ABSTRACT
Hybridoma methods for monoclonal antibody (mAb) cloning are a mainstay of biomedical research, but they are hindered by the need to maintain hybridomas in oligoclonal pools during antibody screening. Here, we describe a system in which hybridomas specifically capture and display the mAbs they secrete: On-Cell mAb Screening (OCMS™). In OCMS™, mAbs displayed on the cell surface can be rapidly assayed for expression level and binding specificity using fluorescent antigens with high-content (image-based) methods or flow cytometry. OCMS™ demonstrated specific mAb binding to poliovirus and rabies virus by forming a cell surface IgG “cap”, as a universal assay for anti-viral mAbs. We produced and characterized OCMS™-enabled hybridomas secreting mAbs that neutralize poliovirus and used fluorescence microscopy to identify and clone a human mAb specific for the human N-methyl-D-aspartate receptor. Lastly, we used OCMS™ to assess expression and antigen binding of a recombinant mAb produced in 293T cells. As a novel method to physically associate mAbs with the hybridomas that secrete them, OCMS™ overcomes a central challenge to hybridoma mAb screening and offers new paradigms for mAb discovery and production.
Introduction
Hybridoma methods have been the backbone of monoclonal antibody (mAb) discovery for over four decades, despite advances in recombinant mAb expression and next-generation DNA sequencing (NGS) of antibody repertoires. mAbs cloned by hybridoma methods can access repertoires distinct from those obtained by other methods and are well suited to manufacture in mammalian cell lines.Citation1−Citation5 Hybridoma methods are technically straightforward. They produce full-length, glycosylated mAbs that maintain their original heavy chain:light chain pairings without the need for recombinant gene expression. However, their major shortcoming is that mAbs are secreted into the cell culture medium, so that hybridomas must be maintained in oligoclonal pools while their secreted mAbs are analyzed separately. This impedes the discovery of rare mAbs because it imposes practical limits on the numbers of cells that can be analyzed, and is a disadvantage compared to yeast display methods, in which mAbs are expressed on the cell surface and can be screened for antigen binding in bulk culture.Citation6 Thus, hybridoma methods are encumbered by a central challenge in biotechnology: how to find and select a single mammalian cell, within a heterogeneous cell population, that secretes a protein of desired expression level, structure, activity, or binding specificity.
Improvements to hybridoma technology have been developed, including automation, but the fundamental challenges posed by standard screening paradigms remain.Citation7 Methods of polyclonal cell culture have been developed whereby mAbs are associated with the cells that secrete them, for example, by trapping secreted mAbs in microdroplets or semi-solid media.Citation8-Citation11 Other methods directly attach mAbs to the hybridomas that secrete them by using secondary antibodies that adhere to the hybridoma surface and capture secreted mAbs. One approach biotinylates the hybridomas and binds anti-IgG secondary antibodies conjugated to avidins.Citation12,Citation13 Another conjugates the secondary antibodies to membrane-anchoring lipophilic molecules.Citation14 However, these methods do not prevent mAbs from binding to nearby cells that did not secrete them. Over-expression of B cell receptor proteins (CD79a, CD79b) in hybridomas can capture and display sufficient IgG to allow screening for antigen binding specificity, but this approach has not been extended to human mAb cloning.Citation15
In this report, we describe a novel hybridoma method for screening polyclonal cell populations to identify and isolate cells that secrete mAbs with desired features and stability of expression: On-Cell mAb Screening (OCMS™). OCMS™ transiently captures and displays mAbs on the hybridoma surface, while preventing mAbs from binding to cells that do not secrete them. OCMS™ streamlines the hybridoma method for human mAb cloning, facilitates novel assays for viral and conformational antigens, and improves real-time assessment of mAb expression by hybridomas and adherent cells expressing recombinant mAbs. OCMS™ will leverage recent advances in high content imaging to improve the discovery of useful mAbs.Citation16,Citation17 Furthermore, as a general method for analyzing proteins secreted by mammalian cells, OCMS™ will have many applications throughout biomedical research and drug development.
Results
Overview of the OCMS™ design
We created a novel system for human mAb cloning in which hybridomas are enabled for on-demand capture and display of the mAbs they produce. Surface-displayed mAbs can be assessed for binding activity and expression by fluorescence imaging. This facilitates identification and isolation of clones that stably express desired IgGs. The system uses an “Anchor-Linker” strategy, in which an “Anchor” protein is expressed on the surface of a fusion partner cell line and maintained by hybridomas after cell fusion. The Anchor contains a tandem scFv specific for rabbit IgG. The “Linker” is a rabbit anti-human IgG antibody (RAH). When present in the culture medium, RAH binds to Anchor molecules on the hybridomas and captures secreted human mAbs. Excess RAH in the cell culture medium is a competitor that prevents mAbs from binding to cells that do not secrete them. The methods presented here create libraries of hybridomas producing human mAbs that can be evaluated in physical association with the cells that make them. This obviates the need to test hybridoma mAbs in assays separate from the hybridoma cultures, as cells expressing mAbs of interest can be readily identified by fluorescence imaging.
Construction and validation of OCMS™ mAb display methods
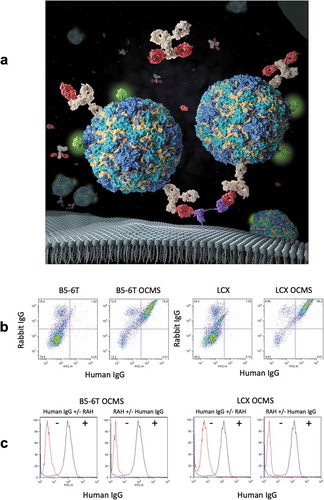
) depicts an immune complex enabled by the OCMS™ method. The tandem single-chain variable fragments (scFvs) in the Anchor capture RAH, which in turn captures and adheres secreted human IgG to the cell surface, where it can be assayed for expression level and binding activity. The Anchor is expressed from a recombinant gene that encodes a kappa Ig leader sequence, a pair of murine scFvs (specific for the rabbit IgG CH1 domain and separated by a Gly-Ser linker), a MYC tag, and a minimal platelet derived growth factor (PDGF) receptor beta transmembrane domain (Supplementary Figures 1–2, Supplementary Table 1).Citation19,Citation20 The Anchor was expressed on two cell lines optimized for fusion to human B cells, B5-6T and LCX, creating the cell lines B5-6T OCMS™ and LCX OCMS™. B5-6T has been described.Citation21 LCX is a next-generation fusion partner that expresses the human telomerase catalytic subunit (hTERT) and a constitutively active gp130 protein (see Materials and Methods). We confirmed functional Anchor expression by incubating the cells with a polyclonal rabbit anti-human IgG (AB16) and the 1B8 human IgG mAb (specific for poliovirus (PV), data not shown). Bound IgGs were detected with allophycocyanin (APC) anti-rabbit IgG (AB5) and Alexa Fluor 488® anti-human IgG (AB1) ()). Approximately 75% of the B5-6T OCMS™ and 86% of the LCX OCMS™ cells were double positive for rabbit IgG and human IgG, compared to less than 2% of the B5-6T and LCX cells. The B5-6T and LCX cells gave some signals in the Rabbit IgG+/human IgG- quadrant (18% and 29%, respectively) due to non-specific binding (see Materials and Methods). We compared human IgG (1B8 mAb) binding in the presence and absence of a designated prototype RAH (AB14), a rabbit mAb specific for anti-human IgG ()), which confirmed that B5-6T OCMS™ and LCX OCMS™ cells require a Linker for human IgG binding.
Figure 1. Structure and expression of a functional tandem scFv anchor on the surface of fusion partner cells. (a) A depiction the OCMS™ method. The anchor is attached to the outer plasma membrane through a minimal PDGF receptor transmembrane domain. The anchor contains two scFv elements (purple) that bind to a rabbit IgG linker, here shown as a monovalent Fab (red). The linker, here shown as a monovalent Fab, captures human IgGs (tan) secreted by the hybridoma cell. Cell surface-bound mAbs can be tested for antigen binding specificity by incubating the cells with a fluorescent antigen, here depicted as a poliovirus labeled with green fluorescent Streptavidin, or for total mAb expression using a fluorescent anti-human IgG secondary (not shown). Cells specifically capture the mAbs they secrete, because mAb binding to non-secreting cells is competed by an excess of the linker in solution. Thus, cells within a polyclonal population can be assessed individually for mAb binding specificity and expression level using fluorescence imaging methods. This image was created by Nicolás Fernández using images from the protein databank archive www.rcsb.org Citation18. (b) B5-6T, B5-6T OCMS™, LCX, and LCX OCMS™ cells were incubated with a polyclonal rabbit anti-human IgG (AB16) and a human IgG mAb (1B8), which were detected with APC anti-rabbit IgG (AB5) and Alexa Fluor 488® anti-human IgG (AB1). (c) (first and third panels) Cells were incubated with the 1B8 mAb, with or without the RAH (the designated prototype rabbit mAb specific for human IgG: AB14), and tested for 1B8 binding with Alexa Fluor 488® anti-human IgG (AB1). (second and fourth panels) Cells were incubated with or without RAH (AB14), then with or without 1B8, and tested for 1B8 binding with Alexa Fluor 488® anti-human IgG (AB1).
