Abstract
Generation of reactive oxygen species is useful for various medical, engineering and agricultural purposes. These include clinical modulation of immunological mechanism, enhanced degradation of organic compounds released to the environments, removal of microorganisms for the hygienic purpose, and agricultural pest control; both directly acting against pathogenic microorganisms and indirectly via stimulation of plant defense mechanism represented by systemic acquired resistance and hypersensitive response. By aiming to develop a novel classes of artificial redox-active biocatalysts involved in production and/or removal of superoxide anion radicals, recent attempts for understanding and modification of natural catalytic proteins and functional DNA sequences of mammalian and plant origins are covered in this review article.
Introduction
Empirically, people have been aware that increased intake of antioxidants in the form of fruits and vegetables may reduce the risk of chronic diseases, chiefly of cancer.Citation1 Reactive oxygen species (ROS) are generated during mitochondrial oxidative metabolism as well as in cellular responses to xenobiotics, cytokines, and bacterial invasion; and oxidative stress in living cells of both animal and plant origins.Citation2 Today, natural plant products are viewed as potential candidates for the cancer chemoprevention due to their antioxidant, anti-inflammatory and antitumor activities and, therefore, intake of such antioxidants are necessary for controlling degenerative reactions produced by ROS and nitrogen species in vivo.Citation1
On the other hand, generation of ROS is useful for various medical, engineering and agricultural purposes namely, clinical modulation of immunological mechanism,Citation3,4 enhanced degradation of organic compounds released to the environments,Citation5 removal of microorganisms for the hygienic purpose,Citation6 and agricultural pest control both directly acting against pathogenic microorganisms and indirectly via stimulation of plant defense mechanism represented by systemic acquired resistance and hypersensitive response.Citation7,8
In this review article, recent approaches for understanding and modification of natural catalytic proteins and functional DNA sequences of mammalian and plant origins are covered, aiming to develop a novel classes of artificial redox-active biocatalysts involved in production and/or removal superoxide anion radicals (O2•−).
In general, catalysts (Cs) can be defined as the set of different types of elements, molecules or compounds as follows:(1) where OCs, ICs, and BCs are organic catalysts, inorganic catalysts, and biocatalysts, respectively.Citation9 OCs can be represented by biological and bio-inspired organic molecules such as guanidine-typeCitation10 or amino acid proline-type catalysts,Citation11 and ICs can be represented by inorganic molecules or complex such as metal-based catalysts.Citation12 While the natures of OCs and ICs can be clearly defined and described based on their chemical properties, the category of BCs simply implies the origins but not the natures of these catalysts. It is trivial that the set {BCs} can be divided into 2 subsets as follows:
(2) where Es and Ns are enzymes and nucleozymes, respectively.
In fact, Es and Ns can be further confirmed as follows:(3)
(4) Moreover, the sets of {Es} and {Ns} can be further divided into subsets as follows:
(5)
(6) where Ens and Nns, are natural enzymes and nucleozymes, respectively, which can be found in or produced by living organisms; and Eas and Nas are artificial enzymes and nucleozyme, respectively, which are now newly designed or engineered in the laboratory. Among the Es, many portion of both Ens and Eas reportedly bind catalytically active metals directly or indirectly (by possessing prosthetic groups such as iron-centered hemes), to form the center of catalytic reactions within the molecules.Citation13 Thus, catalytic activities of natural and artificial metal-binding enzymes can be attributed to the behaviors of bound metals. Therefore, it is natural to obtain the following proposition, P(Es).
(7)
This type of enzymes should be categorized and termed as metalloenzymes. According to recent reviews on metalloenzymes,Citation13,14 an artificial metalloenzyme can be designed de novo by arranging the peptidic sequence composed of 20 natural amino acids. Basically, such de novo designs of metalloproteins can be achieved freely designing the amino acid sequences capable of binding metal ions.Citation13 In order to artificially design or modify the catalytic peptides or proteins, it is much easier to learn from the catalytically active peptidic motifs within the naturally existing active enzymes or proteins as the platforms of engineering.Citation9,15
As reported by Yeung et al.,Citation16 modification of myoglobin (Mb) is one of successful cases in engineering of semi-natural metalloenzyme. Accordingly, natural Mb was re-designed into a functional nitric oxide reductase, by newly forming a non-heme iron binding site in the distal pocket of Mb. Presence of such natural, semi-natural and artificial metalloenzymes consists the elements of conceptual subset of bio-originated catalysts within the set of {ICs} fulfilling the proposition (7):
Similarly to the cases of metalloenzymes, we have been seeking for the cases of metallonucleozymes, in which catalytic activities of natural and artificial metal-binding nucleic acids (DNAs and RNAs) can be attributed to the behaviors of bound metals. Therefore, it is also natural to obtain the following proposition, P(Ns).(8)
In the processes to revise recent progresses, we would like to highlight some examples of metallonucleozymes which may be supporting the proposition (8).
Two propositions listed above (7, 8) can be combined and generalized as follows:(9) By analogy to the cases of metal-binding BCs, we can assume that catalytic actions of some BCs can be attributed to the catalytic mode of actions similar to OCs, since proline, one of natural amino acids composing proteins, is now consider as an active catalyst.Citation11 Thus, following proposition can be arisen.
(10) This proposition will be discussed at the end of this article.
Non-biological catalysts producing O2•−
As an example of {ICs} in definitive proposition (1), we have studied the mechanism of O2•− production by titanium dioxide (TiO2)-based sono-photocatalytic (SPC) system.Citation17 Recently, a variety of ultraviolet (UV)-driven photochemically active catalysts designated as photocatalysts consisting of TiO2 has been developedCitation18-21 and applied for hygiene and antimicrobial purposes.Citation22,23 The likely mechanism of such catalysts involves the generation of ROS on the surface of the catalysts as expected (not fully proven) from the previously proposed models.Citation24 However, no attempt to confer long-lasting chemical properties to the waters (e.g., preparation of waters rich in ROS) has been reported, except for our model, despite of the increasing demands for the use of photocatalysts in various environments including the use in aqueous phase.
Our attempts were firstly, to obtain the data scoring the rate of O2•− production by both sono- and photo-catalytic manner, and secondly, to testify if the sono-photochemical priming of oxygen saturated water results in continuous release of O2•− in the system lasting at least for a half hour or not. For above purposes, a novel water conditioning SPC apparatus () equipped with sheets of TiO2-coated photocatalytic fibers were applied for the preparation of ROS-containing water.Citation17 The apparatuses used in our demonstration was equipped with TiO2-coated fibers and UV-A (360 nm) bulbs enabling the excitation of TiO2, and also with 2 ultrasonic wave (USW) generating devices to assist the reaction of interest. When required, O2 gas was supplied to the system through artificial lung (thus minimizing the impacts of air bubbles) connected with a magnet/roller pump. Monitoring of the dissolved O2 level was necessarily required for enabling the optimal generation of O2•− in the water (5 L) maintained at ca. 20ºC and circulated at 20 L/min.
Figure 1. Generation of ROS by SPC processes. (a, a’) Water conditioning SPC apparatus. Two exPCAW1.2s (WIPO No: WO10/032765; K2R Inc., Kitakyushu, Japan) connected in tandem (A) and the diagram of water conditioning system (a’). (B) Scanning electron microscopic image of the catalytic fiber which was fabricated by finely coating TiO2 over alumina layer of aluminum fibers. (C) Detection of DMPO-OH signal with ESR reflecting the generation of HO•. (D) Monitoring of long lasting redox activity in the SPC-processed water using O2•−-specific CL probe, CLA. (E) Quenching of CLA-CL by Tiron, a scavenger of O2•−. (F) Synergistic impact of UV and USW in generation of O2•−. Images were obtained and modified from.Citation17
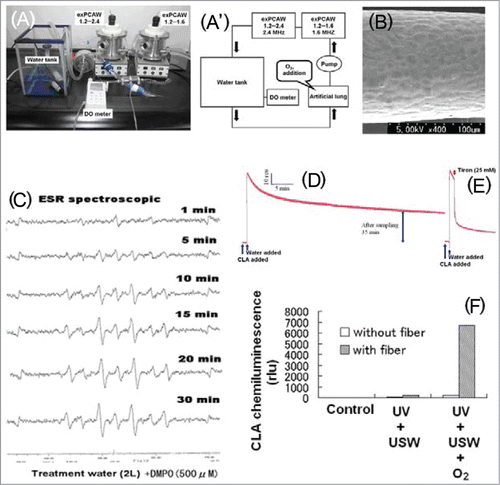
In the model experiments, we detected the generation of hydroxyl radicals (HO•) () and O2•− () as the key members of ROS generated in the water circulated in SPC chambers, through electron spin resonance (ESR) spectroscopy and chemiluminescence (CL) assays, respectively. For CL assays, O2•−-specific CL probe Cypridina luciferin analog (2-Methyl-6-phenil-3,7-dihydromidazo[1,2-a]pyrazin-3-one; designated as CLA;Citation25 was used. For ESR, a spin trapping agent, DMPO (5,5-Dimethyl-1-pyrroline-N-Oxide) that readily forms an adduct with HO• was used. Note that CL reflecting the generation of O2•− can be detected in the water transferred out of the water circulating system, suggesting that some intermediate species or precursors of O2•− can be produced for certain length of time (). In contrast, HO• was detected only when the trapping agent, DMPO was circulated inside the SPC chambers. DMPO added to the water manually sampled out of the apparatus did not provide the signal for HO• (data not shown).
One likely use of the conditioned water is controlling of the biological responses of living plant cells since it has been well documented that various physiological and biochemical events during the plant life cycle, such as germination of seeds, induction of defense mechanism against pathogenic microorganisms and adaptation to severe environments, are controlled by ROS.Citation24 It has been shown that expression of a number of redox-induced stress-related genes is regulated through calcium signaling in plant cells.Citation8 Therefore, pre-treatments of plants with redox-modifying agents that target the calcium channel opening may result in induction of anti-oxidative capacity (conferred by expression of ROS-responsive genes).
We proposed an attempt for conferring anti-oxidative capacities to plants for protesting the damaging impacts of photochemical oxidants or other oxidative stress, by novel water-processing technology which provides redox-active water designed for stimulating the expression of redox-related and defense-related genes in plants, through pre-treatments with moderate oxidative stresses.
