ABSTRACT
As semi-autonomous cell organelles that contain only limited coding information in their own DNA, chloroplasts and mitochondria must import the vast majority of their protein constituents from the cytosol. Respective protein import machineries have been identified that mediate the uptake of chloroplast and mitochondrial proteins and interact with molecular chaperones of the HEAT-SHOCK PROTEIN (HSP) 70 family operating as import motors. Recent work identified unexpected new functions of 2 DnaJ co-chaperones in mitochondrial and chloroplast protein translocation and suggest a common mechanism of reactive oxygen species (ROS) scavenging that shall be discussed here.
Introduction
Chloroplasts and mitochondria are thought to have arisen from endosymbiosis and as a consequence of mass gene relocation events lost most of their own DNA during evolution.Citation1 To sustain their various functions both chloroplasts and mitochondria depend on a large number of cytosolic proteins that have to be imported in a developmentally regulated fashion. It was thus far believed that chloroplasts and mitochondria possess unique protein import machineries with little or no common components.Citation2,3 An exception to the rule is provided by molecular chaperones of the HEAT SHOCK PROTEIN (HSP) 70 family that, in association with other proteins, operate in both organelle types and function as import motors.Citation4,5 Here we report on a unique class of co-chaperones designated DnaJ and DnaJ-like holdasesCitation6,7 that act as key components in both mitochondrial and chloroplast protein translocation and accomplish unique roles in reactive oxygen species (ROS) scavenging and signaling.
Two distinct DnaJ-like co-chaperones with conserved structural features operate in chloroplasts and mitochondria
Chloroplasts and mitochondria need to import over 95% of their protein constituents from the cytosol and do so by virtue of specific protein import machineries dubbed translocases of the outer and inner chloroplast envelope membranes (TOC and TIC) versus translocases of the outer and inner mitochondrial membrane (TOM and TIM), respectively.Citation2,3 To drive the translocation of cytosolic precursor proteins across these multi-protein complexes, chloroplasts and mitochondria make use of import motors that catalyze the unfolding of the precursors in the cytosol, mediate the translocation step and assist in the refolding of the imported proteins inside the organelles. In chloroplasts, the import motor consists of the ATP-driven HEAT-SHOCK PROTEIN 70 (cpHSP70), its membrane anchor proteins TIC40 and TIC110, and a not yet identified nucleotide exchange factor to keep the HSP70 cycle functional.Citation2,6,7 cpDnaJL as a presumed cpHSP70 co-chaperone is a protein of 258 amino acids encoded by At5g23040 in Arabidopsis thaliana.Citation8 cpDnaJL displays 32.9% sequence homology to the protein encoded by At3g51140 and 22.4% sequence homology to the protein encoded by At2g20920.Citation9,10 Homologs of cpDnaJL are present in cyanobacteria, green algae, mosses, gymnosperms, and angiosperms,Citation9,10 suggesting a link to oxygenic photosynthesis. The J-domain of cpDnaJL from Arabidopsis and the cyanobacterium Synechocyotis sp. PCC6803 are distantly related to the J-domain of Tid1, a human mitochondrial homolog of bacterial DnaJ co-chaperones, and other DnaJ proteins (). Lee et al.Citation9 noted the lack of a highly conserved tripeptide, His-Pro-Asp (HPD) in the J-domain of cpDnaJL, which is normally required for the interaction with HSP70.Citation6,7 This observation suggests other sequence motives to be involved in CDF1-HSP70 interactions or that the function of cpDnaJL may be fundamentally different from that of canonical DnaJ co-chaperones. Indeed, cpDnaJL was found to accomplish a role as holdase in chloroplasts and etioplasts.Citation9,10 Holdases are a particular kind of ATP-independent molecular chaperones that bind to protein folding intermediates to prevent their aggregation but without directly refolding them (summarized in refs. Citation9,10).
Figure 1. Structural models of cpDnaJL (CDF1)(A) and mtDnaJ (PAM16)(B). The 3D-modeling was performed using I-TASSER, an online protein structure and function prediction tool,Citation30 with the J-domain of Tid1 as template.Citation31 Despite the limited amino acid sequence identity of cpDnaJL (CDF1)(A) and mtDnaJ (PAM16), the topology of their J-domains is quite similar.