Specific capture and analysis of human igGs secreted by OCMS™ hybridomas
We expressed the Anchor in an established hybridoma cell line, 8C5, which produces a human IgG specific for the rabies virus glycoprotein (GP) (data not shown), to create 8C5 OCMS™. We cultured the 8C5 OCMS™ and B5-6T OCMS™ cells overnight in fresh medium with RAH (AB14) to capture newly secreted IgGs. The next morning, we incubated them with biotinylated rabies GP (courtesy of Dr. Matthias Schnell, Thomas Jefferson University) and visualized the bound rabies GP with Alexa Fluor 488® streptavidin (AB3) and fluorescence microscopy ()). Most of the 8C5 OCMS™ cells exhibited green membrane staining in a “cap” structure, whereas the B5-6T OCMS™ cells did not.
Figure 2. Analysis of human IgGs displayed on the surface of OCMS™ hybridomas. (a) 8C5 OCMS™ (top row) and B5-6T OCMS™ (bottom row) cells were incubated overnight with the RAH (AB14), followed by biotinylated rabies GP and Alexa Fluor 488® streptavidin (green) (AB3), and visualized by fluorescence microscopy. Images are shown with DAPI (blue) (right) or without DAPI (left). Scale bar = 5 µm. (b) The 4G4 OCMS™ (top row) and 8C5 OCMS™ (bottom row) hybridomas were incubated overnight with a rabbit Fab anti-human IgG (AB17), then for an hour with additional rabbit Fab anti-human IgG and Sabin type III PV (left column) or GP (right column), followed by an Alexa Fluor® 488 anti-human IgG (green) (AB2). Nuclei were stained with DAPI (blue). Scale bar = 10 µm. (c) In this mixing experiment, non-secretor OCMS™ cell lines were labeled with CFSE (green) and mixed in a 10:1 ratio with IgG-secreting OCMS™ hybridomas. Cells were incubated overnight with rabbit F(ab’)2 anti-human IgG (AB15) then washed and incubated with biotinylated Protein A (AB12) and APC streptavidin (red) (AB4). (Top row) 8C5 OCMS™ vs. B5-6T OCMS™ CFSE. (Bottom row) 4G4 OCMS™ vs. LCX OCMS™ CFSE. With DAPI (blue) (right column) or without (left column). Scale bar = 5 µm.
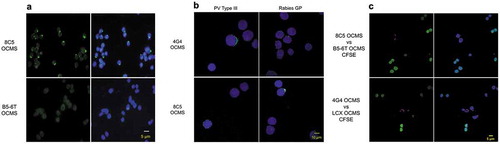
To determine whether surface-displayed mAbs could be tested for antigen-binding specificity, we compared the 8C5 OCMS™ hybridoma to the 4G4 OCMS™ hybridoma (which secretes a mAb specific for PV; see below) ()). Cells were incubated overnight with a rabbit antigen-binding fragment (Fab) anti-human IgG (AB17), then with unlabeled rabies GP or type III Sabin PV and additional AB17. Bound human IgG was detected with Alexa Fluor® 488 anti-human IgG (AB2). In the presence of specific antigen, the cells acquired a human IgG cap: e.g., 8C5 OCMS™ with GP and 4G4 OCMS™ with PV. In contrast, 8C5 OCMS™ and 4G4 OCMS™ did not form a cap with PV and GP antigens, respectively. Instead, IgG labeling formed a punctate, circumferential pattern. These images show that mAbs adhered to the surface of OCMS™ cells can be assessed for antigen binding specificity by fluorescence microscopy. They further demonstrate that mAb binding to polyvalent viral antigens can be revealed by changes in the arrangement of membrane-adherent IgG.
To determine whether mAbs can be prevented from binding to hybridomas that do not secrete them, we performed a mixing experiment. B5-6T OCMS™ cells were labeled with a fluorescent dye (carboxyfluorescein diacetate, succinimidyl ester (CSFE)) and mixed with 8C5 OCMS™ cells at a 10:1 ratio. Cells were cultured overnight with rabbit F(ab’)2 anti-human IgG (AB15). The next day, bound human IgG was detected with biotinylated protein A (AB12) and APC streptavidin (AB4) (), top panels). Whereas most of the 8C5 OCMS™ hybridomas showed human IgG binding, none of the B5-6T OCMS™ cells did. We repeated this experiment with CSFE-labeled LCX OCMS™ cells and the 4G4 OCMS™ hybridoma (which was derived from the LCX OCMS™ cell line), and observed the same specificity (), bottom panels). These data confirm the capability of the OCMS™ system to prevent mAbs from binding to hybridomas that did not secrete them.
Ocms™-enabled hybridomas secreting human anti-PV mABs
To test the ability of the LCX OCMS™ cell line to generate OCMS™-enabled hybridomas expressing useful human IgGs, we cloned human mAbs specific for type III PV. We used B cells from previously described donors P3 and P6, who received multiple doses of oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV), as well as an IPV boost 8 days prior to blood sampling.Citation22 We fused CD27+ peripheral blood mononuclear cells (PBMCs) to the LCX OCMS™ cell line to create hybridomas that we screened by ELISA for IgG binding to Sabin type III PV, following previously described methods.Citation22,Citation23 From 8 hybridoma pools, we established 7 stable clones, all of which were IgG1λ (Supplementary Table 2). Each IgG neutralized both Sabin type III PV and Saukett PV (wild type III), ranging from 1:3,200-1:106 titer of a 1 mg/ml mAb solution, values comparable to those previously obtained with the same B cell samples and the B5-6T fusion partner cell line (Supplementary Table 2).Citation22,Citation23
We used the OCMS™ method to assess these hybridomas for secretion of PV-specific IgGs, testing PV binding after an overnight period of mAb capture. LCX OCMS™, 8C5 OCMS™, or the PV OCMS™ cells were cultured with the rabbit F(ab’)2 anti-human IgG (AB15). The next day, the cells were washed and incubated with biotinylated Sabin type III PV for one hour, after which bound PV was detected with Alexa Fluor 488® streptavidin (AB3) (). All of the PV hybridoma clones had many cells with green fluorescence, often distributed asymmetrically as a cap, whereas the LCX OCMS™ and the 8C5 OCMS™ hybridomas did not.
Figure 3. Binding of type III PV to OCMS™ hybridomas secreting human anti-PV IgG mAbs. (a) Hybridomas were incubated overnight with the rabbit F(ab’)2 anti-human IgG (AB15) and then biotinylated PV. PV was detected with Alexa Fluor 488® streptavidin (green) (AB3). Nuclei were stained with DAPI (blue). Scale bar = 5 µm. (b) Hybridomas were prepared as in (a) but biotinylated PV was detected with APC streptavidin (AB4) and the cells were analyzed by flow cytometry. (c) Hybridomas were incubated overnight with the rabbit F(ab’)2 anti-human IgG (AB15) then biotinylated protein A (AB12). Bound rabbit and human antibodies were then assessed by flow cytometry with the APC F(ab’)2 anti-rabbit IgG (AB5) and Alexa Fluor 488® streptavidin (AB3). (d) The percentages of hybridomas showing binding to PV (green), rabbit IgG (blue), and human IgG (red) were collected into a spider plot.