To assess if the level of ROS produced in the photochemically conditioned water attained and remained at the level actively inducing the responses of living plant cells, we tested the responses of tobacco cell suspension culture (BY-2, expressing aequorin gene) to SPC-conditioned water. Presence of O2·− in the conditioned water-treated cell suspension culture was detected with the CL of CLA (). Similarly, addition of SPC-conditioned water to tobacco BY-2 cells expressing aequorin gene resulted in increase in cytosolic Ca2+ concentration ([Ca2+]c) () and slight increase in cell death (). Similarly, we have shown that addition of nano-sized particles of TiO2 induces the generation of ROS and also an increase in [Ca2+]c in living tobacco cells.Citation26
Figure 2. Biological impact of SPC-treated water. Effect of SPC-processed waters on O2•− production (A), induction of calcium influx into tobacco cells measured with aequorin luminescence (B and C) and cell death assessed with Evans Blue staining (D). (E) Induction of a ROS-responsive gene (PR-gene) expression by SPC-treated water. (F) Proposed use of SPC-treated water for hardening of plants eventually conferring stress tolerance to living plants. Effects of UV, USW and oxygen were compared in (A–D). Arrows in (B) indicate the timing of processed water addition. Data on gene expression analysis is cited from,Citation106 (oral presentation).
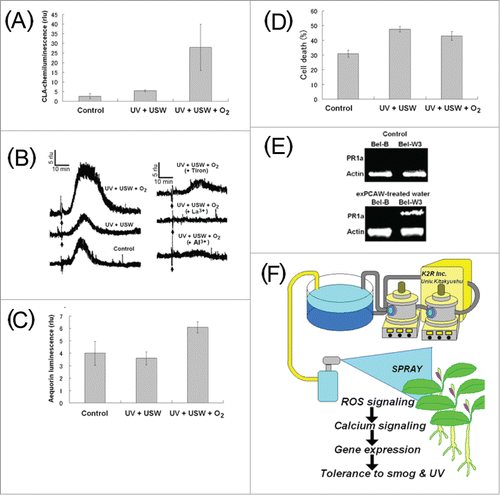
In addition, we observed that treatment of plants of ROS-sensitive tobacco cultivar (Bel-W3) with the SPC-conditioned water resulted in induced expression of PR1a, a ROS-responsive gene known to be involved in pathogenesis response in plants (). Our proposal to use this novel water-treatment technology based on SPC has potential for vaccinating the plants, thus confering the resistance to oxidative stresses as illustrated in .
Learning from the chemico-biological models in plants (1): plant peroxidase as model enzyme
Living plants are sources of a number of enzymes involved in metabolism (production and removal) of ROS.Citation7 In higher plants, 2 major mechanisms are known to be responsible for production of ROS, namely one involving NADPH oxidasesCitation27 and one involving peroxidases.Citation28 A research group in Geneva University metaphorically described that plant enzymes belonging to peroxidases (EC 1.11.1.7) display multiple functions more than a ‘Swiss army knife’.Citation29 In the conventional peroxidase cycle, hydrogen peroxide (H2O2) is used as the common acceptor of electrons (e−), and a variety of compounds function as e−-donating substrates.Citation28 Therefore, regulation of the level of H2O2 and oxidation of a wide range of substrates are the likely functions of plant peroxidases. Furthermore, diversified roles for plant peroxidases also include concomitant generation of ROS coupled to oxidation of phenolics such as salicylates,Citation8,30 and aromatic amines such as phenylethylamine.Citation31,32 As above, it is now accepted that plant peroxidases are capable of catalysis leading to generation of ROS, chiefly O2•−, through oxidation of key substrates through H2O2-dependent conventional peroxidase cycle involving the cyclic formation of redox active enzyme intermediates known as Compounds I and II (path 3→5→4→3 in ).Citation7,8,28 Generation of O2•− via peroxidase cycle involves the H2O2-dependent generation of intermediate radical species such as phenoxyl radicals or aromatic amine radicals which could be detected by ESR spectroscopy by using ascorbic acid as a spin trapper.Citation31-34
Figure 3. Hourglass model that summarizes the inter-conversions among active and inactive forms of peroxidase intermediates. The model was simplified based on earlier works.Citation28,43,106 The numbers on the black balls indicate the formal oxidation states of enzyme and its intermediates. Formation of radical intermediate (A•) further participates in formation of O2•−. This model dissecting 2 distinct cycles initiated by interaction of native POX with acceptors and donors of e−, is often referred to as the hourglass model due to its shape.Citation8,28,106
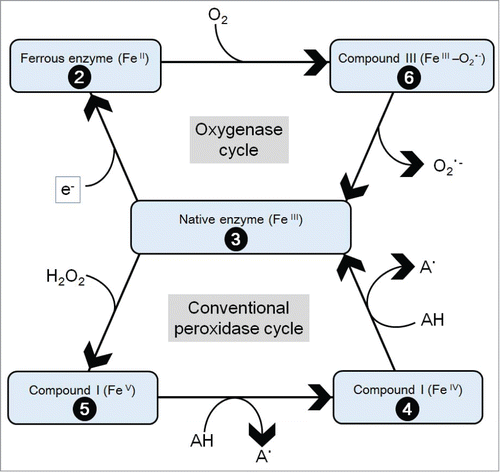
In addition to aforementioned e− acceptor-driven (H2O2-requiring) conventional peroxidative cycle, there is an alternative mechanism for generation of O2•− in an e− donor-dependently driven manner, which should be described as the oxygenation or oxygenase cycle of plant peroxidase (path 3→2→6→3 in ). The most widely known substrate for this H2O2-independent cycle is indole-3-acetic acid (IAA), the principal form of natural auxin in higher plants.Citation35-38
We view that the role of IAA in POX-catalyzed generation of O2•− is one of effective e− donors converting native enzyme into ferrous intermediate in the oxygenation cycle (, upper half). IAA-dependent reduction of native enzyme (with heme at FeIII) into ferrous intermediate (with heme at FeII) is immediately followed by a series of reaction proceeding under the atmospheric O2-rich condition, by which, unstable ferrous complex can be readily converted to O2-bound form of enzyme intermediate known as Compound III in which the state of heme iron can be described as O2-heme-FeII or O2•−-heme-FeIII.Citation39 Then, gradual decay of this complex into native enzyme at heme-FeIII state accompanies the release of O2•− (, upper half) as confirmed with IAA-stimulated horseradish peroxidase (HRP) using O2•−-specific CL probe, CLA.Citation37
Interestingly, medical application of HRP-labeled antibodies and IAA, novel O2•−-generating agents enabling cancer-targeted cell death induction, has been proposed based on the views that transient formation of [POX-IAA-O2] complex results in robust release of O2•−.Citation37 To date, 3 approaches have been reported, namely, HRP-conjugated immuno-labeling of cancer-related molecules,Citation4,40 expression of recombinant HRP in mammalian cells,Citation41 and modulation of the IAA-induced reaction using a fungal molecule.Citation38
Assuming that the hypothetical model mechanism proposed in (upper half) is correct, we should be able to screen or identify some effective e− donors from a variety of single e− reducing agents which target the native enzyme to trigger the onset of oxygenation cycle in plant POXs, eventually leading to a robust and long-lasting burst of O2•− production. After testing a wide range of chemicals, we observed that free ferrous ion (Fe2+) acts as a novel inducer of O2•− production in aid of plant POX, possibly by behaving as an effective e− donor for FeIII-to-FeII conversion of heme in a model POX, HRP. The aim of the present section is to share our novel finding on the Fe-driven O2•− production mechanism involving HRP.
In plants, physiological significance of such peroxidase-mediated redox reaction could be found in the defense mechanism against microbial attacks. Recently, we have proposed a likely role for non-biological inorganic factors such as free ferrous ionCitation42 and nitric oxideCitation43 in reduction of ferric native enzyme into ferrous intermediate protein which readily produces O2•− through the mechanism involving the formation and decay of Compound III (Path 3→2→6→3 in , upper half), thus, possibly contributing to the plant defense mechanism. By making use of the oxygenase cycle in plant peroxidase, modulation of the enzyme activities by doping-chemicals without supplementation of any substrate is one of the artificial regulatory modes of peroxidase.
Learning from the chemico-biological models in plants (2): Plant-derived short peptides H2O2-dependently producing O2•−
Ozone (O3) is a major secondary air pollutant, threatening the living plants, often reaching high concentrations in the urban areas under intensive solar ray. It is generally understood that generation and/or removal of ROS form the key signaling events governing the behaviors of living plants at cellular level under the O3 stresses.Citation44,45 Interestingly, one of key factors required for plant survival under O3 toxicity was shown to be endogenous peroxidaseCitation46 since the level of ROS determines the onset of localized apoptotic cell death in O3-exposed cells.Citation47,48
In addition to plant peroxidases, small peptides behaving similarly to peroxidases reportedly participate in the plant responses to O3.Citation9 As one of O3-induced plant responses, expression of O3-inducible (OI) genes have been identified in saltbush (Atriplex canescens) and few other plants.Citation49 OI gene inducers include some additional chemical and environmental factors such as SO2 and water deficit, suggesting the multiple roles for these genes.Citation49 By No and his colleagues,Citation50 2 isotypes of OI peptides, OI2–2 (158 amino acids) and OI14–3 (119 amino acids), were identified. These peptides possess a common repeat unit (8–10 times repeated in tandem), consisting of hexa-amino acids (Y-G-H-G-G-G). OI peptides are considered to be putative members of glycine-rich proteins designated as GRPs, due to the presence of above repeat unit.Citation50 The secondary structures of most GPRs and OI peptides are rich in β-pleated sheets,Citation49-51 suggests that there is some similarity between prion proteins (PrPs) from animal systems and plant GRPs including OI peptides.
Recently,Citation51 it was suggested that the hexa-repeat in OI peptides acts as a metal-binding motif and synthesized model peptides with the hexa-repeat found in OI peptides are highly active in generation of O2•−, upon addition of H2O2 to the copper-loaded peptides. Interestingly, in the above report, possible mechanism of the reaction and biological consequence of the reactions catalyzed by OI peptides were discussed by analogy to the action of octarepeat peptides derived from human prion protein (PrP) as discussed later.
