ABSTRACT
Controlled generation of reactive oxygen species (ROS) is widely beneficial to various medical, environmental, and agricultural studies. As inspired by the functional motifs in natural proteins, our group has been engaged in development of catalytically active oligo-peptides as minimum-sized metalloenzymes for generation of superoxide anion, an active member of ROS. In such candidate molecules, catalytically active metal-binding minimal motif was determined to be X-X-H, where X can be most amino acids followed by His. Based on above knowledge, we have designed a series of minimal copper-binding peptides designated as GnH series peptides, which are composed of oligo-glycyl chains ended with C-terminal His residue such as GGGGGH sequence (G5H). In order to further study the role of copper binding to the peptidic catalysts sharing the X-X-H motif such as G5H-conjugated peptides, we should be able to score the occupancy of the peptide population by copper ion in the reaction mixture. Here, model peptides with Cu-binding affinity which show intrinsic fluorescence due to tyrosyl residue (Y) in the UV region (excitation at ca. 230 and 280 nm, and emission at ca. 320 nm) were synthesized to score the effect of copper occupancy. Synthesized peptides include GFP-derived fluorophore sequence, TFSYGVQ (designated as Gfp), and Gfp sequence fused to C-terminal G5H (Gfp-G5H). In addition, two Y-containing tri-peptides derived from natural GFP fluorophores, namely, TYG and SYG were fused to the G5H (TYG-G5H and SYG-G5H). Conjugation of metal-binding G5H sequence to GFP-fluorophore peptide enhanced the action of Cu2+ on quenching of intrinsic fluorescence due to Y residue. Two other Y-containing peptides, TYG-G5H and SYG-G5H, also showed intrinsic fluorescence which is sensitive to addition of Cu2+. There was linear relationship between the loading of Cu2+ and the quenching of fluorescence in these peptide, suggesting that Cu2+-dependent quenching of Y-reside-derived fluorescence could be a measure of copper occupancy in the peptides. Lastly, the fate of Y residue in the Cu-loaded peptides under oxidative condition in the presence of H2O2 was discussed based on the Cu/H2O2-dependent changes in fluorescence spectra.
Introduction
Controlled generation of reactive oxygen species (ROS) is widely beneficial to various medical, environmental engineering, and agricultural fields including clinically applied immunological modulations,Citation2,7 degradation of polluting organic compounds,Citation23 inactivation of bacterial cells for the hygienic purpose,Citation19 activated sludge process,Citation22 and direct and indirect agricultural pest controls targeting pathogens and host plants, respectively.Citation16,29
In the last decade, our group has been engaged in development a novel classes of engineered biocatalysts including catalytic oligonucleotides (functional DNA sequences;Citation10 and peptides,Citation11 designed to catalyze the production and/or removal superoxide anion radicals (O2•−) through understanding and modification of natural catalytic proteins of animal and plant origins.
For example, we found that peptides derived from human and mammalian prion proteins (PrPs)Citation14,24 and plant stress-responsive peptidesCitation25 have catalytic nature although they are not considered as enzymes at present. Actually, the kingdoms of plants and animals are rich in such small peptides with high affinity to metal ions which might aid in catalysis. By mimicking such natural peptide, novel series of minimal-sized oligo-peptidic artificial enzymes catalyzing the generation of O2•− in peroxidase-like manner requiring hydrogen peroxide (H2O2) and electron donating substrates such as phenolics or amines were developed.Citation11,21
The first criterion for consisting such minimal peroxidase-like small peptides is the presence of His-containing motif(s) required for binding to metals (chiefly copper), and then, free-form and/or peptide-bound form of substrates fuels the reaction.Citation27 Our preliminary studies on PrP-derived peptides have pointed that His residues (at least single His) are required for anchoring of Cu onto PrP-derived peptides,Citation13,14 and eventually, the catalytically active Cu-binding minimal motif was determined to be tri-peptidic sequence X-X-H, where X can be most amino acids followed by HisCitation12,27 An engineering example of a catalytic peptide sequence sharing XXH motif for development of biosensing and bioengineering materials was reported.Citation21 Accordingly, one of X-X-H motif derivatives, Gly-Gly-His (G-G-H) sequence was introduced onto the glycidyl methacrylate grafted on the polyethylene platform (porous hollow fiber membrane). Chemiluminescence assay reveled that loading of Cu2+ on the peptide-conjugated membrane conferred the catalytic activity to it, thus, catalyzing the generation of O2•− upon addition of a pair of substrates (H2O2 and tyramine).