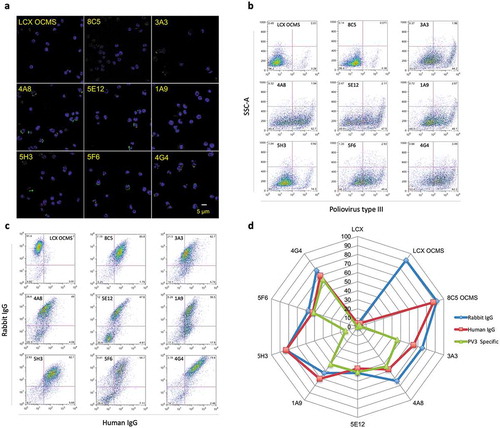
We analyzed these cells by flow cytometry for PV binding, comparing them to the LCX OCMS™ and 8C5 OCMS™ cell lines. We incubated the cells with the rabbit (Fab)2 anti-human IgG (AB15), followed by biotinylated Sabin type III PV and APC streptavidin (AB4). Percent PV binding varied among the hybridomas, from 14.3% to 62.2% (3A3, 44.2%; 4A8, 52.7%; 5E12, 47.5%; 1A9, 48.1%; 5H3, 14.3%; 5F6, 49.4%; 4G4, 62.2%), compared to background levels of ~3% for the LCX OCMS™ and 8C5 OCMS™ cells ()). We next tested Anchor expression and human mAb capture. We cultured the cells with rabbit F(ab’)2 anti-human IgG (AB15) overnight, then labeled the bound AB15 and human IgG with APC F(ab’)2 anti-rabbit IgG (AB5), biotinylated protein A (AB12), and Alexa Flour 488® streptavidin (AB3). The double positive populations ranged from 47.6–85.8%, compared to ~3% for the LCX OCMS: 8C5, 85.8%; 3A3, 62.7%; 4A8, 49.0%; 5E12, 47.6%; 1A9, 56.5%; 5H3, 82.7%; 5F6, 54.7%; 4G4, 79.8% (). Comparing these three measurements in a spider plot allowed a visual, qualitative assessment of the stability of mAb expression, functional Anchor levels, and antigen binding activity (). For example, 5H3 consistently expressed human IgG, but had low PV binding, whereas 4G4, 5F6, and 5E12 each bound IgG and PV at approximately the same percentages. Furthermore, 8C5 OCMS™ consistently bound IgG but had minimal PV binding, LCX OCMS™ bound rabbit IgG only, and LCX bound none of the molecules tested. These data also show that Anchor expression can be readily maintained throughout a mAb cloning experiment, even in the absence of positive selection.
Isolation of a human mAB specific for the N-methyl-D-aspartate receptor by visual screening
We used OCMS™ to clone a human IgG associated with anti-N-methyl-D-aspartate receptor encephalitis (ANRE), a potentially fatal autoimmune disease that can cause psychiatric and cognitive disturbances, movement disorders, autonomic dysfunction, and seizures.Citation24 ANRE is caused by IgGs specific for the ionotropic N-methyl-D-aspartate receptor (NMDAR) in the hippocampus and cortex.Citation25,Citation26 The pathogenic epitope resides on the amino terminal domain (ATD) of the GluN1 NMDAR subunit; it is conformational and depends on mammalian-type glycosylation.Citation27 We previously described a cell line that produces a membrane-anchored ATD, which contains the pathogenic epitopes and can be released from the cell with TeV protease.Citation28 We first tested binding of the ATD to mAbs displayed in OCMS™ immune complexes. LCX OCMS™ cells were first bound to the RAH (AB14) and then to either a human anti-NMDAR mAb (5F5) or the 6A mAb (a human isotype control mAb specific for botulinum toxin).Citation29,Citation30 They were then incubated with biotinylated ATD and Alexa Fluor 488® streptavidin (AB3). Confocal imaging revealed patchy circumferential staining on cells exposed to the 5F5 mAb, but not to the 6A control mAb ().
Figure 4. 3C11 OCMS™, a hybridoma that secretes a human antibody specific for the NMDAR. (a) LCX OCMS™ cells were incubated with the RAH (AB14) and either the 5F5 mAb (NMDAR specific) or 6A mAb (isotype control), followed by the biotinylated NMDAR ATD and Alexa Fluor 488® streptavidin (AB3), and imaged by confocal microscopy. Cells are shown ATD staining only (left column) or merged with DAPI (right column). (b) The 3C11 OCMS™ and 4G4 OCMS™ hybridomas were compared for binding to biotinylated ATD after overnight incubation of the hybridomas with the RAH (AB14). Bound ATD was detected with Alexa Fluor™ 555 streptavidin (AB18). Cells are shown with ATD staining only (left column) or merged with DAPI (right column). (c) Binding of purified 3C11 and 6A human IgGs to the 293T-ATD cell line was compared. Bound human IgG was detected with Alexa Fluor™ 555 anti-human IgG (red) (AB10). ATD was detected with the commercial NR1 mAb (AB20) with Alexa Fluor 488® goat anti-mouse IgG (AB9) (green). Merged images that include DAPI staining (blue) are shown in the right column. (d) The 3C11 and 6A mAbs were compared for binding to cultured 14 day-old primary rat hippocampal neurons. Cells were stained as in 4c except that a different commercial NR1 mAb was used (AB19) (green). Human IgG is shown in red, and merged images that include DAPI staining (blue) are shown in the right column. Scale bars = 10 µm.
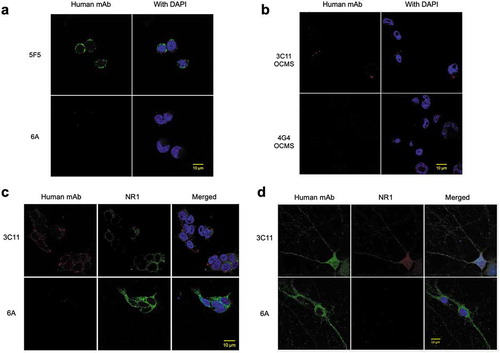
We performed a hybridoma experiment, fusing CD27+ PBMCs from an ANRE patient to LCX OCMS™ cells. We followed standard fusion protocols and plated the fusions in four 96-well plates.Citation29 After hypoxanthine-aminopterin-thymidine (HAT) selection, the resultant hybridoma pools were incubated with RAH (AB14) overnight, followed by biotinylated ATD and Alexa Fluor™ 555 streptavidin (AB18). Cells were viewed by fluorescence microscopy and one well was found to be positive (data not shown). From that well, we used limiting dilution methods to isolate a stable monoclonal hybridoma that expresses a human IgG1λ mAb, 3C11 OCMS™. Confocal images of the 3C11 OCMS™ hybridoma treated as above showed ATD binding as punctate red staining at the cell periphery, in contrast to the 4G4 OCMS™ hybridoma (). We confirmed the antigen binding specificity of the 3C11 mAb by testing it on 293T-ATD cells and primary rat hippocampal neurons. 3C11 bound to both cell types (), whereas 6A did not.
OCMS™ uses: rapid evaluation of recombinant igG expression and antigen binding
The production of recombinant mAbs in mammalian cells is a common feature of drug discovery and development campaigns. To test whether OCMS™ can be used to assess expression and antigen binding of an ectopically expressed mAb in adherent cells, we expressed the Anchor protein in 293T cells, creating 293T OCMS™. We incubated 293T OCMS™ cells with the RAH (AB14) and the 1B8 mAb, followed by APC anti-rabbit IgG (AB5) and Alexa Fluor 488® anti-human IgG (AB1). Flow cytometry showed a double positive population of 83% (). As we observed with the OCMS™ fusion partners (), 293T OCMS™ cells only bound human IgG in the presence of the RAH ().
Figure 5. Expression of a functional tandem scFv Anchor on the surface of 293T cells. (a) 293T cells and 293T OCMS™ cells were analyzed by flow cytometry for capture of rabbit and human IgG, after incubation with the RAH (AB14), the human 1B8 IgG, and detection with secondary reagents APC anti-rabbit IgG (AB5) and Alexa Fluor 488® anti-human IgG (AB1). (b) 293T OCMS™ cells were tested for human IgG (1B8 mAb) binding under the following conditions: (left panel) Human IgG was added, with or without the RAH (AB14), (right panel) RAH (AB14) was added, with or without human IgG. Human IgG was detected by flow cytometry with an Alexa Fluor 488® anti-human IgG (AB1). (c) 293T OCMS™ (left column) or 293T cells (right column) were transfected with plasmids encoding the A12 mAb, which binds types I and II PV, and compared with un-transfected 293T OCMS™ cells (middle column) for capture of human IgG on their outer plasma membrane. Following transfection, cells were cultured in the presence of the RAH (AB14) overnight, then trypsinized, and bound human IgG was detected with an Alexa Fluor 488® anti-human IgG (AB1). Confocal images are shown with DAPI staining (blue) (lower row) and without (upper row). Scale bar = 10 µm. (d) Transfected (TX) (right panel) and untransfected (UT) (left panel) 293T OCMS™ cells were incubated with the RAH (AB14) overnight, removed from the plate with Trypsin, incubated with biotinylated type I poliovirus (PV1) and APC streptavidin (AB4), and analyzed by flow cytometry.
