Abstract
In a recent paper,Citation1 we examined whether oxytocin in the domestic dog modulates the maintenance of close social bonds in non-reproductive contexts. We found that exogenous oxytocin promotes positive social behaviors not only toward conspecifics, but also toward human partners. Here we examined in further detail the effect that oxytocin manipulation has on social play. When sprayed with oxytocin, subjects initiated play sessions more often and played for longer periods of time than when sprayed with saline. Furthermore, after oxytocin nasal intake dogs displayed play signals more often than after saline administration, suggesting that oxytocin enhances dogs' play motivation. To our knowledge, this study provides the first evidence that oxytocin promotes social play in the domestic dog. We use these results to hypothesize on the potential therapeutic use of oxytocin for promoting social behaviors and treating social deficits in the domestic dog.
Over the past several decades, extensive animal research has shown that the hypothalamic neuropeptide oxytocin plays an important role in the regulation of several behaviors associated with sociality, such as sexual behavior, pair-bonding, parental care, peer recognition, and social memory (for reviews see refs.Citation2,3). More recent studies in humans have revealed that oxytocin is also involved in aspects of social cognition including social perception, emotion recognition, sensibility to the experiences of others, and pro-social behaviors (for reviews see refs.Citation4,5).
Building on these findings, researchers have suggested that the manipulation of the oxytocin system may be used as a tool for improving socio-cognitive abilities in individuals with social deficit disorders (e.g. autism spectrum disorder, social anxiety disorder, and borderline personality disorder; for reviews see refs.Citation6,7). In fact, in the last decade the effect of administered oxytocin has been tested in clinical populations, with several of these studies reporting beneficial effects of oxytocin on social attention and emotion recognition in autistic individuals, and a reduction of social anxiety in patients with social phobia and borderline personality disorder (e.g., refs.Citation8-11). While at this point much remains unknown about the lasting effects of these benefits and the extent to which personality and personal history mitigate these beneficial effects, such findings are clearly grounds for optimism about the therapeutic potential of oxytocin.
A similar therapeutic use of oxytocin could be relevant to animal health. This clinical approach could be particularly interesting for companion animals showing behavioral problems related to social deficits. Each year, millions of dogs and cats arrive in animal shelters after abandonment, abuse, or relinquishment by their previous caregivers. The most widely reported reasons for relinquishment of dogs in USA are behavioral problems due to lack of proper socialization and habituation (i.e. fear and aggression directed to strangers or other dogs).Citation12,13 Although in many countries there are approved drugs for the treatment of anxiety related disorders in domestic animals,Citation14 to date there is no drug for the treatment of social deficits. Thus, the development of pharmacotherapies that promote social integration in companion animals may be helpful in the treatment of selected behavior problems.
In a recent paper,Citation1 we showed that nasal intake of oxytocin promotes social bonding in domestic dogs. A total of 16 adult dogs from different breeds participated as subjects in a randomized placebo-controlled experiment (females = 8; male = 8; mean age 6.1 y (SEM = 0.7)). Each dog received a nasal spray of 100μl of oxytocin (40 IU, Peptide institute, Japan) or 100μl of saline solution, depending on the testing condition. All subjects received both conditions and each condition was carried out on different days. After spray intake, dogs stay in the experimental room (11.5 × 6.5m) with their owners and a familiar dog partner, and their behaviors were video recorded for 60 minutes. Owners were instructed to sit quietly in the experimental room and not to actively interact with their dogs; while dogs could move freely in the room (for further details, seeCitation1). We found that oxytocin caused dogs to engage in higher levels of affiliation and approach with their dog partners, suggesting that the experimental intranasal administration of oxytocin in dogs promotes affiliative motivation and facilitates bond formation and maintenance.