In human PrP, His96 centered in G-G-G-T-H-S-Q-W-N sequences is considered as one of Cu-binding sites. Effect of His position on the catalytic activity in PrP-derived peptides was examined by comparing the H-S-Q-W-N (His-started pentapeptide) and the G-G-G-T-H (His-ended pentapeptide).Citation11 While reaction with tyramine (given as a model phenolic substrate) and G-G-G-T-H peptide resulted in robust production of O2•−, the H-S-Q-W-N peptide showed no catalytic activity, suggesting that G-T-H motif within the His-ended pentapeptide is one of X-X-H motif derivatives. As the catalytic activities among G-G-G-T-H and shorter derivatives (G-G-T-H and G-T-H) were compared, the importance of the N-terminal glycyl-chain elongation for maximal redox activity in C-terminal His anchored peptides was implied.Citation11 Furthermore, the likely common structure formed by Cu/X-X-H motif complex found in Cu-binding motifs in human PrP including octarepeat regionCitation9 helical region,Citation27 neurotoxic regionCitation12 was proposed to be semi-planar shape resembling the structure of metal-centered heme in which metallic element is coordinated by planarly arranged four nitrogen atoms, by analogy to the structure of Ni/X-X-H metallopeptides.Citation5,6
As inspired by the natural PrP-derived G-G-G-T-H sequence, we have designed a series of simplified model peptides designated as GnH series peptides, which are composed of oligo-glycyl chains (Gn) ended with C-terminal His residue.Citation11 As expected, importance of the elongated N-terminal Gn chain with anchoring His was confirmed by comparing the GnH series peptides (n = 2, 3, 4, 5 and 10) and Gn series peptides lacking His. Notably, Gn series lacking the metal-binding motif showed no catalytic activity even in the presence of free Cu2+. In GnH series, G3H tetrapeptide showed a detectable increase in production of O2•−, and peptides with longer chain showed higher activity, confirming the importance of N-terminal Gn chain length. Data suggested that the requirement for the N-terminal Gn elongation is nearly fulfilled at between G5 and G10,Citation11 and amazingly, unlikely to conventional enzymes, the catalytic activity in these metal-binding peptides survive the heating treatment such as autoclaving and the repeated freeze and thaw cycles.Citation28
Fluorometry often provides strong approaches for studying the molecular interaction.Citation9 We have previously assessed (1) the quenching of Tb3+ fluorescence by PrP-derived metal-binding peptides and (2) the Cu2+-dependent quenching of intrinsic fluorescence in human PrP octarepeat peptide sequence. Quenching of Tb-fluorescence by interacting peptides implied the important role for His-ended peptidic sequence sharing X-X-H motif (in case of human PrP's octarepeat region, P-Q-H). On the other hand, quenching of intrinsic peptide fluorescence due to the presence of a tryptophan (W) residue by copper ion suggested that classically known H-G-G-G motif in PrPCitation3 forms an active motif in metal binding. Taken together, in the mammalian PrP octarepeat regions, in which P-H-G-G-G-W-G-Q is repeated for four (human) to six (bovine) times, two distinct metal binding motifs, namely, X-X-H motif (in this case, Q-P-H tripeptide sequence) and H-G-G-G motif, could be overlaid by sharing common His residue (Q-P-H-G-G-G) and thus co-existed and synergically capturing the metals.Citation9
In order to further study the role of copper binding to the biocatalysts sharing the X-X-H motif such as GnH series catalytic peptides, we should be able to score the occupancy of the peptide population by copper ion in the reaction mixture. In the present study, we attempted to monitor the binding of copper to GnH catalytic peptides by designing the chimeric molecule fusing fluorescent oligo-peptide sequence derived from green fluorescence protein (GFP) and G5H sequence.