Role of His and Tyr residues in redox-active peptides
As described above, plant-derived OI peptides containing PrP-like repeated sequence consisted of a His- and Tyr-containing hexa-repeat unit repeated for 8–10 times in tandem.Citation50 Accordingly,Citation51 the repeat unit found in the O3-induced peptides shows H2O2-dependent O2•− producing activity by using self-Tyr residues as phonolic substrates required for peroxidative process, thus simply converting H2O2 to O2•− without requirement for additional phenolics or amine as substrates. As mentioned above, 2 OI peptides, OI2–2 and OI14–3, share the identical repeat motif (G-G-G-Y-G-H) which is repeated for 9 and 7 times, respectively. The each repeat unit in OI peptide was shown to form a complex with transition metals, chiefly copper ion in the physiological pH range based on the spectroscopic studies by measuring the cyclic dichroism (CD) and nuclear magnetic resonance (NMR).Citation52 Yokawa et al. have assessed the O2•−-generating activity of metal-binding motif derived from plant OI peptides, by chemically synthesising a series of peptides, namely G-G-G-Y-G-H, Y-G-H-G-G-G, H-G-G-G-Y-G, and G-G-G-F-G-H.Citation51 To prevent the peptide from forming self circularization, amino and carboxyl terminal of each peptide was acetylated and amidated, respectively.
Accordingly, the peptide with His residue located at the carboxyl terminal (G-G-G-Y-G-H) showed the highest activity and that with N-terminal His residue (H-G-G-G-Y-G) showed no activity. Therefore, not only the presence, but also the location of His residue is one of critical requirement for the reaction.Citation51 Similar observation confirming the role for His residue have been reported for the model His-containing Cu-binding peptides as discussed in the below sections of this article.Citation53
By analogy to the putative role for Tyr residue as intra-molecular substrates within some redox active enzymes such as cyclooxygenase-2Citation54 and ribonucleotide reductases,Citation55 in which corresponding reactions proceed via the formation of a tyrosyl radical, the importance of Tyr residue in the repeat unit in OI peptides was assessed. Accordingly, a Tyr-to-Phe (Y-to-F) substituted mutant peptide (G-G-G-F-G-H) was synthesized and used for comparison. As predicted, the Cu/G-G-G-F-G-H complex showed no activity for the generation of O2•− after the addition of H2O2.Citation51 On the other hand, by supplementation of free Tyr as a substrate into the reaction mixture containing Cu/G-G-G-F-G-H complex, the H2O2-dependent O2•−-generating activity was regained. Requirement for free Tyr or Tyr residue in H2O2-dependent O2•−-generating reaction suggest that tyrosyl radical (one form of phenoxy radicals) is transiently formed for one-electron reduction of O2 similarly to the plant peroxidase reaction H2O2-dependently generating O2•− by coupling to oxidation of phenolics (such as salicylic acid) to form phenoxy radicals.Citation33,56
Finding and defining the metal-binding and catalytic motifs within chicken prion proteins
There is a set of proteins which could not be defined by proposition (3)
The case of prion proteins (PrPs) and derived small peptides could be one such example. Generally, PrPs and derived peptides are not considered as enzymes at present, although they are either proteins or peptides having catalytic nature (proposition 11).(11) By admitting that there are proteins or peptides (both natural and artificial) with catalytic activity which can be considered as element of {BCs} in a broad sense as defined below (proposition 12), the phenomena observed with plant OI-peptides and animal PrPs belonging to novel class of BCs can be compared with conventional BCs such as plant peroxidases.Citation9
(12) Actually, the kingdoms of plants and animals are rich in such small peptidic metalloenzymes, belonging to BCs in a broad sense, catalyzing the generation of O2•−.Citation53
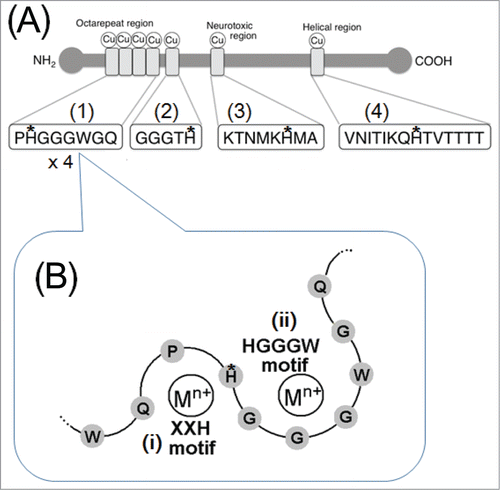
The criteria for consisting a minimal peroxidase-like small peptides is the presence of His-rich motifs required for binding to metals (chiefly copper), and free and/or peptide-bound substrates.Citation51 Similarly, recent studies have shown that peptides derived from human PrP mediates the production of O2•− through oxidation of substrates such as aromatic monoamines or phenolics (mostly, neurotransmitters and their analogs).Citation57 Upon binding to copper at 4 different putative copper-binding motifs (), PrP and derived peptides may gain the catalytic activities as our earlier works have revealed that PrP-derived copper-binding peptides catalyze the generation of O2•− in peroxidase-like manner involving H2O2 as e− acceptor and aromatic amines or phenols as the e− donors.Citation57,58
Figure 4. Copper-binding motifs in human PrP. (A) Putative copper-binding domains present in human PrP. Four peptide sequences corresponding to copper-binding motifs in PrP. (1) The most characterized Cu-binding sequence repeated 6 times in bovine and 4 times in human, mouse and ovine. (2) An additional Cu binding sequence found in the basic region in human and mouse PrPs immediately after the octarepeats. (3) PrPs from mammals and chicken show similar sequences. (4) Well characterized PrP helix 2 sequence.Citation57 (B) Recently proposed overlapping motifs in the human PrP's octarepeat region required for binding to metals. (i) X-X-H motifCitation9,76,77 suggested to bind Tb in the Tb-fluorescence assay. (ii) H-G-G-G-W motifCitation80 suggested to bind Cu in the peptide-fluorescence assay. M2+, metal cations. Histidine residues possibly involved in anchoring of copper are marked with asterisks.
Actions of Cu-bound PrPs are of great importance from the engineering point of view in order to design the novel peptidic metalloenzymes. Apart from engineering purpose, but viewing from the biological and medical points, the importance of metalloproteins in neurobiology has been suggested both as oxidants and antioxidants in neurodegenerative processes in animals.Citation59 Cu is an essential trace element in most living organisms but its redox reactivity often leads to the risk of oxidative damage to the cells and tissues, as observed in the neurodegenerative diseases such as 'prion' disease and Alzheimer, Menkes' and Wilson's diseases all occurring via disorders of Cu metabolism.Citation60–62 Especially, Alzheimer disease and prion disease are 2 of known major conformational diseases, as documented to date.
Deposition of abnormal protein fibrils is a common pathological feature observed in “protein conformational” diseases, including prion dementias and Alzheimer, Parkinson and motor neuron diseases.Citation63 Generation of ROS is now considered as one of key events required for development of conformational diseases. In the cases of accumulation of α-synuclein in Parkinson disease and accumulation of β-amyloid in Alzheimer disease, the evidence for involvement of ROS, chiefly H2O2 and derived HO•, in the neurodegenerative mechanisms have been documented, suggesting that pathogenesis of such neurodegenerative diseases could be attributed to the generation and damaging impacts of ROS which eventually stimulates the formation of abnormal protein aggregates.Citation63,64
PrPs are the only known causative agents for transmissible spongiform encephalopathies in mammalian brains.Citation65 A number of studies have shown that PrPs can form a group of Cu-binding proteins possibly involved in redox reactionsCitation66,67 as human PrP has 4 Cu-binding sites in the “octarepeats” region (PrP 60–91) in which amino acid sequence P-H-G-G-G-W-G-Q appears 4 times in tandem and each repeat possibly binds single Cu2+ at physiological neutral and basic range of pH.Citation68 Similarly, in chicken PrP, the Cu-binding motif analogous to the octarepeats are known as hexa-repeats in which each repeat consist of the 6 amino acids, H-N-P-G-Y-P. In chicken PrP, His residues in hexa-repeat are considered to play a key role in anchoring of Cu.Citation69
Note that both His and Tyr residues can be found in the chicken PrP's hexa-repeat unit. As Tyr-containing peptides could be a target of the redox reaction catalyzed by metal-containing proteins or peptides involved in peroxidative and ROS generating reactions, we synthesized 6 peptides corresponding to Cu-binding region (hexa-repeat) of chicken PrP and examined its catalytic activity for the generation of O2•−. Each of 6 peptides synthesized (N-P-G-Y-P-H, P-G-Y-P-H-N, G-Y-P-H-N-P, Y-P-H-N-P-G, P-H-N-P-G-Y, and H-N-P-G-Y-P) contains both His residue possibly anchoring Cu ion and Tyr residue possibly behaving as a substrate were used for assessing the O2•−generating activity using the O2•−-specific CL of CLA.Citation70 As a results, the generation of O2•− was observed in the presence of hexapeptide, copper and H2O2 without addition of any phenolic substrate since Tyr-residue on the hexapeptide possibly behaves as a substrate for the reaction.
To conclude that requirement of Tyr residue on the peptides in H2O2-dependent generation of O2•−, we also tested the mutation of Tyr resides into Phe residues in 2 model peptides (i.e. N-P-G-F-P-H and F-P-H-N-P-G). As expected, the Y-to-F substitution mutant peptides showed complete loss of H2O2-dependent generation of O2•−. Furthermore, we confirmed that supplementation of free Tyr to the reaction mixture containing the Y-to-F mutant peptides results in production of O2•−. It is conclusive that similarly to plant OI-peptide, both the presence and positions of His and Tyr residues in chicken PrP's hexa-repeat units are highly important for the catalytic modes leading to generation of O2•−.
