ABSTRACT
A sensitive and specific bioanalytical method was required to measure the exposure of a LAGA-mutated surrogate mouse IgG2a monoclonal antibody in mouse plasma, but the lack of highly specific reagents for the LAGA mutant hindered the development of a ligand-binding assay. Equally problematic is that no sensitive unique tryptic peptides suitable for quantitative mass spectrometric analysis could be identified in the mIgG2a complementarity-determining regions. To overcome these challenges, a trypsin alternative pepsin, an aspartic protease, was systematically investigated for its use in digesting the mutated mIgG2a antibody to allow generation of signature peptides for the bioanalytical quantification purpose. After a series of evaluations, a rapid one-hour pepsin digestion protocol was established for the mutated Fc backbone. Consequently, a new pepsin digestion-based liquid chromatography-tandem mass spectrometry (LC/MS/MS) method was successfully developed to support the mouse pharmacokinetic (PK) sample analysis. In brief, robust and reproducible C-terminal cleavage of both leucine and phenylalanine near the double mutation site of the mutated mIgG2a was accomplished at pH ≤2 and 37°C. Combined with a commercially available rat anti-mIgG2a heavy-chain antibody, the established immunoaffinity LC/MS/MS assay achieved a limit of quantitation of 20 ng/mL in the dynamic range of interest with satisfactory assay precision and accuracy. The successful implementation of this novel approach in discovery PK studies eliminates the need for tedious and costly generation of specific immunocapturing reagents for the LAGA mutants. The approach should be widely applicable for developing popular LAGA mutant-based biological therapeutics.
The L235A/G237A (LAGA) mutation of the IgG constant region has been widely used to mitigate monoclonal antibody effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC) in antibody-based biotherapeutics.Citation1,Citation2 In order to better understand how such promising mutated antibody drug candidates interact with targets and trigger the expected mechanism of action in humans, the same type of LAGA mutation has been frequently incorporated to surrogate mouse antibodies and evaluated across chosen syngeneic mouse models even at the early discovery stage.Citation3,Citation4 As such, the analysis of surrogate mouse antibodies in mice becomes an essential step in facilitating mouse-to-human translation of pharmacological effects.
Quantifying mutated mouse IgG isotypes in mouse PK studies imposes challenges to the required bioanalytical method development, especially when recombinant target antigens or highly specific anti-idiotypic antibodies are not available.Citation5 In those studies, the ligand-binding assay tends to be inadequate and the “bottom-up” protein analysis based on mass spectrometry (MS) is generally preferred over intact protein analysis because of its improved sensitivity as well as uncompromised specificity. However, having at least one unique signature peptide per monoclonal antibody, identified in the complementary-determining regions (CDRs) or mutation sites, is a prerequisite for such bioanalyses.
A number of endoproteases have been widely used in the “bottom-up” bioanalytical sample preparation workflow, such as trypsin, lysC, and GluC.Citation6–9 Among them, tryptic digestion has been extensively used due to its widely recognized digestion efficiency, specificity, reproducibility, and MS compatibility. Compared to trypsin, pepsin has primarily been a complementary protease for peptide mappingCitation10,Citation11 and de novo protein sequencing.Citation12–14 Despite its renowned utility in hydrogen/deuterium exchange (HDX) experimentsCitation15–18 and antibody antigen-binding fragment production,Citation19 the use of pepsin in targeted protein quantification has largely been understudied.
With no overlap to tryptic digestion, pepsin frequently cleaves c-terminal of leucine (L), phenylalanine (F), tyrosine (Y), and tryptophan (W) in protein sequences under highly acidic condition (pH ≤ 2).Citation8,Citation9 A small number of reports suggested that distinct surrogate peptides could be produced by pepsin-based proteolysis for quantitative analysis.Citation20,Citation21 For example, by using overnight pepsin digestion followed by the SISCAPA approach, Yang X et al.Citation20 successfully quantified α-tubulin acetylation adduct, which was unachievable using trypsin. In a separate study, it was also demonstrated that pepsin could reach similar quantitative reproducibility compared to trypsin in both online and offline applications.Citation21
Herein, we report the development of a rapid peptic digestion method to quantify the LAGA mutated peptide of an engineered mIgG2a monoclonal antibody dosed in mice. Driven initially by our unsuccessful attempt to produce usable tryptic peptides from mIgG2a CDRs, our study demonstrated a potential cost-effective and reproducible peptic digestion method to measure proteins that were not readily amenable to digestion by trypsin or other popular proteases.
