10.1007/s10529-013-1445-6.
- Gibson TJ, McCarty K, McFadyen IJ, Cash E, Dalmonte P, Hinds KD, Dinerman AA, Alvarez JC, Volkin DB. Application of a high-throughput screening procedure with PEG-induced precipitation to compare relative protein solubility during formulation development with IgG1 monoclonal antibodies. J Pharm Sci. 2011;100:1009–1021. doi:10.1002/jps.22350.
- Garidel P, Kuhn AB, Schafer LV, Karow-Zwick AR, Blech M. High-concentration protein formulations: how high is high? Eur J Pharm Biopharm. 2017;119:353–360. doi:10.1016/j.ejpb.2017.06.029.
- Trevino SR, Scholtz JM, Pace CN. Measuring and increasing protein solubility. J Pharm Sci. 2008;97:4155–4166. doi:10.1002/jps.21327.
- Sormanni P, Amery L, Ekizoglou S, Vendruscolo M, Popovic B. Rapid and accurate in silico solubility screening of a monoclonal antibody library. Sci Rep. 2017;7:8200. doi:10.1038/s41598-017-07800-w.
- Geng SB, Cheung JK, Narasimhan C, Shameem M, Tessier PM. Improving monoclonal antibody selection and engineering using measurements of colloidal protein interactions. J Pharm Sci. 2014;103:3356–3363. doi:10.1002/jps.24130.
- Garidel P. Protein solubility from a biochemical, physicochemical and colloidal perspective. Am Pharm Rev. 2013; December 30. https://www.americanpharmaceuticalreview.com/Featured-Articles/152568-Protein-Solubility-from-a-Biochemical-Physicochemical-and--Colloidal-Perspective/
- Kalonia C, Toprani V, Toth R, Wahome N, Gabel I, Middaugh CR, Volkin DB. Effects of protein conformation, apparent solubility, and protein-protein interactions on the rates and mechanisms of aggregation for an IgG1Monoclonal antibody. J Phys Chem B. 2016;120:7062–7075. doi:10.1021/acs.jpcb.6b03878.
- Maurer RW, Sandler SI, Lenhoff AM. Salting-in characteristics of globular proteins. Biophys Chem. 2011;156:72–78. doi:10.1016/j.bpc.2011.02.002.
- Kumar V, Sharma VK, Kalonia DS. Effect of polyols on polyethylene glycol (PEG)-induced precipitation of proteins: impact on solubility, stability and conformation. Int J Pharm. 2009;366:38–43. doi:10.1016/j.ijpharm.2008.08.037.
- Li L, Kantor A, Warne N. Application of a PEG precipitation method for solubility screening: a tool for developing high protein concentration formulations. Protein Sci. 2013;22:1118–1123. doi:10.1002/pro.2289.
- Toprani VM, Joshi SB, Kueltzo LA, Schwartz RM, Middaugh CR, Volkin DB. A micro-polyethylene glycol precipitation assay as a relative solubility screening tool for monoclonal antibody design and formulation development. J Pharm Sci. 2016;105:2319–2327. doi:10.1016/j.xphs.2016.05.021.
- Hofmann M, Winzer M, Weber C, Gieseler H. Limitations of polyethylene glycol-induced precipitation as predictive tool for protein solubility during formulation development. J Pharm Pharmacol. 2018;70:648–654. doi:10.1111/jphp.12699.
- Ruppert S, Sandler SI, Lenhoff AM. Correlation between the osmotic second virial coefficient and the solubility of proteins. Biotechnol Prog. 2001;17:182–187. doi:10.1021/bp0001314.
- Valente JJ, Payne RW, Manning MC, Wilson WW, Henry CS. Colloidal behavior of proteins: effects of the second virial coefficient on solubility, crystallization and aggregation of proteins in aqueous solution. Curr Pharm Biotechnol. 2005;6:427–436.
- Tessier PM, Lenhoff AM, Sandler SI. Rapid measurement of protein osmotic second virial coefficients by self-interaction chromatography. Biophys J. 2002;82:1620–1631. doi:10.1016/S0006-3495(02)75513-6.
- Moon. YU, Curtis RA, Anderson CO, Blanch HW, Prausnitz JM. Protein—protein interactions in aqueous ammonium sulfate solutions. Lysozyme and Bovine Serum Albumin (BSA). J Solution Chem. 2000;29:19. doi:10.1023/A:1005112927213.
- Alford JR, Kendrick BS, Carpenter JF, Randolph TW. Measurement of the second osmotic virial coefficient for protein solutions exhibiting monomer-dimer equilibrium. Anal Biochem. 2008;377:128–133. doi:10.1016/j.ab.2008.03.032.
- Johnson DH, Wilson WW, DeLucas LJ. Protein solubilization: a novel approach. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;971:99–106. doi:10.1016/j.jchromb.2014.09.003.
- Velev OD, Kaler EW, Lenhoff AM. Protein interactions in solution characterized by light and neutron scattering: comparison of lysozyme and chymotrypsinogen. Biophys J. 1998;75:2682–2697. doi:10.1016/S0006-3495(98)77713-6.