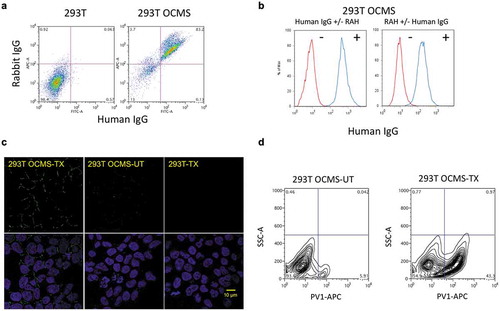
We transfected 293T OCMS™ and 293T cells with plasmids encoding the Ig heavy chain and light chain of the mAb A12, a chimpanzee/human chimeric mAb that binds and neutralizes PV types 1 and 2 (courtesy of Dr. Zhaochun Chen, National Institute of Allergy and Infectious Diseases).Citation31 Two days later, we incubated the cells with RAH (AB14) and Alexa Fluor 488® anti-human IgG (AB1) and examined the cells by confocal microscopy (). Human IgG was visualized by its circumferential staining of the 293T OCMS™ cells, but not the A12-transfected 293T cells or the untransfected 293T OCMS™ cells. Not all of the cells bound human IgG, consistent with the known efficiency of 293T cell transfection and the cell-specific mAb binding seen with the hybridomas. We next compared binding of biotinylated type I PV to 293T OCMS™ cells, with and without A12 plasmid transfection. The day following transfection, cells were cultured with the RAH (AB14) for 24 hours, then removed from the plate with trypsin, incubated with biotinylated Sabin type 1 PV and APC streptavidin (AB4), and analyzed by flow cytometry (). Approximately 43% of the transfected cells bound PV, whereas none of the un-transfected cells did. These results demonstrate that the OCMS™ method can be used to assess recombinant mAbs expressed by adherent cells.
Discussion
Here we describe a novel on-cell assay system to analyze the production and characteristics of mAbs secreted by hybridomas and transiently transfected cells using fluorescent imaging techniques. The essential components of the OCMS™ method are an Anchor protein on the surface of a secreting cell and a Linker molecule that binds the Anchor and captures mAbs secreted by the cell. Once displayed on the cell surface, mAbs can be analyzed for expression level, binding activity, and other characteristics. An important feature of the method is that the Linker also functions as a competitor. Provided in excess in the culture solution, it prevents mAb binding to Anchor molecules on non-secreting cells. This provides specificity to the reaction, so that mAbs secreted by cells within a heterogeneous population can be analyzed individually in association with the cells that make them. Cells expressing mAbs with desired features can be identified by fluorescence imaging techniques.
To generate libraries of OCMS™ hybridomas, we used a fusion partner cell line expressing the Anchor protein, which efficiently maintained expression of the Anchor after cell fusion. Studies of seven OCMS™-enabled PV hybridomas demonstrated how flow cytometry can assess heterogeneous cell populations for Anchor expression, human IgG expression, and antigen binding. Our cloning of a human mAb specific for an NMDAR receptor showed how conventional ELISAs can be replaced by fluorescent imaging with OCMS™. We also used OCMS™ to assess mAb expression and antigen binding by transiently transfected 293T-OCMS™ cells using fluorescent microscopy and flow cytometry.
The OCMS™ method will synergize with high-content (cell-based) imaging methods to create new paradigms for antigen preparation and hybridoma screening.Citation16,Citation17 To clone a human anti-NMDAR mAb, we used a biotinylated, soluble NMDAR ATD antigen expressed in 293T cells and mobilized with TeV protease. This was practical because the amount of antigen needed for OCMS™ is small compared to traditional ELISA methods.Citation28 This procedure for antigen preparation fits well into the work flow for OCMS™ and can be generalized to produce a wide variety of antigens for mAb screening. The OCMS™ method is analogous to yeast display methods of mAb expression and cloning.Citation6 Thus, high throughput competitive binding, epitope complementation, and dissociation rate assays developed for yeast should be adaptable for screening mAbs expressed by OCMS™ hybridomas.Citation32-Citation34 OCMS™ can also be used to assess mAb expression levels by individual cells in a heterogeneous population in real time, using either fluorescence imaging or flow cytometry. This feature should be useful to establish and monitor stable, high expressing cell clones for master cell banks and bioreactor production runs.Citation35
We also found that mAbs expressed by OCMS™ hybridomas can be tested indirectly for antigen binding. When exposed to cells expressing their specific mAbs, two viral antigens (GP and PV) nucleated the formation of polar IgG-containing immune complexes, which could be detected with a fluorescent anti-human IgG secondary antibody. This feature will facilitate hybridoma screening with universal mAb assays, in which mAb binding to a polyvalent antigen is identified by detecting alterations in the distribution of IgG on the cell surface. For example, such an assay could identify mAbs specific for a novel virus without the need to create a customized binding assay. As OCMS™ hybridoma libraries can be created from primary human B cells and screened within 3–4 weeks, this universal anti-viral mAb assay offers a novel paradigm for mAb discovery in response to emerging or epidemic viral threats.
Biotechnology relies heavily on the production of proteins secreted by mammalian cells. Here, we have focused on human mAbs, but OCMS™ provides a general method to analyze proteins secreted by a heterogeneous population of mammalian cells and to identify individual cells that secrete a protein of interest. Lastly, although this study of OCMS™ was performed with basic laboratory equipment, it is well-suited to automation.
Materials and methods
Volunteer blood donors
Two PV-exposed individuals were studied. Donor P3 (age 30–35 years) formerly lived in a PV endemic country and was exposed to multiple doses of OPV. Donor P6 (age > 60) had a possible wild PV infection as well as multiple lifetime exposures to OPV and IPV. They both received a dose of IPV eight days prior to blood sampling. Blood was also obtained from an 18 year-old female diagnosed at the Children’s Hospital of Philadelphia with ANRE. Two anti-NMDAR mAbs from this patient were previously described.Citation29 Work with human blood cells was performed with informed consent, under protocols approved by the Main Line Hospitals Institutional Review Board or the Institutional Review Board of the Children’s Hospital of Philadelphia and consistent with the principles set out in the WMA Declaration of Helsinki and the US Office for Human Research Protections’ Belmont Report (https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/index.html).