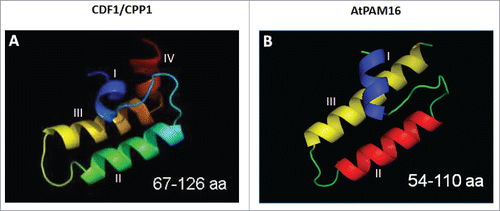
In mitochondria, the import motor needed for the uptake of both presequence-containing and presequence-less cytosolic precursors is composed of mitochondrial HEAT-SHOCK PROTEIN 70 (mtHSP70), its membrane anchor protein TIM44, the homodimeric nucleotide exchange factor GrpE (Mge1), and mtDnaJ (synonymous with PAM16, the presequence translocase-associated motor complex protein of 16 kDa), that forms hetero-dimers with PAM18Citation11,12 (summarized in ). mtDnaJ is highly conserved and particularly shares ≈85% residue conservation in the J-domain with related proteins ().Citation13,14
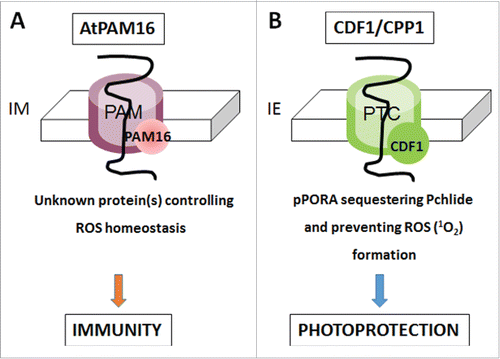
Figure 2. Model on the roles of cpDnaJL (CDF1) and mtDnaJ (PAM16) in chloroplasts (A) and mitochondria (B). A, mtDnaJ (PAM16) is supposed to regulate the translocation of hypothetical protein(s) with functions in ROS scavenging and/or signaling across the inner mitochondrial membrane (IM). As a consequence, ROS production would be kept low and plant immunity be inhibited. (B), cpDnaJL regulates the import of PORA through the Pchlide-dependent translocaon (PTC), spanning both the outer and inner plastid envelope (IE) membrane. Hereby, cpDnaJL's function is that of a holdase permitting the binding and sequestration of Pchlide in a protein-bound and thus non-hazardous form, conferring photoprotection onto etiolated seedlings during greening.
Roles of cpDnaJL and mtDnaJ (PAM16) in protein import
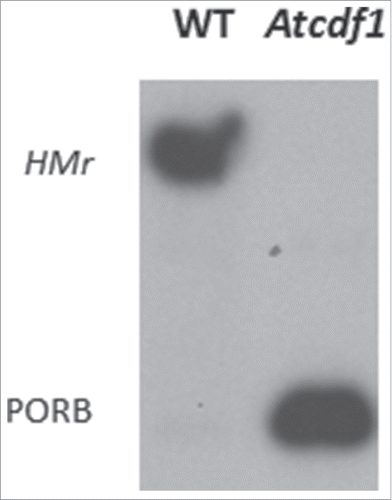
Chloroplasts and mitochondria import both presequence-containing and presequence-less proteins from the cytosol. Import of either type of cytosolic precursor into mitochondria is driven by the Δψ and ATP.Citation3 Hereby, the TIM23 subcomplex in the mitochondrial inner membrane interacts with the presequence translocase-associated motor complex including mtDnaJ.Citation11,12 In chloroplasts, the situation is different. cpDnaJL does not seem to participate in overall import of presequence-containing or presequence-less cytosolic precursors; instead it accomplishes a specific role. cpDnaJL is part of a unique protein import site through which NADPH:protochlorophyllide oxidoreductase (POR) A is specifically importedCitation10 (). Uptake of the pPORA into the plastids is unique in requiring protochlorophyllide (Pchlide) as import trigger.Citation14-17 Time courses over import revealed that the pPORA sequentially binds the tetrapyrrole pigment PchlideCitation10 and thereby lowers its interactions with molecular oxygen provoking the generation of singlet oxygen as cytotoxin and cell death inducer.Citation18,19 The interaction between the translocating pPORA polypeptide chain and Pchlide is enabled by the binding of cpDnaJL that operates independently of cpHSP70 and keeps the pPORA in a state ready to bind PchlideCitation9,10 Once pPORA's transit sequence is removed by the stromal processing peptidase, the mature pPORA loaded with Pchlide interacts with PORB and assembles into larger light-harvesting complexes in the prolamellar body of etiolated plants.Citation20,21 Lack of cpDnaJL, as found after RNA interference-induced gene silencing (RNAi), impaired the establishment of these complexes () and led to overaccumulation of free Pchlide acting as photosensitizer for singlet oxygen formation during greening.Citation10 Measurements with the dansyl-based singlet oxygen quencher DanePy confirmed mass generation of ROS in cpDnaJL-depleted RNAi seedlings.Citation10 Accumulation of singlet oxygen and other ROS explains why young-born sprouts rapidly die during greening.Citation10 Interestingly, similar, essential functions of mtDnaJ in mitochondrial biogenesis and function were deduced from knock-out mutant studies using different PAM16 alleles.Citation12,13 While single mtDnaJ (PAM16) mutants in Arabidopsis had reduced size, double mutants were lethal.Citation12,13 Thus, a common link of mtDnaJ and cpDnaJL function is suggested that shall be discussed here.
