ABSTRACT
There are probably few terms in evolutionary studies regarding neuroscience issues that are used more frequently than ‘behavior', ‘learning', ‘memory', and ‘mind'. Yet there are probably as many different meanings of these terms as there are users of them. Further, investigators in such studies, while recognizing the full phylogenetic spectrum of life and the evolution of these phenomena, rarely go beyond mammals and other vertebrates in their investigations; invertebrates are sometimes included. What is rarely taken into consideration, though, is that to fully understand the evolution and significance for survival of these phenomena across phylogeny, it is essential that they be measured and compared in the same units of measurement across the full phylogenetic spectrum from aneural bacteria and protozoa to humans. This paper explores how these terms are generally used as well as how they might be operationally defined and measured to facilitate uniform examination and comparisons across the full phylogenetic spectrum of life. This paper has 2 goals: (1) to provide models for measuring the evolution of ‘behavior' and its changes across the full phylogenetic spectrum, and (2) to explain why ‘mind phenomena' cannot be measured scientifically at the present time.
Introduction: The phylogenetic tree of life
One must take note of a fundamental dichotomy when approaching any comparative biological issue across the full phylogenetic spectrum. Does one wish to seek an understanding of the most general characteristics of living systems that are common to all life, such as cell membrane structure and function, specific enzymes, genes, metabolic pathways, or to those characteristics that are unique to each group, (i.e. what uniquely sets this group apart from all others)? For example, how does the mammal differ from the bird or reptile? Obviously both approaches, comparative similarities and comparative differences, are of interest since all living systems have characteristics that are common to all life as well as characteristics that are unique to each group. It is these 2 approaches to comparative studies that give meaning to any of the “Phylogenetic Tree of Life” models of evolution that have been proposed, such as that by Woese, ().Citation1,2
Figure 1. Woese's universal 'Phylogenetic Tree of Life Model' determined from rRNA sequence comparisons.Citation1 (Reprinted with permission. © 1987 American Society for Microbiology).
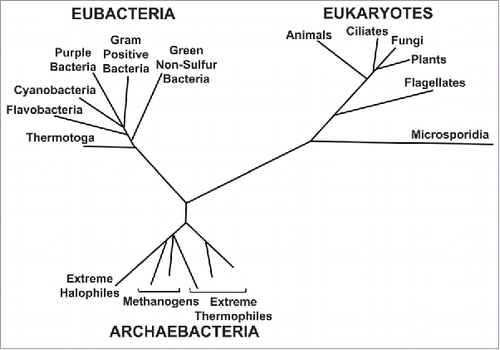
The universe is estimated to have begun about 15 billion years ago; the origin of the planet earth, about 5 billion years ago. The earliest indicators of life on earth suggest life began about 3.5 to 4 billion years ago. The similarities among all present day organisms from bacteria to humans suggest the presence of a common origin from which all known species diverged through the process of evolution.
The Woese model (), which is receiving considerable support, proposes that of the 3 domains of life, the archaebacteria and eubacteria are the least evolved types of cells. They are the closest to the common point of origin of life about 4 billion years ago. Eukaryotes are the most evolved type of single cell. According to Woese, eukaryotes did not begin to diversify (i.e., branch out) until relatively late in evolution. It begins at a time when the eubacteria diversify into those that show oxygenic photosynthesis. This refers to the photosynthetic process which began about 3 billion years ago when bacteria drastically changed the earth's atmosphere by producing oxygen. This change allowed the further multicellular evolution of the eukaryotes. While diversification of eukaryotes continues in unicellular protozoa, multicellular eukaryotes on Woese's phylogenetic tree of life model include plants, animals and fungi. Thus, phylogenetic tree models are schematics to illustrate comparative similarities and differences over time across the entire phylogenetic spectrum, which includes aneural single cell organisms, such as bacteria and protozoa, as well as multicellular plant and animal life.
Many of the phenomena studied in Neuroscience are not unique to nervous systems but also occur in single-cell aneural systems. Thus, sensory transduction and electrical conduction can be seen in protozoa.Citation3,4 If one examines comparative similarities of insects and humans, for example, it is noted that insects (a class of arthropods), and vertebrates (from which humans evolved), are estimated to have diverged about 500 to 600 million years ago, i.e., both insects and vertebrates had a common evolutionary background for over 2 billion years before they diverged on the phylogenetic tree. Thus, when it comes to comparing nervous system functions of all kinds, the evidence shows that humans and insects have much in common. Because of this common origin of life, there is no limit as to which living systems can be compared for both similarities and differences. It is these comparative similarities and differences that give meaning to the concept of the phylogenetic tree of life as well as to the evolutionary significance for survival of the various biological characteristics that are seen.
With respect to the evolution of ‘behavior’, ‘learning’, ‘memory’ and ‘mind', the field of Psychology primarily has been concerned with how to ‘verbalize' and ‘describe' them, (i.e. label them), rather than how to investigate their evolution biologically. The main attempt in this paper is to do just that. The full evolutionary range considered in this paper is described in the Abstract and shown in .
How does one measure the physical universe and behavioral and biological changes in the same metrics?
Measuring the 'physical universe'
Scientists have created and use a series of fundamental measuring units and their derivatives to measure all the biological phenomena of life as well as the entire physical universe. These measuring units and their derivatives are fundamental to scientific research. One set of such fundamental metrics for measurement are the "CGS" units, representing length (‘centimeters'), mass (‘grams'), and time (‘seconds'). Terms, such as ‘density', ‘velocity', acceleration' and ‘force', are ‘derivative measures' of these fundamental ‘CGS' measures.Footnotea Measurements made in the physical universe require a ‘frame of reference' (FOR) from which such measurements are made. Depending on the FOR used, the values of the obtained measures vary. Thus, measuring the speed of a car going past you when you are standing on a corner, and the speed of the same car when you are riding in another car alongside it are different when measured from each of these 2 ‘frames of reference', even though the physical laws underlying both measures are the same. With the exception of the ‘velocity of light' in Einstein's ‘Special Theory of Relativity', which Einstein postulated to be the same regardless of the FOR used, all other measurements in the physical universe vary with the FOR from which such measurements are made.
The fundamental measurements used in biology are these same basic measures. Thus, biological measures of ‘DNA', or an ‘enzyme' are all measureable in these same basic ‘CGS' measures and their derivatives. However, particularly in the field of Psychology, when it comes to some ‘behavioral' and so-called ‘mind' neuroscience issues, the basic terminology used often is not quantifiable in these fundamental measuring units of science or their derivatives.Citation5,6
Measuring the changes in ‘behavior' related to ‘learning and memory' across phylogeny
Like light, ‘behavior' is not stationary in the physical world. The word itself denotes movement over time. Unlike physical objects, such as a table or a chair, ‘behavior' has no specific fixed spatial dimensions at any given point in space/time, but rather is constantly changing its spatial characteristics over time. Considering the dynamics of this phenomenon, finding a fixed physical ‘frame of reference' (FOR) from which to measure it and its changes across the full phylogenetic tree of life is difficult. When it comes to learning and memory, there are 4 basic ‘behaviors and their changes', most commonly studied in the field of Psychology. They are:
Habituation and sensitization
These two learned behavioral changes generally refer to a repetitive external sensory stimulus leading to an effector response change over several stimulus repetitions, (i.e., trials). They can be examined throughout phylogeny from ‘an aneural protozoan contraction magnitude change to a repeated vibratory stimulus', to ‘a human galvanic skin response (GSR) change' to a repeated tone.Citation7,8,9 Thus, the sensory stimulus input and effector output of habituation and sensitization learning can represent either a decrease (habituation) or an increase (sensitization) in effector response magnitude, in a given person or organism to a particular sensory stimulus over initial stimulus repetitions.Footnoteb It has been shown many times that these 2 behavioral changes over repetitive trials (habituation and sensitization) are not merely due to sensory receptor adaptation or effector fatigue. Various theories regarding the possible evolutionary significance of these 2 learned behavioral changes, observed across phylogeny from aneural protozoa to humans and which show remarkable similarity, have been proposed.Citation7,8,Citation10-13,b
Pavlovian (Classical) conditioning
Pavlovian conditioning learning is based on the temporal pairing of 2 stimuli over repeated trials. One of the stimuli (called the unconditioned stimulus or UCS) triggers an effector system reflex, such as salivation to food in the mouth or heart rate increase to a shock. The other stimulus, called the conditional stimulus or CS (e.g., a tone), initially does not trigger this reflex. However, when temporally paired with the UCS, (i.e. when the CS precedes the UCS in onset time over several stimulus presentations (trials)), the CS gradually leads to an increase in effector response magnitude. Commonly used effector system responses in such learning in vertebrates include salivation, GSR, and heart rate change. In order to demonstrate that the effector system change to the CS is based on the ‘temporal order' of onset of the 2 stimuli, and not just to the occurrence of the 2 stimuli per se, a ‘control group' can be used which receives the same number of both stimuli except that the ‘temporal order' of onset presentation of the 2 stimuli is reversed. Thus, UCS onset precedes the CS.