Materials and methods
Chemicals, reagents and apparatus
The test article, Takeda proprietary mouse IgG2a (TR4495) monoclonal antibody, was provided by the Takeda Global Biologics (Cambridge, MA). Trifluoroacetic acid (TFA), Trizma base, Tween 20 were purchased from MilliporeSigma (St. Louis, MO or Milwaukee, IL). C57BL/6J mouse K2EDTA plasma was purchased from BioIVT (Hicksville, NY). Acetonitrile, water, and formic acid (FA) were HPLC grade or better, along with biotinylated rat anti-mIgG2a heavy chain (HC) (MA5–16788, not cross-reactive with other murine immunoglobulin classes or subclasses), and Dynabeads™ M-280 (streptavidin coated), were purchased from Thermo Fisher (Chicago, IL). A stable isotope-labeled surrogate peptide of the mIgG2a LAGA mutant (SIL-AGAPSVF*, heavy labeled phenylalanine [13C915N] +10 Da) was synthesized by Life Technologies (Rockford, IL). Different lots of porcine pepsin were purchased either from Promega (Cat No. V1959) (Madison, WI) or MilliporeSigma (Cat No. P6887) (Saint Louis, MO). Tris buffer (0.2 M, pH 8), 1× phosphate-buffered saline (PBS) buffer (pH 7.4), PBST1 (PBS with 0.01% Tween 20), PBST5 (PBS with 0.05% Tween 20) were prepared in house. KingFisher Flex magnetic beads processor used for immunoaffinity enrichment and HP D300 multidrop dispenser used for direct antibody dispensing, were purchased from ThermoFisher Scientific (Waltham, MA or Pittsburgh, PA).
Preparation of calibration standards (STDs), quality control (QC) samples
STDs at 20, 50, 250, 500, 1000, 2000, 5000, and 10,000 ng/mL, QCs at 20, 60, 800, and 8000 ng/mL, were prepared in C57BL/6J mouse plasma by direct dispensing from a TR4495 stock solution of 3.7 mg/mL (containing 0.3% Tween 20) using an HP D300 dispenser.
Liquid chromatography and MS
A Shimadzu Nexera UHPLC system (Kyoto, Japan) was interfaced to a Sciex 6500 QTRAP mass spectrometer (Framingham, MA). The reversed phase HPLC separation was carried out on a Phenomenex Synergi Fusion column (30 × 2.0 mm, 2.5 μm, 100 Å). Mobile phase A (water containing 0.1% FA) and mobile phase B (acetonitrile containing 0.1% FA) were delivered at a total flow of 0.6 mL/min and at 35°C to the column. For the surrogate peptide analysis, the gradient was as follows: 0 min, 3% B; 0.1 min, 3% B; 2.0 min, 45% B; and 2.01–2.50 min, 80% B; 2.60–3 min, 3% B. Performed under a positive ion electrospray voltage of 5000 V, a source temperature of 600°C, two [M+H]+>[y4]+ transitions, 648.3 > 449.2 for AGAPSVF and 658.3 > 459.2 for its internal standard AGAPSVF* (dwell time of 100 ms and CE = 31 for both), were monitored in the analysis. In addition, GS1, GS2, and curtain gas pressure were set to 60 L/min, 60 L/min, and 30 psi, respectively. Chromatographic data were acquired and processed using Sciex Analyst 1.7.2. Linear calibration curves were established based on peak area ratio (analyte/IS) vs concentration using 1/XCitation2 weighting to enable the assay optimization as well as quantification of unknowns.