Secondary antibodies and labeling reagents
AB1: Alexa Fluor 488® AffiniPure F(ab’)2 Fragment Goat Anti-Human IgG, F(ab’)2 fragment specific (109-546-097; Jackson ImmunoResearch, West Grove, PA) RRID: AB_2337849
AB2: Alexa Fluor® 488 AffiniPure F(ab’)₂ Fragment Goat Anti-Human IgG, Fcγ fragment specific (109-546-098; Jackson ImmunoResearch) RRID: AB_2337850
AB3: Alexa Fluor 488® Streptavidin (016-540-084; Jackson ImmunoResearch) RRID: AB_2337249
AB4: APC Streptavidin (016-130-084; Jackson ImmunoResearch) RRID: AB_2337342
AB5: APC AffiniPure F(ab’)2 fragment Goat anti-rabbit IgG (H + L) (111-136-144; Jackson ImmunoResearch) RRID: AB_2337987
AB6: CellTrace™ CFSE Cell Proliferation Kit, for flow cytometry (C34554; Thermo Fisher, Waltham, MA)
AB7: CY™5 AffiniPure Goat Anti-Mouse IgG (H + L) (115-175-146; Jackson ImmunoResearch) RRID: AB_2338713
AB8: EZ-Link™ Sulfo-NHS-LC-Biotinylation Kit (21327; Thermo Fisher)
AB9: Goat anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (A-11029; Thermo Fisher) RRID: AB2534088
AB10: Goat anti-Human IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 (A-A21433; Thermo Fisher) RRID: AB_2534088
AB11: gp130 mAb (AN-H2) (sc-9994; Santa Cruz Biotechnology, Dallas, TX) RRID: AB_627685
AB12: Pierce™ Protein A, Biotinylated (29989; Thermo Fisher). Nickname biotinylated Protein A
AB13: Rabbit IgG-BIOT (0111-08; SouthernBiotech, Birmingham, AL) RRID: AB _627685
AB14: (RAH) rabbit mAb [H169-1-5] anti-Human IgG Fc (ab125909; Abcam, Cambridge, MA)
AB15: Rabbit F(ab’)2 Anti-Human IgG(H + L)-UNLB (6000-01; SouthernBiotech)
AB16: Rabbit Anti-Human IgG(H + L)-UNLB (6140-01; SouthernBiotech)
AB17: Rabbit Fab Anti-human IgG (H&L) (809-4102; Rockland Immunochemicals, Pottstown, PA)
AB18: Streptavidin, Alexa Fluor™ 555 conjugate (S21381; Thermo Fisher) RRID: AB_2307336
AB19: Anti-NMDAR1 Antibody, clone 54.1 (MAB363; Millipore Sigma, St. Louis, MO) RRID: AB_94946
AB20:Anti-NMDAR1 Antibody, (all splice variants), clone R1JHL (MAB1586; Millipore Sigma)
Expression of the OCMS™ tandem scFv anchor on fusion partner and 293T cell lines
The LCX cell line was created by expressing hTERT and a constitutively active gp130, gp130ΔΔYY, in K6H6/B5 murine/human hybrid cells (ATCC® CRL-1823™).Citation36 Standard retroviral transduction methods were followed with the plasmids pMSCVpuro hTERT and pMSCVhygro gp130ΔΔYY (Takara Bio, Mountain View, CA) (gp130ΔΔYY was synthesized by Genscript, Piscataway, NJ).Citation21,Citation37 Cells expressing gp130ΔΔYY were isolated by fluorescence-activated cell sorting (FACS) using the BD FACSCanto II (Becton Dickinson, Franklin Lakes, NJ, USA) with a gp130 mAb (sc-9994; Santa Cruz Biotechnology) (AB11) (1 µg/million cells) and 1:200 CY™5 goat-anti-mouse IgG (115-175-146; Jackson ImmunoResearch) (AB7) (data not shown). Expression of hTERT was verified by RT-PCR (data not shown).Citation21
A DNA construct encoding the tandem scFv Anchor (Supplementary Figures 1–3) was synthesized. It encodes a kappa Ig leader sequence, a pair of murine scFvs (specific for the rabbit IgG CH1 domain and separated by a Gly-Ser linker), a MYC tag, and a minimal transmembrane domain from PDGF receptor beta (UniProtKB - P09619) (Supplementary Figures 1–3).Citation19,Citation20 The construct was subcloned into the vector pLXSN (Takara Bio) by GenScript, to create pLXSN OCMS™, which was introduced into the LCX, B5-6T, and 8C5 cell lines to create LCX OCMS™, B5-6T OCMS™, and 8C5 OCMS™, respectively, using standard retroviral transduction methods. B5-6T is a previously described fusion partner cell line.Citation21 8C5 is a hybridoma derived from B5-6T that expresses a human mAb specific for the rabies glycoprotein (data not shown). The OCMS™ DNA construct was also subcloned into pMSCVpuro (Takara Bio), creating pMSCVpuro OCMS™, which was expressed in the 293T cell line (ATCC® CRL-3216™) by retroviral transduction with pCL-Ampho.Citation38 Fusion partner cells and hybridomas were cultured in Advanced RPMI-1640 (Thermo Fisher) with 1% fetal bovine serum (FBS; 100–106; Gemini Bio-Products, West Sacramento, CA). 293T cell lines were cultured in DMEM (Thermo Fisher) with 10% FBS (Gemini Bio-Products). Cell populations expressing the Anchor were isolated by FACS following binding to Rabbit IgG-BIOT (0111-08; SouthernBiotech) (AB13) (1 µg/million cells) and 1:200 APC streptavidin (016-130-084; Jackson ImmunoResearch) (AB4).
Capture and display of human mabs on OCMS™ cells
Human IgG binding to the B5-6T OCMS™ and LCX OCMS™ cell lines in the presence of a Linker ()) was evaluated by incubating 106 cells with a 1 µg rabbit Anti-Human IgG(H + L)-UNLB (6140-01; SouthernBiotech) (AB16) and 1 µg 1B8 human IgG mAb (specific for PV; data not shown), followed by 1:200 APC AffiniPure F(ab’)2 fragment Goat anti-rabbit IgG (H + L) (111-136-144; Jackson ImmunoResearch) (AB5) and 1:200 Alexa Fluor 488® AffiniPure F(ab’)2 Fragment Goat Anti-Human IgG, F(ab’)2 fragment specific (109-546-097; Jackson ImmunoResearch) (AB1). Some of the B5-6T and LCX cells sorted into the rabbit IgG+/human IgG- quadrant (18.4% and 29.3%, respectively), which was likely due to non-specific interactions between the cells and antibody reagents, as it was seen without human IgG binding. In ), we used the same procedure to test rabbit IgG and human 1B8 binding to trypsinized 293T OCMS™ cells, but with the RAH (AB14) substituted for the polyclonal rabbit IgG (AB16).
The importance of RAH for human IgG binding to OCMS™ cells was assessed by flow cytometry. 1 × 106 B5-6T OCMS™ and LCX OCMS™ ()), or 293T OCMS™ ()) were incubated with or without 1 µg/ml RAH (AB14) for 1 hour, washed with PBS 1% BSA, incubated with or without 1B8 (1 µg/ml) for 1 hour, washed, then incubated with 1:200 Alexa Fluor 488® AffiniPure F(ab’)2 Fragment Goat Anti-Human IgG, F(ab’)2 fragment specific (109-546-170; Jackson ImmunoResearch) (AB1), and analyzed with the BD FACS Canto II (Becton Dickinson).
Assessment of immune complexes on OCMS™ -enabled cells
Fluorescence microscopy was used to assess viral antigen binding to OCMS™ hybridomas with surface-captured human IgG. 5 × 104 B5-6T OCMS™ and 8C5 OCMS™ cells were plated on round Corning™ BioCoat™ 12 mm #1 German Glass Coverslips (354087, Corning, NY) in 24-well plates in advanced RPMI with 1% FBS ()). Cells were cultured overnight with 1 µg/ml RAH (AB14) at 37°C in 5% CO2, washed with PBS 1% BSA, incubated with 1 µg/ml biotinylated rabies GP (courtesy of Dr. Matthias Schnell, Thomas Jefferson University, Philadelphia, PA) in PBS 1% BSA for 1 hour, washed and incubated with 1:200 Alexa Fluor 488® streptavidin (016-540-084; Jackson ImmunoResearch) (AB3) and analyzed by fluorescence microscopy. The GP was biotinylated with the EZ-Link™ Sulfo-NHS-LC-Biotinylation Kit (21435; Thermo Fisher) (AB8).
PV and GP binding to the 8C5 OCMS™ and 4G4 OCMS™ cells was compared ()). 4G4 OCMS™ makes a mAb specific for PV (). Cells were plated on German Glass Coverslips as above, incubated overnight with monovalent rabbit Fab Anti-human IgG (H&L) (AB17) (809-4102; Rockland Immunochemicals, Pottstown, PA), washed and incubated with additional rabbit Fab anti-human IgG (AB17) and either 1 µg/ml un-modified rabies GP or 10 µL Sabin PV type III (109 TCID50/ml) in PBS 1% BSA for 1 hour, washed and incubated with 1:200 Alexa Fluor® 488 AffiniPure F(ab’) Fragment Goat Anti-Human IgG, Fcγ fragment specific (109-546-170; Jackson ImmunoResearch) (AB2), and then imaged by confocal microscopy.
To assess specific capture of secreted mAbs by hybridomas ()), fusion partner cell lines B5-6T OCMS™ and LCX OCMS™ were labeled with CellTrace™ CFSE Cell Proliferation Kit (C34554; Thermo Fisher) (AB6) and mixed with 8C5 OCMS™ or 4G4 OCMS™, respectively, at 10:1 ratio (non-secreting cells:mAb-secreting hybridomas). Cells were plated on German Glass coverslips as above and incubated overnight with 1 µg/ml rabbit F(ab’)2 anti-human IgG (H + L) (6000-01; SouthernBiotech) (AB15). The cells were washed three times with PBS 1% BSA, incubated for 1 hour at RT with additional rabbit anti-human IgG (AB15) and 1 µg/ml Pierce™ Protein A, Biotinylated (29989; Thermo Fisher) (AB12), then washed and incubated for 1 hour with 1:200 APC streptavidin (016-540-084; Jackson ImmunoResearch) (AB4), and imaged by confocal microscopy.