- Rakel N, Bauer KC, Galm L, Hubbuch J. From osmotic second virial coefficient (B22) to phase behavior of a monoclonal antibody. Biotechnol Prog. 2015;31:438–451. doi:10.1002/btpr.2065.
- Rowe JB, Cancel RA, Evangelous TD, Flynn RP, Pechenov S, Subramony JA, Zhang J, Wang Y. Metastability gap in the phase diagram of monoclonal IgG antibody. Biophys J. 2017;113:1750–1756. doi:10.1016/j.bpj.2017.08.048.
- Jacobs SA, Wu SJ, Feng Y, Bethea D, O’Neil KT. Cross-interaction chromatography: a rapid method to identify highly soluble monoclonal antibody candidates. Pharm Res. 2010;27:65–71. doi:10.1007/s11095-009-0007-z.
- Liu Y, Caffry I, Wu J, Geng SB, Jain T, Sun T, Reid F, Cao Y, Estep P, Yu Y, et al. High-throughput screening for developability during early-stage antibody discovery using self-interaction nanoparticle spectroscopy. MAbs. 2014;6:483–492. doi:10.4161/mabs.27431.
- Baek Y, Zydney AL. Intermolecular interactions in highly concentrated formulations of recombinant therapeutic proteins. Curr Opin Biotechnol. 2017;53:59–64. doi:10.1016/j.copbio.2017.12.016.
- Baek Y, Singh N, Arunkumar A, Borys M, Li ZJ, Zydney AL. Ultrafiltration behavior of monoclonal antibodies and Fc-fusion proteins: effects of physical properties. Biotechnol Bioeng. 2017;114:2057–2065. doi:10.1002/bit.26326.
- Lutz H, Arias J, Zou Y. High concentration biotherapeutic formulation and ultrafiltration: part 1 pressure limits. Biotechnol Prog. 2017;33:113–124. doi:10.1002/btpr.2334.
- Shieu W, Lamar D, Stauch OB, Maa YF. Filling of high-concentration monoclonal antibody formulations: investigating underlying mechanisms that affect precision of low-volume fill by peristaltic pump. PDA J Pharm Sci Technol. 2016;70:143–156. doi:10.5731/pdajpst.2015.005926.
- Shieu W, Torhan SA, Chan E, Hubbard A, Gikanga B, Stauch OB, Maa Y-F. Filling of high-concentration monoclonal antibody formulations into pre-filled syringes: filling parameter investigation and optimization. PDA J Pharm Sci Technol. 2014;68:153–163. doi:10.5731/pdajpst.2014.00973.
- Allmendinger A, Fischer S, Huwyler J, Mahler HC, Schwarb E, Zarraga IE, Mueller R. Rheological characterization and injection forces of concentrated protein formulations: an alternative predictive model for non-Newtonian solutions. Eur J Pharm Biopharm. 2014;87:318–328. doi:10.1016/j.ejpb.2014.01.009.
- Narasimhan C, Mach H, Shameem M. High-dose monoclonal antibodies via the subcutaneous route: challenges and technical solutions, an industry perspective. Ther Deliv. 2012;3:889–900.
- Esfandiary R, Parupudi A, Casas-Finet J, Gadre D, Sathish H. Mechanism of reversible self-association of a monoclonal antibody: role of electrostatic and hydrophobic interactions. J Pharm Sci. 2015;104:577–586. doi:10.1002/jps.24237.
- Kanai S, Liu J, Patapoff TW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab-Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97:4219–4227. doi:10.1002/jps.21322.
- Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94:1928–1940. doi:10.1002/jps.20347.
- Li L, Kumar S, Buck PM, Burns C, Lavoie J, Singh SK, Warne NW, Nichols P, Luksha N, Boardman D. Concentration dependent viscosity of monoclonal antibody solutions: explaining experimental behavior in terms of molecular properties. Pharm Res. 2014;31:3161–3178. doi:10.1007/s11095-014-1409-0.
- Kamerzell TJ, Kanai S, Liu J, Shire SJ, Wang YJ. Increasing IgG concentration modulates the conformational heterogeneity and bonding network that influence solution properties. J Phys Chem B. 2009;113:6109–6118. doi:10.1021/jp9001548.
- Guo Z, Chen A, Nassar RA, Helk B, Mueller C, Tang Y, Gupta K, Klibanov AM. Structure-activity relationship for hydrophobic salts as viscosity-lowering excipients for concentrated solutions of monoclonal antibodies. Pharm Res. 2012;29:3102–3109. doi:10.1007/s11095-012-0802-9.
- He F, Woods CE, Litowski JR, Roschen LA, Gadgil HS, Razinkov VI, Kerwin BA. Effect of sugar molecules on the viscosity of high concentration monoclonal antibody solutions. Pharm Res. 2011;28:1552–1560. doi:10.1007/s11095-011-0388-7.