Optimization of required pepsin concentration and incubation duration
On a 96-well plate, 136 picoliter of TR4495 stock solution was dispensed into 120 µL of 0.1% TFA solution (pH ≈ 2) using the HP D300 dispenser repetitively to obtain a set of neat QC samples (45 identical aliquots) with a total TR4495 amount equivalent to the desired ULOQ level, which was 10 µg/mL by 50 µL of assay volume intended for the mouse PK study sample analysis. After adding 20 µL of IS (SIL AGAPSVF*, 25 ng/mL prepared in 5% acetonitrile in water), the 45 replicates were divided into 3 treatment groups, which were incubated with 20 µL of freshly prepared pepsin (in amounts of 10, 5, or 1 µg dissolved in 20 µL of water) at 37°C for 15, 30, 60, 90, or 120 min, respectively (n = 3). At each scheduled time point, three replicates from each group were removed from the incubator and immediately neutralized through adding 30 µL of Tris buffer (0.2 M, pH 8) plus 30 µL of acetonitrile to each well to deactivate pepsin. The sample plate was centrifuged for 10 min at 3000 rpm and total 3 µL of supernatants were injected for liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis.
Reproducibility test of pepsin digestion across different lots under optimized conditions
Two levels of QC samples (equivalent to 0.1 µg/mL and 10 µg/mL by 50 µL volume) were prepared as described above in order to mimic the downstream digestion process of beads eluates. For the lot-to-lot comparison, 10 µg of pepsin (0.5 mg/mL × 20 µL per well) was prepared using four individual lots of pepsin. There were 3 replicates per level for each pepsin lot and only the optimized peptic digestion time of 60 min was assessed. With the exception of the different pepsin lots used, there were no other sample processing differences across these groups.
Precision and accuracy assessment of 1-hour pepsin digestion protocol for potential discovery bioanalytical study use
Four spiked QC levels, including 20 ng/mL (LLOQ), 60 ng/mL (LQC), 800 ng/mL (MQC), and 8000 ng/mL (HQC), were evaluated for their precision and accuracy using optimized digestion conditions and the surrogate peptic peptide AGAPSVF located at the LAGA double mutation site (n = 6).
Briefly, an aliquot (50 µL) of each spiked mouse plasma sample was transferred to a 96-well Thermo KingFisher plate with 0.25 mg of rat anti-mIgG2a HC coupled M-280 beads preadded to the plate (suspended in 100 µL of PBST1, pH 7.4). Samples and magnetic beads were mixed well, then processed with the previously published 6-cycle immunoaffinity extraction protocol on a KingFisher Flex system.Citation5 After adding 20 µL of internal standard (SIL AGAPSVF*, 25 ng/mL prepared in 5% acetonitrile in water) and 20 µL of pepsin (0.5 mg/mL freshly dissolved in water) to the TR4495-containing eluate (120 µL), the resulting solution was incubated at 37°C for 1 h with shaking (600 rpm). At the end of incubation, the sample was immediately neutralized by adding 30 µL of Tris buffer (0.2 M, pH 8) and 30 µL of acetonitrile to each well to stop the digestion. The sample plate was centrifuged for 10 min at 3000 rpm and total 20 µL of supernatants were injected to LC/MS/MS for analysis.
Quantitative analysis of preclinical study samples using multicycle immunoaffinity extraction followed by pepsin digestion
Plasma samples were collected from tumor-bearing C57BL/6J mice (15 weeks) at non-serial times from 1 to 168 h following a single intravenous administration at 10 mg/kg of TR4495 (3 mice per time point). The in-life protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Takeda. All study samples were kept frozen at −80°C prior to sample preparation following the qualified method conditions described above. PK profiles were plotted using Thermo Watson LIMS 7.6.
