ABSTRACT
The extracellular signal-regulated kinase (ERK1/2) cascade regulates a myriad of functions in multicellular organisms. Scaffold proteins provide critical spatial and temporal control over the specificity of signaling. Shoc2 is a scaffold that accelerates activity of the ERK1/2 pathway. Loss of Shoc2 expression in mice results in embryonic lethality, thus highlighting the essential role of Shoc2 in embryogenesis. In agreement, patients carrying mutated Shoc2 suffer from a wide spectrum of developmental deficiencies. Efforts to understand the mechanisms by which Shoc2 controls ERK1/2 activity revealed the intricate machinery that governs the ability of Shoc2 to transduce signals of the ERK1/2 pathway. Understanding the mechanisms by which Shoc2 contributes to a high degree of specificity of ERK1/2 signaling as well as deciphering the biological functions of Shoc2 in development and human disorders are major unresolved questions.
KEYWORDS:
Introduction
The biochemistry of the canonical extracellular-signal-regulated kinases (ERK1/2) pathway is well studied: growth factor receptors activate Ras GTPases that recruit Raf kinase to phosphorylate MEK (mitogen-activated protein kinase kinase) which in turn phosphorylates ERK.Citation1 However, it is unclear how the signal specificity is achieved within the wide biological responses controlled by the ERK pathway. Scaffolding proteins facilitate interactions and functions of core signaling partners of the ERK pathway and play indispensable roles in controlling the specific ERK1/2 activities. Examples of proteins that assemble the components of the pathway into scaffolding complexes include proteins like kinase suppressor of Ras (KSR), MEK partner 1 (MP1)-p14 (LAMTOR2/3) complex, IQGAP and Shoc2.Citation2
The first reference of Shoc2 in the literature is dated to the late 1990s, when Sur-8/Soc2, suppressor of clear, was isolated in C. elegans genetic screensCitation3-5 that identified several positive regulators of the universal ERK1/2 signaling pathway (e.g. sem-5, soc-1, and ksr-1).Citation6,7 Sur-8 had been predicted to function downstream of Ras and upstream of Raf and to possess no apparent catalytic activity.Citation3,4 Subsequent studies suggested that Sur-8/Shoc2 was capable of enhancing ERK1/2 activity by forming a ternary complex with Ras and Raf-1 kinase in response to stimuli from growth factorsCitation8 and showed that Shoc2 can interact with several Ras isoforms.Citation4 The critical role of Sur-8/Shoc2 in modulating ERK1/2 activity became fully appreciated when Rodriguez-Viciana and co-workers found that silencing of Shoc2 abrogates ERK1/2 activity in various cell types even in the presence of oncogenic Ras and Raf mutations.Citation9 Embryonic mice lethality due to severe heart defects induced by the ablation of Shoc2 and the identification of the Shoc2 mutation in Noonan-like RASopathy patients further emphasized the critical role of Shoc2 in embryonic development.Citation10,11 Clearly, the Shoc2 scaffold plays an essential role in modulating ERK1/2, but our understanding of the mechanism by which Shoc2 controls this pathway is limited. In the present article, we discuss the recent advances in our understanding of Shoc2 function and the mechanisms by which it modulates ERK1/2 signaling.
Domain organization and sequence conservation of Shoc2
Shoc2 is a 65 kDa protein expressed from humans to parasitic microorganisms with the 51% amino acids sequence similarity between P. falciparum and its human homolog. This striking conservation of Shoc2 implies that Shoc2 function is evolutionarily conserved as well.Citation12 All known Shoc2 orthologues are comprised of two major domains: the short, unstructured N-terminal domain followed by the long stretch of leucine-rich repeats (LRRs).Citation3,12 The well preserved N-terminal domain spans 85 amino acids in endothermic vertebrates and encompasses anywhere from 56 to 145 amino acids in other taxa.Citation12 Similar to other proteins that contain tandem arrays of multiple LRRs, Shoc2 is likely to form a horseshoe-shaped solenoid structure, which generates a platform holding several binding partners (i.e. PP1c, SCRIB, Erbin, HUWE1, PSMC5) (). The involvement of each known interacting partner in the regulation of Shoc2 function, assembly of the scaffolding complex, and/or subsequent control over the ERK1/2 signaling is discussed in following section. It is important to note that the last LRR contains a unique stretch of ∼20 amino acids that are responsible for the targeting of Shoc2 to late endosomes.Citation13,14
Figure 1. Schematic model depicting how ERK1/2 activity is transduced through the Shoc2 scaffold. Shoc2 routes Ras-Raf signals of the ERK1/2 pathway. (A) In the complex with Shoc2, PP1c dephosphorylates inhibitory Serine 259 of Raf-1 to facilitate signal transmission. (B) SCRIB competes with Shoc2 for PP1 binding thereby reducing ERK1/2 signaling. (C) Erbin disrupts the formation of Shoc2/Ras/Raf1 complexes to inhibit Shoc2-ERK1/2 signals. (D) Activation of the ERK1/2 pathway leads to an allosteric reversible ubiquitination of Shoc2 and, subsequently of Raf-1, by the E3 ligase HUWE1. HUWE1-mediated ubiquitination of Raf-1 fine-tunes its activity. In addition, ubiquitination triggers accumulation of Shoc2 complexes on the late endosomes/multi-vesicular bodies (LE/MVBs). On endosomes, Shoc2 complexes undergo remodeling by AAA+ ATPase PSMC5. The mechanoenzyme activity of this ATPases is utilized to reduce ubiquitination of Shoc2 and Raf-1 and, possibly, to reactivate the complex.