- Wang S, Zhang N, Hu T, Dai W, Feng X, Zhang X, Qian F. Viscosity-lowering effect of amino acids and salts on highly concentrated solutions of two IgG1 monoclonal antibodies. Mol Pharm. 2015;12:4478–4487. doi:10.1021/acs.molpharmaceut.5b00643.
- Hong T, Iwashita K, Shiraki K. Viscosity control of protein solution by small solutes: a review. Curr Protein Pept Sci. 2018;19:746–758. doi:10.2174/1389203719666171213114919.
- Neergaard MS, Kalonia DS, Parshad H, Nielsen AD, Møller EH, van de Weert M, van de Weert M. Viscosity of high concentration protein formulations of monoclonal antibodies of the IgG1 and IgG4 subclass - prediction of viscosity through protein-protein interaction measurements. Eur J Pharm Sci. 2013;49:400–410. doi:10.1016/j.ejps.2013.04.019.
- Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci. 2010;99:4812–4829. doi:10.1002/jps.22190.
- He F, Becker GW, Litowski JR, Narhi LO, Brems DN, Razinkov VI. High-throughput dynamic light scattering method for measuring viscosity of concentrated protein solutions. Anal Biochem. 2010;399:141–143. doi:10.1016/j.ab.2009.12.003.
- Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JM, Shire SJ, Gokarn YR. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103:69–78.
- Singh SK, Afonina N, Awwad M, Bechtold-Peters K, Blue JT, Chou D, Cromwell M, Krause H-J, Mahler H-C, Meyer BK, et al. An industry perspective on the monitoring of subvisible particles as a quality attribute for protein therapeutics. J Pharm Sci. 2010;99:3302–3321. doi:10.1002/jps.22097.
- Das TK. Protein particulate detection issues in biotherapeutics development–current status. AAPS PharmSciTech. 2012;13:732–746. doi:10.1208/s12249-012-9793-4.
- Philo JS. Is any measurement method optimal for all aggregate sizes and types? AAPS J. 2006;8:E564–71. doi:10.1208/aapsj080365.
- Philo JS. A critical review of methods for size characterization of non-particulate protein aggregates. Curr Pharm Biotechnol. 2009;10:359–372.
- Nowak C, Ponniah G, Neill A, Liu H. Characterization of succinimide stability during trypsin digestion for LC-MS analysis. Anal Biochem. 2017;526:1–8. doi:10.1016/j.ab.2017.03.005.
- Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci. 2010;99:1152–1168. doi:10.1002/jps.21898.
- Yadav S, Shire SJ, Kalonia DS. Viscosity behavior of high-concentration monoclonal antibody solutions: correlation with interaction parameter and electroviscous effects. J Pharm Sci. 2012;101:998–1011. doi:10.1002/jps.22831.
- Kayser V, Chennamsetty N, Voynov V, Helk B, Trout BL. Conformational stability and aggregation of therapeutic monoclonal antibodies studied with ANS and Thioflavin T binding. MAbs. 2011;3:408–411.
- Gaza-Bulseco G, Bulseco A, Chumsae C, Liu H. Characterization of the glycosylation state of a recombinant monoclonal antibody using weak cation exchange chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;862:155–160. doi:10.1016/j.jchromb.2007.12.001.
- Ponniah G, Kita A, Nowak C, Neill A, Kori Y, Rajendran S, Liu H. Characterization of the acidic species of a monoclonal antibody using weak cation exchange chromatography and LC-MS. Anal Chem. 2015;87:9084–9092. doi:10.1021/acs.analchem.5b02385.
- Chumsae C, Gaza-Bulseco G, Sun J, Liu H. Comparison of methionine oxidation in thermal stability and chemically stressed samples of a fully human monoclonal antibody. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:285–294. doi:10.1016/j.jchromb.2006.11.050.
- Teshima G, Li M-X, Danishmand R, Obi C, To R, Huang C, Kung J, Lahidji V, Freeberg J, Thorner L, et al. Separation of oxidized variants of a monoclonal antibody by anion-exchange. J Chromatogr A. 2011;1218:2091–2097. doi:10.1016/j.chroma.2010.10.107.
- Perkins M, Theiler R, Lunte S, Jeschke M. Determination of the origin of charge heterogeneity in a murine monoclonal antibody. Pharm Res. 2000;17:1110–1117.
- Zhang W, Czupryn MJ. Analysis of isoaspartate in a recombinant monoclonal antibody and its charge isoforms. J Pharm Biomed Anal. 2003;30:1479–1490.
- Gandhi S, Ren D, Xiao G, Bondarenko P, Sloey C, Ricci MS, Krishnan S. Elucidation of degradants in acidic peak of cation exchange chromatography in an IgG1 monoclonal antibody formed on long-term storage in a liquid formulation. Pharm Res. 2012;29:209–224. doi:10.1007/s11095-011-0536-0.
- King C, Patel R, Ponniah G, Nowak C, Neill A, Gu Z, Liu H. Characterization of recombinant monoclonal antibody variants detected by hydrophobic interaction chromatography and imaged capillary isoelectric focusing electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1085:96–103. doi:10.1016/j.jchromb.2018.03.049.