Here, we analyze in more detail the episodes of social play that took play during the experimental sessions in order to address the question of whether intranasal administration of oxytocin enhances social play in dogs. Play is a highly plastic and versatile behavior that generally occurs when animals are free from environmental and social stressors.Citation15 Although play is characteristic of early life stages, in many species social play continues into adulthood (e.g. rodents,Citation16 canids,Citation17,18 primatesCitation19,20). Social play is thought to help animals to develop social and emotional flexibility, conflict resolution skills and appropriate reactions to unexpected situations.Citation15,21-24 It could also serve to assess social relationships and/or increase social affinity between individuals. Citation19,25,26 Social play is also associated with immediate benefits for the animals. Recent evidence shows that through play animals reduce tension around stressful situations,Citation27 or turn a stranger into a familiar individual.Citation28 Furthermore, play is frequently used as part of therapies to correct behavioral problems in dogs.Citation29 Thus, the combination of behavioral interventions with pharmacotherapies that promote affiliation in general and play in particular might be a potential fruitful strategy for the treatment of behavior problems in companion animals.
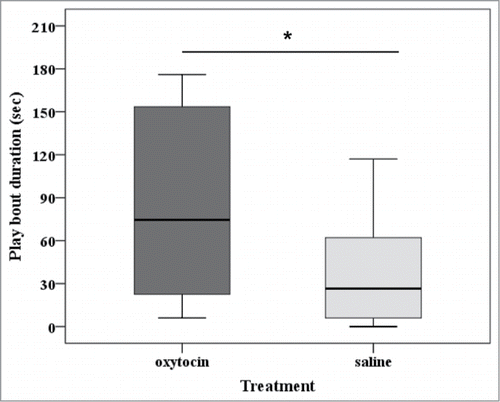
In the present study, all dogs that participated as partners had a friendly relationship with the subjects. Thus, we did not observe any agonistic behavior during the experimental sessions and none of the play sessions resembled or escalated into aggression. A play session started when an individual directed a play signal (including play bow, face paw, and exaggerated approaches and retreatsCitation18,21,30) or any playful behavior (including nipping, inhibited bite, play-chasing, mounting, play-fighting, and play-tacklingCitation18,21,30) toward the partner, and ended when their activity ceased or one of the subjects moved away (>1 m). A new play session was recorded if at least 5 seconds elapsed between the end of the first bout and the start of the new bout. Only play sessions that lasted more than 5 seconds were included in the analysis (N = 74). All subjects but one were involved in at least one social play session (mean duration in seconds = 161.3 ± 52.6 SE), and only one subject engaged in solitary play. Thus, solitary play sessions were excluded from the analyses. The type of treatment dogs were administered with did not affect how often subjects were involved in social play (Wilcoxon signed rank test: z = −1.749, N = 15, p = 0.08), nor how often they engaged in play after receiving a play invitation (z = 0.479, N = 15, p = 0.6384). However, after oxytocin nasal spray intake, dogs initiated play sessions significantly more often than after saline treatment (z = −1.997, N = 15, p = 0.046). We then examined the total amount of time dogs spent playing with their partners. We found that oxytocin treatment was associated with longer play sessions (z = −2.040, N = 15, p = 0.041, ).
Figure 1. Duration of play bouts during the oxytocin and the saline conditions. The box plots represent the median and upper and lower quartiles; and the whiskers indicate the values within 1.5 times the interquartile range. *P < 0.05
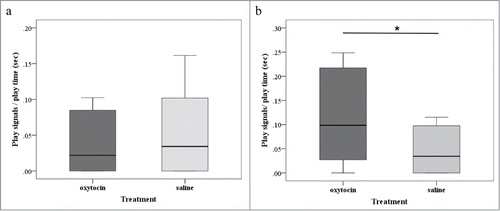
Given that social play is an interaction that requires the active involvement of both partners, the total time that 2 individuals play together depends on both individuals' motivation. Thus, we evaluated the subject's relative role in maintaining the play session by examining the production of play signals that are known to promote and/or facilitate play interactions.Citation18,31 We did not find any effect of treatment type on the frequency of play signals received (z = −0.255, N = 15, p = 0.799, ). However, after oxytocin nasal intake dogs directed play signals to their play-mates significantly more often than after saline treatment (z = −2.090, N = 15, p = 0.037, ). These findings suggest that the experimental administration of oxytocin had positive effects on dogs' motivation to interact in a playful way with conspecifics. Play has long been identified as a potential welfare indicator,Citation15 a facilitator of social-emotional learning,Citation22 and an implicitly rewarding behavior.Citation32 Thus, increasing play interactions could be beneficial for dogs. Previous resultsCitation1 have provided behavioral evidence that exogenous oxytocin promotes positive social behaviors in the domestic dog. The present study extends this data showing that oxytocin manipulation also enhances play motivation, suggesting that this pharmacological intervention may be used to help manage a range of animal behavioral problems. The reader should note, however, that the positive effects of oxytocin on social approach, general affiliationCitation1 and play (this study) were observed in healthy dog participants. Hence, a key question is whether administration of oxytocin can influence social behaviors in animals with behavioral disorders. Caution is therefore warranted in the interpretation of our results, and further studies on whether and how oxytocin influences social interactions in clinic populations are clearly necessary.