This ‘control group' shows no continuous increase in response magnitude to the CS over repetitive trials.Citation14,15 Thus, the learning associated with ‘Pavlovian conditioning' is a function of the ‘temporal order' of onset of the 2 paired stimuli. In addition to the ‘temporal order' being critical for Pavlovian conditioning, it also has been shown that the ‘temporal interval' between CS and UCS onset time is critical. Thus, when the temporal interval between the CS and UCS onset time increases or decreases by as little as .5 seconds it can increase or decrease the magnitude of Pavlovian conditioning in humans over trials considerably.Citation14
‘Instrumental‘ or ‘self-initiated' learned behavior
This ‘behavioral learning', unlike habituation, sensitization, and Pavlovian conditioning which depend on specific external stimuli to trigger specific effector responses, is self-initiated. It is generally studied in a laboratory-created environment in which a rat, for example, is placed in a box with a bar in it (Skinner box) and, if the rat presses the bar during its explorative behavior, it receives a pellet of food; or if it is placed in a T-maze, where it can make a choice to turn right or left. If the correct choice is made it receives a pellet of food.Citation16-18 The change in the behavior over trials in these ‘devices' is based on the nature of the ‘reward'. If, for example, pressing the bar in the Skinner box or turning right in a T-maze leads to positive reinforcement such as food, the rat's learned behavioral changes improve more rapidly each time it is placed in this ‘device', in order to achieve the reward as soon as possible.
Instrumental learned behavior also can occur to negative reinforcement such as a shock to the feet. Thus, a rat can be placed in the ‘white side' of a ‘hurdle box' with a shock grid on the floor and a ‘hurdle' to jump over. When the shock is turned on the rat will learn over repeated trials to jump over the hurdle to escape the shock. Thus, the rat learns, each time it is placed in the ‘hurdle box', over trials to avoid the shock more rapidly. It is predicted that if the rat is shocked outside of the hurdle box, and then placed in the ‘white side' of the ‘hurdle box', it will not immediately jump over the hurdle.Citation16 If this is proven, it will show that it is not the shock per se, but the pattern of stimulation and response sequence that determines the changes in behavior over trials. Thus, just as with habituation, sensitization and Pavlovian conditioning, it is likely that instrumental learned behavioral changes involve a repeated temporal sequence of stimulus input and effector response changes that lead, over trials, to acquired memory.
Role of ‘time' in learning and memory
As noted, learning involves linking 2 or more repetitive ‘stimulus' and ‘response' events, (i.e., ‘trials'), over time. The perception of time is based on the linking or connecting of at least 2 non-simultaneously occurring events—a first and a second. It is the ability of the organism to connect these 2 non-simultaneously occurring events that give it the perception of time. It is the second occurring event that serves as the unit to measure time. Without time perception, there could be no learning, and without learning, there could be no "acquired memory." Thus, the concept of learning involves the association, i.e., the linking of non-simultaneously occurring external stimulus and organism response events. It would appear that the primary transition from ‘learning' to ‘acquired memory storage' in all 4 types of learning (habituation, sensitization, Pavlovian conditioning and Instrumental learning) is that of converting a repeated sequence of serial events into a 3D structural state for storage in the system.Citation16
Exploration of the ‘dilemma' of how to compare ‘instrumental learning and memory' across the phylogenetic tree of life
In habituation, sensitization and Pavlovian conditioning, the exact sensory stimulus input and effector response elements are very ‘specifiable'. However, in ‘instrumental learning', the ‘specific external stimulus input' that is responsible for the ‘self-initiated behavior and its changes,' and the exact nature of the ‘effector responses and their changes' over trials, are often not ‘specifiable'.Citation17 This is the dilemma of studying ‘instrumental learning and memory' across the full phylogenetic tree of life.
‘Instrumental learning' commonly involves a rat or a pigeon, which often is placed in a given laboratory ‘device', and either presses a bar in a ‘Skinner box', turns right or left in a ‘T-maze' or is placed in a ‘hurdle box'.Citation16-18 After the appropriate ‘device behavior' has been ‘self-initiated', the motivation to continue it is based on the nature of the ‘reinforcer' or ‘reward' at the end of the task, (i.e., either a positive or a negative one). Thus, a positive reward, such as food, can lead to a rapid change in the behavior leading to it, while a negative reinforcer, such as a shock, leads to a rapid change in the behavior of escaping and avoiding it.
As noted, pressing a bar in a ‘Skinner box' or turning right or left in a ‘T-maze' can lead to a positive reward such as food. Several repetitions of these procedures lead to a shorter time to achieve the reward over repetitive trials. Generally the physical parameters of ‘instrumental behavior', and a learned change in them over trials, are not measured in terms of specific ‘biological effector system changes' to ‘specific repetitive external stimulus' presentations, as they are in habituation, sensitization and Pavlovian conditioning, but rather by the ‘context' or ‘device' in which such behavior occurs as, for example, pressing a bar in a ‘Skinner box', or turning right or left at the choice point in a ‘T-maze'. A ‘T-maze', or a ‘Skinner box with a bar to press', commonly are the contexts used to define such behavior and its changes.Citation17 Thus, the exact meaning of the term ‘instrumental behavior and its change' in such situations varies with the ‘context' or ‘device' in which it is examined.
While the physical boundaries of such ‘instrumental (self-initiated) behaviors' and their changes are not fixed and sharply defined in terms of biological effector measures, (e.g., skeletal muscle changes), of the person or organism, it also is the case that its measured outcome is often not ‘behavior' per se, but the ‘effect of the behavior' on the environment it is in. Thus, ‘pressing a bar' in a ‘Skinner box' is not a description of behavior per se, but the effect of an organism's behavior on the environment it is in. So-called ‘bar press behavior', for example, could mean a ‘foreleg press', a ‘hind leg press', a ‘right or left leg press' or a 'both-legs' press—or even a nose or beak press. All lead to the same effect, (i.e. ‘pressing a bar'). However, the press of the bar is the ‘result of the behavior', not the ‘behavior' itself. Often, the nearest we come to measuring ‘an instrumental component of the behavior' in ‘effector system' measures is to view a ‘bird nest' or a ‘spider web' as it is being built. But even in these cases the so-called ‘behavior' being measured is often after they are built, (i.e., the ‘nest' or 'web), and not ‘the behavior itself' as they are being built.
