Abstract
What bees learn during pollen collection, and how they might discriminate between flowers on the basis of the quality of this reward, is not well understood. Recently we showed that bees learn to associate colors with differences in pollen rewards. Extending these findings, we present here additional evidence to suggest that the strength and time-course of memory formation may differ between pollen- and sucrose-rewarded bees. Color-naïve honeybees, trained with pollen or sucrose rewards to discriminate colored stimuli, were found to differ in their responses when recalling learnt information after reversal training. Such differences could affect the decision-making and foraging dynamics of individual bees when collecting different types of floral rewards.
Abbreviations
| GEE | = | generalized estimating equations |
The ability of bees to learn the location of profitable flower patches and the features of highly rewarding flowers is an adaptation to efficiently exploit flowers as a variable food source.Citation1-5 This learning-based flower constancy has been widely studied in nectar-foraging pollinatorsCitation5-7 and is likely to have been a powerful evolutionary driver in the dominance of nectar as the main reward type in modern angiosperms.Citation8-10 However, insect pollination pre-dates the emergence of nectar-producing organs, and not all flowers provide a nectar reward.Citation8,9,Citation11-13 This raises the question whether pollen alone is sufficient to reinforce learning in pollinating insects, and whether such learning processes differ from those which occur during reinforcement with sucrose solution.
Social bees collect pollen as a protein source for the brood and queen, and species such as honeybees and bumblebees are polylectic, meaning they forage for pollen from a range of different plant species. However, despite being pollen generalists, honeybees and bumblebees are known to preferentially collect the pollen of certain plants over others.Citation14-17 Such preferences can develop during the lifetime of a foraging individual,Citation18 although the sensory and neural mechanisms involved are currently unknown. Recently we demonstrated a role for learning in the development of pollen preferences, finding that individual bumblebees are able to discriminate pollen that has been artificially diluted with α-cellulose to varying degrees, and can associate such differences with the presence of a contextual colored cue.Citation19 This kind of learning may be mediated by reward mechanisms other than those implicated in sucrose-rewarded learning, since pollen is not ingested but is actively packed into the leg corbiculae during collection. In further experiments we compared the learning and recall of color memories in both sucrose and pollen-rewarded bees trained under the same experimental conditions. Given that color learning in bees is fast, and strong long-term memories can already be formed after 3 color-reward pairings, we opted to use a color-reversal conditioning paradigm.Citation20,21 We expected that bees would learn better when rewarded with sucrose than with pollen, since it is known from studies with sucrose-rewarded bees that individuals prevented from imbibing a reward show lower levels of acquisition.Citation22-24
Small colonies were housed within a flight net.Citation19 Color-naïve honeybees were hatched in an incubator and kept in a color-neutral environment within the flight net to prevent the formation of foraging-related color experiences and thus achieve equal levels of color exposure during the experiment. Pollen was provided in the dark, inside gray opaque boxes which prevented exposure to the color of pollen. Bees were observed entering the boxes to collect pollen, or feeding from a gray sucrose feeder, and were marked individually. Subsequently they were conditioned with their respective feeders to fly back and forth between the flight net and a testing arena connected by a wide Plexiglas corridor with sliding doors. To begin with, a bee's initial color preference was tested in the absence of rewards, by recording the number of approaches an individual bee made to 2 colored stimuli (Blue/Yellow) mounted on the surface of a gray box (). Two-tailed paired t-tests were used to compare bees' choices to each stimulus. In the color-learning task that followed, stimuli were mounted on the surface of 2 gray boxes (), and pollen-rewarded bees had to learn to enter and leave the box via entrance tubes positioned at the center of the rewarding color (e.g. Blue). The end of the tubes in the center of the unrewarding color (e.g., Yellow), and all tubes used when training sucrose-rewarded bees, were covered in mesh to prevent access to the box. The mesh still permitted diffusion of the pollen odor, meaning both pollen and sucrose-rewarded bees were exposed to pollen odor during training. Sucrose-foraging bees were rewarded with droplets of sucrose (30% w:w) at a distance of approximately 1 cm from the entrance of the tube. Tubes of the unrewarded color contained a drop of water to prevent bees using water vapor as a cue. All bees returned to the hive after each training trial to unload the rewards and returned to the entrance of the testing arena voluntarily to continue foraging in subsequent trials. After each test trial bees also returned to the flight net and could enter the hive. We therefore assume that bees of each forager type were equally satiated and motivated to forage.