Materials and methods
Peptides and chemicals
Model peptides with Cu-binding affinity which show intrinsic fluorescence in the UV region were synthesized to score the effect of copper occupancy. The peptides were obtained from the custom peptide service department of Sigma Genosys Japan, Ishikari, Hokkaido, Japan. The amino acid sequences of the peptides chemically synthesized were purified on high pressure liquid chromatography prior to the experimental use. Other chemicals such as CuSO4 and salts for buffer used in this study were of reagent grade purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Synthesized peptides include GFP-derived fluorophore sequence, TFSYGVQ (designated as Gfp), and Gfp sequence fused to GGGGGH, thus, designated as Gfp-G5H. Note that the synthesized peptide sequences corresponding to GFP fluorophore do not show green fluorescence without post-translational process for developing the molecular rigidity in living cells. Instead, intrinsic fluorescence due to presence of the tyrosyl residue (Y) can be expected. Therefore, two Y-containing tri-peptide sequences found in natural GFP fluorophores, namely, TYG and SYG were fused to the Cu-binding GGGGGH sequence (thus, designated as TYG-G5H and SYG-G5H, respectively).
Fluorometric analysis
Intrinsic fluorescence from the 30 µM peptides with and without loading of copper ions were detected in potassium phosphate buffer (50 mM, pH 7.0) using a fluorescence spectrophotometer (F-4500 Hitachi High-Technol. Co., Tokyo). The three-dimensional (3D) spectral measurement of fluorescence was carried out at the excitation wavelength between 200 and 700 nm with 5 nm intervals and emission wavelength between 200 and 700 nm with 5 nm intervals.
To perform full occupancy of peptide with metals, the concentrations of metals of interest tend to be higher than the peptide concentration as previously observed in fluorescence measurements with prion-derived oligo-peptides.Citation9,13 High concentration of metals in phosphate-based reaction mixture often cause a slight turbidity which sensitively interferes with fluorescence measurement by developing an intensive diagonal band of scattered light over the fluorescence contour plot. To avoid such situation, peptides needs to be maintained at around 30 µM (so that metal concentration attaining full occupancy can be lowered) as described in our previous reports.Citation9,13
Results and discussion
Enhanced Cu2+-dependent changes in intrinsic fluorescence spectra of a GFP-fluorophore peptide conjugated with metal binding sequence
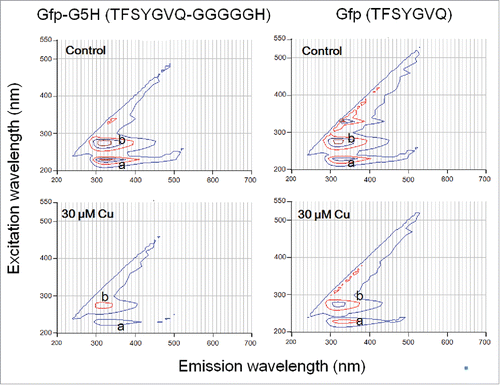
GFP fluorophore-derived peptides tested here showed non-green intrinsic fluorescence at UV region (). Upon excitation at 230 nm (peak a) and 280 nm (peak b), both Gfp-G5H (TFSYGVQ-GGGGGH) and Gfp (TFSYGVQ) showed fluorescence emission at around 320 nm. Fluorescence signals at the peak a (230 nm excitation/320 nm emission) by 30 μM Gfp-G5H and Gfp showed tendency to be quenched in the presence of 30 μM Cu2+ by 61.6 % and 32.0 %, respectively. Under the same conditions, the fluorescence signals at the peak b (280 nm excitation/320 nm emission) by Gfp-G5H and Gfp showed Cu2+-dependent quench by 48.5 % and 22.7 %, respectively. These data suggested that presence of Cu may partially quench the fluorescence of Gfp-heptapeptide lacking the Cu-binding motif due to non-specific interaction. Addition of C-terminal G5H sequence doubled the extent of fluorescence quenching induced by CuSO4, thus suggesting the impact of selective binding of copper onto the G5H sequence conjugated to the sequence with fluorophore. Therefore, we can expect that Cu-dependent quenching of UVC-excited UVA fluorescence by Gfp-G5H can be used as a measure of Cu-occupancy in G5H domain.