Figure 2. Frequency of play signals received (A) and given (B) during the oxytocin and the saline experimental conditions. The box plots represent the median and the upper and lower quartiles; and the whiskers indicate the values within 1.5 times the interquartile range. *P < 0.05.
In summary, this study provides the first evidence that administration of oxytocin increases dogs' motivation to play with conspecifics and refines our knowledge about the behavioral effects of exogenous oxytocin in dogs. A more thorough understanding of this peptide and its behavioral consequences in healthy individuals may help to design pharmacological interventions aimed at promoting social behavior in companion animals whose social skills have been compromised, such as dogs with poor socialization at earlier life stages. Further investigation is clearly necessary to further test oxytocin's behavioral effects on clinical populations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the dogs and their owners for their participation in the study. We are also grateful to Darby Proctor for useful comments on a previous version of the manuscript.
Funding
The present study was approved by the Ethics Committee of Azabu University (Japan) (No. 130304–2). This research was supported by the JSPS Research Fellowships for Foreign Researchers (No. P10311) and the MEXT Grant-in-aid for Scientific Research (No. 26380981) to T. R., the MEXT Grant-in-aid for Challenging Exploratory Research (No. 23650132) to T. H., and the Grant-in-Aid for Scientific Research on Innovative Areas (No. 4501) to T.K. and T. H.
References
- Romero T, Nagasawa M, Mogi K, Hasegawa T, Kikusui T. Oxytocin promotes social bonding in dogs. Proc Natl Acad Sci USA 2014; 111:9085-90; PMID:24927552; http://dx.doi.org/10.1073/pnas.1322868111
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol 2009; 30:534-47; PMID:19481567; http://dx.doi.org/10.1016/j.yfrne.2009.05.004
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010; 65:768-79; PMID:20346754; http://dx.doi.org/10.1016/j.neuron.2010.03.005
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci 2010; 15:301-9.
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol 2009; 30:548-57; PMID:19505497; http://dx.doi.org/10.1016/j.yfrne.2009.05.005
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm Behav 2012; 61:340-50; PMID:22206823; http://dx.doi.org/10.1016/j.yhbeh.2011.12.010
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psych 2013; 3:e258; http://dx.doi.org/10.1038/tp.2013.34
- Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA 2010; 107:4389-94; PMID:20160081; http://dx.doi.org/10.1073/pnas.0910249107
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psych 2010; 67:692-4; http://dx.doi.org/10.1016/j.biopsych.2009.09.020
- Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, Oishi M, Kimura T, Onaka T, Ozono K, Taniike M. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol 2013; 23:123-7; PMID:23480321; http://dx.doi.org/10.1089/cap.2012.0048
- Stavropoulos KK, J CL. Research review: Social motivation and oxytocin in autism—implications for join attention development and intervention. J Child Psychol Psych 2013; 54:603-18; http://dx.doi.org/10.1111/jcpp.12061
- Salman MD, Hutchison J, Ruch-Gallie R, Kogan L, New JCJ, Kass PH, Scarlett JM. Behavioral reasons for relinquishment of dogs and cats to 12 shelters. J Appl Anim Welf Sci 2010; 3:93-106; http://dx.doi.org/10.1207/S15327604JAWS0302_2