Confocal microscopy
Cells were processed for confocal microscopy by washing three times with PBS 1% BSA and fixing with 4% paraformaldehyde (PFA) in PBS for 15 minutes at RT. After fixation, cells were washed 3 times with PBS 1% BSA, once with PBS, and once with dH2O. Then the coverslips were mounted with ProLong® Gold Antifade reagent with DAPI (P36935; Thermo Fisher) or without DAPI (P36934; Thermo Fisher). Slides were imaged with a C2+ Nikon confocal microscope with 63x/1.3 NA oil objective; images were analyzed with ImageJ software (https://imagej.nih.gov/ij/).
Hybridoma generation and screening
Human monoclonal antibodies were cloned following previously described methods.Citation22,Citation29 PBMCs were stored frozen in 90% FBS (Gemini Bio-Products) and 10% DMSO (Sigma-Aldrich, St. Louis, MO) under liquid nitrogen until needed. Prior to cell fusion, CD27+ PBMCs were isolated with anti-CD27 magnetic beads (Miltenyi Biotec, Auburn, CA) per manufacturer’s instructions and cultured for 8 days in Advanced RPMI supplemented with 10% FBS, Human UltraCD40L (Multimeric Biotherapeutics, La Jolla, CA), cytokines and other growth factors. Cultured cells were electrofused to the LCX OCMS™ heteromyeloma cell line in a 1:3 ratio, PBMCs to LCX OCMS™ cells, and cells were seeded at 1000 PBMCs/well in 96-well plates. Nascent hybrid cells were selected with HAT (Sigma-Aldrich) in Advanced RPMI + 1% fetal calf serum.
We performed 2 cell fusions with PBMCs from the PV-immune subjects, P3 and P6. We fused 5 × 105 CD27+ PBMCs to LCX OCMS™ cells and plated in five 96-well plates at 1000 PBMCs/well. PV-specific mAbs were identified by whole PV ELISA.Citation22 A total of 8 pools with OD450 2.0 or greater were selected from the P3 and P6 samples, of which 7 gave rise to stable monoclonal hybridomas following limiting dilution cloning. PV mAb neutralization titers were determined using the microneutralization test.Citation39,Citation40 We used the PV strains Sabin US reference stock NC2 (Type 3) and wild type Saukett (Type 3), provided by Dr. Emmanuel Vidor, Sanofi-Pasteur.
To screen for expression of anti-NMDAR mAbs, we used a soluble NMDAR ATD derived from the ionotropic NMDAR GluN1 subunit (NR1), as produced by 293T-ATD cells.Citation28 The 293T-ATD cells were plated at 2 × 105 cells/well in 12-well plates. The next day, they were trypsinized, washed with PBS, and treated with 25 μg rTEV Protease (4469; R&D Systems, Minneapolis, MN) with Xpert Protease inhibitor cocktail solution (P3100–001; GenDEPOT, Barker, TX) in PBS for 10 min. The cells were centrifuged in Eppendorf tubes at 3000 rpm for 10 min at 4°C, then the supernatant was collected and dialyzed against cold PBS overnight. Protein concentration was measured using the NanoDrop 1000 (Thermo Fisher) and the ATD was visualized on a Coomassie-stained SDS:PAGE gel. The ATD was biotinylated with the EZ-LinkTM Sulfo-NHS-Biotin kit (21326; Thermo Fisher) (AB8).
Four x 105 CD27+ PBMCs from an ANRE patient were fused as described above and plated in four 96-well plates at 1000 cells/well. Approximately 70% of the wells produced viable hybridomas. Hybridomas were screened 14 days following cell fusion. Cells were incubated overnight with 1 µg/ml RAH (AB14) and 1 µg/ml biotinylated ATD in fresh cell culture medium, followed without washing by 1:200 Streptavidin, Alexa Fluor™ 555 conjugate (S21381; Thermo Fisher) (AB18). The wells were visualized by fluorescence microscopy and 1 well was found positive. The plates were also screened using the 293T ATD whole cell ELISA, with concordant results (data not shown).Citation29 The positive hybridoma pool (3C11) was subcloned 3 times by limiting dilution.
Following subcloning, all hybridomas were adapted to medium with 5% Ultra Low IgG FBS (Life Technologies, Grand Island, NY), and then incubated for 5 days in 500-ml roller bottles. Filtered supernatants were purified over protein G-Sepharose (Life Technologies). Antibody concentrations were determined using the NanoDrop spectrophotometer (Thermo Fisher). IgG heavy chain and light chain subtypes were determined by ELISA as described.Citation22
PV and igG binding by OCMS™ -enabled hybridomas
To test PV binding to OCMS™ hybridomas by fluorescence microscopy ()), cells were incubated overnight with 1 µg/ml rabbit F(ab’)2 anti-human IgG (H + L) (AB15) in culture medium. The cells were washed and incubated with 20 µL biotinylated Sabin type III PV (8 × 105 PFU) in PBS 1% BSA, and binding was detected with 1:200 Alexa Fluor 488® Streptavidin (016-540-084; Jackson ImmunoResearch) (AB3). PV was biotinylated with the EZ-Link™ Sulfo-NHS-LC-Biotinylation Kit (21435; Thermo Fisher) (AB8). To assess binding of PV specific IgGs on the surface of PV OCMS™ hybridomas by flow cytometry ()), 5× 105 PV OCMS™ cells were plated in 6-well plates in culture medium with 1 µg/ml rabbit F(ab’)2 Anti-Human IgG(H + L) (AB15). After overnight incubation at 37°C in 5% CO2, cells were washed three times with PBS 1% BSA, incubated with 50 µL biotinylated PV (2 × 106 PFU) for 1 hour at 37°C in 5% CO2, washed three times, then incubated with 1:200 APC streptavidin (016-130-084; Jackson ImmunoResearch) (AB4). Human and rabbit IgG binding to the PV OCMS™ cell lines ()) were assessed by incubating 5 × 105 cells overnight with 1 µg/ml rabbit F(ab’)2 Anti-Human IgG (H + L) (6000-01; SouthernBiotech) (AB15), followed by washing and then 1 µg/ml Pierce™ Protein A, Biotinylated (29989; Thermo Fisher) (AB12) for 1 hour. Cells were washed and incubated with 1:200 Alexa Fluor 488® Streptavidin (016-540-084; Jackson ImmunoResearch) (AB3) and 1:200 APC AffiniPure F(ab’)2 fragment Goat anti-rabbit IgG (H + L) (111-136-144; Jackson ImmunoResearch) (AB5) for one hour, and then analyzed by flow cytometry.
A human mAB specific for the NMDAR ATD
Binding of the NMDAR ATD to mAbs on OCMS™ cells was tested using the LCX OCMS™ cell line and a human anti-NMDAR mAb, 5F5, previously cloned in this laboratory.Citation29 We cultured 75,000 LCX OCMS™ cells/well on German Glass Coverslips in a 24-well plate at 37°C and 5% CO2. The following day, we added 1 μg/ml RAH (AB14) in PBS 1% BSA and incubated for 1 hour at RT, followed by 2 washes and either the 5F5 mAb or the 6A control mAb at 1 μg/ml. After one hour, the cells were washed and incubated with biotinylated ATD antigen (1 μg/ml) for one hour, then washed and incubated with 1:50 Alexa Fluor 488® streptavidin (016-540-084; Jackson ImmunoResearch) (AB3), and analyzed by confocal microscopy ()).
The antigen-binding specificity of the 3C11 OCMS™ mAb was tested in comparison to the 4G4 OCMS™ mAb ()). 75,000 3C11 OCMS™ and 4G4 OCMS™ cells were plated on German Glass Coverslips in 24-well plates in advanced RPMI with 1% FBS and 1 µg/ml RAH (AB14). After an overnight incubation at 37°C, cells were washed with PBS 1% BSA and incubated with 1 µg/ml biotinylated ATD for one hour. ATD antigen on the surface of OCMS™ hybridomas was then detected with 1:200 Streptavidin, Alexa Fluor™ 555 conjugate (S21381; Thermo Fisher) (AB18) and confocal microscopy ()).