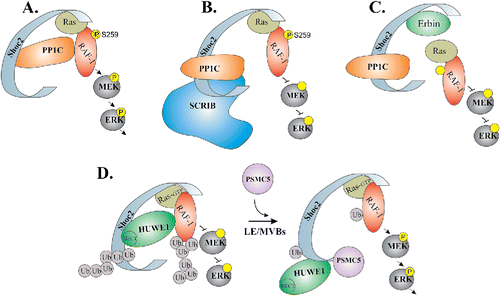
Shoc2 as a scaffolding protein - threshold control of ERK1/2 signaling
Initially, scaffolding properties have been inferred for Shoc2 due to the apparent absence of an enzymatic activity and its ability to tether Ras and Raf-1 in close proximity. This notion of Shoc2 being a molecular platform that recruits enzymes to fine-tune signals transmitted through the scaffolding complex has since been supported by the several following studies. One such enzyme that interacts with Shoc2 is the catalytic subunit of the protein phosphatase 1 (PP1c) ().Citation9 PP1c, known for its ability to cooperate with various regulatory proteins,Citation15 forms a holoenzyme with Shoc2. Owing to the high specificity of the Shoc2 interaction with M-Ras, the Shoc2-PP1c holoenzyme is targeted to stimulate Raf-1 kinase activity by dephosphorylating the S259 inhibitory site of Raf-1. The M-Ras/Shoc2/PP1c complex then activates Raf-1 recruited by other Ras proteins or Ras family GTPases.Citation9 It should be noted that the exclusivity of M-Ras binding to Shoc2 was challenged by others, and a consensus in regard to whether distinct Ras isoforms (H-Ras, K-Ras and N-Ras) form a direct complex with Shoc2 has yet to be reached. An unexpected feature of the M-Ras/Shoc2/PP1c complex was later revealed by Young et al. (2013), who showed that the M-Ras and Shoc2 dimer competes for PP1c binding with Scribbled Homolog (SCRIB) ().Citation16 SCRIB, a member of the LAP (LRR and PDZ) proteins family, is a known regulator of the ERK1/2 pathway.Citation17 Young et al. showed that SCRIB antagonizes Shoc2-mediated Raf-1 dephosphorylation through a mechanism involving competition for PP1 molecules within the same scaffolding complex. Competition of Shoc2 and SCRIB for PP1c binding allows for the sophisticated mechanisms controlling the frequency and amplitude of Shoc2-transduced ERK1/2 activity thereby affecting establishment of cell polarity and tumorigenic growth.Citation16
Erbin (also known as ERBB2IP) is another member of the LAP protein family and a Shoc2 interacting partner.Citation18,19 Analogous to SCRIB, Erbin is a suppressor of the growth factor-induced ERK1/2 activity. Erbin dampens ERK1/2 activity by sequestering Shoc2 and disrupting the Ras and Raf-1 interaction in keratinocytes and cardiomyocytes ().Citation19-21 Both SCRIB and Erbin appear to provide an essential mechanism to control signaling strength of Shoc2-ERK1/2 activation.