Catalytic activity in human prion-derived peptides
To date, key involvement of trace elements, chiefly of Cu, in prion disease has been well documented.Citation71,73 Until recently, 2 opposing roles for Cu-bound PrPs have been proposed and discussed, namely the role of bound copper as an anti-oxidant element and contrary as a pro-oxidant element enhancing the neurodegenerative process.Citation74 In both cases, Cu-binding sequences highly preserved in PrPs play key roles in generationCitation57 or removal of ROS.Citation72
A series of works conducted by our group suggested that 4 distinct peptide sequences corresponding to 7 putative copper-binding sites containing metal anchoring His residues (His61, His69, His77, His85, His96, His111, and His187) in human PrP function as catalytic motifs active for O2•− generation through reactions with aromatic monoamines.Citation57 Furthermore, phenol-dependent O2•− generation catalyzed by several PrP-derived copper-binding peptides was recently assessed using various phenolics as substrates such as free phenolics, free Tyr,Citation53,75 solubilized polymers with phenolic groups (i.e., polyvinyl phenol which is a polymer with multiple phenolic groups, chain length, 12–58 mer,Citation9 and Tyr residues on peptide chains.Citation58 Since supplementation of H2O2 is required for oxidation of amines or phenols by these copper-centered peptides, the modes of reactions were considered to be peroxidase-like.Citation9,57
Among PrP-derived and related Cu-binding motifs ever examined, H2O2-dependent O2•−-generating activity was most active in a truncated helical sequence (V-N-I-T-K-Q-H-T-V-T-T-T-T) which is highly analogous to original (wildtype) PrPs’ helical sequence.Citation57,75
Two distinct metal-binding motifs overlaid in the PrP octarepeat region
Our earlier studies have revealed that His residues (at least single His) are required for anchoring copper on PrP-derived peptides,Citation57,76 and consequently, the catalytically active copper-binding motif within PrP-derived peptides was determined to be X-X-H, where X can be any amino acids followed by His residue.Citation9,77
In human PrP, His96 is located between G-G-G-T and S-Q-W-N sequences. To examine the positional effect of His on the catalytic activity in the derived peptides, comparison of the His-started H-S-Q-W-N pentapeptide and the His-ended G-G-G-T-H pentapeptide was carried out.Citation53 While reaction with tyramine (given as model substrate) and G-G-G-T-H peptide resulted in robust production of O2•−, the H-S-Q-W-N peptide showed no catalytic activity. By assuming that G-T-H motif within the G-G-G-T-H pentapeptide is one of X-X-H motif derivatives, experimental comparison of the catalytic activities among G-G-G-T-H pentapeptide and shorter derivatives (G-G-T-H and G-T-H) were performed and the data obtained clearly suggested the importance of the N-terminal glycyl-chain elongation for manifesting the maximal redox activity in C-terminal His anchored peptides.
Example of artificial enzyme based on XXH motif was developed,Citation78 demonstrating that Cu-binding peptides with X-X-H motif conjugated to organic materials could form a novel class of biosensing and bioengineering tools. Accordingly, a tripeptide, Gly-Gly-His (G-G-H, one of X-X-H motif derivatives) was introduced onto the glycidyl methacrylate-grafted porous hollow fiber membrane made on the polyethylene platform by radiation-induced graft polymerization. After loading of Cu2+ on the membrane, CL assay was performed to testify the catalytic activity of the membrane, generating O2•− upon addition of H2O2 and tyramine as the paired substrates. Inokuchi et al.Citation79 have studied the minimal motifs required for binding of metals within human PrP, by assessing (1) the peptide-dependent quenching of Tb3+ fluorescence and (2) the Cu2+-dependent quenching of intrinsic fluorescence in human PrP octarepeat-derived peptides. Nobel assays based on the quenching of Tb-fluorescence by interacting peptides emphasized the role of His-ended peptides sharing X-X-H motif. The obtained data clearly supported the view that an intact X-X-H motif located at C-termini of peptides, is desirable as the site of metal chelation. In the case of human PrP's octarepeat unit, P-Q-H motif rather than N-terminal H-G-G-G-W motif was shown to be active in metal binding.
Empirically, N-terminal His-started oligo-peptides derived from human PrP have been used as models for Cu-binding in earlier in vitro studies. These study suggested that the actual least motif in the octarepeats necessarily required for binding of Cu consists of 5 amino acids H-G-G-G-WCitation80 or 4 amino acids H-G-G-G.Citation68
Interestingly, the role of His-started motif (H-G-G-G-W) was supported by the Cu-dependent peptide fluorescence quenching assay.Citation79 Among the octapeptide sequences examined, the P-H-G-G-G-W-G-Q peptide was shown to be the most sensitive to the low Cu concentration although this sequence lacks the presence of intact metal-binding X-X-H motif.
Taken together, in the mammalian PrP octarepeat regions, in which P-H-G-G-G-W-G-Q is repeated for 4 (human) to 6 (bovine) times, 2 distinct metal binding motifs, namely, X-X-H motif and H-G-G-G motif, could be overlaid by sharing common His residue and thus co-existed ().
Role of Tyr residues within human PrP, the likely targets of catalysis
Human PrP-derived catalytic model peptides all showed requirements for addition of aromatic substrates in order to produce O2•− in the presence of H2O2Citation53,57 while the studies with the Cu-binging motifs in plant OI-peptides and chicken PrP are strongly indicating that Tyr residues presented on the peptide chains are the likely target of redox relay eventually converting H2O2 into O2•−.Citation9,70 Since free Tyr (among the active phenolics examined) is a good substrates for human PrP-derived catalytic short peptides, we assume that the modes of catalytic actions among the plant-derived, chicken-derived and human-derived copper-binding peptides described above may not differ much.
In case of non-peptidic free Tyr given as a model substrate, the presence of phenolic moiety, but not the amino and carboxyl groups, was shown to be important in the interaction with Cu-bound PrP-derived peptide.Citation58 Therefore, it is tempting to testify if the Tyr residues flanking on peptidic chains or proteins function as putative targets of human PrP's copper-binding motifs.
In fact, human PrP possesses several Tyr-resides being exposed to the external media and events involving such Tyr residue may play a pivotal role in development of prion dementias, as recent reports suggested that helix H1 of human PrP and its 2 flanking loops (highly rich in Tyr residues) are subjected a transition into a β sheet-like structure during forced conformational conversion of the intrinsic cellular form of PrP (PrPc) into the scrapie form of PrP (PrPsc) (Bertho et al., 2008). By definition, conversion of the PrPC into PrPSC is a fundamental event observable upon onset of prion disease development. Yokawa et al.Citation58 have reported an attempt to testify if the Tyr residues on PrP or derived peptides can be used as the substrate for a human PrP-derived Cu-bound catalytic peptide. In their experiments, the Cu-bound V-N-I-T-K-Q-H-T-V-T-T-T-T helical peptide was used as a model catalyst H2O2-dependently producing O2•−. On the other hand, the tested putative substrates include (1) tyrosyl-tyrosyl-arginine tripeptide (Y-Y-R) which appears twice in the PrP's Tyr-rich region (DYEDR-YYR-ENMHRYPNQV-YYR-PMDEY) and (2) longer peptide sequences corresponding to the Tyr-rich region in human PrP (D-Y-E-D-R-Y-Y-R-E-N-M-H-R).
Compared to free Tyr, Y-Y-R tripeptide was shown to be much more active in production of O2•−, confirming that both free form and peptide-integrated forms of Tyr can be recognized by the Cu-loaded catalytic peptide.Citation58 Although the reactivity of longer peptide sequences corresponding to the Tyr-rich region in human PrP (D-Y-E-D-R-Y-Y-R-E-N-M-H-R) was obviously lower than free Tyr, comparison with the Y-to-F substitution mutant (D-F-E-D-R-F-F-R-E-N-M-H-R) confirmed that Tyr-rich long peptides are favored for production of O2•−. These data suggest that the Tyr residues presenting on the intra- and inter-PrP molecules could be the target of the Cu-bound PrP-catalyzed reaction.
Synthesis of novel metalloenzymes with peptides and their substrate preferences
Among human PrP-related Cu-binding model peptides, the octarepeat unit (P-H-G-G-G-W-G-Q) was shown to be active in aromatic monoamine (AMA)-dependent O2•− generation using phenylethylamine as model substrate,Citation57 by mimicking the plant AMA-utilizing enzymes sensitive to monoamine oxidase inhibitors.Citation31 On the other hand, a helical motif V-N-I-T-K-Q-H-T-V-T-T-T-T undecapeptide and G-G-G-T-H pentapeptide, both derived from human PrP, showed negligible AMA-dependent activity while performing much greater phenol-dependent O2•− generating activities.Citation53,57,58 Based on above knowledge, substrate specificity of novel metalloenzymes can be properly designed. Based on the results with PrP-derived peptides, our group has designed a series of novel peroxidative biocatalysts as discussed below.
By analogy to G-G-G-T-H, a phenol-oxidizing catalytic pentapeptide derived from human PrP, we have designed a series of simplified model peptides (GnH series peptides) which are composed of oligoglycyl chains ended with C-terminal His. To test the importance of the elongated N-terminal glycyl chain and anchoring His residue, both GnH series peptides varied in N-terminal glycyl chain length (n = 2, 3, 4, 5 and 10) and oligoglycyl peptides lacking His (Gn series) were synthesized.
As expected, Gn series showed no catalytic activity since these sequence lack the motif for binding to catalytically important Cu2+. Within the GnH series, catalytic activity of the minimal Cu-binging motif (G2H tripeptide) was hardly detected despite the Okobira model.Citation78 Probably, in the Okobira model, in addition to G-G-H sequence, the supporting chains of glycidyl methacrylate on which G-G-H is grafted may playing a role similarly to N-terminal elongating oligo-Gly chain. In GnH series, G3H tetrapeptide showed a detectable increase in production of O2•−, confirming the importance of N-terminal Gly elongation. It can be generalized that the catalytic performance in GnH series can be ca. 3-fold enhanced by single amino acid elongation (addition of N-terminal Gly residue, allowing elongation from G2H to G3H, G3H to G4H, and G4H to G5H). However, further elongation from G5H hexapeptide to G10H undecapeptide resulted in only ca. 3-fold of enhancement suggesting that the requirement for the N-terminal elongation is nearly fulfilled. These data suggest that the presence of the C-terminal His is the primary requirement for catalytic performance, and N-terminal elongation contributes to the enhancement of the catalytic activity.
Although involvement of Cu and generation of ROS are analogous to tyrosinase which oxidizes Tyr and polyphenols with concomitant release of O2•−,Citation59 the roles played by H2O2 are largely different in the GnH series metalloenzymes. While H2O2 is often regarded as an inhibitor of the tyrosinase reaction,Citation81 the GnH series metalloenzymes require the presence of H2O2 as co-substrate. On the other hand, plant peroxidases are shown to be active in generation of O2•− upon oxidation of various phenolics and monoamines in the presence of H2O2,Citation28,33 suggesting that the modes of reactions catalyzed by PrP-derived peptides and artificial GnH series metalloenzymes are analogous to the modes of peroxidase reactions.