‘Behavior' as a ‘concept', in such situations, is ill-defined and tenuous. Exactly what it is that the brain is ‘processing' and ‘storing' when it comes to such ‘self-initiated instrumental behavior and its change over time' is generally unknown, since the term itself, ‘self-initiated behavior', is tenuous in its definition and, as noted, is often defined by its ‘methodological impact' or effect on the environment rather than on measureable effector system changes within the organism over time. Furthermore, joint and skeletal muscle activity, which often are the basis of the so-called ‘instrumental behaviors' may change their spatial and temporal characteristics continuously over time and are not generally measured in the CGS units that can be (and are) used to define and measure other physical and biological changes. Thus, we are left with the fundamental and disconcerting question as to whether such ‘learned self-initiated behavioral information', as it is stored in the brain, represents a sequence of specific effector system responses to the stimulus situation (i.e., the ‘device') the animal is placed in, or does it only represent the final outcome or goal of such actions, (i.e., the ‘bar press' or a ‘correct turn' in a T-maze)? If it is a combination of both, then what are the specific physical characteristics of what it is, (i.e., specific stimuli and responses), that are encoded in the brain and stored as ‘self-initiated learned behavior' and memory?Citation17 Clearly, in order to establish a physical relationship between (a) the initial ‘cause' (i.e., the specific sensory input in the ‘device' responsible for the learned behavioral changes) and (b) the ‘effect', (i.e., the learned motor behavioral changes (often involving muscle contractions) and how it is stored as a memory and has evolved throughout phylogeny, it is necessary to measure both (a) sensory ‘cause' and (b) ‘effect' in the same physical units, e.g., nerve impulses, when possible. The absence of such ‘dimensional equivalence' in cause and effect measures prevents specifically knowing the neural bases of the ‘self-initiated instrumental learning', its memory, and how it has evolved over time. In the next 2 sections (IV & V) ‘model systems' will be reviewed that show how it may be possible to compare all 4 types of learning and memory across the full phylogenetic spectrum.
A suggested 'model system' of how both the evolution of habituation and sensitization learning and Pavlovian conditioning can be compared across phylogeny in organisms with nervous systems
The hypothetical model system shown in indicates how such a simple approach may be useful in answering fundamental questions about the ‘neural bases' of habituation, sensitization and Pavlovian conditioning across phylogeny in organisms with a nervous system.Citation19 It represents a simple way to help determine the relative contributions that primary sensory input, motor output, efferent sensory gating, and afferent sensory feedback play in the evolution of learning and memory. All sensory input and motor output are measured in the same units, (i.e., nerve impulses). Thus, as shown, if one were to record from the motor nerve which goes directly to an effector such as a muscle and sever this distally from the effector as in (b), it should be possible to eliminate afferent sensory feedback from the active effector system. Correspondingly, if one stimulates primary sensory nerve input directly, bypassing the primary sensory receptors as in (c), it should be possible to separate out the effects of efferent gating (i.e., inhibition of sensory input) at the receptor level. By stimulating the cut primary sensory nerve directly and recording from the cut motor nerve output as in (d), it should be possible to remove the roles of both efferent gating at the sensory receptor level and afferent sensory feedback in the learning and memory. Thus, in principle, it should be possible to determine what the relative contributions all these factors make to any learning and memory storage that occurred. All biological changes are measured in the same CGS units, namely, parameters of nerve discharges. Thus, one can stimulate and monitor known sensory inputs, as well as monitor interneuron activity and motor outputs. Questions can be asked and answered about rates and magnitude of learning, retention,—in fact, the questions that are normally asked about behavioral learning and memory in any system—except that we now know specifically what is coming into the system and being processed, and what is going out to the effector system. With such a hypothetical model system it may be possible to answer questions of how any nervous system may encode the various non-simultaneous temporal sequences of events that form the bases of habituation and sensitization learning, as well as that of Pavlovian conditioning.Citation16
Figure 2. A 'simple schematic model' of how behavioral learning and memory (Pavlovian conditioning, habituation and sensitization) can be examined in the nervous system across phylogeny where the sensory input, learning and memory processing, and motor output all can be measured in the same dimensionally equivalent metrics of nerve impulses. It also may serve as a model for part of an organism's nervous system, e.g., a 'ganglion', where the sensory and motor systems to it are intact. (a) Usual learning system of an intact organism: REC. (sensory receptor); AFF. (sensory nerve); INT. integrator (ganglion or brain); EFF.(motor nerve) to an effector such as a muscle. (b) Bypassing the role of 'afferent sensory feedback' from the effector response by recording directly from the cut efferent nerve. (c) Bypassing the role of 'efferent gating', i.e. inhibition of primary sensory input to the system, by stimulating the cut sensory nerve directly rather than stimulating it through its sensory receptors. (d) Bypassing the roles of both primary sensory receptor input and proprioceptive feedback from effector output in learning and memory by cutting the sensory nerve and eliminating efferent gating, i.e., inhibition of sensory input through sensory receptors, and cutting the motor nerve and eliminating afferent sensory feedback from the effector response.Citation19 (Reprinted with permission from Elsevier).
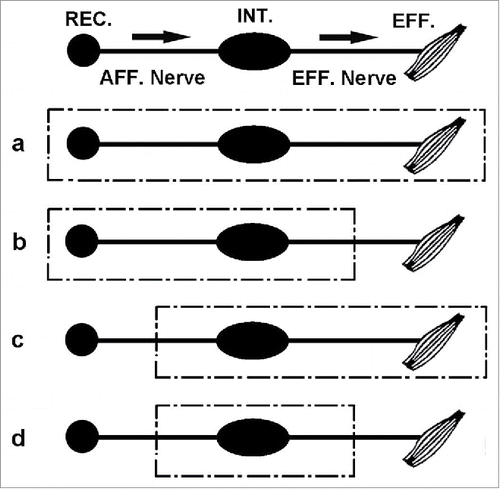
The purpose of utilizing a model system is to simplify the variables involved in the analysis. Fundamental to the use of a model system is the notion that the variables and their interactions in the model also apply to the intact and more complex biological system that is being modeled. The hypothetical model in shows how such an approach can be useful in answering fundamental questions about the evolution of learning and memory across the phylogenetic spectrum in organisms with a nervous system. It would be desirable to know and separate out, for example, the relative contributions that primary sensory input, motor output, efferent gating, and afferent sensory feedback play in the evolution of habituation, sensitization and Pavlovian conditioning learning and their memory. The approaches shown in make it possible to ascertain for a given nervous system, at any level of the phylogenetic tree, what the relative contributions such factors play in learning and memory storage. In principle, they allow us to ask as we ascend the evolutionary tree whether or not, and in what ways, phenomena such as feedback and gating are involved in learning and memory storage. One might expect, for example, that at lower phylogenetic levels there is relatively little difference in learning and memory for a given nervous system between stimulating the primary sensory nerve directly and recording from the motor nerve as in example (d), and stimulating through its sensory receptors and recording its effector output as in example (a) (assuming that there is relatively little, if any, contribution from feedback and gating processes in learning in this system). However, as we ascend the phylogenetic tree, we may well expect to see a marked difference in learning and memory when we have eliminated afferent sensory feedback and efferent gating from consideration.
A suggested ‘model system’ of how the use of a ‘negative reinforcer' may allow ‘instrumental learning' to be quantified and measured in the same dimensionally equivalent CGS units as that used for ‘Pavlovian conditioning and habituation and sensitization', across phylogeny
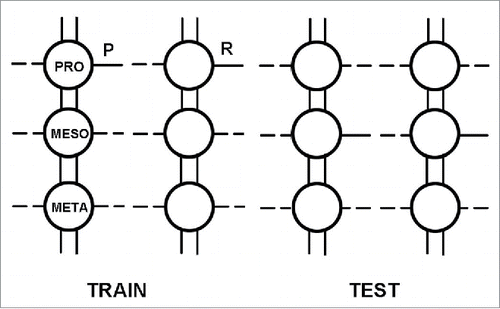
In an insect each pair of the 3 pairs of legs is connected to a thoracic ganglion and its peripheral nerves as shown in . In the cockroach, a thoracic ganglion may contain as few as a thousand neurons. Each ganglion can be viewed as a ‘mini-brain' when it comes to learning and memory.Citation15
Figure 3. Schematic dorsal view of the 3 thoracic ganglia of the cockroach (prothoracic, mesothoracic and metathoracic) showing the bilateral peripheral nerves of each ganglion going to a pair of legs, as well as the 2 connectives between the ganglia. This system can be used for studying learning and memory in the same ganglion, as well as studying the transfer of such learning-related information from ganglion one to ganglion two and three. The actual escape-avoidance learning procedure used in the first prothoracic ganglion is shown in .Citation19 (Reprinted with permission from Elsevier).