ERK1/2 signals transduced by Shoc2 are fine-tuned through Shoc2 partnering with the proteins of the ubiquitin machinery: the HECT-domain E3 ubiquitin ligase HUWE1 and the AAA+ ATPase PSMC5.Citation13,22 HUWE1 and PSMC5 provide mechanism for the negative feedback that modifies the amplitude of Shoc2-mediated ERK1/2 signaling through allosteric post-translation modifications (). Shoc2 ubiquitination is the first post-translational modification reported for Shoc2. The diversity of ubiquitin chains ligated to Shoc2 (K63, K48, K6, K11)Citation22 (unpublished data) indicates that this ubiquitination plays an active role in controlling Shoc2 function. Triggered by the activation of the ERK1/2 pathway, ubiquitination of Shoc2 by HUWE1 is a prerequisite for the subsequent ubiquitination of the Raf-1 kinase by the same E3 ligase.Citation22 The role of HUWE1 in controlling functions and levels of numerous essential proteins and cellular processes is reviewed in detail in Bernassola et al. 2008.Citation23 In the context of the Shoc2–Raf-1 scaffolding module, HUWE1 regulates the amplitude of ERK1/2 signal as well as the stability of both partners. Given that elevated levels of HUWE1 or somatic mutations have been found in several human pathologies,Citation24-27 it would be reasonable to expect that functions of Shoc2 and Raf-1, the dynamics of the ERK1/2 signaling pathway and the signaling network response are altered and contribute to these conditions.
An additional protein of the ubiquitin machinery involved in regulating Shoc2-mediated ERK1/2 signaling is the AAA+ ATPase (ATPase associated with diverse cellular activities) PSMC5. PSMC5 (rpt6 or Sug1), widely known for its essential role in the 19S proteasome,Citation28,29 also functions as an unfoldase/remodeler of APIS (AAA Proteins Independent of 20S) complexes.Citation30,31 In the Shoc2 assembly, PSMC5 has a unique role that does not involve control over stability of Shoc2 or its known partners. Here, PSMC5 governs the mechanism that controls dynamics of the Shoc2 transmitted ERK1/2 signals through remodeling of the Shoc2 complexes, sequestration of HUWE1 from the complex and modulating ubiquitination of Shoc2. Experiments using ATPase activity deficient mutants of PSMC5 demonstrated that ATP-fueled mechano-activity of PSMC5 is required for the remodeling of the Shoc2 complex in a spatially defined manner. Namely, PSMC5 remodels Shoc2 complexes and modulates ubiquitination of Shoc2 and Raf-1 when targeted to endosomes. By incorporating AAA+ PSMC5 unfoldase, Shoc2 complex acquires an additional layer of control over the amplitude of the ERK1/2 signals transmitted through the module. While HUWE1-mediated ubiquitination of Shoc2 and Raf-1 allows for the dynamic range of Raf-1 activity to be fine-tuned, PSMC5 controls the ability of HUWE1 to modify the noncatalytic scaffold Shoc2 thereby actively modulating the assembly of molecules in the complex.Citation13,22
The role of Shoc2 in various biological processes
Given that aberrations in the mechanisms that control timing and amplitude of ERK1/2 signals have critical implications for numerous cellular processes, several studies have explored the biological significance of Shoc2. As mentioned above, the loss of Sur-8/Shoc2 in C. elegans was not lethal.Citation3 While the total ablation of Shoc2 in mice led to an early embryonic lethality, its endothelial knock-out resulted in multiple cardiac defects (e.g., the reduced mesenchyme proliferation phase during the atrioventricular canal (AVC) development progressing to the formation of hypoplastic endocardial cushions) and late embryonic lethality.Citation10 Demonstrating an essential role of Shoc2 in embryogenesis, this study had a surprising conclusion: Shoc2 is able to regulate AVC development in an ERK-independent manner.Citation10 The role of Shoc2 in differentiation and proliferation of neuronal progenitor cells (NPCs) was examined using lentivirus-mediated Shoc2 knock-down.Citation32 In this model, Shoc2 acted as an anti-differentiation factor that stimulated ERK signals to NPCs proliferation and self-renewal.Citation32 The ability of Shoc2 to increase self-renewal was attributed to the cellular mechanisms controlling the protein stability of Shoc2. The authors of this study also suggested that Shoc2 might be a potential stem cell marker for NPCs.