Among hydroxybenzoic acids (HBAs) and benzoic acid (BA), 2-HBA (salicylic acid) and BA were shown to be poor substrates for O2•−-generating reactions catalyzed by G-G-G-T-H pentapeptide and GnH series metalloenzymes.Citation53 In contrast, 3-HBA and 4-HBA were shown to be good substrates, suggesting that the presence of phenolic moieties with m- or p-positioned OH group is required for generation of O2•−, notably differed from the plant peroxidase which favors 2-HBA.Citation56 When dihydroxybenzoic acids (DHBAs) were used as model substrates, inutility of o-positioned OH group was also observed using 2,6-DHBA. However, PrP-derived peptides (G-G-G-T-H and V-N-I-T-K-Q-H-T-V-T-T-T-T), plant OI-peptides, and GnH series metalloenzymes showed O2•− generating activity upon addition of other DHBAs with o-positioned OH (2,3-DHBA, 2,4-DHBA, and 2,5-DHBA), indicating that the presence of o-positioned OH does not interfere with the roles for active OH groups at m- and p-positions.Citation9,53,58
Kinetic analysis performed with GnH series metallopeptides
G5H hexapeptide, one of GnH series artificial enzyme was selected for kinetic analyses based on the O2•−-specific CL with CLA.Citation53 By calibrating the yield of O2•− using KO2 as standard, the rate of O2•− production proceeding in the presence of tyramine was assessed. With Lineweaver-Burk analysis, Km and Vmax for the G5H hexapeptide-catalyzed production of O2•− in the presence of tyramine were determined to be 0.42 mM and 0.12 mmol / mg peptide / min, respectively.
Enhanced thermo-stability in novel metalloenzymes
It has been reported that prion-infected brain tissues or homogenates hardly lose their infectivity even after severe heat treatmentCitation82 and repeated freezing and thawing.Citation83 Yokawa et alCitation84 have hypothesized that redox activities reflected by the generation of ROS in PrP-derived Cu-binding peptides may also show thermo-stability, therefore, PrP-derived peptide may strive through heating and/or repeated freezing and thawing cycles. It is noteworthy that tyramine-dependent O2•−-generating activity found in the peptides corresponding to the Cu-binding motifs in human PrP (G-G-G-T-H, V-N-I-T-K-Q-H-T-V-T-T-T-T), showed extreme thermo-stability surviving under heat-incubation (90°C, 100 min), autoclaving, and repeated freezing/thawing cycles, despite most enzymes and proteins are sensitive to high temperature and repeated freezing.
Newly designed metallo-peptides, G5H hexapeptide and G10H undecapeptide were also used for thermo-stability assessment.Citation53 When the peptides (G5H and G10H) were incubated in the absence of copper, any loss of the catalytic activity was observed even after thermal denaturing treatments. In contrast, Cu-bound form of G5H hexapeptide showed some extent of the loss in catalytic activity (ca. 20%) after autoclaving (121°C, 20 min) but this peptide survived the 100 min of heating at 90°C and 10-time repeated freezing and thawing cycles.
Signal transduction-sensitive artificial enzyme
Protein phosphorylation is associated with most cell signaling and developmental processes in eukaryotes. Owing to the introduction of phosphate, a bulky and highly charged group, the phosphorylating events often results in a drastic changes in the properties of proteins (mostly enzymes), eventually modulating the activity of enzymes or protein-protein interaction properties.Citation85
In order to design a novel (probably first) signal transduction-sensitive artificial enzyme, a chimeric bio-catalyst designated as ErkG5H () was constructed,Citation15 by fusing G5H, a PrP-inspired metalloenzymeCitation53 with Tyr-containing Erk1/2 MAP kinase (MAPKK) substrate sequence (erk1/2 residues, 182–187 MAPKK phosphorylation sites) consisting of F-L-T-E-Y-V-A.Citation86 Using this molecule, Tyr residue-assisted conversion of H2O2 to O2•− was successfully achieved without supplementation of phenolic substrates (), due to the presence of a single Tyr-residue vicinal to Cu-binding catalytic motif (G5H).
Figure 5. A novel phosphorylation-sensitive chimeric biocatalyst designated as ErkG5H. (A) Proposed design of the novel phosphorylation-sensitive metalloenzyme. (B) Phosphorylated and non-phosphorylated model peptides examined. (C) CLA-CL reflecting the H2O2-dependent O2•−-generating reaction catalyzed by ErkG5H and its phosphorylated derivatives. (D) Comparison of O2•−-generating activity among the ErkG5H and its phosphorylated derivatives (error bars, SE, n = 4). (E) Positional effect of phosphorylation against the Cu-Tyr interaction. Circled “P” stands for a phosphate residue (A, B, E). Graphs and illustrations originally appeared inCitation9,15 were modified.
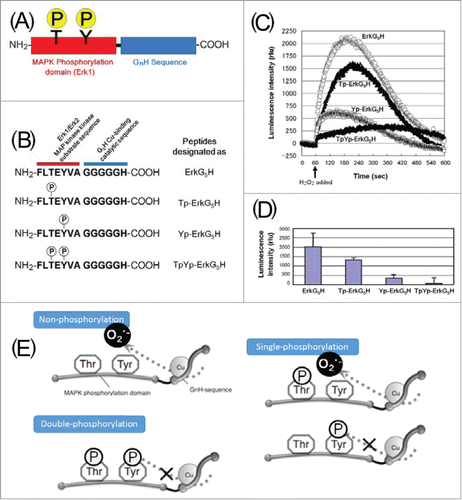
Then, ErkG5H, consisting of (i) Tyr-containing substrate mimic region and (ii) the catalytic region, was used to study the impact of amino acid phosphorylation, which could be the first demonstration of the phosphorylation-regulated artificial enzyme.Citation15 Within short peptide sequence F-L-T-E-Y-V-A-G-G-G-G-G-H, 2 amino acid residues, namely, Thr and Tyr residues, can be the sites of phosphorylation since the original sequence was derived from the phosphorylation domain within MAPKK.Citation86
In order to examine the phosphorylation-sensitiveness in ErkG5H molecule, O2•−-generating catalytic activities in non-phosphorylated and phosphorylated peptides (Thr-phosphorylated, Tyr-phosphorylated, and Thr and Tyr double phosphorylated) were compared.Citation15 Catalytic activity of ErkG5H was completely lost in the double phosphorylated sample (). Even by single phosphorylation at Tyr residue alone, catalytic activity of ErkG5H was mostly lost. In contrast, single phosphorylation at Thr residue resulted in partial inhibition only. Positional impact of phosphorylation determining the catalytic activity is summarized in . This work provided the first implication that phosphorylation-controllable artificial enzyme can be synthesized.
Interaction between DNA and redox-active metals
It is well known that oxidative damage to genomic DNA is promoted in the presence of ROS such as HO•, which can be generated via Fenton-type or Harbor-Weiss-type reactions in the presence of the Cu and Fe ions.Citation87 It is also known that the metal-mediated oxidative damage to DNA is further enhanced by coexisting ascorbic acid or H2O2.Citation88 Such oxidative DNA fragmentation and subsequent chromosomal dysfunction may play key roles in apoptotic cell death mechanisms in mammalian cells.Citation89 Through the DNA-degrading reactions in the system containing Cu(II) + H2O2 or Cu(II) + ascorbate, the production of a large amount of HO• at physiological pH condition via Harbor-Weiss-like reaction has been recorded, by monitoring the level of 8-hydroxyguanosine which is a reliable biomarker for HO•-dependent oxidative damage to guanosine residues on DNA.Citation88
It is known that Cu2+ strongly binds to the guanosine and cytidine bases at physiological pH, eventually perturbing the A-T base pairs and disrupting the double-helical structure of DNA.Citation90 In addition, specific regions on DNA, so called Z-DNA structure-like micro-domains, show much higher affinity to binding of Cu2+, especially at the base guanine.Citation91
HO• generated in the biological system hardly migrates even a short distance in aqueous phase due to its hyper-reactivity toward neighboring molecules such as water molecules, suggesting that HO• generated at the site vicinal to DNA likely results in enhanced degradation of or damages to DNA. Accordingly, the complex between Cu and aforementioned Z-DNA domains readily results in oxidative damaging to DNA chains.Citation91
Aiming at prevention of the Cu-mediated DNA damages, which is highly important from a gerontological point of view, Yokawa et al.Citation92 have reported their attempt in which copper-binding tripeptides with X-X-H motif found within PrP-derived catalytic peptides, prevented the degradation of DNA proceeding in the presence of Cu(II) + H2O2 and Cu(II) + ascorbate. The likely mechanism of peptide-mediated prevention of DNA degradation may involve the chelation of the Fenton catalysts such as Fe and Cu ions.
According to Kageenishi et al.,Citation77 PrP-derived redox-inert copper-binding octapeptide, K-T-N-M-K-H-M-A, effectively protected the plant cells from the toxicity of copper which initiates the apoptotic cell death development in plant cells.
Protection of plant cells by oligo-DNA interacting with metals
Treatments of living plants with excess of transition metals reportedly coincide the production of, molecular responses to, and cellular damages by ROS members such as O2•−, H2O2 and HO•, possibly via direct e− transfer involving metal cations, formation of catalytic complex with natural chelating agents such as small peptides, or as a consequence of metal-mediated inhibition of metabolic reactions.Citation93,97 It has been well documented that homeostasis and signaling crosstalk involving ROS and other signaling events such as the changes in [Ca2+]c are crucial to plant responses and adaptation to abiotic environmental factors threatening the plants, thus determining the fate of plant cells under stressful conditions.Citation46–48,92,98–100
We have previously testified the effect of a copper-binding PrP-derived peptide () on protection of tobacco BY-2 cells from Cu toxicity.Citation77 By analogy, Iwase et al.Citation101 have shown that oligo DNAs derived from copper-binding motifs function as effective plant cell-protecting agents preventing the toxicity of copper. Accordingly, addition of GC-rich double-stranded DNA fragments (such as CGCGCG hexamer), prior to treatment with copper ions, effectively blocked both the copper-induced calcium signaling event and resultant programmed cell death. It is known that CGCGCG DNA hexamer is one of minimal GC-rich Z-DNAs.Citation102
In case of plant cell protection by Cu-binding peptides, ca. 5 to 10-fold higher concentrations of peptides compared to that of Cu2+ were required for significantly blocking both the Ca2+ influx and cell death induced by copper, simply due to removal of copper with excess of Cu-binding molecule.Citation77 In contrast, DNA-based protection of plant cells from Cu toxicity requires much lower concentrations of oligo-DNA (between 1/10 and 1/3 of Cu2+ concentration) suggesting that there would be alternative mechanism of plant protection performed by added DNA fragments.