- Santora LC, Krull IS, Grant K. Characterization of recombinant human monoclonal tissue necrosis factor-alpha antibody using cation-exchange HPLC and capillary isoelectric focusing. Anal Biochem. 1999;275:98–108. doi:10.1006/abio.1999.4275.
- Zhang T, Bourret J, Cano T. Isolation and characterization of therapeutic antibody charge variants using cation exchange displacement chromatography. J Chromatogr A. 2011;1218:5079–5086. doi:10.1016/j.chroma.2011.05.061.
- Alvarez M, Tremintin G, Wang J, Eng M, Kao Y-H, Jeong J, Ling VT, Borisov OV. On-line characterization of monoclonal antibody variants by liquid chromatography-mass spectrometry operating in a two-dimensional format. Anal Biochem. 2011;419:17–25. doi:10.1016/j.ab.2011.07.033.
- Ponniah G, Nowak C, Neill A, Liu H. Characterization of charge variants of a monoclonal antibody using weak anion exchange chromatography at subunit levels. Anal Biochem. 2017;520:49–57. doi:10.1016/j.ab.2016.12.017.
- Liu H, Ren W, Zong L, Zhang J, Wang Y. Characterization of recombinant monoclonal antibody charge variants using WCX chromatography, icIEF and LC-MS/MS. Anal Biochem. 2019;564-565:1–12. doi:10.1016/j.ab.2018.10.002.
- Tsai PK, Bruner MW, Irwin JI, Ip CC, Oliver CN, Nelson RW, Volkin DB, Middaugh CR. Origin of the isoelectric heterogeneity of monoclonal immunoglobulin h1B4. Pharm Res. 1993;10:1580–1586.
- Ma S, Nashabeh W. Analysis of protein therapeutics by capillary electrophoresis. Chromatographia. 2001;122:s75–s89.
- Ma S. Analysis of protein therapeutics by capillary electrophoresis: applications and challenges. Dev Biol (Basel). 2005;122:49–68.
- Janini G, Saptharishi N, Waselus M, Soman G. Element of a validation method for MU-B3 monoclonal antibody using an imaging capillary isoelectric focusing system. Electrophoresis. 2002;23:1605–1611. doi:10.1002/1522-2683(200206)23:11<1605::AID-ELPS1605>3.0.CO;2-O.
- Li N, Kessler K, Bass L, Zeng D. Evaluation of the iCE280 Analyzer as a potential high-throughput tool for formulation development. J Pharm Biomed Anal. 2007;43:963–972. doi:10.1016/j.jpba.2006.09.024.
- He XZ, Que AH, Mo JJ. Analysis of charge heterogeneities in mAbs using imaged CE. Electrophoresis. 2009;30:714–722. doi:10.1002/elps.200800636.
- Wu J, Pawliszyn J. Dual detection for capillary isoelectric focusing with refractive index gradient and absorption imaging detectors. Anal Chem. 1994;66:6. doi:10.1021/ac00078a018.
- He Y, Isele C, Hou W, Ruesch M. Rapid analysis of charge variants of monoclonal antibodies with capillary zone electrophoresis in dynamically coated fused-silica capillary. J Sep Sci. 2011;34:548–555. doi:10.1002/jssc.201000719.
- Shi Y, Li Z, Qiao Y, Lin J. Development and validation of a rapid capillary zone electrophoresis method for determining charge variants of mAb. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;906:63–68. doi:10.1016/j.jchromb.2012.08.022.
- Espinosa-de la Garza CE, Perdomo-Abundez FC, Padilla-Calderon J, Uribe-Wiechers JM, Perez NO, Flores-Ortiz LF, Medina-Rivero E. Analysis of recombinant monoclonal antibodies by capillary zone electrophoresis. Electrophoresis. 2013;34:1133–1140. doi:10.1002/elps.201200575.
- He Y, Lacher NA, Hou W, Wang Q, Isele C, Starkey J, Ruesch M. Analysis of identity, charge variants, and disulfide isomers of monoclonal antibodies with capillary zone electrophoresis in an uncoated capillary column. Anal Chem. 2010;82:3222–3230. doi:10.1021/ac9028856.
- Gahoual R, Beck A, Francois YN, Leize-Wagner E. Independent highly sensitive characterization of asparagine deamidation and aspartic acid isomerization by sheathless CZE-ESI-MS/MS. J Mass Spectrom. 2016;51:150–158. doi:10.1002/jms.3735.
- Gahoual R, Busnel JM, Beck A, Francois YN, Leize-Wagner E. Full antibody primary structure and microvariant characterization in a single injection using transient isotachophoresis and sheathless capillary electrophoresis-tandem mass spectrometry. Anal Chem. 2014;86:9074–9081. doi:10.1021/ac502378e.
- Farnan D, Moreno GT. Multiproduct high-resolution monoclonal antibody charge variant separations by pH gradient ion-exchange chromatography. Anal Chem. 2009;81:8846–8857. doi:10.1021/ac901408j.