Addressing the potential role of Shoc2 in tumorigenesis, several studies examined the ability of Shoc2 to increase ERK1/2 signals in various types of malignant cells (e.g. pancreatic, colon, breast and non-small cell lung carcinoma, melanoma, neuroblastoma, fibrosarcoma, and hepatoma cells).Citation9,33,34 Using siRNA silencing of Shoc2, Rodriguez-Viciana et al. demonstrated that Shoc2 was able to accelerate ERK1/2 activity even in cancer cells that carry tumorigenic K-Ras, N-Ras and B-Raf mutations.Citation9 While deciphering mechanisms responsible for the acquired resistance of melanomas to the B-Raf inhibitor vemurafenib (PLX4032), Kaplan et. al found that Shoc2 mediates reactivation of the ERK1/2 pathway in cells with constitutively active N-Ras.Citation35 Shoc2 facilitated melanoma-acquired vemurafenib resistance by altering signaling connections and re-routing oncogenic N-Ras signals to Raf-1.Citation35 Activity of ERK1/2 transduced by Shoc2 was shown to contribute to the malignant properties of different tumor cells via regulating contact inhibition, anchorage-independent proliferation and orientation of the microtubule-organizing center of these cells.Citation16 We showed that Shoc2 modulates ERK1/2 signals to cell motility and attachment, in part, through regulating expression of the protein of extracellular matrix - lectin galactoside-binding soluble 3-binding protein (LGALS3BP).Citation34,36 This secreted glycoprotein forms oligomers in the extracellular milieu and promotes cell adhesion to matrix proteins. High levels of LGALS3BP in serum and tumor tissue are associated with a shorter survival in patients with various malignancies.Citation37,38
Germline mutations in genes encoding proteins of the ERK1/2 pathway trigger RASopathies – developmental disorders characterized by distinct facial features, developmental delays, cardiac defects, growth delays, neurologic issues, gastrointestinal difficulties and predisposition to certain types of cancers.Citation39,40 In 2009 Cordeddu and coworkers reported that the c.4A>G missense mutation in the SHOC2 gene leads to a distinctive RASopathy termed Noonan-like syndrome with loose anagen hair.Citation11 c.4A>G, p.S2G substitution subsequently leads to a protein with the abnormally added myristate fatty acids that is aberrantly targeted to the plasma membrane. The first group of patients carrying c.4A>G mutation were reported to have an unusual combination of features where reduced growth associated with growth hormone deficiency, cognitive deficits, distinctive hyperactive behavior, and a unique hair anomaly (i.e., loose anagen hair). These findings were then expanded from distinctive craniofacial dysmorphisms and a wide spectrum of congenital heart defects to variable neurocognitive impairments, brain anomalies, epilepsy, severe hydrops fetalis and even Moyamoya syndrome, and also questioned the association of Shoc2 mutations with ‘loose anagen hair’ pointing to its speculative nature.Citation41-47 The biological complexity of Shoc2 S2G patients was explored using transcriptome analysis of peripheral blood mononuclear cells.Citation48 A large transcriptional signature characterized for the Shoc2 S2G mutation indicated a unique, Shoc2-specific route for the signaling it controls. Interestingly, the alterations in the expression of transcription factors (TFs) found in Shoc2 (S2G) patients had very little overlap with identities of TFs expression affected by the depletion of Shoc2, emphasizing the selective effect point mutation may have on ERK1/2 activity.Citation34,48
A different Shoc2 substitution (c.519G>A; p.M173I) was recently reported by Hannig et al. (2014) in patients with the mild Noonan-like RASopathy.Citation49 De-novo occurrence of this Shoc2 mutation and a typical clinical phenotype suggested the mutation to be pathogenic, while the analysis of the molecular mechanisms underlying the Noonan-like pathology pointed to an impaired ability of the Shoc2 M173I mutant to interact with PP1C. It is worth mentioning that a similar substitution of the methionine 173 (c. 517A>G, p.M173V) was found in Noonan syndrome patient by the Kuechler et al.,Citation50 but no underlying molecular mechanisms were proposed. Recognizing a clinical variability in patients with the Shoc2-linked RASopathy, it is likely that individuals with no molecularly confirmed mutations carry different or already known Shoc2 substitutions.Citation51
Conclusions and closing remarks
Scaffolding proteins are reported to have critical roles in an increasing number of biological signaling processes.Citation52 Some of these scaffolds show surprising dynamics/diversity in the ways in which they bring multiple binding partners together to facilitate their interactions and functions that are beyond a simple tethering mechanism to increase the efficiency of interaction.Citation53
Initial insights regarding the structural and functional studies of Shoc2 have started to expose the intricacy of the mechanisms controlling function of the scaffold and assembly of the complex. Advances made in the past few years have emphasized the central position of Shoc2 in the ERK1/2 pathway and cellular processes it controls. Nevertheless, several gaps remain to be filled in order to understand the intricacy of the Shoc2 machinery and biological activities controlled by the module. First, it would be important to identify all the proteins assisting in the process of the Shoc2 scaffold assembly and remodeling. Moreover, there is the question of the stimuli that activate assembly and control subcellular distribution of the Shoc2 scaffolding complex. The final effects of Shoc2 and Raf-1 ubiquitination are yet to be understood. Further research regarding the possible contribution of Shoc2 interactions to skin diseases, like psoriasis, or cardiac hypertrophy as well as more insights on Shoc2-ERK1/2 physiological substrates and cellular outcomes will determine whether Shoc2 is an attractive therapeutic target. Finally, additional studies of Shoc2 will provide fundamental insights into our understanding of the mechanisms by which other scaffolds may be controlled.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs. Matthew Gentry, Stacy Smith and members of Galperin lab for critical reading of the manuscript and helpful discussions.
