Abstract
Cycad biology, ecology, and horticulture decisions are not supported by adequate research, and experiments in cycad physiology in particular have been deficient. Our recent report on free sugar content in a range of cycad taxa and tissues sets the stage for developing continued carbohydrate research. Growth and development of cycad pollen, mediation of the herbivory traits of specialist pollinators, and support of expensive strobilus behavioral traits are areas of cycad pollination biology that would benefit from a greater understanding of the role of carbohydrate relations.
Cycads represent the most threatened group of plants worldwide.Citation1 Global research trends have been heavily focused on disciplines such as taxonomy and phylogeny, and research into cycad physiology has not received adequate attention.Citation2 As a result, we studied various aspects of free sugar content and reported that fructose, glucose, and sucrose were abundant in tissues from species representing every described cycad genera, and sugar content and stoichiometry varied greatly among organs.Citation3 The importance of these carbohydrates as forms of carbon storage, components of signaling, or regulators of cycad metabolism have not been determined to date.
Cycads and insects have developed sophisticated pollination mutualisms and specialist antagonistic relationships. A greater understanding of how non-structural carbohydrates influence these relationships may improve conservation efforts for the many threatened cycad species. Here we focus on pollination biology and how the inclusion of carbohydrate studies could aid in efforts to reach that goal.
Pollen
Carbohydrates play critical roles during pollen growth and development, then in the final phase prior to dispersal carbohydrates change to prepare pollen for dispersal.Citation4 Following dispersal and during pollen storage, the various metabolites comprising the carbohydrate pool change in relative proportion, a behavior that is understood as an adaptation for sustaining pollen viability in time.Citation5 Sucrose in particular may be of particular importance during pollen storage, as variations among species for pollen desiccation tolerance have been linked to sucrose content.Citation6 Sucrose may replace water to preserve native protein structures and spacing between phospholipids in the plasma-membrane during dehydration.Citation7 The role of starch and sugars in pollen development, dispersal, and maintenance of viability has not been determined for any member of the Cycadales, and is therefore a focus of needed research.
Several authors have conducted robust surveys and noted that starchy pollen occurred disproportionately among anemophilous species.Citation8-10 Representatives from the Cycadales were absent from these surveys and this should be corrected, as cycad pollination studies have only recently illuminated the sophisticated pollination syndromes that characterize the threatened plant group. Although arthropods were collected from reproductive structures on various cycad taxa in the past, the scientific community largely ignored their potential role as pollinators due to the established belief that all cycads were anemophilous.Citation11 The pendulum swung in the 1980s and 1990s when extensive experimental evidence confirmed mutualisms between cycad species and specialist insect pollinators.Citation2,12,13 More recently, ambophily has been confirmed or proposed for several Cycas species where wind has been shown to augment insect pollination.Citation14-17 Furthermore, variations in aggregation tendencies and settling velocity have been reported for pollen from various cycad species.Citation14,15 This is an opportune time to add members of the Cycadales to studies that have linked the relationship of pollen carbohydrates to insect versus wind pollination strategies.
Herbivory
The insect pollinators of cycads gather on male cones, where the adults socialize, mate, and use the post-dispersal male strobilus tissue as larvae food ().Citation2,13,14,18 The study of idioblasts within cycad sporophyll tissues has illuminated intricacies in how general tissue chemistry may interact with pollinator feeding behaviors. For Zamia furfuracea, these idioblasts remained intact in the male sporophyll tissue but appeared to release their contents into female sporophyll tissue prior to ovule receptivity.Citation19,20 The authors concluded that toxins remained sequestered within the idioblasts for male tissue only, such that the pollinators could feed on parenchyma tissue around the idioblasts or consume intact idioblasts which protected insect metabolism from the toxins after consumption. Many but not all cycad species studied in this context exhibited similar idioblast traits.Citation21 For example, Cycas rumphii and Stangeria eriopus strobili did not contain identifiable idioblasts. In contrast, Macrozamia lucida and Microcycas calocoma exhibited idioblast breakdown prior to pollination stage for both male and female sporophyll tissues. The interactions among carbohydrates, nutrients, and toxins within cycad strobili tissues in relation to these idioblasts may prove to be of crucial importance for developing a full understanding of how pollinators feed on the plant's reproductive tissues while enacting effectual pollination. Conservationists in need of improved knowledge of various pollination syndromes within the Cycadales will require more studies within this context.