Instrumental learning is explored using ‘shock escape/avoidance learning' in the cockroach. The ‘behavior' examined is that of ‘flexion and extension of the coxal-trochanter joint in a particular leg, such as that innervated by the first prothoracic ganglion', of the cockroach. In addition to examining learning per se in a particular ganglion, the ‘transfer' of such learning-related information between the thoracic ganglia of the cockroach also is discussed. This approach represents one way to explore how instrumental learning and memory may possibly be encoded in the nervous system, and its evolution compared across phylogeny in organisms with a nervous system.Citation19-23
The 3 thoracic ganglia (prothoracic, mesothoracic, and metathoracic, in rostro-caudal order) are interconnected by paired ventral connectives which are the neuronal pathways through which interganglionic communication can occur.Citation24 Peripheral branches of the sensory and motor nerves of the first thoracic ganglion are confined to the ipsilateral half of the ganglion that they innervate and are not present in the connectives between the first and second ganglia. Thus, it would appear that whatever information is transferring from a ganglion (from ganglion one to ganglion two or three) is doing so by ‘interneurones' and not by primary sensory or motor neuron branches.
The so-called ‘yoked control' procedure for studying such ‘shock avoidance learning' was first developed by Brady in his seminal studies on the executive monkey syndrome in 1958.Citation25 It was first used to study escape/avoidance learning in the insect ventral nerve cord by Horridge in 1962.Citation26 The ‘initial training' procedure involves connecting the corresponding prothoracic legs (ganglion one) of 2 decapitated insects in a ‘series circuit' as shown in . One of the 2 animals, called the positional or ‘P' animal, activates the circuit when its prothoracic leg lead is in the saline dish. When this occurs, both animals receive shocks until the ‘P' animal flexes and lifts its leg lead out of the saline dish. The other animal of the pair, called the random or ‘R' animal (yoked control) has no control over the shock and receives a shock whenever the ‘P' animal's leg lead is extended into its own saline dish. Thus, the ‘R' animal receives shocks when its prothoracic leg lead is in a variety of different positions, while the ‘P' animal, which controls the shock, is only shocked when its own leg lead is extended into its saline dish. Hence, both ‘P and R' legs receive an identical pattern and number of shocks during this ‘initial training'.
Figure 4. During 'initial training' the 'left prothoracic ‘P' and ‘R' legs' are wired in series and the tarsal lead of P, upon extension into the saline dish, initiates shock pulses to both P and R legs with the same current, and these pulses are recorded as an indication of P's leg position and activity. When the tarsal lead of R makes contact with its saline dish during the 'initial training period', no shock is given to either leg, but the pulses initiated by R are recorded just as they are for P as an indication of R's leg position and activity during initial training. During the 'testing period' the legs are now wired in parallel and each leg can, upon extension into its own saline dish, initiate shock only to itself. The shocks initiated by each leg are recorded. There have been several variations of this procedure. Even with these procedural variations there has been great consistency in the results obtained. The advantages of this particular procedure are: (1) the legs of P and R are identically wired and stimulated; (2) no alteration of the actual wiring on the P and R legs themselves is required in going from training to testing; (3) R's leg position can be recorded during training. Abbreviations used: G, glass rod that the cockroach is attached to by its dorsal surface; A, animal; W, wax dab to insulate the tarsal lead from the leg; SD, saline dish; S1, S2, stimulators; Synch., synchronous frequency output from both P (S1) and R (S2) stimulators; R1 , R2, polygraph recorders for pulses initiated by P and R tarsal leads when they make contact with the saline.Citation22,23 (Reprinted with permission from Elsevier).
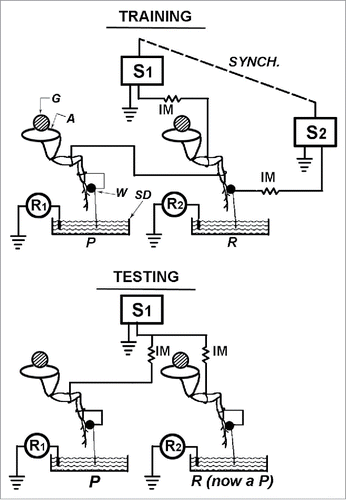
Following a 30–45 minute ‘initial training period', the prothoracic legs of both animals are reconnected in a ‘parallel circuit' for a ‘testing period' (). Each animal now receives a shock independently of the other when its own prothoracic leg lead is extended into its own saline dish. If any learned association between leg extension and shock occurs during ‘initial training', then during the ‘ test period' the ‘P' member of the pair would be expected to initiate fewer shocks than the former ‘R' member. This is predicted because during the training period leg lead extension always was associated with shock in the ‘P' animals, and only ‘randomly' in the ‘R' animals. The data Horridge presents for 2 groups of insects (the cockroach and the locust) show that the ‘P' animals, as a group, initiate significantly fewer shocks than the former ‘R' animals during the test period. He interpreted this as a demonstration of shock avoidance learning by the insect ventral nerve cord. It also has been shown that even a ‘single isolated prothoracic ganglion preparation', (i.e., a decapitated cockroach, with both of its posterior ventral nerve connectives cut before any training) also is capable of showing this same learning.Citation15,27
In addition to ‘shock escape/avoidance learning' in a ‘single thoracic ganglion', it has been shown that such learning-related information in both ‘P' and ‘R' preparations can be transferred from one thoracic ganglion to another as schematized in .Citation19,20,28 Horridge used a yoked control procedure to train the first prothoracic leg of a decapitated ‘P' cockroach, in series with its yoked ‘R' partner, to flex its leg lead to avoid shock, but later tested the third metathoracic leg (which never received any shocks directly during initial training). One can study transfer of learning-related information from the first ganglion to the third, as Horridge did, or from the first ganglion to the second as others have done.Citation20,26 Horridge found a significant difference in the number of shocks received by the third legs (metathoracic) during ‘testing' such that the third leg of the ‘P' animal showed a greater degree of shock avoidance than the third leg of the former ‘R' animal, when each could now control its own shock occurrence independently by flexing its own third leg out of its saline dish. During the first 10 minutes of' ‘testing' the former ‘P' (prothoracic) preparation showed a continuous decrement in number of shocks received by its third leg. This decrement was more rapid than that shown by its first leg during the ‘initial training' period. The former R (prothoracic) preparation, however, showed an increase in number of shocks received by its third leg during the ‘initial testing period'. This was followed by a later decrease similar to that seen in the ‘P' third leg. Thus, both ‘P' and ‘R' learning-related information can transfer from the first (prothoracic) ganglion to the third (metathoracic) ganglion in the decapitated cockroach. These same results also were observed in ‘transfer' from the first prothoracic to the second mesothoracic ganglion.Citation20
The behavioral characteristics associated with ‘transfer of learning-related information' from one thoracic ganglion to another include: (1) If both connectives from the first to the second thoracic ganglia are cut prior to training the first leg, no transfer occurs. If both connectives are cut right after training, transfer already has occurred. This indicates that transfer is mediated by the ventral interganglionic nerve connectives;Citation29 (2) it has been shown that transfer between ganglia occurs equally well if only one connective between the ganglia is left intact during initial training. It makes no difference whether the intact connective is on the same or opposite side as the leg being initially trained. Thus, whatever information is represented in each connective, it is redundant with respect to its ability to produce a significant difference during testing in a posterior ganglion in either the right or left leg; (3) both ‘P' and ‘R' information transfer from one ganglion to another, (i.e., information related to shock avoidance learning) (P)) as well as information related to the behavior described in mammals as ‘learned helplessness', (i.e. (R)), when shock occurs irrespective of what the animal does during the initial training period.Citation20,21 Thus, we not only see more rapid learning in the third leg receiving the ‘transferred information' from the ‘P' (prothoracic) trained leg, but we see interference, (i.e., learned helplessness), in the ability of the third leg receiving information transferred from its ‘R' (prothoracic) leg to show such initial shock avoidance learning; (4) ‘behavioral rectification of transfer' also is observed, i.e., learning-related information appears to transfer from anterior to posterior ganglia , i.e. from ganglion one to 2 and 3, but not from ganglion 2 to one.Citation30,31
How is instrumental learning-related information coded for ‘transfer' from one ganglionic site in the cockroach to another ganglionic site?