Purified 3C11 was tested for binding to the 293T-ATD cell line as previously described ()).Citation28 50,000 293T-ATD cells were plated on German Glass Coverslips in 24-well plates overnight, then incubated for one hour with 5 µg/ml 3C11 or the isotype control 6A.Citation30 The cells were washed with PBST followed by 10 µg/ml Goat anti-Human IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 (A-A21433; Thermo Fisher) (AB10). After 1 hour, the cells were washed again and incubated with 5 μg/mL Anti-NMDAR1 Antibody, (all splice variants), clone R1JHL (MAB1586; Millipore Sigma) (AB20), then washed and incubated with Goat anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (A-11029; Thermo Fisher) (AB9). Cells were examined by confocal microscopy.
3C11 and 6A were tested for binding to primary rat hippocampal neurons obtained from the Cellular Neuroscience Core Facility at the Children’s Hospital of Pennsylvania.Citation29 Cells were grown in Neurobasal Medium supplemented with 200 mmol/L GLUTAMAX and 2% B-27 Supplement (Thermo Fisher). 105 cells/well were plated in 24-well plates on German Glass Coverslips and cultured for 14 days. Cells were washed with PBST, fixed with 4% PFA in PBS, then blocked with PBS 1% BSA, 10% goat serum (PBS+G + B). Cells were then incubated with 5 µg/ml 3C11 or 6A, followed by washing and then 10 µg/ml Goat anti-Human IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 (A-A21433; Thermo Fisher) (AB10). After 1 hour, the wells were washed and incubated for one hour with 1 μg/mL Anti-NMDAR1 Antibody, clone 54.1 (MAB363; Millipore Sigma) (AB19), which binds the extracellular loop between transmembrane regions III & IV of the NMDAR, followed by washing and then one hour with the Goat anti-Mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (A-11029; Thermo Fisher, RRID:AB2534088) (AB9). Cells were examined by confocal microscopy ()).
Antibody and antigen binding to 293T OCMS™ cells
To test capture of expressed IgG by 293T OCMS™ and 293T cells ()), cells were plated at 3 × 104 cells/well on German Glass Coverslips in 24-well plates in DMEM with 10% FBS. The following day, cells were transfected with 1 µg each A12 IgG heavy and light chain expression plasmids (courtesy of Dr. Zhaochun Chen, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) with X-tremeGENE 9 DNA transfection reagent (Sigma Aldrich).Citation31 One day after transfection, medium was replaced with 1 ml fresh medium with 1 µg/ml RAH (AB14), and the cells were incubated for 24 hours at 37°C. The cells were washed 3 times with PBS 1% BSA, incubated for 1 hour with 1:200 Alexa Fluor 488® AffiniPure F(ab’)2 Fragment Goat Anti-Human IgG, F(ab’)2 fragment specific (109-546-097; Jackson ImmunoResearch) (AB1), and then analyzed by confocal microscopy.
To test capture of PV by A12-expressing 293T OCMS™ cells ()), 18–24 hours before transfection, 293T OCMS™ cells were plated at 1 × 105 cells/well in 6-well plates in 2 ml DMEM with 10% FBS. The following day, cells were transfected with 1 µg each of A12 heavy and light chain plasmids with X-tremeGENE 9 DNA Transfection Reagent, (06 365 787 001; SigmaAldrich). The next day, the culture medium was replaced with fresh medium and 1 µg/ml RAH (AB14), and incubated 24 hours at 37°C in 5% CO2. Cells were removed from the plate with trypsin, washed with PBS 1% BSA, and incubated with biotinylated Sabin type I (2x106 PFU/ml) in PBS 1% BSA (US Reference Stock NA4) for 1 hour at 37°C in 5% CO2. After 3 washes with PBS 1% BSA, cells were incubated with 1:200 APC streptavidin (016-130-084; Jackson ImmunoResearch) (AB4) and analyzed by flow cytometry.
Abbreviations
| Anchor | = | the tandem scFv specific for rabbit IgG, which is expressed on the outer plasma membrane of a cell |
| ANRE | = | anti-NMDA receptor encephalitis |
| ATD | = | NMDA receptor amino terminal domain |
| GP | = | rabies glycoprotein |
| IPV | = | inactivated polio vaccine |
| Linker | = | a rabbit secondary antibody specific for human IgG |
| mAb | = | monoclonal antibody |
| MYC | = | c-myc epitope tag |
| NGS | = | Next Generation (DNA) Sequencing |
| NMDAR | = | N-methyl-D-aspartate receptor |
| OCMS™ | = | On-Cell mAb Screening |
| OPV | = | oral polio vaccine |
| PBMCs | = | peripheral blood mononuclear cells |
| PDGF | = | platelet-derived growth factor |
| PFA | = | paraformaldehyde |
| PV | = | poliovirus |
| RAH | = | “Rabbit anti-Human”—a rabbit mAb specific for Human IgG Fc (AB14) |
| SA | = | streptavidin. |
Author Contributions
RDP, RS: conceptualization, methodology, investigation, writing-review and editing. ABV, DK, FHA, CDK: methodology, investigation. DRL: resources; funding acquisition. GCP: writing-review and editing. KC: supervision. SKD: conceptualization, methodology, funding acquisition, project administration, supervision, visualization, writing-original draft preparation, review and editing.
The) image was created by Nicolás Fernández (www.ilustracionesnaturaleza.com) using images from the Protein Databank Archive (www.rcsb.org).Citation18 IgG: PDB ID: 1HZH.Citation41 Streptavidin: PDB ID: 1SWE.Citation42 Poliovirus: PDB ID 1PVC.Citation43
Disclosure of Potential Conflicts of Interest
SKD and RDP state a conflict of interest related to their role as inventors on pending patent applications filed by the Lankenau Institute for Medical Research, which claim methods described in this report, and the associated OCMS™ trademark. The other authors state no conflict of interest for this work.
Supplemental Material
Download MS Word (814.6 KB)Acknowledgments
This work was supported by the World Health Organization, the PATH Foundation, NIH grant R21 AI 119368 (SKD), NIH grant R21 NS 088148 (David R. Lynch (PI), Children’s Hospital of Pennsylvania, and SKD), NIH grant NCI 5 P30 CA-56036 (Bioimaging Shared Resource of the Sidney Kimmel Cancer Center, Thomas Jefferson University), and the Lankenau Institute for Medical Research. SKD holds the Joseph and Ray Gordon Chair for Clinical Oncology and Research and GCP holds the Havens Chair for Biomedical Research at the Lankenau Institute for Medical Research. Thanks to Dr. Matthias Schnell, Thomas Jefferson University, Philadelphia, PA, for the rabies GP and to Dr. Zhaochun Chen, National Institute of Allergy and Infectious Diseases, for the A12 mAb expression plasmids.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–97.
- Smith SA, Crowe, Jr. JE Jr. Use of human hybridoma technology to isolate human monoclonal antibodies. Microbiol Spectr. 2015;3. doi:10.1128/microbiolspec.AID-0027-2014.
- Rossant CJ, Carroll D, Huang L, Elvin J, Neal F, Walker E, Benschop JJ, Kim EE, Barry ST, Vaughan TJ. Phage display and hybridoma generation of antibodies to human CXCR2 yields antibodies with distinct mechanisms and epitopes. MAbs. 2014;6:1425–38. doi:10.4161/mabs.34376.
- Foltz IN, Gunasekaran K, King CT. Discovery and bio-optimization of human antibody therapeutics using the XenoMouse® transgenic mouse platform. Immunol Rev. 2016;270:51–64. doi:10.1111/imr.12409.
- Spencer S, Bethea D, Raju TS, Giles-Komar J, Feng Y. Solubility evaluation of murine hybridoma antibodies. MAbs. 2012;4:319–25. doi:10.4161/mabs.19869.
- Feldhaus MJ, Siegel RW, Opresko LK, Coleman JR, Feldhaus JM, Yeung YA, Cochran JR, Heinzelman P, Colby D, Swers J, et al. Flow-cytometric isolation of human antibodies from a nonimmune saccharomyces cerevisiae surface display library. Nat Biotechnol. 2003;21:163–70. doi:10.1038/nbt785.
- Layton D, Laverty C, Nice EC. Design and operation of an automated high-throughput monoclonal antibody facility. Biophys Rev. 2013;5:47–55. doi:10.1007/s12551-012-0095-6.