Figure 1. The microlepidopteran Anatrachyntis sp. is a specialist pollinator of Cycas micronesica. Left: microstrobilus tissue is tunneled and consumed by larvae (arrows) immediately after pollen dispersal. Right: within days the entire microstrobilus is reduced to frass and pupation (arrow) heralds in a new generation of pollinators. The role of cycad sugars in mediating this mutualism is unknown.
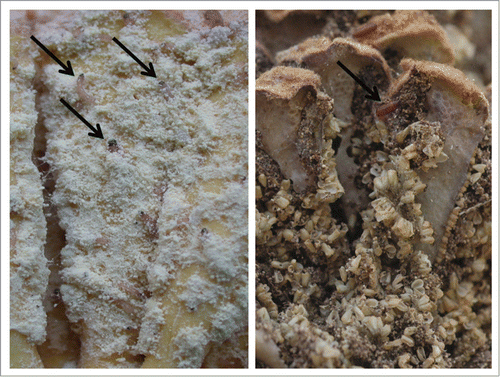
The majority of cycad pollinators consume strobilus tissue, but direct consumption of cycad pollen may occur for some cycad pollinators.Citation22,23 Cumulative research on pollen digestion has not progressed enough to discern generalities from idiosyncracies.Citation24 The addition of cycads to studies that determine carbohydrate and nutritional quality of pollen will greatly improve this line of research whether the mutualisms represent ancient associations that pre-dated angiosperms or examples where the contemporary mutualisms were recently derived from an initial antagonistic relationship.Citation14,25,26
Strobilus Behavior
The reproductive structures of cycad species exhibit thermogenesis and volatile emissions at the time of pollination, traits that mediate pollinator behavior and maintain pollinator specificity.Citation13,27,28 Research is accumulating in areas such as modeling the plant behavior Citation29,30 and parsing the influence of specific volatiles on insect behavior.Citation31,32 Enacting synchronized thermogenesis and volatile biosynthesis is an exceedingly expensive plant behavior, and no studies have determined the tissues of residence, quantities, and stoichiometric relations of the carbohydrate reserves that are mobilized to fund those activities.
Cycad ovules are large structures that greatly exceed the size of their pollinators. Droplets have been documented at the micropyle location on cycad ovules, and these droplets contain metabolites that may provide a reward for pollinators.Citation33 Studies that marked pollinators then used deposition of the markers to track pollinator behavior have confirmed that the pollen they vector is trapped by the ovule droplets.Citation23,25,34 This remarkable female strobilus behavior represents a system that would be interesting to study in relation to specificity of pollinator attraction, diversity of sugar rewards, and cycad phylogeny. Droplets may exhibit taxon-specific carbohydrates that are suited to attracting and nourishing specific specialist pollinators.
Conclusions
Our recent report of fructose, glucose, and sucrose content in cycad tissuesCitation3 sets the stage for designing continued research on how non-structural carbohydrates are involved in cycad pollination biology. Moreover, cycad horticulturists routinely harvest, store, and ship pollen prior to its use for successful pollination. Improved understanding of how carbohydrates and other factors influence pollen viability and longevity would improve protocols for artificial pollination.Citation35 Finally, the risks associated with coextinctions are very real during this phase of the Anthropocene, and species with complex life history traits, such as cycads, appear to be at greater risk for direct involvement in coextinctions.Citation36 An increase in knowledge of how cycad carbohydrates influence successful pollination relationships may help reduce the risks of coextinctions in these mutualisms that support contemporary cycad biology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, Carpenter KE, Chanson J, Collen B, Cox NA, et al. The impact of conservation on the status of the world's vertebrates. Science 2010; 330:1503-9; PMID:20978281; http://dx.doi.org/10.1126/science.1194442
- Norstog KJ, Nicholls TJ. The biology of the cycads. Ithica, New York: Taylor & Francis. 1997; 363.