Figure 3. Lack of POR-containing higher molecular mass (HMr) complexes in prolamellar bodies of etiolated Arabidopsis seedlings that had been depleted of cpDnaJL (CDF1) by RNAi (Atcdf1), as compared to etiolated wild-type (WT) seedlings. Note the complete lack of HMr complexes as well as drastically reduced levels of PORB, which cannot stably accumulate in the absence of PORA, in Atcdf1 plants.
Common aspects of DnaJ function in mitochondria and chloroplasts
cpDnaJL and mtDnaJ are both essential for organelle biogenesis. In case of cpDnaJL, incapability to sequester Pchlide in a protein-bound form favors generation of singlet oxygen and other ROS that cause seedling lethality during greening.Citation10 Because cpDnaJL not only binds PORA but also its constitutively expressed counterpart PORB, the growth phenotype also manifests in green plants, as reported by Lee et al.Citation9 who used virus-induced gene silencing and dexamethasone-induced gene silencing to pinpoint the role of cpDnaJL in green plants. In case of mtDnaJ, however, lack of an essential protein needed for the import of both presequence-containing and presequence-less cytosolic precursors would lead to depletions in the mitochondrial proteome and pleiotropic defects in mitochondrial activity. Huang et al.,Citation13 however, proposed a completely different scenario in which mtDnaJ is supposed to play a specific role in the import of a protein regulating plant viability and immunity.
Huang et al.Citation13 carried out a feed-forward genetic screen for mutants of nucleotide-binding and leucine-rich repeat domain (NB-LRRs) proteins that serve as immune receptors.Citation22 In a mutant snc1-enhancing (muse) screen for such NB-LRRs, a component was identified termed MUSE5 that is an ortholog of mtDnaJ.Citation13 Interestingly, single mtDnaJ mutants had enhanced resistance against virulent pathogens, suggesting that mtDnaJ may normally operate to sequester a cell death regulator in the mitochondrial compartment. Candidate proteins accomplishing such role could be the heme binding proteins localized in the different mitochondrial inner membrane complexes and being potential ROS generators.Citation23 If so, this situation would resemble that seen for cpDnaJL that by its activity in the Pchlide-dependent plastid import pathway of PORA lowers the level of potential photosensitizers for ROS generation and thereby prevents cell death. Other targets of mtDnaJ could be thus far unknown cell death proteins. Last but not least, mtDnaJ could be involved in controlling the biogenesis and assembly of cytochrome c that has a well-established role in apoptotic cell death regulation. R protein-mediated immunity in fact involves the release of cytochrome c from mitochondria to the cytosol, triggering cell death and boosting ROS production (see. ref. Citation13; for references). In addition, multiple sources and types of ROS were implicated in the development of the hypersensitive response (HR) to deter pathogens.Citation24
Interestingly, another mutant allele of mtDnaJ (Atpam16-2, originally named txr1-1) was isolated from a forward genetic screen for mutants resistant to the phytotoxin and herbicide thaxtomin A from the potato scab-inducing species Streptomyces scabies.Citation25 This mutant was resistant to exogenously administered thaxtomin A normally provoking severe growth defects.Citation25 A hypothesis put forth by Huang et al.Citation13 is that thaxtomin A may be targeting a mitochondrial matrix protein that, as we propose here, relies on mtDnaJ for import or may bind and inactivate mtDnaJ (AtPAM16) itself. It was further hypothesized that such targeting may serve as a virulence strategy to release a death signal from mitochondria and assists the killing of host cells so that the pathogen can consume the plant's photosynthates.Citation13
Another, interesting link between mtDnaJ and ROS is provided by the work of Scheibel et al.Citation26 who noticed differential effects of thaxtomin on root growth in seedlings undergoing photomorphogenesis or skotomorphogenesis. While root growth was reduced by more than 50% by 100 nM thaxtomin in wild-type seedlings germinating in white light (photomorphogenesis), root growth was unaffected in seedlings developing in darkness (skotomorphogenesis). Because root development in either case was normal in the thaxtomin-resistant mutant (trx1/Atpam16), a light-dependent relocation of ROS themselves or ROS-derived signals controlling root growth was anticipated.Citation26 Alternatively, the insensitivity to the herbicide could be due to altered ROS perception mechanisms rendered inactive by the mutation. Several studies have demonstrated that root meristem growth and differentiation are regulated by ROS production and distribution.Citation27-29 Thus, failure to correctly localize a protein involved in ROS metabolism would likely affect the root phenotype and may even restore the correct growth pattern. Huang et al.Citation13 in fact proposed a positive regulatory role of mitochondria during immune responses through ROS generation and that this circuit is counteracted by mtDnaJ. As said before, a possible, mtDnaJ-dependent mechanism could be to control the import of a nuclear-encoded negative regulator of plant immunity that may bind and scavenge ROS. Failure to sequester this factor would lead to enhanced disease susceptibility. If correct, this scenario would resemble the mechanism by which cpDnaJL controls ROS homeostasis in plastids. Proteomics and other approaches are required to define the exact role of mtDnaJ in the import of such immune regulators and/or ROS scavengers and to compare it with that of cpDnaJL.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Margulis L. Origin of eukaryotic cells. New Haven, CT: Yale University Press; 1970.