Figure 1. Test apparatus. (A) Bees had to learn to enter the boxes via a tube positioned in the center of the correct color to access the reward. For sucrose-rewarded bees, a drop of sucrose was provided inside the entrance tubes. For the unrewarded color and sucrose-rewarded bees, the ends of the entrance tubes were covered with mesh permit the odor to diffuse out. Arrangement of the stimuli was changed after each trial. (B) Prior to and following training bees color preferences were tested in the absence of rewards.
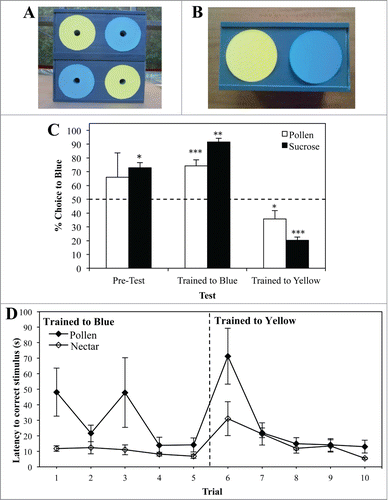
In the first experiment, pollen- (n = 7) and sucrose-foraging (n = 6) bees were individually trained in a bout of 5 trials in which Blue was the rewarding color (Blue+), followed by a further 5 Yellow-rewarded (Yellow+) trials. Color preferences were re-tested following each 5 trial bout, revealing that under our experimental conditions, naïve honeybees could learn to associate either a pollen or sucrose reward with a colored stimulus, and that their preferences shifted quickly following a reversal in the rewarding color (). We did not find any striking differences in the choices of each forager type during unrewarded color tests. Interestingly, the time taken to enter a correctly rewarding tube (search time) during acquisition differed to some extent (). General estimating equation (GEE) modeling was used to test whether bees adjusted their search behavior toward the rewarding color within each 5-trial bout, with search time as the response variable, and forager type and trial or bout number as factors. Pollen foragers exhibited significantly longer latencies in finding the correct rewarding stimulus than sucrose-rewarded foragers during training (GEE, Forager type, X21 = 16.176, p < 0.001). However, for both groups, latency decreased within each bout (Least significant difference (LSD) contrast, Pollen, T1–T5 p = 0.028; T6–T10 p = 0.001; Sucrose T1–T5 p = 0.009; T6–T10 p = 0.020), and by the fifth trial of each bout foragers from both groups exhibited similar search times.
In a second experiment, a new cohort of bees (n = 12) were trained for 20 trials in a color-reversal learning task, where bouts of 5 Blue+ and 5 Yellow+ trials were alternated (). Bees' color preferences were tested before training to assess spontaneous preferences. After training, memory recall of the 2 colors was tested; immediately after the fourth training bout and following a delay (‘rest’) of one hour to distinguish interference effects arising from the most recently trained color (Yellow+).
Figure 2. Pollen (white bars, mean% approaches to blue +/− SE, N = 15) and sucrose-rewarded (black bars, N = 12) bees showed a strong preference for blue prior to training (A). The rewarded color was switched every 5 trials, for 20 trials, after which bees from both groups had a preference for Yellow, the last rewarded color (Test 1). After a one-hour rest however, pollen rewarded bees chose both colors equally (N = 6), whereas sucrose-rewarded bees (N = 6) strongly preferred Blue (Test 2). In the final bout of training to yellow, both groups preferred this color (Test 3). During each 5-trial bout, both pollen (white diamonds, mean latency +/− SE) and sucrose-rewarded bees (black diamonds) showed a reduction in search time between the first and last trial (B), though pollen-rewarded bees exhibited longer search times immediately following a color reversal.
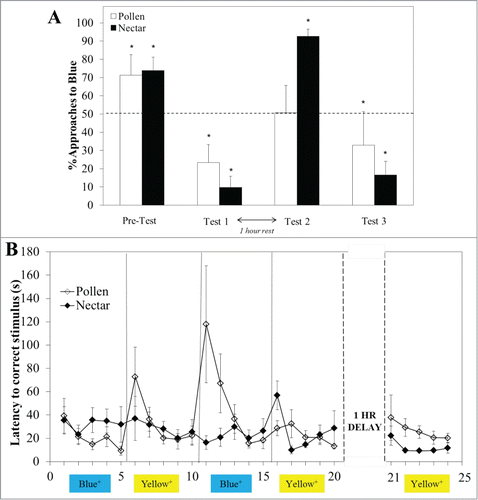
Both pollen and sucrose foragers had a strong initial preference for Blue (, Pre-Test, 2-tailed paired t-test, Pollen-reward t14 = 3.262 p = 0.006; Sucrose-reward t11 = 5.836, p < 0.001). When re-tested after the fourth training bout (Yellow+) of the color-reversal task, both pollen and sucrose-rewarded bees showed a strong preference for Yellow (, Test 1, 1-tailed paired t test, Pollen t5 = 3.376, p = 0.020; Sucrose t5 = 4.417, p = 0.010), which indicates that the most recent color task (Yellow+) determined immediate memory recall in both groups. Though overall search times did not differ (GEE, Forager type X21 = 0.808, p = 0.369), during the reversal task pollen and sucrose-rewarded bees responded differently to switches in rewarding color. While sucrose-rewarded bees exhibited little difference in search times between the first trial of a new bout and the last trial of the preceding bout (, LSD contrast, ns), pollen rewarded bees exhibited a significant increase in time taken to find the rewarding color in all but the final switch (LSD contrast, T5–T6 p = 0.009, T10–T11 p = 0.031, T15–T16 p = 0.493). By the fifth trial of each bout, there was no difference in the search times of the 2 groups.