- Duffy DL, Kruger KA, Serpell JA. Evaluation of a behavioral assessment tool for dogs relinquished to shelters. Prev Vet Med 2014; PMID:25457136
- Simpson BS, Papich MG. Pharmacologic management in veterinary behavioral medicine. Veterinary Clinics of North America: Small Animal Practice 2003; 33:365-404; http://dx.doi.org/10.1016/S0195-5616(02)00130-4
- Held SDE, Spinka M. Animal play and animal welfare. Anim Behav 2011; 81:891-9; http://dx.doi.org/10.1016/j.anbehav.2011.01.007
- Pellis SM. Sex-differences in play fighting revisited: traditional and nontraditional mechanisms for sexual differentiation in rats. Arch Sex Behav 2002; 31:11-20; http://dx.doi.org/10.1023/A:1014070916047
- Bauer EB, Smuts BB. Cooperation and competition during dyadic play in domestic dogs, Canis familiaris. Anim Behav 2007; 73:489-99; http://dx.doi.org/10.1016/j.anbehav.2006.09.006
- Horowitz L. Attention to attention in domestic dog (Canis familiaris) dyadic play. Anim Cogn 2009; 12:107-18; PMID:18679727; http://dx.doi.org/10.1007/s10071-008-0175-y
- Pellis SM, Iwaniuk AN. Adult-adult play in primates: comparative analyses of its origin, distribution and evolution. Ethology 2000; 106:1083-104; http://dx.doi.org/10.1046/j.1439-0310.2000.00627.x
- Palagi E, Paoli T. Play in adult bonobos (Pan paniscus): modality and potential meaning. Am J Phys Anthropol 2007; 134:219-25; PMID:17596855; http://dx.doi.org/10.1002/ajpa.20657
- Bekoff M. Social play behavior. Bioscience 1984; 34:228-33; http://dx.doi.org/10.2307/1309460
- Spinka M, Newberry RC, Bekoff M. Mammalian play: training for the unexpected. Q Rev Biol 2011; 76:141-68; http://dx.doi.org/10.1086/393866
- Smith EFS. Does play matter: functional and evolutionary aspects of animal and human play. Behav Brain Sci 1982; 5:139-55; http://dx.doi.org/10.1017/S0140525X0001092X
- Palagi E, Cordoni G, Borgognini Tarli TS. Immediate and delayed benefits of play behaviour: new evidence from chimpanzees (Pan troglodytes). Ethology 2004; 110:949-62; http://dx.doi.org/10.1111/j.1439-0310.2004.01035.x
- Palagi E. Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): Implications for natural social systems and interindividual relationships. Am J Phys Anthropol 2006; 129:418-26; PMID:16323189; http://dx.doi.org/10.1002/ajpa.20289
- Ciani F, Dall'Olio S, Stanyon R, Palagi E. Social tolerance and adult play in macaque societies: a comparison with different human cultures. Anim Behav 2012; 84:1313-22; http://dx.doi.org/10.1016/j.anbehav.2012.09.002
- Palagi E, Paoli T, Tarli SB. Short-term benefits of play behavior and conflict prevention in Pan paniscus. Int J Primatol 2006; 27:1257-70; http://dx.doi.org/10.1007/s10764-006-9071-y
- Antonacci D, Norscia I, Palagi E. Stranger to familiar: wild strepsirhines manage xenophobia by playing. PLoS ONE 2010; 5:e13218; PMID:20949052; http://dx.doi.org/10.1371/journal.pone.0013218
- Landsberg GM, Hunthausen WL, Ackerman LJ. Behavior problems of the dog and cat. Elsevier Health Sciences, 2012.
- Abrantes R. Dog language. Napierville, Illinois: Wakan Tanka Publishers, 1997.
- Bekoff M. The development of social interaction, play, and meta-communication in mammals: an ethological perspective. Q Rev Biol 1972; 47:412-34; http://dx.doi.org/10.1086/407400
- Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play-behavior in rats. Neurosci Biobehav Rev 1997; 21:309-26; PMID:9168267; http://dx.doi.org/10.1016/S0149-7634(96)00020-6