Because transfer of learning-related information occurs through interneurones in the connectives between the trained and tested ganglia, this indicates that the learning-related information transfer likely involves ‘coded' primary sensory and motor information.Citation32 This system offers a unique opportunity (which needs to be explored further) to examine how such nerve impulse information can be coded or sequenced temporally to transfer ‘instrumental avoidance learning-related information' from one ganglionic site to another.Citation30
What is the learning-related information that is coded for transfer, and, what is the nature of the code used to transfer such information?Citation21,33–35 Is it possible to reveal a pattern or code of impulses traversing the connectives between the first to second or third ganglion associated with the statements "lift the leg to avoid shock," "extend the leg to avoid shock" or "the leg will be shocked independently of its movement"? What constitutes a "bit" or "unit" of such transferred CNS information relevant to ‘avoidance learning' (P information) and ‘learned helplessness' (R information) learning?
It is very likely that the basic coding mechanisms for transfer of learning-related information, (e.g., instrumental, habituation and sensitization, and Pavlovian conditioning), used in the ventral nerve cord of the insect is widely distributed throughout the animal kingdom, and likely includes the more complex mammalian nervous system since, as previously noted, insects and vertebrates only diverged about 500–600 million years ago. In further support of this possibility is the finding, in several studies in insects, that suggests there is both a short-term memory (STM) and a long-term memory (LTM) component of shock-avoidance learning in the thoracic ganglia of the cockroach.
Thus, in the cockroach, the initial avoidance learning (STM) appears to dissipate within one hour after learning. However, after 2 or more hours the (LTM) memory returns. A similar time course is seen in shock avoidance learning and memory in the rat.Citation36 While still to be proven, it would appear that the bases of both STM and LTM is similar in both the insect and mammal. STM and LTM undoubtedly occur in all types of learning and memory.
are ‘models' of how neuronal structure and function, which are remarkably similar throughout phylogeny, can be used to explore the evolution of ‘behavior', 'learning' and 'memory' across the full phylogenetic range of nervous systems.
The measurement of ‘mind’: The paradox of measuring ‘the last transition’
The previous discussion suggests a way of approaching comparative studies of simple effector system learned behaviors across phylogeny which, hopefully, would provide useful insights into their underlying similarities and differences, as well as their evolution across phylogeny. In such learning and memory ‘cause' (sensory input) and ‘effect' (motor output) can be measured in equivalent metrics, such as CGS units and their derivatives. Thus, both sides of the ‘cause' and ‘effect' relation are connected through a dimensionally balanced equation. However, when it comes to comparing ‘mind processes' and their changes across the full phylogenetic tree, such as ‘thoughts', ‘sensory perceptions' or ‘emotional feelings', this opens a whole new domain beyond ‘simple effector system learned behaviors'.
This section contains some repetition of earlier statements to clarify in each example discussed the problems associated with trying to separate ‘mind' and ‘brain' phenomena and the interaction between them. With respect to ‘mind perceptions', in organisms with a nervous system, there are 2 ‘effects in the brain’ following any given ‘causal sensory stimulus' such as light spectra, sound frequencies or chemoreceptor input. They are: (1) the activation of specific sensory nerves and other brain nerve potential activity, as well as other physical changes in the brain some of which can be measured by CT, fMRI and PET scans, and (2) the ‘mind perception effect' itself. Both ‘effects’ are caused by the same external sensory stimulus. However, while both the brain nerve potentials and brain scan ‘effects’ can be measured in the same physical measures as that of the causal sensory stimulus, (e.g., CGS measures and their derivatives), the ‘mind perception' itself, (i.e., the ‘last transition’ from, for example, nerve activity in any given sensory nerve and its network to a specific ‘mind perception,’ such as ‘a color', ‘a sound', ‘a taste' and ‘a smell’) cannot be measured in these same CGS units. Thus, although the physical changes in each ‘sensory system' causing these different “mind perceptions” are measureable in the same physical units (e.g., nerve potentials), the ‘mind perceptions' themselves are not.
Thus, when it comes to ‘mind properties,’ ‘cause’ and ‘effect’ do not appear measureable in the same CGS physical measures as they can be in the ‘simple effector system learned behaviors’ discussed previously.Footnotec It would appear that the properties of the ‘mind’ itself cannot be measured in the very same physical dimensions and scales that are used to measure the entire physical universe, (i.e., mass, space, time and their derivatives). We can compare 2 ‘forces,’ 2 ‘masses,’ 2 ‘genes’ and 2 ‘proteins’ in these CGS measures but how do we compare 2 ‘mind perceptions', such as 2 ‘thoughts,’ 2 ‘feelings,’ 2 ‘tastes’ and 2 ‘smells’ in these same measures? What are their physical boundaries in mass, time, shape, size and density?
Asking what the boundaries are of any ‘mind phenomenon' is like asking what the boundaries are of the universe. Physicists speculate and theorize on the possible multi-dimensionality of the universe beyond our measureable 4 dimensional space-time universe.Citation37,38 However, to qualify as ‘Scientific', any predictions from such theoretical speculations must be tested or testable in the physical measures used in our 4 dimensional space-time universe. The same situation would hold for any ‘speculations' about the possible role of other dimensionalities in the universe as those regarding the measurement of mind. Thus, just as in Physics, any ‘postulates' about the measurement of ‘mind' based on dimensionalities beyond our 4 dimensional universe would have to be tested or testable in the physical measures used in our 4 dimensional `space-time universe'. Otherwise, they are outside the realm of science.
Generally the fundamental units of measurement that the ‘Scientist' uses for the physical universe are based on a ‘ratio scale' of physical measurement.Citation5,6 This involves a measurement scale with an absolute zero and equal intervals between measures. Thus, the difference between 1 and 2 g is the same interval magnitude as between 99 and 100 g. A 10 g measure is twice a 5 g measure since we start from the same zero level. Not all physical measures in the universe, however, are ‘ratio scale' measures. Sometimes ‘interval scale' measures are used. In addition to ratio and interval scale measures, other scale measures often used, particularly for ‘psycho-physical measures’ (i.e., ‘mind perceptions') utilize an ‘ordinal scale'. Thus, an individual may be asked to quantify a degree of his/her perception of ‘painfulness' on a scale between 1 and 10 when 10 is greater than one. However, there is no ‘absolute zero' so we can't quantify that a rating of 10 is twice that of 5, and further, the intervals between ratings are not equal, and therefore we can't quantify that the difference in ‘pain' between a rating of 4 and 5 is the same as the difference in ‘pain' between a rating of 8 and 9 for an individual and certainly not between individuals. Ratio scale units of measurement and interval scale measurements measure quantity and the units within each scale are equal, and they are the fundamental physical units used to measure both biological phenomena as well as the entire physical universe. However, ordinal scale units of measurement as used in ‘psycho-physical measurements', measure quality, and are the units commonly used to measure ‘perceptions' (or so-called ‘mind phenomena'). Such ordinal scale measures represent ‘more or less of something.’ Thus, measures of ‘thought’, ‘feeling', and ‘pain' have no precise physical dimensions for measurement in the space-time dimensions and metrics of our physical universe.