- Marler TE, Lindström, AJ. Free sugar profile in cycads. Front Plant Sci 2014; 5:526. doi: 10.3389/fpls.2014.00526; http://dx.doi.org/10.3389/fpls.2014.00526
- Pacini E, Guarnieri M, Nepi M. Pollen carbohydrates and water content during development, presentation, and dispersal: a short review. Protoplasma 2006; 228:73-7; PMID:16937057; http://dx.doi.org/10.1007/s00709-006-0169-z
- Guarnieri M, Speranza A, Nepi M, Artese D, Pacini E. Ripe pollen carbohydrate changes in Trachycarpus fortunei: the effect of relative humidity. Sexual Plant Reproduction 2006; 19:117-24; http://dx.doi.org/10.1007/s00497-006-0027-3
- Hoekstra FA, Crowe LM, Crow JH. Differential desiccation sensitivity of corn and Pennisetum pollen linked to their sucrose contents. Plant Cell Envrion 1989; 12:83-91; http://dx.doi.org/10.1111/j.1365-3040.1989.tb01919.x
- Buitink J, Leprince O. Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 2004; 48:215-28; PMID:15157771; http://dx.doi.org/10.1016/j.cryobiol.2004.02.011
- Calvino EM. Le sostanze de riserva dei pollini e il loro significato, filogenetico, ecologico, embriologico. Nuovo Giornale Botanico Italiano 1952; 59:1-26; http://dx.doi.org/10.1080/11263505209436475
- Baker HG, Baker I. Starch in angiosperm pollen grains and its evolutionary significance. Amer J Bot 1979; 66:591-600; http://dx.doi.org/10.2307/2442509
- Baker HG, Baker I. Some evolutionary and taxonomic implications of variation in the chemical reserves of pollen. In: Mulcahy DL, Ottaviano E, eds. Pollen: biology and implications for plant breeding. New York: Taylor & Francis, 1983:43-52.
- Chamberlain CJ. Gymnosperms: structure and evolution. Chicago: Taylor & Francis. 1935; 484.
- Stevenson DW, Norstog KJ, Fawcett PKS. 1998. Pollination biology of cycads, In: Owens SJ, Rudall PJ, eds. Reproductive Biology. Taylor & Francis, 1998:277-94.
- Terry I, Tang W, Taylor AS, Donaldson JS, Singh R, Vovides AP, Jaramillo AC. An overview of cycad pollination studies. Mem New York Bot Gard 2012; 106:352-94.
- Hall J. The ecology of cycads: living representatives of an ancient plant lineage and their interactions with animals [dissertation]. Australia: Univ Queensland; 2011. 259 p.
- Hamada TS. The role of wind in pollination of the endangered Cycas micronesica K.D. Hill (Cycadaceae) on Guam [thesis]. Mangilao: Univ Guam; 2013. 155 p.
- Kono M, Tobe H. 2007. Is Cycas revoluta (Cycadaceae) wind- or insect-pollinated? Amer J Bot 2007; 94:847-55; http://dx.doi.org/10.3732/ajb.94.5.847
- Terry I, Roe M, Tang W, Marler TE. Cone insects and putative pollen vectors of the endangered cycad, Cycas micronesica. Micronesica 2009; 41:83-99.