- Yokoyama WM, Christensen M, Dos Santos G, Miller D, Ho J, Wu T, Dziegelewski M, Neethling FA. Production of monoclonal antibodies. Curr Protoc Immunol. 2013 Oct 1;102:Unit 2.5. doi:10.1002/0471142735.im0205s102. https://www.ncbi.nlm.nih.gov/pubmed/24510488.
- Kenney JS, Gray F, Ancel MH, Dunne JF. Production of monoclonal antibodies using a secretion capture report web. Biotechnology. 1995;13:787–90.
- Weaver JC, McGrath P, Adams S. Gel microdrop technology for rapid isolation of rare and high producer cells. Nat Med. 1997;3:583–85.
- El Debs B, Utharala R, Balyasnikova IV, Griffiths AD, Merten CA. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc Natl Acad Sci U S A. 2012;109:11570–75. doi:10.1073/pnas.1204514109.
- Carroll S, Al-Rubeai M. ACSD labelling and magnetic cell separation: a rapid method of separating antibody secreting cells from non-secreting cells. J Immunol Methods. 2005;296:171–78. doi:10.1016/j.jim.2004.11.007.
- Manz R, Assenmacher M, Pfluger E, Miltenyi S, Radbruch A. Analysis and sorting of live cells according to secreted molecules, relocated to a cell-surface affinity matrix. Proc Natl Acad Sci U S A. 1995;92:1921–25.
- Kida A, Iijima M, Niimi T, Maturana AD, Yoshimoto N, Kuroda S. Cell surface-fluorescence immunosorbent assay for real-time detection of hybridomas with efficient antibody secretion at the single-cell level. Anal Chem. 2013;85:1753–59. doi:10.1021/ac303067k.
- Price PW, McKinney EC, Wang Y, Sasser LE, Kandasamy MK, Matsuuchi L, Milcarek C, Deal RB, Culver DG, Meagher RB. Engineered cell surface expression of membrane immunoglobulin as a means to identify monoclonal antibody-secreting hybridomas. J Immunol Methods. 2009;343:28–41. doi:10.1016/j.jim.2009.01.005.
- Mattiazzi Usaj M, Styles EB, Verster AJ, Friesen H, Boone C, Andrews BJ. High-content screening for quantitative cell biology. Trends Cell Biol. 2016;26:598–611. doi:10.1016/j.tcb.2016.03.008.
- Fraietta I, Gasparri F. The development of high-content screening (HCS) technology and its importance to drug discovery. Expert Opin Drug Discov. 2016;11:501–14. doi:10.1517/17460441.2016.1165203.
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28:235–42.
- Shen Z, Mernaugh RL, Yan H, Yu L, Zhang Y, Zeng X. Engineered recombinant single-chain fragment variable antibody for immunosensors. Anal Chem. 2005;77:6834–42. doi:10.1021/ac0507690.
- Ho M, Pastan I. Display and selection of scFv antibodies on HEK-293T cells. Methods Mol Biol. 2009;562:99–113. doi:10.1007/978-1-60327-302-2_8.
- Adekar SP, Jones RM, Elias MD, Al-Saleem FH, Root MJ, Simpson LL, Dessain, SK. A human monoclonal antibody that binds serotype A botulinum neurotoxin. Hybridoma. 2005;2008(27):11–17.
- Puligedda RD, Kouiavskaia D, Al-Saleem FH, Kattala CD, Nabi U, Yaqoob H, Bhagavathula VS, Sharma R, Chumakov K, Dessain SK. Characterization of human monoclonal antibodies that neutralize multiple poliovirus serotypes. Vaccine. 2017;35:5455–62. doi:10.1016/j.vaccine.2017.03.038.
- Puligedda RD, Kouiavskaia D, Adekar SP, Sharma R, Devi Kattala C, Rezapkin G, Bidzhieva B, Dessain SK, Chumakov K. Human monoclonal antibodies that neutralize vaccine and wild-type poliovirus strains. Antiviral Res. 2014;108:36–43. doi:10.1016/j.antiviral.2014.05.005.
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091. doi:10.1016/S1474-4422(08)70224-2.
- Kreye J, Wenke NK, Chayka M, Leubner J, Murugan R, Maier N, Jurek B, Ly L-T, Brandl D, Rost BR, et al. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain. 2016;139:2641–52. doi:10.1093/brain/aww208.
- Malviya M, Barman S, Golombeck KS, Planaguma J, Mannara F, Strutz-Seebohm N, Wrzos C, Demir F, Baksmeier C, Steckel J, et al. NMDAR encephalitis: passive transfer from man to mouse by a recombinant antibody. Ann Clin Transl Neurol. 2017;4:768–83. doi:10.1002/acn3.444.
- Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci. 2012;32:11082–94. doi:10.1523/JNEUROSCI.0064-12.2012.
- Sharma R, Al-Saleem FH, Puligedda RD, Rattelle A, Lynch DR, Dessain SK. Membrane-bound and soluble forms of an NMDA receptor extracellular domain retain epitopes targeted in auto-immune encephalitis. BMC Biotechnol. 2018;18:41. doi:10.1186/s12896-018-0440-3.
- Sharma R, Al-Saleem FH, Panzer J, Lee J, Puligedda RD, Felicori LF, Kattala CD, Rattelle AJ, Ippolito G, Cox RH, et al. Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis. Ann Clin Transl Neurol. 2018;5:935–51. doi:10.1002/acn3.592.
- Adekar SP, Jones RM, Elias MD, Al-Saleem FH, Root MJ, Simpson LL, Dessain SK. Hybridoma populations enriched for affinity-matured human IgGs yield high-affinity antibodies specific for botulinum neurotoxins. J Immunol Methods. 2008;333:156–66. doi:10.1016/j.jim.2008.01.015.
- Chen Z, Fischer ER, Kouiavskaia D, Hansen BT, Ludtke SJ, Bidzhieva B, Makiya M, Agulto L, Purcell RH, Chumakov K. Cross-neutralizing human anti-poliovirus antibodies bind the recognition site for cellular receptor. Proc Natl Acad Sci U S A. 2013;110:20242–47. doi:10.1073/pnas.1320041110.
- Feldhaus MJ, Siegel RW. Yeast display of antibody fragments: a discovery and characterization platform. J Immunol Methods. 2004;290:69–80. doi:10.1016/j.jim.2004.04.009.
- Tillotson BJ, Goulatis LI, Parenti I, Duxbury E, Shusta EV, Ho M. Engineering an anti-transferrin receptor ScFv for pH-sensitive binding leads to increased intracellular accumulation. PLoS One. 2015;10:e0145820. doi:10.1371/journal.pone.0145820.
- Puri V, Streaker E, Prabakaran P, Zhu Z, Dimitrov DS. Highly efficient selection of epitope specific antibody through competitive yeast display library sorting. MAbs. 2013;5:533–39. doi:10.4161/mabs.25211.
- Priola JJ, Calzadilla N, Baumann M, Borth N, Tate CG, Betenbaugh MJ. High-throughput screening and selection of mammalian cells for enhanced protein production. Biotechnol J. 2016;11:853–65. doi:10.1002/biot.201500579.
- Carroll WL, Thielemans K, Dilley J, Levy R. Mouse x human heterohybridomas as fusion partners with human B cell tumors. J Immunol Methods. 1986;89:61–72.
- Sommer J, Effenberger T, Volpi E, Waetzig GH, Bernhardt M, Suthaus J, Garbers C, Rose-John S, Floss DM, Scheller J. Constitutively active mutant gp130 receptor protein from inflammatory hepatocellular adenoma is inhibited by an anti-gp130 antibody that specifically neutralizes interleukin 11 signaling. J Biol Chem. 2012;287:13743–51. doi:10.1074/jbc.M111.349167.
- Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–05.
- Machida K, Cheng KT, Sung VM, Shimodaira S, Lindsay KL, Levine AM, Lai M-Y, Lai MMC. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc Natl Acad Sci U S A. 2004;101:4262–67. doi:10.1073/pnas.0303971101.
- WHO. Polio laboratory manual. Geneva (Switzerland): World Health Organization; 2004.
- Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–59. doi:10.1126/science.1061692.
- Freitag S, Le Trong I, Klumb L, Stayton PS, Stenkamp RE. Structural studies of the streptavidin binding loop. Protein Sci. 1997;6:1157–66. doi:10.1002/pro.5560060604.
- Filman DJ, Syed R, Chow M, Macadam AJ, Minor PD, Hogle JM. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. Embo J. 1989;8:1567–79.