- Paila YD, Richardson LG, Schnell DJ. New insights into the mechanism of chloroplast protein import and its integration with protein quality control, organelle biogenesis and development. J Mol Biol 2015; 427(5):1038-60; PMID:25174336; http://dx.doi.org/10.1016/j.jmb.2014.08.016
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem 2007; 76:723-49; PMID:17263664; http://dx.doi.org/10.1146/annurev.biochem.76.052705.163409
- Flores-Pérez Ú, Jarvis P. Molecular chaperone involvement in chloroplast protein import. Biochim Biophys Acta 2013; 1833:332-40; http://dx.doi.org/10.1016/j.bbamcr.2012.03.019
- Schulz C, Schendzielorz A, Rehling P. Unlocking the presequence import pathway. Trends Cell Biol 2015; 25(5):265-75; PMID:25542066; http://dx.doi.org/10.1016/j.tcb.2014.12.001
- Qiu J, Li GW, Sui YF, Song HP, Si SY, Ge W. Heat-shocked tumor cell lysate-pulsed dendritic cells induce effective anti-tumor immune response in vivo. World J Gastroenterol 2006; 12:473-8; PMID:16489653
- Rajan VB, D'ISilva P. Arabidopsis thaliana J-class heat shock proteins: cellular stress sensors. Funct Integr Genomics 2009; 9:433-46; PMID:19633874; http://dx.doi.org/10.1007/s10142-009-0132-0
- Kawai-Yamada M, Saito Y, Jin L, Ogawa T, Kim KM, Yu LH, Tone Y, Hirata A, Umeda M, Uchimiya H. A novel Arabidopsis gene causes Bax-like lethality in Saccharomyces cerevisiae. J Biol Chem 2005; 280:39468-73; PMID:16192270; http://dx.doi.org/10.1074/jbc.M509632200
- Lee JY, Lee HS, Song JY, Jung YJ, Reinbothe S, Park YI, Lee SY, Pai HS. CHAPERONE-LIKE PROTEIN of POR1 plays a role in stabilization of light-dependent protochlorophyllide oxidoreductase in Nicotiana benthamiana and Arabidopsis. Plant Cell 2013; 25:1-17; http://dx.doi.org/10.1105/tpc.113.250110
- Reinbothe S, Gray J, Rustgi S, von Wettstein D, Reinbothe C. Cell growth defect factor 1 is crucial for the substrate-dependent plastid import of NADPH:protochlorophyllide oxidoreductase A in Arabidopsis thaliana. Proc Natl Acad Sci USA 2015; 112:5838-43; PMID:25901327; http://dx.doi.org/10.1073/pnas.1506339112
- van der Laan M, Hutu DP, Rehling P. On the mechanism of preprotein import by the mitochondrial presequence translocase. Biochim Biophys Acta 2010; 1803:732-9; PMID:20100523; http://dx.doi.org/10.1016/j.bbamcr.2010.01.013
- Pais JE, Schilke B, Craig E. A. Reevaluation of the role of the Pam18:Pam16 interaction in translocation of proteins by the mitochondrial Hsp70-based import motor. Mol Biol Cell 2011; 22:4740-9; PMID:22031295; http://dx.doi.org/10.1091/mbc.E11-08-0715
- Huang Y, Chen X, Liu Y, Roth C, Copeland C., McFarlane HE, Huang S, Lipka V, Wiermer M, Li X. Mitochondrial AtPAM16 is required for plant survival and the negative regulation of plant immunity. Nature Commun 2013; 4:2558
- Chen X, Ghazanfar B, Khan AR, Hayat S, Cheng Z. Comparative analysis of putative orthologues of mitochondrial import motor subunit: Pam18 and PAM16 in plants. Plos One 2013; 8(10):e78400; PMID:24194927; http://dx.doi.org/10.1371/journal.pone.0078400
- Reinbothe S, Runge S, Reinbothe C, van Cleve B, Apel K. Substrate-dependent transport of the NADPH: protochlorophyllide oxidoreductase into isolated plastids. Plant Cell 1995; 7:161-72; PMID:7756827; http://dx.doi.org/10.1105/tpc.7.2.161