As noted, all measureable properties of the physical universe throughout all science endeavors are primarily properties of ‘quantity', (i.e. the amount of something). We measure size, such as length, in centimeters and mass in grams and amount of time in seconds, and electrical activity in volts and amperes. But, as noted, properties of ‘mind' itself are not ‘quantity' properties. They are ‘quality' properties and therefore are not measureable in the physical units or their derivatives that are used to measure the physical universe. ‘Mind properties' (‘perceptions') are often referred to as (‘sensations') i.e., those ‘qualities' associated with specific sensory system stimulation such as sound, light, touch, taste and smell. While it is true one can intensify the ‘quality' of each of these ‘mind experiences' by increasing the ‘quantity' of their ‘cause', (i.e., the ‘sensory input’), the ‘mind experiences' themselves, (i.e., the ‘effects'), are not measureable in the same physical units of science. To summarize: the same scale of measurement cannot be used for both the ‘mind' and ‘brain' functions because ‘mind and brain' are not measurable in the same dimensionally equivalent physical units.
A question which will arise in the reader's mind is this: How can one say ‘mind properties', such as a ‘perception of a red car', are not measureable in the CGS units of science when I can take a tape measure and go out and measure the length, width, and height of the car? I can put the car on a scale and weigh it and I can measure the speed at which it moves. So what are we talking about when we say ‘mind properties' are not measureable in the same physical CGS units?
An answer to this is that a person ‘perceives' an object (e.g., a red car) and ‘infers' that this ‘perception' is caused by an object outside of its body. The person's visual system detects the spectra and angles of the light reflecting from the car and, accordingly, it activates various neural pathways in the ‘brain' and a ‘perception of the red car' emerges in the ‘mind'. However, the ‘human mind' itself cannot be measured in the very units used to measure the physical basis of its perceptions. Thus, the measures of ‘brain’ and ‘mind’ (cause and effect) cannot be connected through a mathematical function as ‘cause' and ‘effect' relations are connected in all physical measurements throughout the universe. Thus, the units in which we can measure ‘brain functions' (i.e., nerve impulses, EEG patterns, transmitters, inhibitory and excitatory graded potentials, etc.) are all basic physical units or their derivatives, but the ‘mind phenomena'(perceptions/sensations) that may emerge such as erotic feelings including ‘love', ‘along with elated feelings', such as viewing a beautiful sunset, smelling a rose or listening to a moving Beethoven symphony, cannot be measured in these units, even though physical changes in body and brain which may be associated with these ‘mind phenomena' can be. Thus, nerve impulses, transmitters, inhibitory and excitatory nerve activity, patterns of EEG, etc. may well be associated, or the actual ‘cause', of a particular ‘mind phenomenon' (whether brief or continuous), but the array of ‘mind phenomena' themselves (the effects just described) are not measureable in the same CGS units in which one measures their associated neural changes.
When one writes about ‘mind' and ‘consciousness' and their dimensionless nature, one is at a loss for precise words, (e.g. nouns and verbs), to physically define a phenomenon (or phenomena) such as ‘mind perceptions' which are dimensionless in CGS units of measurement. How is one to physically describe and measure them? Specifically, you the reader, must realize that the processes you are going through while reading these lines and pondering on the definitions of ‘mind' and ‘consciousness' are precisely the ineffable phenomena we are talking about. We are conscious of our mind's experiences and they are as real as reality can be, and yet, because of their physically dimensionless nature, these experiences cannot be verbalized in the concrete language of scientific communication nor measured and described in CGS units and their derivatives.
In summary, "That which you, the reader, experience as a ‘mind phenomenon' (e.g., a ‘thought' or a ‘feeling' as you read this text, which is as real as reality can be) cannot be measured in the CGS units or its derivative measures of ‘Science'. However, that which causes, or is closely associated with your mind experience of a ‘thought' or ‘feeling' (for example, ‘nerve impulses') can be measured in the CGS and derivative measures of ‘Science', but cannot itself be directly experienced as a ‘mind phenomenon'. Stimulation of the brain, by whatever cause, may or may not initiate a ‘mind perception', but when and if it does, it is the ‘mind perception' and not the ‘neural activity' underlying it that is perceived. Thus, you and I are not aware of nerve impulses or their changes. What we are aware of is the result of a ‘transition’ from nerve impulse changes to a ‘mind experience.’”Citation39,Footnoted
All ‘cause' and ‘effect' relations in the physical universe are connected through dimensionally balanced equations of physical variables. Thus, when measuring ‘momentum', for example, both sides of the equation are measured in the same CGS units and their derivatives. However, when it comes to the relationship between a ‘mind phenomenon', such as a ‘thought' or ‘feeling' which can initiate ‘brain neural activity' or ‘vice-versa', their ‘cause' and ‘effect' relationship cannot both be measured in the same CGS measures and their derivatives as can be done in all other ‘cause' and ‘effect' relations in the physical universe.
The mystery of "labeled lines"
We know that ‘human brain activity' can initiate a variety of ‘human mind experiences' as noted by direct stimulation of our different sensory systems. It also has been shown that ‘human mind activity' can initiate specific ‘human brain activity' and other bodily changes. Libet addresses this and other issues related to it.Citation39 Two lines of evidence have shown that ‘thoughts associated with words' can initiate specific brain changes. Thus, ‘a verbal thought', such as ‘lift a glass of water', can initiate an EEG pattern of activity, and this pattern can be recorded and used to initiate a robotic device to carry out this function. This has been shown in humans.Citation40,41 It is useful for quadriplegics to use to initiate robotic device activity to meet their needs. This is a clear example of a ‘specific verbal thought' initiating ‘specific brain activity'. A second line of evidence also supports the notion that ‘specific verbal thoughts' can initiate brain and bodily changes. It is called the ‘placebo effect' and is a powerful example of the causal relation between ‘mind' and ‘brain', i.e., where ‘specific verbal thoughts' cause a change in ‘brain activity' leading to certain bodily function changes. It has been shown, for example, that telling someone that a certain ‘pill' (placebo) taken regularly over time will lower his/her blood pressure, and it does, shows that such ‘specific verbal thoughts' can produce a change in the ‘brain' followed by a ‘change in bodily function', (i.e., lowering of blood pressure).Citation42 At this point it is not clear how to distinguish a ‘specific verbal thought' from a ‘non-verbal mind phenomenon', such as, a ‘mood' or a ‘feeling'. Are these 2 types of mind experiences, (i.e., verbal and non-verbal), in the same category of a mind experience', or are they 2 separate classes of mind phenomena? This is yet to be determined. However, the results show that a ‘specific verbal thought' can initiate identifiable brain and bodily changes.