Results and discussion
Method development
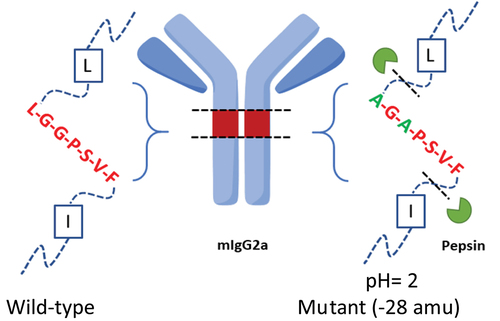
Identification of surrogate peptides for mIgG2a antibody (TR4495) quantification. Analyzing surrogate mIgG2a in mouse plasma demands extensive bioanalytical method development especially when highly specific target antigens or anti-idiotypic antibodies are not available. In this particular case, due to the high background levels of endogenous mouse immunoglobulin presented in the matrix, the identification of analyte unique peptides was essential, but none of the tryptic peptides from the mIgG2a antibody CDRs demonstrated the desired specificity and sensitivity during the initial test. Therefore, efforts shifted toward in silico digestion analysis using Skyline for potential alternative proteases. We found that a unique peptic peptide AGAPSVF extended over the LAGA mutation site could be a more favorable choice because it could serve as a shared signature across all LAGA mutants. A simplified scheme is shown in . As a result, pepsin-based digestion was prioritized for further LC/MS/MS method optimization.
Figure 1. A scheme shows the two pepsin cleavage sites in mIgG2a LAGA double mutants (right) and mass shift of 28 amu compared to its wild type counterpart (left, with ragged N-term).
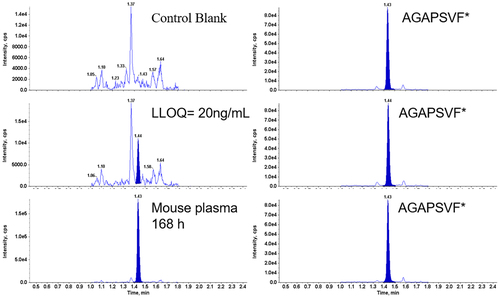
First, approximately 2 µg of neat TR4495 reference material was digested with pepsin at 37°C for 1 h. The resulted antibody digest was neutralized and injected to LC/MS/MS to confirm the detection of peptide AGAPSVF by monitoring a large number of transition pairs derived from two charge states including [M+H]1+ and [M +2 H]2+ suggested by the Skyline software. The collision energy (CE) values of the three most intensive mass transitions were further optimized. Based on both sensitivity and selectivity, two mass transitions, including 648.3 > 449.2 (CE = 31, quantifier [M+H]+>[y4]+, 1.5× higher) and 648.3 > 483.3 (CE = 25, qualifier, [M+H]+>[b6]+) were monitored in the final analysis. The above MS parameters were later confirmed after the synthetic stable isotope-labeled AGAPSVF* became available. Representative chromatograms of a control blank sample, a 20 ng/mL (LLOQ) spiked mouse plasma sample, and an in vivo mouse PK sample are shown in .
Figure 2. Representative LC/MS/MS chromatograms of the surrogate peptide AGAPSVF and stable isotope labeled AGAPSVF* from a C57BL/6J mouse plasma control blank (top panels), a spiked LLOQ sample (middle panels), a mouse PK study 168 h plasma sample after dosing (bottom panels).
Optimization of concentration and time required to achieve rapid digestion of TR4495 backbone using pepsin. In recent efforts to streamline bottom-up protein quantification workflow via LC/MS, rapid digestion has replaced overnight digestion in many applications.Citation22 A number of trypsin and/or lysC-based commercial rapid digestion kits have also been developed by vendors to meet researcher’s throughput needs.
For the same consideration, pepsin was tested for possible fast digestion of TR4495 (10 µg/mL × 50 µL = 0.5 µg) under three high enzyme-to-substrate (E/S) ratios, including 2:1, 10:1, and 20:1 (w/w) for up to 2 h. Since immunoaffinity enrichment was the selected sample cleanup workflow and high E/S ratios were used, it was assumed the digestion of magnetic bead eluates would be similar to the digestion of surrogate neat samples used in the experiment.