Designing the DNA-based catalytic molecules removing O2•−
In addition to simple Cu2+ removal model, the DNA-Cu complex examined was shown to possess the O2•−-scavenging catalytic activity, suggesting that DNA-mediated protection of the cells from Cu toxicity is due to removal of O2•−.Citation101 For assessing the catalytic activity hidden in the Cu-binding DNA oligomers, 4 different sources of O2•− was employed, namely, (i) short pulse of O2•− increase by injection of potassium superoxide (KO2, dissolved in dry DMSO), (ii) slow release of O2•− in the presence of copper ions and H2O2 (through known path for Cu2+-mediated conversion of H2O2 to O2•− via HO2), (iii) addition of SPC-activated O2•−-rich medium which was passed through the SPC reactor equipped with TiO2-coated alumina fiber, ultra violet light source, and ultrasonic generator designated as Ex-PCAW (as described in ), and (iv) xanthine oxidase.
Through analysis of O2•− removal by DNA-Cu complex, the mode of DNA-Cu complex was experimentally proven to be highly similar to the catalytic mode of SOD-like molecules (unpublished results). Kinetic analysis evaluated the Vmax for the O2•−-degrading action of the DNA-Cu complex, performed in the presence of various concentrations of KO2 or SPC-dependently produced O2•−, to be between 1.155 and 1.675 nmol / mg DNA / sec.
It is conclusive that the primary role of GC-rich oligo DNA is the trapping of toxic copper ions. Furthermore, upon binding to Cu, such metallonucleic acid complex may show catalytic activity for removal of O2•−. As the Vmax values obtained from independent assays fell in similar range, we now understand that GC-rich double-stranded DNA forms some catalytic complex for removal of O2•−. This type of molecules could be a good model for designing novel metal-centered artificial nucleozymes, thus fulfilling the criterion predicted by definitive proposition (8):(8)
Organic catalyst-like biocatalysts
Propositions (1) to (6), (12) and (13) are merely definitive. Predictions by propositions (7) to (9), of the cases of biocatalysts acting upon binding to catalytically active metals, such as copper-centered metallo-enzymes, peptides and nucleic acids, were examined and proven through discussion up to here, thus confirming the generalized proposition (9).
Contrary, our knowledge on the cases fulfilling the proposition (10) is yet to be covered.(10) As this proposition predicts that there could be biocatalysts showing catalytic activity due to the action of guanidine-like or amino acid Pro-like catalytic domains without involvement of the action of metals. In addition, the model presented in a series of classical works by Kunitake and his colleagues on imidazole-containing enzyme-like catalytic polymers could be also considered.Citation103,104
Interestingly, Pro-containing octarepeat-peptides from human PrP shows self-catalytic generation of O2•−, showing spiky CLA-CL upon mixture with media lacking metals. Since this phenomenon can be silenced by replacing Pro with other groups (Inokuchi et al., unpublished results), this topic may provide more clues to the development of OC-type BCs in the near future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant of Regional Innovation Strategy Support Program implemented by Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- Kumar M, Kumar S, Kaur S. Role of ROS and COX-2/iNOS inhibition in cancer chemoprevention: a review. Phytochem Rev 2012; 11:309-37; http://dx.doi.org/10.1007/s11101-012-9265-1
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012; 24:981-90; PMID:22286106; http://dx.doi.org/10.1016/j.cellsig.2012.01.008
- Bogdan C, Röllinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 2000; 12:64-76; PMID:10679404; http://dx.doi.org/10.1016/S0952-7915(99)00052-7
- Folkes LK, Greco O, Dachs GU, Stratfoed MRL, Wardman P. 5-Fluoroindole-3-acetic acid:a prodrug activated by a peroxidase with potential for use in targeted cancer therapy. Biochem Pharmacol 2002; 63:265-72; PMID:11841802; http://dx.doi.org/10.1016/S0006-2952(01)00868-1
- Werner JJ, McNeill K, Arnold WA. Environmental photodegradation of mefenamic acid. Chemosphere 2005; 58:1339-46; PMID:15686751; http://dx.doi.org/10.1016/j.chemosphere.2004.10.004
- Laroussi M, Leipold F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrometry 2004; 233:81-6; http://dx.doi.org/10.1016/j.ijms.2003.11.016
- Yoshioka H, Bouteau F, Kawano T. Discovery of oxidative burst in the field of plant immunity: looking back at the early pioneering works and towards the future development. Plant Signal Behav 2008; 3:153-5; PMID:19513209; http://dx.doi.org/10.4161/psb.3.3.5537
- Kawano T, Bouteau F. Crosstalk between intracellular and extracellular salicylic acid signaling events leading to long-distance spread of signals. Plant Cell Rep 2013; 32:1125-38; PMID:23689257; http://dx.doi.org/10.1007/s00299-013-1451-0
- Yokawa K, Kagenishi T, Furukawa H, Kawano T. Comparing the superoxide-generating activities of plant peroxidase and the action of prion-derived metallopeptides: towards the development of artificial redox enzymes. Curr Topics Peptide Protein Res 2011; 12:35-50
- Nagasawa K. Total synthesis of marine cyclic guanidine compounds and development of novel guanidine type asymmetric organocatalysts. Yakugaku Zasshi 2003; 123:387-98; PMID:12822483; http://dx.doi.org/10.1248/yakushi.123.387
- Jarvo ER, Miller SJ. Amino acids and peptides as asymmetric organocatalysts. Tetrahedron 2002; 58:2481; http://dx.doi.org/10.1016/S0040-4020(02)00122-9
- Pardieck DL, Bouwer EJ, Stone AT. Hydrogen peroxide use to increase oxidant capacity for in situ bioremediation of contaminated soils and aquifers: a review. J Contam Hydrol 1992; 9:221-42; http://dx.doi.org/10.1016/0169-7722(92)90006-Z
- Rosati F, Roelfes G. Artificial Metalloenzymes. Chem Cat Chem 2010; 2:916-27.
- Lu Y, Yeung N, Sieracki N, Marshall NM. Design of functional metalloproteins. Nature 2009; 460:855-62; PMID:19675646; http://dx.doi.org/10.1038/nature08304
- Kawano T. Learning from prion-derived peptides for designing novel phosphorylation-sensitive peptide probes. Med Hypotheses 2011; 77:159-61; PMID:21665374; http://dx.doi.org/10.1016/j.mehy.2011.03.008
- Yeung N, Lin YW, Gao YG, Zhao X, Russel BS, Lei L, Miner KD, Robinson H, Lu Y. Rational design of a structural and functional nitric oxide reductase. Nature 2009; 462:1079-82; PMID:19940850; http://dx.doi.org/10.1038/nature08620
- Lin C, Tanaka K, Tanaka L, Kawano T. Chemiluminescent and electron spin resonance spectroscopic measurements of reactive oxygen species generated in water treated with titania-coated photocatalytic fibers. Bioluminescence and Chemiluminescence, 2008. In: Eds, Kricka LJ, Stanley PE. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2008:225-8.
- Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C 2000; 1:1-21; http://dx.doi.org/10.1016/S1389-5567(00)00002-2
- Miao L, Tanemura S, Kondo Y, Iwata M, Toh S, Kaneko K. Microstructure and bactericidal ability of photocatalytic TiO2 thin films prepared by rf helicon magnetron sputtering. Appl Surf Sci 2004; 238:125-31; http://dx.doi.org/10.1016/j.apsusc.2004.05.193
- Tanemura S, Miao L, Wunderlich W, Tanemura M, Mori Y, Toh S, Kaneko K. Fabrication and characterization of anatase/rutile-TiO2 thin films by magnetron sputtering: a review. Sci Technol Adv Mater 2005; 6:11-7; http://dx.doi.org/10.1016/j.stam.2004.06.002
- Farahani N, Kelly PJ, West G, Ratova M, Hill C, Vishnyakov V. Photocatalytic activity of reactively sputtered and directly sputtered titania coatings. Thin Solid Films 2011; 520:1464-9; http://dx.doi.org/10.1016/j.tsf.2011.09.059
- Jiang D, Zhang S, Zhao H. Photocatalytic degradation characteristics of different organic compounds at TiO2 nanoporous film electrodes with mixed anatase/rutile phases. Environ Sci Technol 2007; 41:303-8; PMID:17265963; http://dx.doi.org/10.1021/es061509i
- Caballero L, Whitehead KA, Allen NS, Verran J. Inactivation of E.coli on immobilized TiO2 using fluorescent light. J Photochem Photobiol A 2009; 202:92-8; http://dx.doi.org/10.1016/j.jphotochem.2008.11.005
- Kagenishi T, Yokawa K, Lin C, Tanaka K, Tanaka L, Kawano T. Chemiluminescent and bioluminescent analysis of plant cell responses to reactive oxygen species produced by newly developed water conditioning apparatus equipped with titania-coated photocatalystic fibers. Bioluminescence and Chemiluminescence, 2008. In: Eds, Kricka LJ, Stanley PE, Singapore: World Scientific Publishing Co. Pte. Ltd., 2008:27-30.
- Nakano M, Sugioka K, Ushijima Y, Goto T. Chemiluminescence probe with Cypridina luciferin analog, 2-methyl-6-phenyl-3,7-dihydroimidazo[1,2-a]pyrazin-3-one, for estimating the ability of human granulocytes to generate O2-. Anal Biochem 1986; 159:363-9; PMID:3030158; http://dx.doi.org/10.1016/0003-2697(86)90354-4
- Tran D, Kadono T, Meimoun P, Kawano T, Bouteau F. TiO2 nanoparticles induce ROS generation and cytosolic Ca2+ increases on tobacco cells: A chemiluminescence study. Luminescence 2010; 25:140-2.