Figure 1. Different quenching action of copper ion against intrinsic tyrosine fluorescence in GFP-derived fluorophore sequence with and without fusing to copper binging sequence hexapeptide. Peptides (30 μM) used were Gfp-G5H (TFSYGVQ-GGGGGH) and Gfp (TFSYGVQ). Peaks of tyrosine fluorescence (emission at ca. 320 nm) were observed with excitation at 230 nm (a) and 280 nm (b). Quenching of fluorescence in the presence of 30 μM CuSO4 was assessed.
Intrinsic fluorescence in tyrosine-containing peptides showed sensitivity to Cu
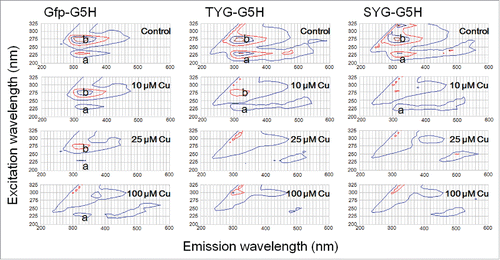
The intrinsic fluorescence signals (the peaks a and b) from the three G5H-conjugated GFP-derived peptides (30 μM of Gfp-G5H, TYG-G5H, and SYG-G5H) plotted on 3-dimentional contour graphs were compared. Data showed that the intrinsic fluorescence by all peptides can be quenched in the presence of CuSO4 (10, 25 and 100 μM; ). Among three peptides examined, SYG-G5H was most sensitive to lower range of CuSO4 concentration (10 μM, molar ratio to peptide: ca. 0.17–0.33). The fluorescent signals from TYG-G5H also showed higher sensitivity to Cu as compared to the signals from Gfp-G5H.
Figure 2. Effect of copper ion on quenching of intrinsic fluorescence signals by three GFP fluorophore-derived oligo-peptides conjugated with copper-binding hexapeptide motif. Peptides (30 μM) used were Gfp-G5H (TFSYGVQ-GGGGGH), TYG-G5H (TYG-GGGGGH), and SYG-G5H (SYG-GGGGGH). In the absence of CuSO4, two typical peaks of tyrosine fluorescence (emission at ca. 320 nm) were observed with excitation at 230 nm (a) and 280 nm (b). Quenching of fluorescence in the presence of 10, 25 and 100 μM CuSO4 was assessed.
Linear relationship between loading of Cu2+ and quenching of fluorescence
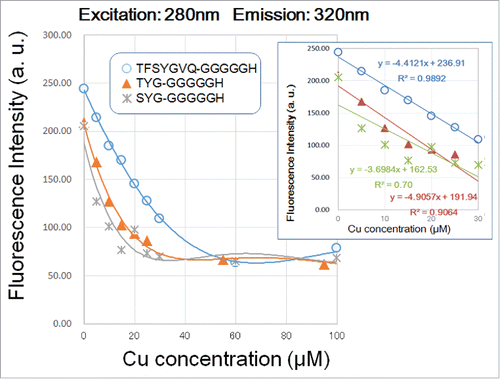
Among three peptides examined, only Gfp-G5H showed linear decrease in fluorescent signal along with occupancy with copper in the range between 0.17 and 1.33 of molar ratios of Cu2+ over peptide (). Note that within this range of copper concentration, the squared correlation coefficients (r2) for Cu-dependent quenching of fluorescence signals at the peaks a and b in Gfp-G5H were 0.982 and 0.989, respectively (). On the other hand, r2 for regressions corresponding to Cu-dependent quenching of fluorescence signals by TYG-G5H and SYG-G5H ranged at relatively low scores between 0.700 and 0.906 ( ), suggesting that linear relationship between the quenching of TYG- and SYG-conjugated molecules are less significant compared to the Gfp-conjugated molecule. Therefore, we can conclude that the fluorometric kinetics reported by Gfp-conjugated G5H peptide is most proportional to the copper occupancy in G5H sequence.