While still controversial as to its exact meaning, the expression ‘Labeled Lines' is as near as the field of Neuroscience can get at present to explaining the ‘cause' and ‘effect' relationship between ‘brain’ and ‘mind’. It generally is used to note how the exact same physical parameters of neurons and synapses, (i.e., nerve potentials, transmitters, etc.), in different sensory systems, can produce such radically different ‘mind experiences' ('sensations') as sight, sound, taste and smell. It has been said that if the primary ‘auditory' and ‘optic' nerves of the ‘12 cranial nerves' of the ‘head and neck' could be crossed, we would hear lightning and see thunder.Citation39 ‘Facial nerve #7' of the ‘12 cranial nerves' has sensory input of both ‘touch' and ‘taste' receptors. If these 2 sensory inputs were crossed, would the ‘mind experience' be the ‘touch of taste' and the ‘taste of touch'? Clearly ‘mind experiences' are remarkably different from their similar underlying nerve activity. Recent evidence even suggests that if a person is born ‘sightless', the visual cortex remains functional as a ‘visual site' and can learn to convert other sensory inputs, such as ‘sound', into a ‘visual-like' experience.Citation43
Conclusions
Based on any of the proposed models of the ‘phylogenetic tree of life', studies of "learning and memory" will need to be done to determine how such ‘behaviors and their changes', have evolved across the full phylogenetic spectrum—from single cell aneural organisms, such as bacteria and protozoa, to humans. It is suggested that for uniformity as to the meaning of the phrase "behavior and its change" across the full phylogenetic tree, such studies use the phrase, "effector measures," such as body movement, muscle contraction, glandular secretion or motor nerve/cell potential" changes that are either ‘self-initiated instrumental behavior' or a response to specific identifiable stimuli such as in ‘habituation and sensitization', and 'Pavlovian conditioning'.
‘Cell potential measures', such as transduction of a stimulus into a sensory receptor input and an effector system change, allow ‘cause’ and ‘effect’ to be measured in the same physical CGS units for simple effector system learning and memory in both neural and aneural systems across phylogeny.
In studies of ‘mind phenomena', the ‘causes’ in all of the sensory systems of the human brain can be measured in the same fundamental neural changes. However, the final ‘effect’ or ‘last transition', (i.e., the ‘mind phenomenon' itself" in each sensory system) such as the ‘color red', the ‘sound of a bell', the ‘taste of ice-cream', the ‘smell of a rose', ‘feeling of a cold temperature’, ‘the touch of silk' as well as mood changes, cannot be measured in these same units. However, the same broad approach to comparative studies that has been discussed for ‘behavioral learning and memory', and which include systems from aneural single cell organisms to humans, still needs to be pursued with respect to ‘comparative mind processes.’ After all, if any phylogenetic tree of life model regarding evolution is correct, then any questions regarding ‘mind processes' in humans, for example, must consider their evolution from single cell aneural organisms to humans, regardless of the present paradox of whether they can be scientifically measured. This keeps open the possibility of future insights into their measurability so that further exploration continues to be done over time.
When one examines the behaviors, and their changes, seen in eukaryotic protozoa, such as in Paramecia, Spirostoma and Stentor, for example, one observes an enormous range of behavioral choices in any given situation (see refs. Citation3, 4, 7, 8, 9, 10, 11, 16, 19, 24, 44). Thus, avoidance behavior, ciliary directional change behavior and contraction behavior are but a few of the range of behaviors and their changes that can occur at any given point in time depending on the situation. Such a range of ‘behavioral choices' would suggest ‘decision-making' or ‘mind-like processes' as in human beings. Thus, the evolution of such ‘mind-like processes' need to be considered and explored throughout phylogeny from aneural prokaryotes, such as ‘bacteria', as well as in protozoan eukaryotes, such as ‘Spirostomum' and 'Paramecium', as well as in ‘humans'.
If one accepts a ‘phylogenetic tree of life' model as truly representing the evolution of all life (animal and plant), then one must explore how to measure the major evolutionary phenomena of ‘learning and memory' observed throughout phylogeny. Do the learned behavioral changes of ‘habituation, sensitization, Pavlovian conditioning and instrumental learning' also occur in ‘plants' as well as in ‘organisms'? Can one use comparable metrics in both ‘plants' and ‘organisms' in order to compare the evolution of these behavioral phenomena throughout the ‘full' phylogenetic tree of life?
Since ‘plant-life' is part of Woese's full phylogenetic tree of life model as shown in , one must question, and explore, the extent to which all ‘attributes' of ‘behavior, learning, memory and mind', observed (or speculated about) across phylogeny in ‘organisms', are noted in ‘plant-life'.Citation3,44,Citation45,46,47,48 (Note the article by P.B. Applewhite on ‘plant and animal behavior' in ref. Citation3). Further exploration of ‘behavior', 'learning', 'memory' and 'mind' in ‘plants' is absolutely necessary in order to give full meaning to a ‘phylogenetic tree of life'.
Abbreviations
| CGS | = | One set of metrics used for physical measurements (Centimeters, Grams, Seconds) |
| CS | = | a conditional (mild) stimulus in Pavlovian (Classical) Conditioning |
| CT | = | computer-assisted tomography |
| EEG | = | Electroencephalogram |
| EMG | = | Electromyogram |
| FOR | = | Frame of Reference |
| GSR | = | Galvanic Skin Response |
| LTM | = | long-term memory |
| MRI | = | magnetic resonance imaging |
| PET | = | positron emission tomography |
| SCL | = | Skin Conductance Level (palmar) |
| STM | = | short-term memory |
| UCS | = | unconditioned stimulus in Pav. Cond |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are greatly indebted to Dr. Akiko Iida-Klein for her excellent work on formatting the figures for this paper, and for the important insights and comments of Dr. Herschel Knapp. Dr. Knapp is a Research Analyst/Statistician at Dignity Health in the Greater Los Angeles area and is involved in mental health care. We also would like to thank the United States Government through the Veterans Administration Greater Los Angeles Healthcare System for its support in the preparation of this paper.
Notes
a Another set of such fundamental metrics are the "MKS" (meter, kilogram, second) units. These metrics have been upgraded to include other measures and are now known as the "SI" units.
b The word ‘effector' can, for example, refer to a muscle contraction, glandular secretion or a body movement in an aneural protozoan, all of which may change in magnitude over trials.Citation9
c The transition from the neural activity in an activated sensory system, such as optical or auditory, to a 'mind perception' can be viewed as a ‘correlation' rather than a ‘cause and effect' relation. However, the evidence seen throughout the activity in any sensory system strongly suggests a ‘cause and effect' relation throughout the system.
d Many scientists are trying to study the "mind" and have wrestled with this "hard problem" as David Chalmers has dubbed it (ref. Citation48). We have tried to clarify in this paper what we believe is an important reason for the impasse.
References
- Woese CR. Bacterial evolution. Microbiol Rev 1987; 51(2):221-71.
- Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci 1990; 87:4576–9; http://dx.doi.org/10.1073/pnas.87.12.4576
- Eisenstein EM. (ed.). Aneural organisms in neurobiology. New York & London: Plenum Press, 1975.
- Wood DC. Electrophysiological correlates of the response decrement produced by mechanical stimuli in the protozoan, Stentor coeruleus. J Neurobiol 1970; 2:1–11; PMID:4333386; http://dx.doi.org/10.1002/neu.480020102
- Serway RA, Jewett JW Jr. Principles of Physics, 4th Ed., 2006. Brooks/Cole-Thomson Learning, Publisher.
- Stevens SS. Mathematics, Measurement, and Psychophysics. In: Stevens SS (ed.) Handbook of experimental psychology. Ch.1.New York: John Wiley and Sons, Inc., 1951: pp.1–49.
- Eisenstein EM, Eisenstein DL, Sarma JSM, Knapp H, Smith JC. Some new speculative ideas about the behavioral homeostasis theory as to how the simple learned behaviors of habituation and sensitization improve organism survival throughout phylogeny. Commun Integr Biol 2012 May1; 5(3):233–9; http://dx.doi.org/10.4161/cib.19480
- Eisenstein EM, Eisenstein D, Smith, James C. The evolutionary significance of habituation and sensitization across phylogeny: A behavioral homeostasis model. Integ Physiol Behav Sci 2001; 36(4):251–65; http://dx.doi.org/10.1007/BF02688794
- Hamilton TC, Thompson JM, Eisenstein EM. Quantitative analysis of ciliary and contractile responses during habituation training in Spirostomum ambiguum. J Behav Biol 1974; 12(3):393–407; http://dx.doi.org/10.1016/S0091-6773(74)91601-0
- Clark KB. Origins of learned reciprocity in solitary ciliates searching grouped 'courting' assurances at quantum efficiencies. Biosystems 2010; 99(1):27–41; http://dx.doi.org/10.1016/j.biosystems.2009.08.005
- Eisenstein EM, Eisenstein D. A behavioral homeostasis theory of habituation and sensitization: II. Further developments and predictions. Rev Neurosci 2006; 17:533–57; PMID:17180878; http://dx.doi.org/10.1515/REVNEURO.2006.17.5.533
- Groves PM, Thompson RF. A dual-process theory of habituation: Neural mechanisms. In: Peeke HVS, Herz MJ. (eds.), Habituation: physiological substrates. V2. New York & London: Academic Press, 1973: pp.175–205.