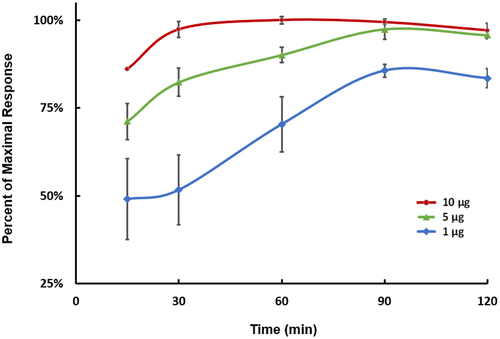
As shown in , in the group with 20:1 of E/S ratio (10 µg pepsin per well), the peak area ratio of signature peptide AGAPSVF to internal standard reached a plateau in approximately 30–60 min. After 60 min of incubation, the response of AGAPSVF was stabilized, then started to trend down gradually after 90 min. Compared to the other two groups with 50% or 10% of pepsin, respectively, the standard deviation of the high pepsin group was also significantly smaller at earlier time points, which indicated more robust proteolysis was achieved in those replicates. Based on the preliminary test, 10 µg of pepsin with 60 min incubation time was used as the standard conditions for further assessment.
Figure 3. Optimization of pepsin digestion conditions. Neat TR4495 (10 µg/mL × 50 µL) was incubated with 1, 5, or 10 µg of pepsin at 37°C for 15, 30, 60, 90, or 120 min, respectively (n = 3). The data represent the average of three analytical replicates, normalized to the maximum surrogate peptide response achieved using 10 µg of pepsin and a 60-minute incubation at 37°C. Error bars represent the standard deviations.
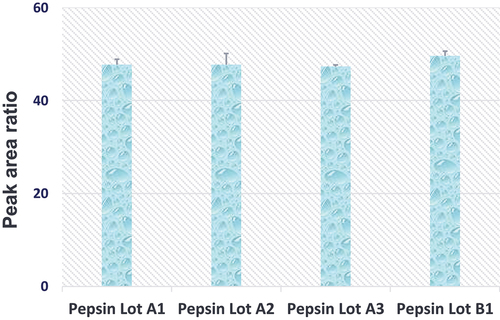
Quantitative assessment of pepsin digestion variability across lots. Since the impurity and activity levels of proteases could differ significantly across lots as a result of distinctive manufacturing processes, it was beneficial to examine the enzyme performance consistency and identify at least one backup vendor in order to ensure reliable long-term assay support for projects. In the experiment, four lots of pepsin from two different vendors were compared side-by-side. As shown in , with the same amounts of substrate (TR4495) and enzyme (pepsin), very comparable peak area ratios were observed on LC/MS after 60 min of incubation. Therefore, it was concluded that pepsin produced by both vendors was similar in terms of performance for the quantitative analysis of TR4495.
Figure 4. Neat TR4495 (10 µg/mL x 50 µL) were digested with 4 different lots of porcine pepsin (10 µg) at 37°C for 60 min. Peak area ratios (analyte/IS) are the average of three analytical replicates, and error bars represent the standard deviations.
Considerations of immunoaffinity enrichment reagents. Bioanalytical methods that use an immunoaffinity enrichment technique coupled with LC/MS platform have been routinely used to analyze biomarkers or therapeutic proteins with increased efficiency, specificity, and sensitivity.Citation5,Citation22,Citation23 In order to leverage these advantages and effectively extract TR4495 from mouse plasma, three commercially available biotinylated anti-mIgG2a secondary antibodies were compared and rat anti-mIgG2a HC was selected because of its better performance in binding capacity (data not shown). The detailed multicycle immunoaffinity enrichment method used in this study was reported previously.Citation5
Precision and accuracy assessment of the new pepsin digestion enabled immunoaffinity LC/MS/MS assay. In order to assess the overall performance of the integrated rapid pepsin digestion workflow, four spiked QC levels, including 20 ng/mL (LLOQ), 60 ng/mL (LQC), 800 ng/mL (MQC), and 8000 ng/mL (HQC) were evaluated for their precision and accuracy (n = 6) against a linear calibration curve ranging from 20 ng/mL to 10,000 ng/mL. As shown in , the assay accuracy at all tested levels was within ± 20% with imprecision less than 25% (LLOQ), which verified that the assay performance met the acceptance criteria for typical discovery stage PK sample bioanalysis.