Figure 3. Effect of copper concentration on quenching of 230 nm excitation/3;20 nm emission signals by three G5H-conjugated GFP fluorophore-derived oligo-peptides. Peptides (30 μM) used were as in . Quenching of fluorescence was performed with 5–100 and 100 μM CuSO4 was assessed. Three different symbols represent the data points obtained. Curves were merely approximation of the response (note that they are not regression curves). In the inset, linear relationships between the remitted range of Cu concentration (up to 40 μM) and the decrease in peptidic fluorescent signals are shown.
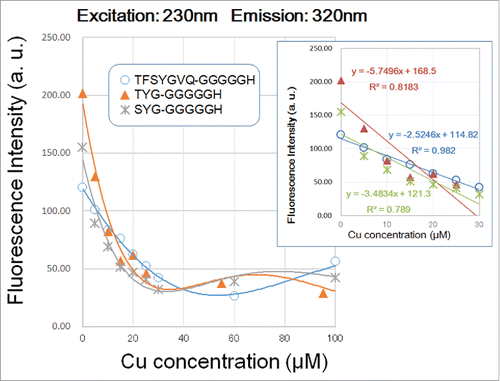
Figure 4. Effect of copper concentration on quenching of 280 nm excitation/320 nm emission signals by three G5H-conjugated GFP fluorophore-derived oligo-peptides. Peptides (30 μM) used were as in . Quenching of fluorescence was performed as in . In the inset, linear relationships between the remitted range of Cu concentration (up to 40 μM) and the decrease in peptidic fluorescent signals are shown.
Previously, catalytic nature of G5H hexapeptide was assessed.Citation11 It has been shown that catalytic activity in G5H peptide requires the binding of copper to it. Furthermore, Michaelis constant (Km) for O2•− production using tyramine as a model substrate for Cu/G5H complex (0.15 mM) was determined to be 0.24 mM. Then, Vmax at molar basis and weight basis were determined to be 52.91 mmol (O2•−) mmol (peptide)−1 min−1 and 0.12 mmol (O2•−) mg (peptide)−1 min−1, respectively.Citation11 With molar-basis comparison, the catalytic activity looks weak, however, due to its low molecular weight characteristics, weight-basis comparison of the catalytic activity reaches applicable range which is almost 1/6 of purified horseradish peroxidase.Citation11 It is obvious that this type of approach for creating heat-stable biocatalysts require further innovation. Therefore, we expect that the use of Gfp-fused G5H peptide for quantification of Cu-binding to G5H motif may contribute to further engineering of the GnH-based catalytic peptides.
Fate of tyrosine residue after oxidative reaction
Up to here we mostly discussed the role of His-ended metal binding motif in novel class of catalytic peptides with aid by fluorescent signal which could be attributed to the presence of Tyr residue. We view that Tyr residue has an additional important role in designing the peptidic O2•−-generating catalysts.Citation15
Reportedly, supplementation of structurally similar free catecholamine-related chemicals (tyramine or phenylethylamine), a free amino acid Tyr (Y), or Tyr-rich oligopeptides (such as tyrosyl-tyrosyl-arginine, YYR) as model substrates (instead of typical peroxidase substrates such as phenolics or amines) to the reaction mixture containing Cu-bound peptides sharing X-X-H motif such as Cu/VNITKQHTVTTTT (helical Cu-binding motif in mammalian PrP;Citation24 Cu/G;GGTH (short Cu-binding motif in human PrP;Citation14 Cu/G;GGGGH (artificial catalyst;Citation11 Cu/GGGFGH,Citation25 or Cu/NPGFPHCitation26 resulted in H2O2-dependent O2•−-generation. Notably, Y residue-containing peptides with X-X-H motif including Cu/GGGYGH (plant ozone-inducible peptide sequence;Citation25 Cu/NPGYPH (chicken PrP hexa-repeat sequence;Citation26 and Cu/FLTEYVA-GGGGGH (Erk1/Erk2 MAP kinase substrate sequence fused with metal binding sequence designated as ErkG5H;Citation15 showed catalytic activity for H2O2-dependent O2•− generation without supplementation of any free phenolic substrates. These knowledges suggest that Y-residue mimics the role for free phenolics in H2O2-dependent O2•− generating reactions.