- Yokawa K, Kagenishi T, Kawano T, Mancuso S, Baluška F. Illumination of Arabidopsis roots induces immediate burst of ROS production. Plant Signal Behav 2011; 6:1457-61; PMID:21957498
- Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 2003; 21:829-37; PMID:12789499
- Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Reports 2005; 24:255-65; PMID:15856234; http://dx.doi.org/10.1007/s00299-005-0972-6
- Kawano T, Furuichi T, Muto S. Controlled free salicylic acid levels and corresponding signaling mechanisms in plants. Plant Biotechnol 2004; 21:319-35; http://dx.doi.org/10.5511/plantbiotechnology.21.319
- Kawano T, Pinontoan R, Uozumi N, Miyake C, Asada K, Kolattukudy PE, Muto S. Aromatic monoamine-induced immediate oxidative burst leading to an increase in cytosolic Ca2+ concentration in tobacco suspension culture. Plant Cell Physiol 2000; 41:1251-8; PMID:11092910; http://dx.doi.org/10.1093/pcp/pcd052
- Kawano T, Pinontoan R, Uozumi N, Morimitsu Y, Miyake C, Asada K, Muto S. Phenylethylamine-induced generation of reactive oxygen species and ascorbate free radicals in tobacco suspension culture: mechanism for oxidative burst mediating Ca2+ influx. Plant Cell Physiol 2000; 41:1259-66; PMID:11092911; http://dx.doi.org/10.1093/pcp/pcd053
- Kawano T, Muto S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco suspension culture. J Exp Bot 2000; 51:685-93; PMID:10938860; http://dx.doi.org/10.1093/jexbot/51.345.685
- Gozzo F. Systemic acquired resistance in crop protection: from nature to a chemical approach. J Agric Food Chem 2003; 51:4487-503; PMID:14705870; http://dx.doi.org/10.1021/jf030025s
- Gazarian IG, Lagrimini LM. Anaerobic stopped-flow studies of indole-3-acetic acid oxidation by dioxygen catalyzed by horseradish peroxidase C and anionic tobacco peroxidase at neutral pH: catalase effect. Biophys Chem 1998; 72:231-7; PMID:17029711; http://dx.doi.org/10.1016/S0301-4622(98)00098-2
- Savitsky PA, Gazaryan IG, Tishkov VI, Lagrimini LM, RuzGas T, Gorton L. Oxidation of indole-3-acetic acid by dioxygen catalyzed by plant peroxidases: specificity for the enzyme structure. Biochem J 1999; 340:579-83; PMID:10359640; http://dx.doi.org/10.1042/0264-6021:3400579
- Kawano T, Kawano N, Hosoya H, Lapeyrie F. Fungal auxin antagonist hypaphorine competitively inhibits indole-3-acetic acid-dependent superoxide generation by horseradish peroxidase. Biochem Biophys Res Commun 2001; 288:546-51; PMID:11676477; http://dx.doi.org/10.1006/bbrc.2001.5800
- Kawano T. Possible use of indole-3-acetic acid and its antagonist tryptophan betaine in controlled killing of horseradish peroxidase-labeled human cells. Medic Hypoth 2003; 60:664-6; PMID:12710900
- Kawano T, Kawano N, Lapeyrie F. A fungal auxin antagonist, hypaphorine prevents the indole-3-acetic aciddependent irreversible inactivation of horseradish peroxidase: inhibition of Compound III-mediated formation of P-670. Biochem Biophys Res Commun 2002; 294:553-9; PMID:12056802; http://dx.doi.org/10.1016/S0006-291X(02)00513-2
- Folkes LK, Wardman P. Oxidative activation of indole-3-acetic to cytotoxic species as potential new role for plant auxins in cancer therapy. Biochem Pharmacol 2001; 61:129-36; PMID:11163327; http://dx.doi.org/10.1016/S0006-2952(00)00498-6
- Dai M, Liu J, Chen D-E, Rao D-E, Tang Z-J, Ho W-Z, Dong C-Y. Tumor-targeted gene therapy using Adv-AFP-HRPC/IAA prodrug system suppresses growth of hepatoma xenografted in mice. Cancer Gene Therapy 2012; 19:77-83; PMID:21959967; http://dx.doi.org/10.1038/cgt.2011.65
- Kimura M, Umemoto Y, Kawano T. Hydrogen peroxide-independent generation of superoxide by plant peroxidase: hypotheses and supportive data employing ferrous ion as a model stimulus. Front Plant Sci 2014; 5:285; PMID:25071789
- Kawano T, Tanaka S, Kadono T, Muto S. Salicylic acid glucoside acts as a slow inducer of oxidative burst in tobacco suspension culture. Z Naturforsch 2004; 59c:684-92; PMID:15540602
- Evans NH, McAinsh MR, Hetherington AM, Knight MR. ROS perception in Arabidopsis thaliana: the ozone-induced calcium response. Plant J 2005; 41:615-26; PMID:15686524; http://dx.doi.org/10.1111/j.1365-313X.2004.02325.x
- Sandermann H. Ecotoxicology of ozone: bioactivation of extracellular ascorbate. Biochem Biophys Res Commun 2008; 366:271-4; PMID:18082136; http://dx.doi.org/10.1016/j.bbrc.2007.12.018
- Kadono T, Yamaguchi Y, Furuichi T, Hirono M, Garrec J-P, Kawano T. Ozone-induced cell death mediated with oxidative and calcium signaling pathways in tobacco Bel-W3 and Bel-B cell suspension cultures. Plant Signal Behav 2006; 1:312-22; PMID:19517002; http://dx.doi.org/10.4161/psb.1.6.3518
- Kadono T, Tran D, Errakhi R, Hiramatsu T, Meimoun P, Briand J, Iwaya-Inoue M, Kawano T, Bouteau F. Increased anion channel activity is an unavoidable event in ozone-induced programmed cell death. PLoS One 2010; 5:e13373; PMID:20967217; http://dx.doi.org/10.1371/journal.pone.0013373
- Tran D, Kadono T, Molas ML, Errakhi R, Briand J, Biligui B, Kawano T, Bouteau F. A role for oxalic acid generation in ozone-induced signalization in Arabidopsis cells. Plant Cell Environ 2013; 36:569-78; PMID:22897345; http://dx.doi.org/10.1111/j.1365-3040.2012.02596.x
- Showalter AM. Structure and function of plant cell wall proteins. Plant Cell 1993; 5:9-23; PMID:8439747; http://dx.doi.org/10.1105/tpc.5.1.9
- No EG, Flagler RB, Swize MA, Cairney J, Newton RJ. cDNAs induced by ozone from Atriplex canescens (saltbush) and their response to sulfur dioxide and water-deficit. Physiol Plant 1997; 100:137-46; http://dx.doi.org/10.1111/j.1399-3054.1997.tb03464.x
- Yokawa K, Kagenishi T, Kawano T. Superoxide generation catalyzed by the ozone-inducible plant peptides analogous to prion octarepeat motif. Plant Signal Behav 2011; 6:477-82; PMID:21350332; http://dx.doi.org/10.4161/psb.6.4.14744
- Kamiya M, Kumaki Y, Nitta K, Ueno T, Watanabe Y, Yamada K, Matsumoto T, Hikichi K, Matsushima N. Copper binding to plant ozone-inducible proteins (OI2-2 and OI14-3). Biochem Biophys Res Commun 2004; 314:908-15; PMID:14741723; http://dx.doi.org/10.1016/j.bbrc.2003.12.158
- Kagenishi T, Yokawa K, Kadono T, Uezu K, Kawano T. Copper-binding peptides from human prion protein and newly designed peroxidative biocatalysts. Z Naturforsch 2011; 66c:182-90; PMID:21630593; http://dx.doi.org/10.5560/ZNC.2011.66c0182
- Li W, Wu S, Ahmad M, Jiang J, Liu H, Nagayama T, Rose ME, Tyurin VA, Tyurina YY, Borisenko GG, et al. The cyclooxygenase site, but not the peroxidase site of cyclooxygenase-2 is required for neurotoxicity in hypoxic and ischemic injury. J Neurochem 2010; 113:965-77; PMID:20236388; http://dx.doi.org/10.1111/j.1471-4159.2010.06674.x
- Boal AK, Cotruvo JA Jr, Stubbe J, Rosenzweig AC. Structural basis for activation of class Ib ribonucleotide reductase. Science 2010; 329:1526-30; PMID:20688982; http://dx.doi.org/10.1126/science.1190187
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: The earliest events in salicylic acid signal transduction. Plant Cell Physiol 1998; 39:721-30; http://dx.doi.org/10.1093/oxfordjournals.pcp.a029426
- Kawano T. Prion-derived copper-binding peptide fragments catalyze the generation of superoxide anion in the presence of aromatic monoamines. Int J Biol Sci 2007; 3:57-63; http://dx.doi.org/10.7150/ijbs.3.57
- Yokawa K, Kagenishi T, Goto K, Kawano T. Free tyrosine and tyrosine-rich peptide-dependent superoxide generation catalyzed by a copper-binding, threonine-rich neurotoxic peptide derived from prion protein. Int J Biol Sci 2009; 5:53-63; PMID:19158988
- Opazo C, Inés-Barría M, Ruiz FH, Inestrosa NC. Copper reduction by copper binding proteins and its relation to neurogenerative diseases. Biometals 2003; 16:91-8; PMID:12572668; http://dx.doi.org/10.1023/A:1020795422185
- Rotilio G, Carr MT, Rossi L, Ciriolo MR. Copper-Dependent Oxidative Stress and Neurodegeneration. IUBMB Life 2000; 50:309-14; PMID:11327325; http://dx.doi.org/10.1080/15216540051081074
- Vassallo N, Herms J. Cellular prion protein function in copper homeostasis and redox signalling at the synapse. J Neurochem 2003; 86:538-44; PMID:12859667; http://dx.doi.org/10.1046/j.1471-4159.2003.01882.x
- Rossi L, Lombardo MF, Ciriolo MR, Rotilio G. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem Res 2004; 29:493-504; PMID:15038597; http://dx.doi.org/10.1023/B:NERE.0000014820.99232.8a
- Tabner BJ, Turnbull S, El-Agnaf O, Allsop D. Production of reactive oxygen species from aggregating proteins implicated in Alzheimer's disease, Parkinson's disease and other neurodegenerative diseases. Curr Top Med Chem 2001; 1:507-17; PMID:11895127; http://dx.doi.org/10.2174/1568026013394822
- Allsop D, Mayes J, Moore S, Masad A, Tabner BJ. Metal-dependent generation of reactive oxygen species from amyloid proteins implicated in neurodegenerative disease. Biochem Soc Trans 2008; 36:1293-2198; PMID:19021543; http://dx.doi.org/10.1042/BST0361293
- Jeffray M, McGovern G, Goodsir CM, Brown KL, Bruce ME. Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy. J Pathol 2000; 191:323-32; PMID:10878556; http://dx.doi.org/10.1002/1096-9896(200007)191:3%3c323::AID-PATH629%3e3.0.CO;2-Z
- Aronoff-Spencer E, Burns CS, Avdievich NI, Gerfen GJ, Peisach J, Antholine WE, Ball HL, Cohen FE, Prusiner SB, Millhauser GL. Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry 2000; 39:13760-71; PMID:11076515; http://dx.doi.org/10.1021/bi001472t
- Burns CS, Aronoff-Spencer E, Legname G, Prusiner SB, Antholine WE, Gerfen GJ, Peisach J, Millhauser GL. Copper coordination in the full-length, recombinant prion protein. Biochemistry 2003; 42:6794-903; PMID:12779334; http://dx.doi.org/10.1021/bi027138+
- Bonomo RP, Imperllizzeri G, Pappalardo G, Rizzarelli E, Tabbi G. Copper(II) binding modes in the prion octapeptide PHGGGWGQ: a spectroscopic and voltammetric study. Chemistry 2000; 6:4195-205; PMID:11128284; http://dx.doi.org/10.1002/1521-3765(20001117)6:22%3c4195::AID-CHEM4195%3e3.0.CO;2-2
- Stanczak P, Luczkowski M, Juszczyk P, Grzonka Z, Kozlowski H. Dalton Trans 2004; 14:2102; PMID:15249945; http://dx.doi.org/10.1039/b405753h
- Yokawa K, Kagenishi T, Kawano T. Cypridina luciferin analog, CLA as a probe for the enzymatic reaction of superoxide generation catalyzed by copper-binding hexapeptide found in chicken prion protein. Luminescence 2010; 25:139
- Sauer H, Dagdanova A, Hescheler J, Wartenberg M. Redox-regulation of intrinsic prion expression in multicellular prostate tumor spheroids. Free Radic Biol Med 1999; 27:1276-83; PMID:10641721; http://dx.doi.org/10.1016/S0891-5849(99)00164-1
- Wong BS, Brown DR, Pan T, Whiteman M, Liu T, Bu X, Li R, Gambetti P, Olesik J, Rubenstein R, et al. Oxidative impairment in scrapie-infected mice is associated with brain metals perturbations and altered antioxidant activities. J Neurochem 2001; 79:689-98; PMID:11701772; http://dx.doi.org/10.1046/j.1471-4159.2001.00625.x
- Watt NT, Taylor DR, Gillott A, Thomas DA, Perera WS, Hooper NM. Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. J Biol Chem 2005; 280:35914-21; PMID:16120605; http://dx.doi.org/10.1074/jbc.M507327200
- Koga S, Nakano M, Tero-Kubota S. Generation of superoxide during the enzymatic action of tyrosinase. Arc Biochem Biophys 1992; 292:570-5; PMID:1309977; http://dx.doi.org/10.1016/0003-9861(92)90032-R
- Yokawa K, Kagenishi T, Kawano T. Use of Cypridina luciferin analog for assessing the monoamine oxidase-like superoxide-generating activities of two peptide sequences corresponding to the helical copper-binding motif. Bioluminescence and Chemiluminescence, 2008. In: Eds, Kricka LJ, Stanley PE. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2008:83-6.