Table 1. Intra-assay accuracy and precision of the integrated pepsin digestion workflow for the quantification of TR4495.
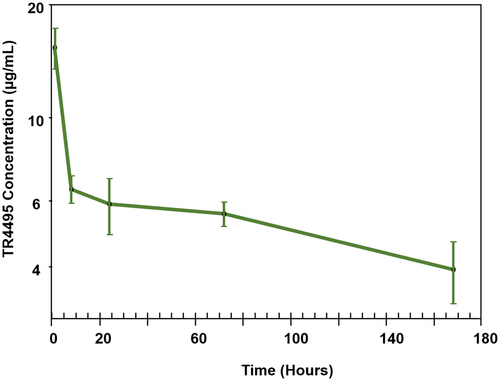
Implementation of pepsin as an alternative protease in support of quantitative discovery bioanalysis. The qualified pepsin digestion-based immunocapture LC/MS/MS assay was used to support a TR4495 single-dose mouse PK study and the resulting concentration–time profile is shown in . The same pepsin digestion strategy was transferred to an external contract research organization and applied to the quantification of other proprietary LAGA mutant-containing molecules, including antibody-drug conjugates, to support regulatory filing (data not shown). In the mentioned studies, pepsin was able to replace trypsin and enabled a generic mIgG2a LAGA mutant assay to be developed for discovery bioanalytical purpose. Without the need of extensive assay development, this approach can also be applied to a series of antibody-based modalities carrying the same mutation in their peptide sequences.
Figure 5. PK profile of TR4495 in C57BL/6J mice. Data are the average of three mice at each time point (1, 8, 24, 72, and 168 h), and error bars represent the standard deviations.
In summary, a rapid peptic digestion method was optimized for selectively quantifying LAGA-mutated mIgG2a monoclonal antibody in mouse plasma. The reproducible cleavage of both leucine and phenylalanine from the C-terminal by pepsin under low pH conditions presented a unique opportunity for the immunoaffinity LC/MS/MS application because of the readily compatible upstream acidic protein elution step. In combination with a previously published multicycle immunoaffinity workflow, we were able to quantify several surrogate mIg2a mutants from discovery mouse PK studies with good sensitivity and reproducibility (only data for TR4495 was shown). In principle, the generic assay would allow the evaluation of plasma exposure of most LAGA mutated mIgG2a bound to diverse targets of interest. Additionally, the switch from trypsin to pepsin not only made the future method development effortless but also significantly reduced the proteolytic enzyme cost for bottom-up protein quantification. It was estimated approximately one US dollar of pepsin can be used to process ten 96-well plates in the protocol described herein.
Although trypsin remains the enzyme of choice for many known protein analytes, it has been demonstrated that commercially available pepsin is a valuable tool that could be used to address certain challenges in quantitative protein analysis.
Conclusions
A novel rapid pepsin digestion strategy was seamlessly incorporated into the immunoaffinity LC/MS/MS workflow to enable sensitive and reproducible quantification of mutated mIgG2a monoclonal antibody in mouse plasma. The new one-hour rapid peptic digestion procedure is not only a unique and cost-effective solution for mIgG2a LAGA mutant analysis, but it can also be a valuable technique for general protein quantification in discovery bioanalysis.
Abbreviations
| ADC | = | antibody-drug conjugate |
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| CDR | = | complementary-determining region |
| CE | = | collision energy |
| CRO | = | contract research organization |
| FA | = | formic acid |
| HC | = | heavy chain |
| HDX | = | hydrogen/deuterium exchange |
| HQC | = | high quality control |
| LAGA | = | L235A/G237A |
| LLOQ | = | lower limit of quantification |
| LQC | = | low quality control |
| MQC | = | medium quality control |
| MS | = | mass spectrometry |
| PBS | = | phosphate-buffered saline |
| PK | = | pharmacokinetic |
| SISCAPA | = | stable isotope standards and capture by anti-peptide antibodies |
| TFA | = | trifluoroacetic acid |
| ULOQ | = | upper limit of quantification |
Acknowledgments
The authors thank Qing Xu and Jiejin Chen from the Protein Sciences group at Takeda Boston for providing mIgG2a antibody TR4495. The authors also want to thank Michelle Ganno-Sherwood, Dongmei Zhang, Natasha Iartchouk, and Adnan Abu-Yousif from the ODDU group for providing the in vivo study samples. Funding for the research work was provided by Takeda Development Center Americas, Inc.