The above view was supported by Y-to-F mutation in chicken PrP sequenceCitation24 and plant ozone-inducible peptide sequenceCitation25 by which Tyr residues on catalytic peptides were replaced with Phe residues. The difference was merely the presence and absence of the OH group on the aromatic ring. Moreover, masking of Y-residue (at OH group) in ErkG5H through tyrosyl phosphorylation in Erk1/Erk2 MAP kinase substrate moiety of ErkG5H peptide was performed, and catalytic activity for H2O2-dependent O2•− generation was largely lost in the resultant Y-phosphorylated peptide.Citation15 To date, ErkG5H is the only artificial catalyst which can be attenuated by phosphorylation event.
Involvement of free Y or Y residue in the Cu/peptide complex-catalyzed H2O2-dependent generation of O2•− suggests that a phenoxy radical derived from Y (tyrosyl radical, Y•) can be formed in aid of single electron reduction of molecular oxygen, by analogy to plant enzymes H2O2-dependently generating O2•− by coupling to oxidation of phenolics (such as salicylic acid) to form phenoxy radicals.Citation17,18
There would be two distinct models for the fate of Y residue in model Cu/peptide complex. One likely model is that Y-residue is simply consumed and oxidized by the Cu-bound catalytic center in the presence of H2O2 as observed for various free phenolics.Citation11 Another likely model is that Y residue lasts longer by repeatedly participating the reaction as shuttle for transferring electron, by analogy to the putative intra-molecular substrate-like roles for Y residues within ribonucleotide reductases.Citation1 and cyclooxygenase-2,Citation20 in which corresponding reactions proceed via transient formation of Y• and recycling of Y.
To obtain a clue to this view, we examined the fate of Y-dependent fluorescence in Gfp-G5H, TYG-G5H, and SYG-G5H peptides after addition of Cu and H2O2 (). Ratio of H2O2 concentration (1 mM) over Cu/peptide concentration (30 μM) was set at excess level since higher range of H2O2 concentration has been employed in the previous studies using G5H-based catalysts.
Figure 5. Changes in UV-excited fluorescence contour spectra in three peptides after addition of copper and/or excess hydrogen peroxide. Peptides used were as in , namely Gfp-G5H, TYG-G5H and SYG-G5H. Each peptide (30 μM) was treated with none, either or both of CuSO4 (30 μM) or/and H2O2 (1 mM). Numbers after (a) and (b) shown with each spectrum represent the relative changes in fluorescence intensities at peaks a (230 nm excitation/320 nm emission) and b (280 nm excitation/320 nm emission), respectively.
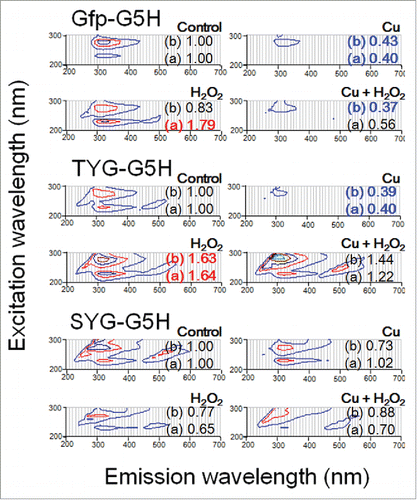
Addition of copper to three peptides largely lowered the fluorescence signals as described earlier in this report. Addition of H2O2 to SYG-G5H lowered the fluorescence signals. Contrary, addition of H2O2 enhanced the fluorescence at both peaks a and b in TYG-G5H and the peak a in Gfp-G5H. The reason why two peaks of Y fluorescence in different peptides showed different sensitivity to H2O2 should be attributed to the fact that even a monomer of phenolic compound often possesses multiple fluorophores within the molecule despite its simple structure as in the case of ferulic acid.Citation4,8