- Wagner-Rousset E, Fekete S, Morel-Chevillet L, Colas O, Corvaia N, Cianferani S, Guillarme D, Beck A. Development of a fast workflow to screen the charge variants of therapeutic antibodies. J Chromatogr A. 2017;1498:147–154. doi:10.1016/j.chroma.2017.02.065.
- Joshi V, Kumar V, Rathore AS. Rapid analysis of charge variants of monoclonal antibodies using non-linear salt gradient in cation-exchange high performance liquid chromatography. J Chromatogr A. 2015;1406:175–185. doi:10.1016/j.chroma.2015.06.015.
- Haverick M, Mengisen S, Shameem M, Ambrogelly A. Separation of mAbs molecular variants by analytical hydrophobic interaction chromatography HPLC: overview and applications. MAbs. 2014;6:852–858.
- Kohli N, Jain N, Geddie ML, Razlog M, Xu L, Lugovskoy AA. A novel screening method to assess developability of antibody-like molecules. MAbs. 2015;7:752–758. doi:10.1080/19420862.2015.1048410.
- Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Prediction of aggregation prone regions of therapeutic proteins. J Phys Chem B. 2010;114:6614–6624. doi:10.1021/jp911706q.
- Estep P, Caffry I, Yu Y, Sun T, Cao Y, Lynaugh H, Jain T, Vásquez M, Tessier PM, Xu Y. An alternative assay to hydrophobic interaction chromatography for high-throughput characterization of monoclonal antibodies. MAbs. 2015;7:553–561. doi:10.1080/19420862.2015.1016694.
- Boyd D, Kaschak T, Yan B. HIC resolution of an IgG1 with an oxidized Trp in a complementarity determining region. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:955–960. doi:10.1016/j.jchromb.2011.03.006.
- Chaderjian WB, Chin ET, Harris RJ, Etcheverry TM. Effect of copper sulfate on performance of a serum-free CHO cell culture process and the level of free thiol in the recombinant antibody expressed. Biotechnol Prog. 2005;21:550–553. doi:10.1021/bp0497029.
- Lacy ER, Baker M, Brigham-Burke M. Free sulfhydryl measurement as an indicator of antibody stability. Anal Biochem. 2008;382:66–68. doi:10.1016/j.ab.2008.07.016.
- Chumsae C, Gaza-Bulseco G, Liu H. Identification and localization of unpaired cysteine residues in monoclonal antibodies by fluorescence labeling and mass spectrometry. Anal Chem. 2009;81:6449–6457. doi:10.1021/ac900815z.
- Xiang T, Chumsae C, Liu H. Localization and quantitation of free sulfhydryl in recombinant monoclonal antibodies by differential labeling with 12C and 13C iodoacetic acid and LC-MS analysis. Anal Chem. 2009;81:8101–8108. doi:10.1021/ac901311y.
- Brych SR, Gokarn YR, Hultgen H, Stevenson RJ, Rajan R, Matsumura M. Characterization of antibody aggregation: role of buried, unpaired cysteines in particle formation. J Pharm Sci. 2010;99:764–781. doi:10.1002/jps.21868.
- Huh JH, White AJ, Brych SR, Franey H, Matsumura M. The identification of free cysteine residues within antibodies and a potential role for free cysteine residues in covalent aggregation because of agitation stress. J Pharm Sci. 2013;102:1701–1711. doi:10.1002/jps.23505.
- Lilyestrom WG, Yadav S, Shire SJ, Scherer TM. Monoclonal antibody self-association, cluster formation, and rheology at high concentrations. J Phys Chem B. 2013;117:6373–6384. doi:10.1021/jp4008152.
- Arora J, Hu Y, Esfandiary R, Sathish HA, Bishop SM, Joshi SB, Middaugh CR, Volkin DB, Weis DD. Charge-mediated Fab-Fc interactions in an IgG1 antibody induce reversible self-association, cluster formation, and elevated viscosity. MAbs. 2016;8:1561–1574. doi:10.1080/19420862.2016.1222342.
- Singh SN, Yadav S, Shire SJ, Kalonia DS. Dipole-dipole interaction in antibody solutions: correlation with viscosity behavior at high concentration. Pharm Res. 2014;31:2549–2558. doi:10.1007/s11095-014-1352-0.
- Bumbaca D, Wong A, Drake E, Reyes AE 2nd, Lin BC, Stephan J-P, Desnoyers L, Shen B-Q, Dennis MS. Highly specific off-target binding identified and eliminated during the humanization of an antibody against FGF receptor 4. MAbs. 2011;3:376–386.
- Nishi H, Miyajima M, Wakiyama N, Kubota K, Hasegawa J, Uchiyama S, Fukui K. Fc domain mediated self-association of an IgG1 monoclonal antibody under a low ionic strength condition. J Biosci Bioeng. 2011;112:326–332. doi:10.1016/j.jbiosc.2011.06.017.