Disclosure statement
All authors were employees of Takeda Development Center Americas, Inc.and may have hold stocks of the company directly or indirectly through mutual funds when the research study was conducted.
Additional information
Funding
References
- Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-engineering for modulated effector functions—improving antibodies for cancer treatment. Antibodies. 2020;9(4):64. doi:10.3390/antib9040064.
- Szapacs M, Jian W, Spellman D, Cunliffe J, Verburg E, Kaur S, Kellie J, Li W, Mehl J, Qian M, et al. 2022 white paper on recent issues in Bioanalysis: ICH M10 BMV guideline & global harmonization; hybrid assays; oligonucleotides & ADC; non-liquid & rare matrices; regulatory inputs (part 1A – recommendations on mass spectrometry, chromatography and sample preparation, novel technologies, novel modalities, and novel challenges, ICH M10 BMV guideline & global harmonization part 1B - Regulatory agencies’ inputs on regulated bioanalysis/BMV, biomarkers/CDx/BAV, immunogenicity, gene & cell therapy and vaccine). Bioanalysis. 2023;15(16):955–6. doi:10.4155/bio-2023-0167.
- Hutchins JT, Kull FC, Bynum J, Knick VC, Thurmond LM, Ray P. Improved biodistribution, tumor targeting, and reduced immunogenicity in mice with a gamma 4 variant of campath-1H. Proc Natl Acad Sci. 1995;92(26):11980–84. doi:10.1073/pnas.92.26.11980.
- Chen X, Song X, Li K, Zhang T. FcγR-Binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.00292.
- Dong L, Bebrin N, Piatkov K, Abdul-Hadi K, Iwasaki S, Qian MG, Wei D. An automated multicycle immunoaffinity enrichment approach developed for sensitive mouse IgG1 antibody drug analysis in mouse plasma using LC/MS/MS. Anal Chem. 2021;93(16):6348–54. doi:10.1021/acs.analchem.1c00698.
- Hansen K, Szarka S, Escoffier E, Berthet A, Venet J, Collet-Brose J, Hepburn S, Wright M, Wheller R, Nelson R, et al. Glu-C, an alternative digestive enzyme for the quantitative LC-MS/MS analysis of an IgG-based antibody biotherapeutic. Bioanalysis. 2018;10(13):997–1007. doi:10.4155/bio-2017-0259.
- Fung EN; Bryan P; Kozhich A. Techniques for quantitative LC-MS/MS analysis of protein therapeutics: advances in enzyme digestion and immunocapture. Bioanalysis. 2016;8(8):847–56. doi:10.4155/bio.16.24.
- Tsiatsiani L, Heck AJ. Proteomics beyond trypsin. FEBS j. 2015;282(14):2612–26. doi:10.1111/febs.13287.
- Giansanti P, Tsiatsiani L, Low TY, Heck AJ. Six alternative proteases for mass spectrometry-based proteomics beyond trypsin. Nat Protoc. 2016;11(5):993–1006. doi:10.1038/nprot.2016.057.
- Schlecht J, Jooß K, Moritz B, Kiessig S, Neusüß C. Two-dimensional capillary zone electrophoresis-mass spectrometry: intact mAb charge variant separation followed by peptide level analysis using In-capillary digestion. Anal Chem. 2023;95(8):4059–66. doi:10.1021/acs.analchem.2c04578.
- Guapo F, Strasser L, Millán-Martín S, Anderson I, Bones J. Fast and efficient digestion of adeno associated virus (AAV) capsid proteins for liquid chromatography mass spectrometry (LC-MS) based peptide mapping and post translational modification analysis (PTMs). J Pharm Biomed Anal. 2022;207:114427. doi:10.1016/j.jpba.2021.114427.