- Thompson RF, Berry SD, Rinaldi PC, Berger TW. Habituation and the orienting reflex: The dual-process theory revisited. In: International conference on orienting reflex in humans. Hillsdale, New Jersey: Lawrence Erlbaum Assoc., 1979: pp. 21-60.
- Eisenstein EM. Magnitude of conditioning as a function of CS-UCS interval: An attempt to condition several response systems concurrently. Ph.D. Thesis, UCLA (University of California, Los Angeles) USA, 1962.
- Eisenstein EM. Learning and memory in isolated insect ganglia. Advances Insect Physiol 1972; 9: 111–81; http://dx.doi.org/10.1016/S0065-2806(08)60276-3
- Eisenstein EM. The use of invertebrate systems for studies on the bases of learning and memory. In: Quarton GC, Melnechuk T, Schmitt FU (eds.), The Neurosciences—A study program. New York: Rockefeller University Press, 1967: pp. 653–665.
- Osgood CE. Method and theory in experimental psychology. New York: Oxford Univ. Press, 1953.
- Skinner BF. The behavior of organisms. An experimental analysis. New York: Appleton-Century-Crofts, Inc., 1938.
- Eisenstein EM. Selecting a model system for neurobiological studies of learning and memory. Beh Brain Res 1997; 82: 121–32; http://dx.doi.org/10.1016/S0166-4328(97)80982-5
- Eisenstein EM, Carlson AD, Harris JT. A ganglionic model of learned helplessness. Integr Physiol Behav Sci 1997; 32(3):265–71; http://dx.doi.org/10.1007/BF02688624
- Eisenstein EM. Assessing the influence of pharmacological agents on shock avoidance learning in simpler systems. Brain Res 1968; 11: 471–80; PMID:5712005; http://dx.doi.org/10.1016/0006-8993(68)90141-8
- Eisenstein EM. A comparison of 'activity' and 'position' response measures of avoidance learning in the cockroach, P. americana. Brain Res 1970; 21:143–7; PMID:5433110; http://dx.doi.org/10.1016/0006-8993(70)90031-4
- Eisenstein EM. Methods and material for investigating shock avoidance learning in the cockroach. Exp Physiol Biochem 1972; 5:257–72.
- Eisenstein EM, Lovell KL, Reep RL, Barraco DA, Brunder DG. Cellular and Behavioral studies of learning and memory in simpler systems. (Presentation at the 28th International Congress of Physiological Sciences, Budapest, 1980). In: Feher O, Joo F, (eds.), Cellular Analogues of conditioning and neural plasticity. Adv. Physiol. Sci., Pergamon Press, 1981; 36:263–73.
- Brady JV, Porter RW, Conrad G, Mason JW. Avoidance behavior and the development of gastro-duodenal ulcers. J Exp Anal Behav 1958; 1(1):69–72; http://dx.doi.org/10.1901/jeab.1958.1-69
- Horridge GA. Learning of leg position by the ventral nerve cord in headless insects. Proc Royal Soc B 1962; 157: 33–52; http://dx.doi.org/10.1098/rspb.1962.0061
- Eisenstein EM, Cohen MJ. Learning in an isolated prothoracic insect ganglion. Animal Behaviour 1965; 13(1):104–8; http://dx.doi.org/10.1016/0003-3472(65)90079-5
- Reep RL, Eisenstein EM, Tweedle CD. Neuronal pathways involved in transfer of information related to leg position learning in the cockroach, P. americana. Physiol Behav 1980; 24(3):501–13; http://dx.doi.org/10.1016/0031-9384(80)90244-9
- Harris JT. Learning in roach ganglia. Ph.D. Thesis, Tufts University, MA, USA, 1971.
- Eisenstein EM, Reep RL. Behavioral and cellular studies of learning and memory in insects. In: Kerkut, G.A., Gilbert, L.I. (eds.) Comprehensive insect physiology, biochemistry and pharmacology. Oxford: Pergamon Press, 1985; 9: 513–547.
- Harris JT, Eisenstein EM. Transfer of learned information between ganglia in the insect ventral nerve cord. Behav Brain Res 1999; 103: 211–7; PMID:10513589; http://dx.doi.org/10.1016/S0166-4328(99)00039-X
- Perkel DH, Bullock TH. Neural coding. Neurosci Res Progress Bulletin 1968; 6(3):221–348.
- Chen S, Cai D, Pearce K, Sun PY-W, Roberts AC, Glanzman DL. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia californica. Elife 2014; 3:e03896;http://dx.doi.org/10.7554/eLife.03896
- Eisenstein EM, Kemeny G, Skurnick ID, Harris JT. Transfer of information within the cockroach central nervous system during shock avoidance learning: Is there a nerve impulse code(s) for learned information? Internat. Journ. Neuroscience 1972; 3:93–8; PMID:4349280.
- Pak K-Y, Harris CL. Evidence for a molecular code for learned shock-avoidance in cockroaches. Comp Biochem Physiol 1975; 52A: 141–4.
- Eisenstein EM. The retention of shock avoidance learning in the cockroach, P. americana. Brain Res 1970; 21:148–50; PMID:5433111; http://dx.doi.org/10.1016/0006-8993(70)90032-6
- Crease RP, Mann CC. The second creation. New York: Macmillan Publ. Co., 1986.
- Greene B. Listening to the Big Bang. Smithsonian 2014 May; 45(2):19–26.
- Libet B. Mind time. Cambridge, MS: Harvard Univ. Press, 2004.
- Leuthardt EC, Gaona C, Sharma M, Szrama N, Roland J, Freudenberg Z, Solis J, Breshears J, Schalk G. Using the electrocorticographic speech network to control a brain-computer interface in humans. J Neural Eng 2011; 8(3):036004; http://dx.doi.org/10.1088/1741-2560/8/3/036004
- Reardon S. Disabled patients mind-meld with robots. Science Now 2011, Sept.6
- Miller NE, Dworkin BR. Critical issues in therapeutic applications of biofeedback. In: Schwartz GE, Beatty J. (eds.). Biofeedback, theory and research. New York: Acad. Press, 1977: Ch. Six, pp.129–161.
- Sumner T. Computer program allow the blind to "see with sound." Science Now, 2014, March 6.
- Kunita I, Kuroda S, Ohki K, Nakagaki T. Attempts to retreat from a dead-ended long capillary by backward swimming in Paramecium. Front Microbiol 2014; 5:270; PMID:24966852; http://dx.doi.org/10.3389/fmicb.2014.00270
- Spang A, Saw JH, Jergensen SL, Zavemba-Niedzwiedzka K, Martijn J, Lind AE, van Eijk R, Schieper C, Guy L, Ettema TJG. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 2015; 521(7551):173–9; PMID:25945739; http://dx.doi.org/10.1038/nature/14447
- Baluska F, Mancuso S. Deep evolutionary origins of neurobiology. Commun Integr Biol 2009 Jan-Feb; 2(1):60–5; http://dx.doi.org/10.4161/cib.2.1.7620
- Darwin CR. In: The power of movements in plants. London: John Murray, 1880.
- Chalmers DJ. Facing up to the problem of consciousness. J Consciousness Studies 1995; 2(3):200–19.