- Kawano T. Quenching and enhancement of terbium fluorescence in the presence of prion-derived copper-binding peptides. ITE Lett 2006; 7:383-385.
- Kagenishi T, Yokawa K, Kuse M, Isobe M, Bouteau F, Kawano T. Prevention of copper-induced calcium influx and cell death by prion-derived peptide in suspension-cultured tobacco cells. Z Naturforsch 2009; 64:411-7; PMID:19678548
- Okobira T, Kadono T, Kagenishi T, Yokawa T, Kawano T, Uezu K. Copper-binding peptide fragment-containing membrane as a biocatalyst by radiation-induced graft polymerization. Sens Mater 2011; 23:207-18.
- Inokuchi R, Yokawa K, Okobira T, Uezu K, Kawano T. Fluorescence measurements revealed two distinct modes of metal binding by histidine-containing motifs in prion-derived peptides. Curr Topics Peptide Protein Res 2012; 13:111-8.
- Burns CS, Aronoff-Spencer E, Dunham CM, Lario P, Avdievich NI, Antholine WE, Olmstead MM, Vrielink A, Gerfen GJ, Peisach J, et al. Molecular features of the copper binding sites in the octarepeat domain of the prion protein. Biochemistry 2002; 41:3991-4001; PMID:11900542; http://dx.doi.org/10.1021/bi011922x
- Wood JM, Schallreuter KU. Studies on the reactions between human tyrosinase, superoxide anion, hydrogen peroxide and thiols. Biochim Biophys Acta 1991; 1074:378-85; PMID:1653610; http://dx.doi.org/10.1016/0304-4165(91)90088-X
- Kitamoto T. Food and Drug Safety. Tokyo: Springer-Verlag; 2005:1.
- Castilla J, Saa P, Soto C. Detection of prions in blood. Nat Med 2005; 11:982-5; PMID:16127436
- Yokawa K, Kagenishi T, Kawano T. Thermo-stable and freeze-tolerant nature of aromatic monoamine-dependent superoxide-generating activity of human prion-derived Cu-binding peptides. Biosci Biotechnol Biochem 2009; 73:1218-20; http://dx.doi.org/10.1271/bbb.90012
- Stülke J. More than just activity control: phosphorylation may control all aspects of a protein's properties. Mol Microbiol 2010; 77:273-5; PMID:20497498; http://dx.doi.org/10.1111/j.1365-2958.2010.07228.x
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem 1995; 270:14843-186; PMID:7797459; http://dx.doi.org/10.1074/jbc.270.25.14843
- Luo Y, Henle ES, Linn S. Oxidative Damage to DNA Constituents by iron-mediated Fenton reactions: the Deoxycytidine family. J Biol Chem 1996; 271:21167-76; PMID:8702887; http://dx.doi.org/10.1074/jbc.271.35.21167
- Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J 1991; 273:601-4; PMID:1899997
- Higuchi Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem Pharmacol 2003; 66:1527-35; PMID:14555231; http://dx.doi.org/10.1016/S0006-2952(03)00508-2
- Tajmir-Riahi HA, Langlais M, Savoie R. A laser Raman spectroscopic study of the interaction of calf-thymus DNA with Cu(II) and Pb(II) ions: metal ion binding and DNA conformational changes. Nucleic Acids Res 1988; 16:751-62; PMID:3340554; http://dx.doi.org/10.1093/nar/16.2.751
- Geierstanger BH, Kagawa TF, Chen SL, Quigley GJ, Ho PS. Base-specific binding of copper(II) to Z-DNA. The 1.3-Å single crystal structure of d(m5CGUAm5CG) in the presence of CuCl2. J Biol Chem 1991; 266:20185-91; PMID:1939079
- Yokawa K, Kagenishi T, Kawano T. Prevention of oxidative DNA degradation by copper-binding peptides. Biosci Biotechnol Biochem 2011; 75:1377-9; PMID:21737913
- Dietz KJ, Baier M, Krämer U. Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. Heavy metal stress in plants. In:Prasad MNV, Hagemeyer J, ed. Berlin-Heidelberg: Springer-Verlag; 1999:73-97.
- Drążkiewicz M, Skórzyńska-Polit E, Krupa Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals 2004; 17:379-87; PMID:15259358; http://dx.doi.org/10.1023/B:BIOM.0000029417.18154.22
- Tewari RK, Kumar P, Sharma PN. Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta 2006; 223:1145-53; PMID:16292566; http://dx.doi.org/10.1007/s00425-005-0160-5
- Bona E, Marsano F, Cavaletto M, Berta G. Proteomic characterization of copper stress response in Cannabis sativa roots. Proteomics 2007; 7:1121-30; PMID:17352425; http://dx.doi.org/10.1002/pmic.200600712
- Gonzalez A, Vera J, Castro J, Dennett G, Mellado M, Morales B, Correa JA, Moenne A. Co-occurring increases of calcium and organellar reactive oxygen species determine differential activation of antioxidant and defense enzymes in Ulva compressa (Chlorophyta) exposed to copper excess. Plan Cell Environ 2010; 33:1627-40; PMID:20444222; http://dx.doi.org/10.1111/j.1365-3040.2010.02169.x
- Lin C, Kadono T, Suzuki T, Yoshizuka K, Furuichi T, Yokawa K, Kawano T. Mechanism for temperature-shift-responsive acute Ca2+ uptake in suspension-cultured tobacco and rice cells. Cryobiol Cryotechnol 2006; 52:83-9; http://dx.doi.org/10.1016/j.cryobiol.2005.10.004
- Kunihiro S, Hiramatsu T, Kawano T. Involvement of salicylic acid signal transduction in aluminum-responsive oxidative burst in Arabidopsis thaliana cell suspension culture. Plant Signal Behav 2011; 6:611-6; PMID:21447999; http://dx.doi.org/10.4161/psb.6.5.14895
- Tran D, El-Maarouf-Bouteau H, Rossi M, Biligui B, Briand J, Kawano T, Mancuso S, Bouteau F. Post-transcriptional regulation of GORK channels by superoxide anion contributes towards increases in outward rectifying K+ currents. New Phytol 2013; 198:1039-48; PMID:23517047; http://dx.doi.org/10.1111/nph.12226
- Iwase J, Furukawa H, Hiramatsu T, Bouteau F, Mancuso S, Tanaka K, Okazaki T, Kawano T. Protection of tobacco cells from oxidative copper toxicity by catalytically active metal-binding DNA oligomers. J Exp Bot 2014; 65:1391-402; PMID:24659609; http://dx.doi.org/10.1093/jxb/eru028
- Ban C, Ramakrishnan B, Sundaralingam M. Crystal structure of the self-complementary 5'-purine start decamer d(GCGCGCGCGC) in the Z-DNA conformation. I. Biophys J 1996; 71:1215-21; PMID:8873995; http://dx.doi.org/10.1016/S0006-3495(96)79350-5
- Kunitake T, Shumada F, Aso C. Imidazole catalyses in aqueous systems. I. The enzyme-like catalysis in the hydrolysis of phenyl ester by imidazole-containing copolymers. J Amer Chem Soc 1969; 91:2716-23; http://dx.doi.org/10.1021/ja01038a051
- Shinkai S, Kunitake T. Imidazole catalyses in aqueous systems. X. Enzyme-like catalytic hydrolyses of phenyl esters by polymers containing imidazole and other anionic functions. Polymer J 1975; 7:387-96; http://dx.doi.org/10.1295/polymj.7.387
- Kawano T, Hiramatsu T, Yokawan K, Tanaka L, Tanaka K. Responses of living plants to increasing UV and ozone. Can we protect the crops and flora from environmental stresses through bioengineering approaches? Proceedings of 3rd Japan–Taiwan Joint International Symposium on Environmental Science and Technology 2008 2008:116-22.
- Takayama A, Kadono T, Kawano T. Heme redox cycling in soybean peroxidase: hypothetical model and supportive data. Sensors and Materials 2012; 24:87-97.