After Test 1, which immediately followed the fourth training bout, bees returned to the colony flight net, but all food sources were removed from the net to prevent foraging. Following a one hour ‘rest’, bees were allowed to return to the test arena for a second unrewarded preference test (Test 2). Two completely different choice patterns were observed between pollen and sucrose-rewarded bees. While pollen foragers chose both colors equally (, Test 2, t5 = 1.452, p = 0.210), sucrose-rewarded bees strongly preferred Blue (t5 = 5.531, p = 0.033). This was not the result of a lowered motivation to forage as bees in both groups were equally fast in finding the rewarding stimulus in the first trial of a final Yellow+ training bout (LSD contrast, T21 p = 0.424) and showed a strong preference for Yellow in the final test (, Test3, Pollen t5 = 4.596, p = 0.030; Sucrose t5 = 4.992, p = 0.035). It is also unlikely that the sucrose-rewarded bees experienced memory loss and reverted to the spontaneous preference. Previous studies have shown that in sucrose-rewarded bees, reversal conditioning within the same context leads to the formation of memories for both trained colors.Citation23-25 We conclude that these bees formed associations of different strengths for the Blue and Yellow stimuli which influenced their choices in a test situation that was ambiguous with respect to the given color task. It is possible that the first-learned color was consolidated rapidly during the initial training trials, forming a more robust memory and influencing memory formation for the second color in the next phase of the reversal training.Citation25-27 MenzelCitation25 showed that for sucrose-rewarded bees, between 1 to 5 trials of learning one color were sufficient to interfere with subsequent acquisition of a second color association in the same context. These interferences disappeared after extended training and bees chose both colors equally. After a long delay, where the setup was removed for the whole of the next day and installed again on the second day after training, he observed that bees still chose both colors equally, though interference effects reappeared during the initial bouts of the continued reversal training. While our results do not fully mirror those findings, as our experiments differed to some degree in procedures, and we used naïve bees, they are nevertheless consistent with this and other previous work showing that successive conditioning of 2 colors leads to varying interactions during learning and memory recall.Citation25,27
The present findings provide preliminary evidence to suggest that differences exist in the learning mechanisms of bees rewarded with sucrose or pollen. While there are no indications that pollen-rewarded color learning is much slower or worse than in sucrose-rewarded bees, the distinct performances in memory recall hint at variations in the time course or strength of memory formation with these 2 reinforcers. This could influence how flexible pollen and sucrose foragers may be when responding to variations in floral resources. One could speculate that learning 2 colors equally might enable pollen foragers to more flexibly switch between several learned flower colors when a preferred flower becomes temporarily unavailable or rare which would be advantageous given the costs of learning to handle new flowers. Since handling is generally considered to be less important in determining foraging success in nectar foragers, the temporarily stronger fixation on a particular color due to faster consolidation of the first-established memory could be less costly than for pollen foragers. To be able to test such scenarios, however, future studies should elucidate the learning mechanisms underlying pollen rewards further and investigate how pollen-rewarded learning influences decision-making, the foraging dynamics of individual foragers and potentially the regulation of task partitioning in bee colonies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank R. Menzel for continuous support, P. Knoll and L. Goss for advice and help in setting up the single-cohort colonies.
Funding
We gratefully acknowledge funding by the BBSRC (BB/I009329/1).
References
- Ribbands CR. The foraging method of individual honey-bees. J Anim Ecol 1949; 18:47-66; http://dx.doi.org/10.2307/1581
- Grant V. The flower constancy of bees. Botanical Review 1950; 16:379-98; http://dx.doi.org/10.1007/BF02869992
- von Frisch K. The Dance Language and Orientation of Bees. Cambridge, MA: Harvard University Press, 1967.