To the combination of Cu and H2O2, three peptides responded differently. Response to the Cu/H2O2 co-treatment in Gfp-G5H was almost identical to the response to Cu alone. Changes in SYG-G5H were less obvious. The fluorescence intensities at 230 nm excitation/320 nm emission and 280 nm excitation/320 nm emission corresponding to the peaks a and b in Cu/H2O2 co-treated TYG-G5H were seemed to be maintained at higher level compared to control. Note that the peak excitation wavelength at peak b fluorescence slightly shifted from 280 to 290 nm, therefore the product of peptide-catalyzed redox reaction challenging Y-residue under Cu/H2O2 co-treatment must be no-longer intact Y residue. The case in TYG-G5H suggests that after possible formation of Y• via Cu/peptide-catalyzed H2O2-dependent reaction, recycling of Y did not sufficiently occur thus a spectral change (shift in the excitation peak) was observed.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant of Regional Innovation Strategy Support Program implemented by Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Funding
This work was supported by a grant of Regional Innovation Strategy Support Program implemented by Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- Boal AK, Cotruvo JA, Jr, Stubbe J, Rosenzweig AC. Structural basis for activation of class Ib ribonucleotide reductase. Science 2010; 329:1526-30; PMID:20688982; http://dx.doi.org/10.1126/s;cience.1190187
- Bogdan C, Röllinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 2000; 12:64-76; PMID:10679404; http://dx.doi.org/10.1016/S;0952-7915(99)00052-7
- Burns CS, Aronoff-Spencer E, Dunham CM, Lario P, Avdievich NI, Antholine WE, Olmstead MM, Vrielink A, Gerfen GJ, Peisach J, et al. Molecular features of the copper binding sites in the octarepeat domain of the prion protein. Biochemistry 2002; 41:3991-4001; PMID:11900542; http://dx.doi.org/10.1021/b;i011922x
- Djikanović D, Kalauzi A, Jeremić M, Mićić M, Radotić K. Deconvolution of fluorescence spectra: Contribution to the structural analysis of complex molecules. Colloids Surfaces B: Biointerfaces 2007; 54:188-92; http://dx.doi.org/10.1016/j;.colsurfb.2006.10.015
- Fang YY, Claussen CA, Lipkowitz KB, Long EC. Diastereoselective DNA cleavage recognition by Ni(II)Gly-Gly-His-derived metallopeptides. J Am Chem Soc 2006; 128:3198-207; PMID:16522100; http://dx.doi.org/10.1021/j;a0569757
- Fang YY, Ray BD, Claussen CA, Lipkowitz KB, Long EC. Ni(II).Arg-Gly-His-DNA interactions: investigation into the basis for minor-groove binding and recognition. J Am Chem Soc 2004; 126:5403-12; PMID:15113212; http://dx.doi.org/10.1021/j;a049875u
- Folkes LK, Greco O, Dachs GU, Stratfoed MRL, Wardman P. Five-Fluoroindole-3-acetic acid: a prodrug activated by a peroxidase with potential for use in targeted cancer therapy. Biochem Pharmacol 2002; 63:265-72; PMID:11841802; http://dx.doi.org/10.1016/S;0006-2952(01)00868-1
- Inokuchi R, Takaishi H, Kawano T. Fluorometric quantification of ferulic acid concentrations based on deconvolution of intrinsic fluorescence spectra. Environ Control Biol 2016; 54:57-64; http://dx.doi.org/10.2525/e;cb.54.57
- Inokuchi R, Yokawa K, Okobira T, Uezu K, Kawano T. Fluorescence measurements revealed two distinct modes of metal binding by histidine-containing motifs in prion-derived peptides. Curr Topics Peptide Protein Res 2012; 13:111-8
- Iwase J, Furukawa H, Hiramatsu T, Bouteau F, Mancuso S, Tanaka K, Okazaki T, Kawano T. Protection of tobacco cells from oxidative copper toxicity by catalytically active metal-binding DNA oligomers. J Exper Bot 2014; 65:1391-402; http://dx.doi.org/10.1093/j;xb/e;ru028
- Kagenishi T, Yokawa K, Kadono T, Uezu K, Kawano T. Copper-binding peptides from human prion protein and newly designed peroxidative biocatalysts. Z Naturforsch 2011; 66c:182-90; http://dx.doi.org/10.5560/Z;NC.2011.66c0182
- Kagenishi T, Yokawa K, Kuse M, Isobe M, Bouteau F, Kawano T. Prevention of copper-induced calcium influx and cell death by prion-derived peptide in suspension-cultured tobacco cells. Z Naturforsch 2009; 64c:411-7