- Sun T, Reid F, Liu Y, Cao Y, Estep P, Nauman C, Xu Y. High throughput detection of antibody self-interaction by bio-layer interferometry. MAbs. 2013;5:838–841. doi:10.4161/mabs.26186.
- Sule SV, Dickinson CD, Lu J, Chow CK, Tessier PM. Rapid analysis of antibody self-association in complex mixtures using immunogold conjugates. Mol Pharm. 2013;10:1322–1331. doi:10.1021/mp300524x.
- Sule SV, Sukumar M, Weiss W, Marcelino-Cruz AM, Sample T, Tessier PM. High-throughput analysis of concentration-dependent antibody self-association. Biophys J. 2011;101:1749–1757. doi:10.1016/j.bpj.2011.08.036.
- Tessier PM, Sandler SI, Lenhoff AM. Direct measurement of protein osmotic second virial cross coefficients by cross-interaction chromatography. Protein Sci. 2004;13:1379–1390. doi:10.1110/ps.03419204.
- Xu Y, Roach W, Sun T, Jain T, Prinz B, Yu T-Y, Torrey J, Thomas J, Bobrowicz P, Vásquez M, et al. Addressing polyspecificity of antibodies selected from an in vitro yeast presentation system: a FACS-based, high-throughput selection and analytical tool. Protein Eng Des Sel. 2013;26:663–670. doi:10.1093/protein/gzt047.
- Datta-Mannan A, Chow CK, Dickinson C, Driver D, Lu J, Witcher DR, Wroblewski VJ. FcRn affinity-pharmacokinetic relationship of five human IgG4 antibodies engineered for improved in vitro FcRn binding properties in cynomolgus monkeys. Drug Metab Dispos. 2012;40:1545–1555. doi:10.1124/dmd.112.045864.
- Hotzel I, Theil FP, Bernstein LJ, Prabhu S, Deng R, Quintana L, Lutman J, Sibia R, Chan P, Bumbaca D, et al. A strategy for risk mitigation of antibodies with fast clearance. MAbs. 2012;4:753–760. doi:10.4161/mabs.22189.
- Kelly RL, Geoghegan JC, Feldman J, Jain T, Kauke M, Le D, Zhao J, Wittrup KD. Chaperone proteins as single component reagents to assess antibody nonspecificity. MAbs. 2017;9:1036–1040. doi:10.1080/19420862.2017.1356529.
- Kelly RL, Sun T, Jain T, Caffry I, Yu Y, Cao Y, Lynaugh H, Brown M, Vásquez M, Wittrup KD, et al. High throughput cross-interaction measures for human IgG1 antibodies correlate with clearance rates in mice. MAbs. 2015;7:770–777. doi:10.1080/19420862.2015.1043503.
- Alexander AJ, Hughes DE. Monitoring of IgG antibody thermal stability by micellar electrokinetic capillary chromatography and matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 1995;67:3626–3632.
- Andya JD, Maa YF, Costantino HR, Nguyen PA, Dasovich N, Sweeney TD, Hsu CC, Shire SJ. The effect of formulation excipients on protein stability and aerosol performance of spray-dried powders of a recombinant humanized anti-IgE monoclonal antibody. Pharm Res. 1999;16:350–358.
- Fesinmeyer RM, Hogan S, Saluja A, Brych SR, Kras E, Narhi LO, Brems DN, Gokarn YR. Effect of ions on agitation- and temperature-induced aggregation reactions of antibodies. Pharm Res. 2009;26:903–913. doi:10.1007/s11095-008-9792-z.
- Franey H, Brych SR, Kolvenbach CG, Rajan RS. Increased aggregation propensity of IgG2 subclass over IgG1: role of conformational changes and covalent character in isolated aggregates. Protein Sci. 2010;19:1601–1615. doi:10.1002/pro.434.
- Hawe A, Kasper JC, Friess W, Jiskoot W. Structural properties of monoclonal antibody aggregates induced by freeze-thawing and thermal stress. Eur J Pharm Sci. 2009;38:79–87. doi:10.1016/j.ejps.2009.06.001.
- Jiskoot W, Beuvery EC, de Koning AA, Herron JN, Crommelin DJ. Analytical approaches to the study of monoclonal antibody stability. Pharm Res. 1990;7:1234–1241.
- Joubert MK, Luo Q, Nashed-Samuel Y, Wypych J, Narhi LO. Classification and characterization of therapeutic antibody aggregates. J Biol Chem. 2011;286:25118–25133. doi:10.1074/jbc.M110.160457.
- Luo Q, Joubert MK, Stevenson R, Ketchem RR, Narhi LO, Wypych J. Chemical modifications in therapeutic protein aggregates generated under different stress conditions. J Biol Chem. 2011;286:25134–25144. doi:10.1074/jbc.M110.160440.
- Telikepalli SN, Kumru OS, Kalonia C, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Structural characterization of IgG1 mAb aggregates and particles generated under various stress conditions. J Pharm Sci. 2014;103:796–809. doi:10.1002/jps.23839.