- Heinrich B. Resource partitioning among some eusocial insects – bumblebees. Ecology 1976; 57:874-89; http://dx.doi.org/10.2307/1941054
- Waser NM. Flower constancy – definition, cause, and measurement. Am Nat 1986; 127:593-603; http://dx.doi.org/10.1086/284507
- Goulson D. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect Plant Ecol, Evol Syst 1999; 2:185-209; http://dx.doi.org/10.1078/1433-8319-00070
- Chittka L, Thomson JD, Waser NM. Flower constancy, insect psychology, and plant evolution. Naturwiss 1999; 86:361-77; http://dx.doi.org/10.1007/s001140050636
- Crepet WL. Insect pollination – paleontological perspective. Bioscience 1979; 29:102-8; http://dx.doi.org/10.2307/1307746
- Crepet WL, Friis EM, Nixon KC, Lack AJ, Jarzembowski EA. Fossil evidence for the evolution of biotic pollination [and discussion]. Philos Trans R Soc Lond B Biol Sci 1991; 333:187-95; http://dx.doi.org/10.1098/rstb.1991.0067
- Grimaldi D. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Annals of the Missouri Botanical Garden 1999; 86:373-406; http://dx.doi.org/10.2307/2666181
- Pettitt JM, Beck CB. Seed from the upper devonian. Science 1967; 156:1727-9; PMID:17813034; http://dx.doi.org/10.1126/science.156.3783.1727
- Colin LJ, Jones CE. Pollen energetics and pollination modes. Am J Bot 1980; 67:210-5; http://dx.doi.org/10.2307/2442644
- Simpson BB, Neff JL. Evolution and diversity of floral rewards. In: Jones CE, Little RJ, eds. Handbook of Experimental Pollination Biology. New York: Van Nostrand Reinhold, 1983:142-59.
- Schmidt JO. Pollen foraging preferences of honey bees (Hymenoptera, Apidae). Southwestern Entomologist 1982; 7:255-9
- Levin MD, Bohart GE. Selection of oollens by honey bees. Am Bee J 1955; 95:392-3
- Doull KM. The relative attractiveness to pollen-collecting honeybees of some different pollens. J Apicult Res 1966; 5:9-13
- Hanley ME, Franco M, Pichon S, Darvill B, Goulson D. Breeding system, pollinator choice and variation in pollen quality in British herbaceous plants. Funct Ecol 2008; 22:592-8; http://dx.doi.org/10.1111/j.1365-2435.2008.01415.x
- Robertson AW, Mountjoy C, Faulkner BE, Roberts MV, Macnair MR. Bumble bee selection of Mimulus guttatus flowers: the effects of pollen quality and reward depletion. Ecology 1999; 80:2594-606; http://dx.doi.org/10.1890/0012-9658(1999)080%5b2594:BBSOMG%5d2.0.CO;2
- Nicholls E, Hempel de Ibarra N. Bees associate colour cues with differences in pollen rewards. J Exp Biol 2014; 217:2783-8; PMID:24855678; http://dx.doi.org/10.1242/jeb.106120
- Menzel R. Das Gedächtnis der Honigbiene für Spektralfarben. Z vergl Physiol 1968; 60:82-102; http://dx.doi.org/10.1007/BF00737097
- Menzel R, Erber J, Masuhr T. Learning and memory in the honeybee. In: Barton-Browne L, ed. Experimental Analysis of Insect Behaviour. Berlin: Springer, 1974:195-217.
- Bitterman ME, Menzel R, Fietz A, Schafer S. Classical-conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 1983; 97:107-19; PMID:6872507; http://dx.doi.org/10.1037/0735-7036.97.2.107
- Sandoz JC, Hammer M, Menzel R. Side-specificity of olfactory learning in the honeybee: US input side. Learn Mem 2002; 9:337-48; PMID:12359841; http://dx.doi.org/10.1101/lm.50502
- Wright GA, Mustard JA, Kottcamp SM, Smith BH. Olfactory memory formation and the influence of reward pathway during appetitive learning by honey bees. J Exp Biol 2007; 210:4024-33; PMID:17981870; http://dx.doi.org/10.1242/jeb.006585
- Menzel R. Das Gedächtnis der Honigbiene fuer Spektralfarben. Z vergl Physiol 1969; 63:290-309; http://dx.doi.org/10.1007/BF00298164
- Menzel R. Behavioural access to short-term memory in bees. Nature 1979; 281:368-9; PMID:481598; http://dx.doi.org/10.1038/281368a0
- Cheng K, Wignall AE. Honeybees (Apis mellifera) holding on to memories: response competition causes retroactive interference effects. Anim Cogn 2006; 9:141-50; PMID:16374626; http://dx.doi.org/10.1007/s10071-005-0012-5