- Kawano T. Quenching and enhancement of terbium fluorescence in the presence of prion-derived copper-binding peptides. ITE Lett 2006; 7:383-5
- Kawano T. Prion-derived copper-binding peptide fragments catalyze the generation of superoxide anion in the presence of aromatic monoamines. Int J Biol Sci 2007; 3:57-63; http://dx.doi.org/10.7150/i;jbs.3.57
- Kawano T. Learning from prion-derived peptides for designing novel phosphorylation-sensitive peptide probes. Medical Hypotheses 2011; 77:159-61; PMID:21665374; http://dx.doi.org/10.1016/j;.mehy.2011.03.008
- Kawano T, Bouteau F. Crosstalk between intracellular and extracellular salicylic acid signaling events leading to long-distance spread of signals. Plant Cell Reports 2013; 32:1125-38; PMID:23689257; http://dx.doi.org/10.1007/s;00299-013-1451-0
- Kawano T, Muto S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco suspension culture. J Exper Bot 2000; 51:685-93; http://dx.doi.org/10.1093/j;exbot/5;1.345.685
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: The earliest events in salicylic acid signal transduction. Plant Cell Physiol 1998; 39:721-30; http://dx.doi.org/10.1093/o;xfordjournals.pcp.a029426
- Laroussi M, Leipold F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrometry 2004; 233:81-6
- Li W, Wu S, Ahmad M, Jiang J, Liu H, Nagayama T, Rose ME, Tyurin VA, Tyurina YY, Borisenko GG, et al. The cyclooxygenase site, but not the peroxidase site of cyclooxygenase-2 is required for neurotoxicity in hypoxic and ischemic injury. J Neurochem 2010; 113:965-77; PMID:20236388; http://dx.doi.org/10.1111/j;.1471-4159.2010.06674.x
- Okobira T, Kadono T, Kagenishi T, Yokawa T, Kawano T, Uezu K. Copper-binding peptide fragment-containing membrane as a biocatalyst by radiation-induced graft polymerization. Sens Mater 2011; 23:207-18
- Sakai Y, Fukase T, Yasui H, Shibata M. An activated sludge process without excess sludge production. Water Sci Technol 1997; 36:163-70; http://dx.doi.org/10.1016/S;0273-1223(97)00704-X
- Werner JJ, McNeill K, Arnold WA. Environmental photodegradation of mefenamic acid. Chemosphere 2005; 58:1339-46; PMID:15686751; http://dx.doi.org/10.1016/j;.chemosphere.2004.10.004
- Yokawa K, Kagenishi T, Goto K, Kawano T. Free tyrosine and tyrosine-rich peptide-dependent superoxide generation catalyzed by a copper-binding, threonine-rich neurotoxic peptide derived from prion protein. Int J Biol Sci 2009a; 5:53-63
- Yokawa K, Kagenishi T, Kawano T. Superoxide generation catalyzed by the ozone-inducible plant peptides analogous to prion octarepeat motif. Plant Signal Behav 2011a; 6:477-82; http://dx.doi.org/10.4161/p;sb.6.4.14744
- Yokawa K, Kagenishi T, Kawano T. Cypridina luciferin analog, CLA as a probe for the enzymatic reaction of superoxide generation catalyzed by copper-binding hexapeptide found in chicken prion protein. Luminescence 2010; 25:139
- Yokawa K, Kagenishi T, Furukawa H, Kawano T. Comparing the superoxide-generating activities of plant peroxidase and the action of prion-derived metallopeptides: towards the development of artificial redox enzymes. Curr Topics Peptide Protein Res 2011b; 12:35-50
- Yokawa K, Kagenishi T, Kawano T. Thermo-stable and freeze-tolerant nature of aromatic monoamine-dependent superoxide-generating activity of human prion-derived Cu-binding peptides. Biosci Biotechnol Biochem 2009b; 73:1218-20; http://dx.doi.org/10.1271/b;bb.90012
- Yoshioka H, Bouteau F, Kawano T. Discovery of oxidative burst in the field of plant immunity: looking back at the early pioneering works and towards the future development. Plant Signal Behav 2008; 3:153-5; PMID:19513209; http://dx.doi.org/10.4161/p;sb.3.3.5537