- Reinbothe S, Quigley F, Gray J, Schemenewitz A, Reinbothe C. Identification of plastid envelope proteins required for the import of protochlorophyllide oxidoreductase (POR) A into the chloroplast of barley. Proc Natl Acad Sci USA 2004; 101:2197-202; PMID:14769934; http://dx.doi.org/10.1073/pnas.0307284101
- Kim C, Apel K. Substrate-dependent and organ-specific chloroplast protein import in planta. Plant Cell 2004; 16:88-98; PMID:14688290; http://dx.doi.org/10.1105/tpc.015008
- Reinbothe C, Pollmann S, Reinbothe S. Singlet oxygen links photosynthesis to translation and plant growth. Trends Plant Sci 2010; 15:499-506; PMID:20580304; http://dx.doi.org/10.1016/j.tplants.2010.05.011
- Kim C, Apel K. Singlet oxygen-mediated signaling in plants: moving from flu to wild type reveals an increasing complexity. Photosynth Res 2013; 116:455-64; PMID:23832611; http://dx.doi.org/10.1007/s11120-013-9876-4
- Reinbothe C, Lebedev N, Reinbothe S. A protochlorophyllide light-harvesting complex involved in de-etiolation of higher plants. Nature 1999; 397:80-4 http://dx.doi.org/10.1038/16283
- Reinbothe C, El Bakkouri M, Buhr F, Muraki N, Nomata J, Kurisu G, Fujita Y, Reinbothe S. Chlorophyll biosynthesis: spotlight on protochlorophyllide reduction. Trends Plant Sci 2010; 15:614-24; PMID:20801074; http://dx.doi.org/10.1016/j.tplants.2010.07.002
- Maekawa T, Kufer TA, Schulze-Lefert P. NLR functions in plant and animal immune systems: so far and yet so close. Nat Immunol 2011; 12:817-26; PMID:21852785; http://dx.doi.org/10.1038/ni.2083
- Quinlan CL, Perovoshchikova IV, Hey-Mogensen M. Sites of reactive oxygen species generation by mitochondria. Redox Biol 2013; 1:304-12; PMID:24024165; http://dx.doi.org/10.1016/j.redox.2013.04.005
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol 1994; 105:467-72; PMID:12232215
- Bischoff V, Cookson SJ, Wu S, Scheible WR. Thaxtomin A affects CESA-complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J Exp Bot 2009; 60:955-65; PMID:19269997; http://dx.doi.org/10.1093/jxb/ern344
- Scheible WR, Fry B, Kochevenko A, Schindelasch D, Zimmerli L, Somerville S, Loria R, Somerville CR. An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 2003; 15(8):1781-94; PMID:12897252; http://dx.doi.org/10.1105/tpc.013342
- Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010; 143(4):606-16; PMID:21074051; http://dx.doi.org/10.1016/j.cell.2010.10.020
- Sundaravelpandian K, Chandrika NN, Schmidt W. PFT1, a transcriptional mediator complex subunit, controls root hair differentiation through reactive oxygen species (ROS) distribution in Arabidopsis. New Phytol 2013; 197(1):151-61; PMID:23106228; http://dx.doi.org/10.1111/nph.12000
- Lin CY, Huang LY, Chi WC, Huang TL, Kakimoto T, Tsai CR, Huang HJ. Pathways involved in vanadate-induced root hair formation in Arabidopsis. Physiol Plant 2015; 153(1):137-48; PMID:24833217; http://dx.doi.org/10.1111/ppl.12229
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 2010; 5:725-38; PMID:20360767; http://dx.doi.org/10.1038/nprot.2010.5
- Lo JF, Hayashi M, Woo-Kim S, Tian B, Huang JF, Fearns C, Takayama S, Zapata JM, Yang Y, Lee JD. Tid1, a cochaperone of the heat shock 70 protein and the mammalian counterpart of the Drosophila tumor suppressor l(2)tid is critical for early embryonic development and cell survival. Mol Cell Biol 2004; 24:2226-36; PMID:14993262; http://dx.doi.org/10.1128/MCB.24.6.2226-2236.2004