- Van Buren N, Rehder D, Gadgil H, Matsumura M, Jacob J. Elucidation of two major aggregation pathways in an IgG2 antibody. J Pharm Sci. 2009;98:3013–3030. doi:10.1002/jps.21514.
- Zhang A, Singh SK, Shirts MR, Kumar S, Fernandez EJ. Distinct aggregation mechanisms of monoclonal antibody under thermal and freeze-thaw stresses revealed by hydrogen exchange. Pharm Res. 2012;29:236–250. doi:10.1007/s11095-011-0538-y.
- Liu H, Gaza-Bulseco G, Lundell E. Assessment of antibody fragmentation by reversed-phase liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:13–23. doi:10.1016/j.jchromb.2008.10.015.
- Dillon TM, Bondarenko PV, Rehder DS, Pipes GD, Kleemann GR, Ricci MS. Optimization of a reversed-phase high-performance liquid chromatography/mass spectrometry method for characterizing recombinant antibody heterogeneity and stability. J Chromatogr A. 2006;1120:112–120. doi:10.1016/j.chroma.2006.01.016.
- Cordoba AJ, Shyong BJ, Breen D, Harris RJ. Non-enzymatic hinge region fragmentation of antibodies in solution. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:115–121. doi:10.1016/j.jchromb.2004.12.033.
- Dillon TM, Bondarenko PV, Speed Ricci M. Development of an analytical reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry method for characterization of recombinant antibodies. J Chromatogr A. 2004;1053:299–305.
- Gaza-Bulseco G, Liu H. Fragmentation of a recombinant monoclonal antibody at various pH. Pharm Res. 2008;25:1881–1890. doi:10.1007/s11095-008-9606-3.
- Xiang T, Lundell E, Sun Z, Liu H. Structural effect of a recombinant monoclonal antibody on hinge region peptide bond hydrolysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;858:254–262. doi:10.1016/j.jchromb.2007.08.043.
- Kroon DJ, Baldwin-Ferro A, Lalan P. Identification of sites of degradation in a therapeutic monoclonal antibody by peptide mapping. Pharm Res. 1992;9:1386–1393.
- Zhang YT, Hu J, Pace AL, Wong R, Wang YJ, Kao YH. Characterization of asparagine 330 deamidation in an Fc-fragment of IgG1 using cation exchange chromatography and peptide mapping. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;965:65–71. doi:10.1016/j.jchromb.2014.06.018.
- Paborji M, Pochopin NL, Coppola WP, Bogardus JB. Chemical and physical stability of chimeric L6, a mouse-human monoclonal antibody. Pharm Res. 1994;11:764–771.
- Cohen SL, Price C, Vlasak J. Beta-elimination and peptide bond hydrolysis: two distinct mechanisms of human IgG1 hinge fragmentation upon storage. J Am Chem Soc. 2007;129:6976–6977. doi:10.1021/ja0705994.
- Arosio P, Rima S, Morbidelli M. Aggregation mechanism of an IgG2 and two IgG1 monoclonal antibodies at low pH: from oligomers to larger aggregates. Pharm Res. 2013;30:641–654. doi:10.1007/s11095-012-0885-3.
- Sharma DK, Oma P, Pollo MJ, Sukumar M. Quantification and characterization of subvisible proteinaceous particles in opalescent mAb formulations using micro-flow imaging. J Pharm Sci. 2010;99:2628–2642. doi:10.1002/jps.22046.
- Ghazvini S, Kalonia C, Volkin DB, Dhar P. Evaluating the role of the air-solution interface on the mechanism of subvisible particle formation caused by mechanical agitation for an IgG1 mAb. J Pharm Sci. 2016;105:1643–1656. doi:10.1016/j.xphs.2016.02.027.
- Kiese S, Papppenberger A, Friess W, Mahler HC. Shaken, not stirred: mechanical stress testing of an IgG1 antibody. J Pharm Sci. 2008;97:4347–4366. doi:10.1002/jps.21328.
- Mahler HC, Muller R, Friess W, Delille A, Matheus S. Induction and analysis of aggregates in a liquid IgG1-antibody formulation. Eur J Pharm Biopharm. 2005;59:407–417. doi:10.1016/j.ejpb.2004.12.004.
- Serno T, Carpenter JF, Randolph TW, Winter G. Inhibition of agitation-induced aggregation of an IgG-antibody by hydroxypropyl-beta-cyclodextrin. J Pharm Sci. 2010;99:1193–1206. doi:10.1002/jps.21931.
- Eppler A, Weigandt M, Hanefeld A, Bunjes H. Relevant shaking stress conditions for antibody preformulation development. Eur J Pharm Biopharm. 2010;74:139–147. doi:10.1016/j.ejpb.2009.11.005.
- Kueltzo LA, Wang W, Randolph TW, Carpenter JF. Effects of solution conditions, processing parameters, and container materials on aggregation of a monoclonal antibody during freeze-thawing. J Pharm Sci. 2008;97:1801–1812. doi:10.1002/jps.21110.