- Dupré M, Duchateau M, Sternke-Hoffmann R, Boquoi A, Malosse C, Fenk R, Haas R, Buell AK, Rey M, Chamot-Rooke J. De Novo sequencing of antibody light chain proteoforms from patients with multiple myeloma. Anal Chem. 2021;93(30):10627–34. doi:10.1021/acs.analchem.1c01955.
- Liu X, Dekker LJM, Wu S, Vanduijn MM, Luider TM, Tolić N, Kou Q, Dvorkin M, Alexandrova S, Vyatkina K, et al. De Novo protein sequencing by combining top-down and bottom-up tandem mass spectra. J Proteome Res. 2014;13(7):3241–48. doi:10.1021/pr401300m.
- Guthals A, Gan Y, Murray L, Chen Y, Stinson J, Nakamura G, Lill JR, Sandoval W, Bandeira N. De Novo MS/MS sequencing of native human antibodies. J Proteome Res. 2017;16(1):45–54. doi:10.1021/acs.jproteome.6b00608.
- Kish M, Smith V, Lethbridge N, Cole L, Bond NJ, Phillips JJ. Online fully automated system for Hydrogen/Deuterium-exchange mass spectrometry with millisecond time resolution. Anal Chem. 2023;95(11):5000–08. doi:10.1021/acs.analchem.2c05310.
- Anacleto J, Lento C, Sarpe V, Maqsood A, Mehrazma B, Schriemer D, Wilson DJ. Apparatus for automated continuous hydrogen deuterium exchange mass spectrometry measurements from milliseconds to hours. Anal Chem. 2023;95(9):4421–28. doi:10.1021/acs.analchem.2c05003.
- Song D, Sun H, Ma L, Liu J, Gao Y, Zhang Q, Xiao P, Sun K, Shen M, Wang X, et al. In-vitro diagnostic reagent evaluation of commercially available cardiac troponin I assay kits using H/D exchange mass spectrometry for antibody-epitope mapping. Anal Chem. 2023;95(4):2278–84. doi:10.1021/acs.analchem.2c03946.
- Vorauer C, Wrigley MS, Rincon Pabon JP, Watson MJ, Mundorff CC, Weis DD, Guttman M. Rapid assessment of pepsin column activity for reliable HDX-MS studies. J Am Soc Mass Spectrom. 2021;32(9):2386–90. doi:10.1021/jasms.1c00080.
- Valedkarimi Z, Nasiri H, Aghebati-Maleki L, Abdolalizadeh J, Esparvarinha M, Majidi J. Production and characterization of anti-human IgG F(ab’)2 antibody fragment. Hum Antibodies. 2018;26(4):171–76. doi:10.3233/HAB-180336.
- Yang X, Naughton SX, Han Z, He M, Zheng YG, Terry AV Jr, Bartlett MG. Mass spectrometric quantitation of tubulin acetylation from pepsin-digested rat brain tissue using a novel stable-isotope standard and capture by anti-peptide antibody (SISCAPA) method. Anal Chem. 2018;90(3):2155–63. doi:10.1021/acs.analchem.7b04484.
- Ahn J, Cao M-J, Yu YQ, Engen JR. Accessing the reproducibility and specificity of pepsin and other aspartic proteases. Biochim Et Biophys Acta (BBA) - Proteins Proteomics. 2013;1834(6):1222–29. doi:10.1016/j.bbapap.2012.10.003.
- Sugimoto H, Wei D, Dong L, Ghosh D, Chen S, Qian MG. Perspectives on potentiating immunocapture-LC-MS for the bioanalysis of biotherapeutics and biomarkers. Bioanalysis. 2018;10(20):1679–90. doi:10.4155/bio-2018-0205.
- Wei D, Horton KL, Chen J, Dong L, Chen S, Abdul-Hadi K, Zhang TT, Casson CN, Shaw M, Shiraishi T, et al. Development of a highly sensitive hybrid LC/MS assay for the quantitative measurement of CTLA-4 in human T cells. Molecules. 2023;28(8):3311. doi:10.3390/molecules28083311.