- Marler, TE. Cycad mutualist offers more than pollen transport. Amer J Bot 2010; 97:841-5; http://dx.doi.org/10.3732/ajb.0900251
- Norstog KJ, Fawcett PKS. Insect-cycad symbiosis and its relation to the pollination of Zamia furfuracea (Zamiaceae) by Rhopalotria mollis (Curculionidae). Amer J Bot 1989; 76:1380-94; http://dx.doi.org/10.2307/2444562
- Vovides AP, Norstog KJ, Fawcett PKS, Duncan W, Nash RJ. Histological changes during maturation in male and female cones of Zamia furfuracea L. fil. (Zamiaceae, Cycadales) and their significance in relation to pollination biology. Bot J Linn Soc 1993; 111:241-52; http://dx.doi.org/10.1111/j.1095-8339.1993.tb01901.x
- Vovides AP. Cone idioblasts of eleven cycad genera: morphology, distribution, and significance. Bot Gaz 1991; 152:91-8; http://dx.doi.org/10.1086/337867
- Hall JA, Walter GH, Bergstrom DM, Machin P. Pollination ecology of the Australian cycad Lepidozamia peroffskyana (Zamiaceae). Austral J Bot 2004; 52:333-43; http://dx.doi.org/10.1071/BT03159
- Terry LI, Walter GH, Donaldson J, Snow E, Forster PI, Machin PJ. Pollination of Australian Macrozamia cycads (Zamiaceae): effectiveness and behavior of specialist vectors in a dependant mutualism. Amer J Bot 2005; 92:931-40; http://dx.doi.org/10.3732/ajb.92.6.931
- Roulston TH, Cane JH. Pollen nutritional content and digestibility for animals. Plant Systematics and Evolution 2000; 222:187-209; http://dx.doi.org/10.1007/BF00984102
- Donaldson JS. Is there a floral parasite mutualism in cycad pollination? The pollination biology of Encephalartos villosus (Zamiaceae). Amer J Bot 1997; 84:1398-406; http://dx.doi.org/10.2307/2446138
- Schneider D, Wink M, Sporer F, Lounibos P. Cycads: their evolution, toxins, herbivores and insect pollinators. Naturwissenschaften 2002; 89:281-94; PMID:12216856; http://dx.doi.org/10.1007/s00114-002-0330-2
- Terry I, Moore CJ, Walter GH, Forster PI, Roemer RB, Donaldson JS, Machin PJ. Association of cone thermogenesis and volatiles with pollinator specificity in Macrozamia cycads. Pl Syst Evol 2004; 243:233-47; http://dx.doi.org/10.1007/s00606-003-0087-x
- Donaldon JS. Hot and smelly sex! Theoretical considerations of the adaptive significance of cone thermogenesis and volatile odors in cycad reproduction. Mem New York Bot Gard 2007; 97:308-25.
- Roemer R, Terry I, Walter G, Marler TE. Comparison of experimental measurements and predictions form biophysical models of thermogenic events in cones of Macrozamia lucida, M. macleayi, and Cycas micronesica. Mem New York Bot Gard 2012; 106:302-17.
- Roemer RB, Terry LI, Marler TE. Cone thermogenesis and its limits in the tropical Cycas micronesica (Cycadaceae): Association with cone growth, dehiscence, and post-dehiscence phases. Amer J Bot 2013; 100:1981-90; http://dx.doi.org/10.3732/ajb.1300047
- Terry I, Walter GH, Moore C, Roemer R, Hull C. Odour-mediated push-pull pollination in cycads. Science 2007; 318:70; PMID:17916726; http://dx.doi.org/10.1126/science.1145147
- Suinyuy TN, Donaldson JS, Johnson SD, Bösenberg JD. Role of cycad cone volatile emissions and thermogenesis in the pollination of Encephalartos villosus Lem.: Preliminary findings from studies of plant traits and insect responses. Mem New York Bot Gard 2012; 106:318-34.
- Tang W. Nectar-like secretions in female cones of cycads. The Cycad Newsl 1993; 16 (2):10-13.
- Procheş Ş, Johnson SD. Beetle pollination of the fruit-scented cones of the South African cycad, Stangeria eriopus. Amer J Bot 2009; 96:1722-30; http://dx.doi.org/10.3732/ajb.0800377
- Tang W. Collecting and storing cycad pollen. The Cycad Newsl 1985; 8(2):10-2.
- Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS. Species coextinctions and the biodiversity crisis. Science 2004; 305:1632; PMID:15361627; http://dx.doi.org/10.1126/science.1101101