- Singh SR, Zhang J, O’Dell C, Hsieh MC, Goldstein J, Liu J, Srivastava A. Effect of polysorbate 80 quality on photostability of a monoclonal antibody. AAPS PharmSciTech. 2012;13:422–430. doi:10.1208/s12249-012-9759-6.
- Nowak C, Ponniah G, Cheng G, Kita A, Neill A, Kori Y, Liu H. Liquid chromatography-fluorescence and liquid chromatography-mass spectrometry detection of tryptophan degradation products of a recombinant monoclonal antibody. Anal Biochem. 2016;496:4–8. doi:10.1016/j.ab.2015.12.004.
- Liu H, Gaza-Bulseco G, Zhou L. Mass spectrometry analysis of photo-induced methionine oxidation of a recombinant human monoclonal antibody. J Am Soc Mass Spectrom. 2009;20:525–528. doi:10.1016/j.jasms.2008.11.011.
- Chaudhri A, Zarraga IE, Kamerzell TJ, Brandt JP, Patapoff TW, Shire SJ, Voth GA. Coarse-grained modeling of the self-association of therapeutic monoclonal antibodies. J Phys Chem B. 2012;116:8045–8057. doi:10.1021/jp301140u.
- Ali SA, Hassan MI, Islam A, Ahmad F. A review of methods available to estimate solvent-accessible surface areas of soluble proteins in the folded and unfolded states. Curr Protein Pept Sci. 2014;15:456–476.
- Agrawal NJ, Helk B, Kumar S, Mody N, Sathish HA, Samra HS, Buck PM, Li L, Trout BL. Computational tool for the early screening of monoclonal antibodies for their viscosities. MAbs. 2016;8:43–48. doi:10.1080/19420862.2015.1099773.
- Buck PM, Chaudhri A, Kumar S, Singh SK. Highly viscous antibody solutions are a consequence of network formation caused by domain-domain electrostatic complementarities: insights from coarse-grained simulations. Mol Pharm. 2015;12:127–139. doi:10.1021/mp500485w.
- Chaudhri A, Zarraga IE, Yadav S, Patapoff TW, Shire SJ, Voth GA. The role of amino acid sequence in the self-association of therapeutic monoclonal antibodies: insights from coarse-grained modeling. J Phys Chem B. 2013;117:1269–1279. doi:10.1021/jp3108396.
- Obrezanova O, Arnell A, de la Cuesta RG, Berthelot ME, Gallagher TRA, Zurdo J, Stallwood Y. Aggregation risk prediction for antibodies and its application to biotherapeutic development. MAbs. 2015;7:352–363. doi:10.1080/19420862.2015.1007828.
- Van Walle I, Gansemans Y, Parren PW, Stas P, Lasters I. Immunogenicity screening in protein drug development. Expert Opin Biol Ther. 2007;7:405–418. doi:10.1517/14712598.7.3.405.
- Bumbaca Yadav D, Sharma VK, Boswell CA, Hotzel I, Tesar D, Shang Y, Ying Y, Fischer SK, Grogan JL, Chiang EY, et al. Evaluating the use of antibody variable region (Fv) charge as a risk assessment tool for predicting typical cynomolgus monkey pharmacokinetics. J Biol Chem. 2015;290:29732–29741. doi:10.1074/jbc.M115.692434.
- D’Atri V, Fekete S, Beck A, Lauber M, Guillarme D. Hydrophilic interaction chromatography hyphenated with mass spectrometry: a powerful analytical tool for the comparison of originator and biosimilar therapeutic monoclonal antibodies at the middle-up level of analysis. Anal Chem. 2017;89:2086–2092. doi:10.1021/acs.analchem.6b04726.
- Periat A, Fekete S, Cusumano A, Veuthey JL, Beck A, Lauber M, Guillarme D. Potential of hydrophilic interaction chromatography for the analytical characterization of protein biopharmaceuticals. J Chromatogr A. 2016;1448:81–92. doi:10.1016/j.chroma.2016.04.056.
- Sorensen M, Harmes DC, Stoll DR, Staples GO, Fekete S, Guillarme D, Beck A. Comparison of originator and biosimilar therapeutic monoclonal antibodies using comprehensive two-dimensional liquid chromatography coupled with time-of-flight mass spectrometry. MAbs. 2016;8:1224–1234. doi:10.1080/19420862.2016.1203497.
- Stoll D, Danforth J, Zhang K, Beck A. Characterization of therapeutic antibodies and related products by two-dimensional liquid chromatography coupled with UV absorbance and mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1032:51–60. doi:10.1016/j.jchromb.2016.05.029.
- Stoll DR, Harmes DC, Danforth J, Wagner E, Guillarme D, Fekete S, Beck A. Direct identification of rituximab main isoforms and subunit analysis by online selective comprehensive two-dimensional liquid chromatography-mass spectrometry. Anal Chem. 2015;87:8307–8315. doi:10.1021/acs.analchem.5b01578.