Funding
Support for this work was provided by the National Cancer Institute (R00CA126161 to EG), the National Institute of General Medical Sciences (R01GM113087 to EG) and the American Cancer Society (RSG-14-172-01-CSM to EG). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIGMS.
References
- Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol 1999; 71:479-500; PMID:10354710; http://dx.doi.org/10.1016/S0079-6107(98)00056-X
- Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal 2009; 21:462-9; PMID:19091303; http://dx.doi.org/10.1016/j.cellsig.2008.11.013
- Sieburth DS, Sun Q, Han M. SUR-8, a conserved Ras-binding protein with leucine-rich repeats, positively regulates Ras-mediated signaling in C. elegans. Cell 1998; 94:119-30; PMID:9674433; http://dx.doi.org/10.1016/S0092-8674(00)81227-1
- Li W, Han M, Guan KL. The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf. Genes Dev 2000; 14:895-900; PMID:10783161.
- Selfors LM, Schutzman JL, Borland CZ, Stern MJ. soc-2 encodes a leucine-rich repeat protein implicated in fibroblast growth factor receptor signaling. Proc Natl Acad Sci U S A 1998; 95:6903-8; PMID:9618511; http://dx.doi.org/10.1073/pnas.95.12.6903
- Eastburn DJ, Han M. Ras Signaling In C. Elegans, in RAS Family GTPases 2006; 199-225.
- Vikis H, Guan KL. Regulation of the Ras-MAPK pathway at the level of Ras and Raf. Genet Eng (N Y) 2002; 24:49-66; PMID:12416300; http://dx.doi.org/10.1007/978-1-4615-0721-5_3
- Matsunaga-Udagawa R, Fujita Y, Yoshiki S, Terai K, Kamioka Y, Kiyokawa E, Yugi K, Aoki K, Matsuda M. The scaffold protein Shoc2/SUR-8 accelerates the interaction of Ras and Raf. J Biol Chem 2010; 285:7818-26; PMID:20051520; http://dx.doi.org/10.1074/jbc.M109.053975
- Rodriguez-Viciana P, Oses-Prieto J, Burlingame A, Fried M, McCormick F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol Cell 2006; 22:217-30; PMID:16630891; http://dx.doi.org/10.1016/j.molcel.2006.03.027
- Yi J, Chen M, Wu X, Yang X, Xu T, Zhuang Y, Han M, Xu R. Endothelial SUR-8 acts in an ERK-independent pathway during atrioventricular cushion development. Dev Dynamics 2010; 239:2005-13; http://dx.doi.org/10.1002/dvdy.22343
- Cordeddu V, Di Schiavi E, Pennacchio LA, Ma'ayan A, Sarkozy A, Fodale V, Cecchetti S, Cardinale A, Martin J, Schackwitz W, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genetics 2009; 41:1022-6; PMID:19684605; http://dx.doi.org/10.1038/ng.425
- Jeoung M, Abdelmoti L, Jang ER, Vander Kooi CW, Galperin E. Functional Integration of the Conserved Domains of Shoc2 Scaffold. PloS one 2013; 8:e66067; PMID:23805200; http://dx.doi.org/10.1371/journal.pone.0066067
- Jang ER, Jang H, Shi P, Popa G, Jeoung M, Galperin E. Spatial control of Shoc2-scaffold-mediated ERK1/2 signaling requires remodeling activity of the ATPase PSMC5. J Cell Sci 2015; 128:4428-41; PMID:26519477; http://dx.doi.org/10.1242/jcs.177543
- Galperin E, Abdelmoti L, Sorkin A. Shoc2 is targeted to late endosomes and required for Erk1/2 activation in EGF-stimulated cells. PloS One 2012; 7:e36469; PMID:22606262; http://dx.doi.org/10.1371/journal.pone.0036469
- Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 2010; 35:450-8; PMID:20399103; http://dx.doi.org/10.1016/j.tibs.2010.03.002
- Young LC, Hartig N, Muñoz-Alegre M, Oses-Prieto JA, Durdu S, Bender S, Vijayakumar V, Vietri Rudan M, Gewinner C, Henderson S, et al. An MRAS, SHOC2, and SCRIB complex coordinates ERK pathway activation with polarity and tumorigenic growth. Mol Cell 2013; 52:679-92; PMID:24211266; http://dx.doi.org/10.1016/j.molcel.2013.10.004
- Elsum IA, Martin C, Humbert PO. Scribble regulates an EMT polarity pathway through modulation of MAPK-ERK signaling to mediate junction formation. J Cell Sci 2013; 126:3990-9; PMID:23813956; http://dx.doi.org/10.1242/jcs.129387
- Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adélaïde J, Margolis B, Birnbaum D. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol 2000; 2:407-14; PMID:10878805; http://dx.doi.org/10.1038/35017038
- Dai P, Xiong WC, Mei L. Erbin inhibits RAF activation by disrupting the sur-8-Ras-Raf complex. J Biol Chem 2006; 281:927-33; PMID:16301319; http://dx.doi.org/10.1074/jbc.M507360200
- Harmon RM, Simpson CL, Johnson JL, Koetsier JL, Dubash AD, Najor NA, Sarig O, Sprecher E, Green KJ. Desmoglein-1/Erbin interaction suppresses ERK activation to support epidermal differentiation. J Clin Invest 2013; 123:1556-70; PMID:23524970; http://dx.doi.org/10.1172/JCI65220
- Rachmin I, Tshori S, Smith Y, Oppenheim A, Marchetto S, Kay G, Foo RS, Dagan N, Golomb E, Gilon D, et al. Erbin is a negative modulator of cardiac hypertrophy. Proc Natl Acad Sci U S A 2014; 111:5902-7; PMID:24711380; http://dx.doi.org/10.1073/pnas.1320350111
- Jang ER, Shi P, Bryant J, Chen J, Dukhande V, Gentry MS, Jang H, Jeoung M, Galperin E. HUWE1 is a molecular link controlling RAF-1 activity supported by the Shoc2 scaffold. Mol Cell Biol 2014; 34(19):3579-93; PMID:25022756; http://dx.doi.org/10.1128/MCB.00811-14
- Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 2008; 14:10-21; PMID:18598940; http://dx.doi.org/10.1016/j.ccr.2008.06.001
- Yoon SY, Lee Y, Kim JH, Chung AS, Joo JH, Kim CN, Kim NS, Choe IS, Kim JW. Over-expression of human UREB1 in colorectal cancer: HECT domain of human UREB1 inhibits the activity of tumor suppressor p53 protein. Biochem Biophys Res Commun 2005; 326:7-17; PMID:15567145; http://dx.doi.org/10.1016/j.bbrc.2004.11.004
- Confalonieri S, Quarto M, Goisis G, Nuciforo P, Donzelli M, Jodice G, Pelosi G, Viale G, Pece S, Di Fiore PP. Alterations of ubiquitin ligases in human cancer and their association with the natural history of the tumor. Oncogene 2009; 28:2959-68; PMID:19543318; http://dx.doi.org/10.1038/onc.2009.156
- Isrie M, Kalscheuer VM, Holvoet M, Fieremans N, Van Esch H, Devriendt K. HUWE1 mutation explains phenotypic severity in a case of familial idiopathic intellectual disability. Eur J Med Genet 2013; 56:379-82; PMID:23721686; http://dx.doi.org/10.1016/j.ejmg.2013.05.005
- Kurokawa M, Kim J, Geradts J, Matsuura K, Liu L, Ran X, Xia W, Ribar TJ, Henao R, Dewhirst MW, et al. A network of substrates of the E3 ubiquitin ligases MDM2 and HUWE1 control apoptosis independently of p53. Sci Signal 2013; 6:ra32; PMID:23652204.
- Bar-Nun S, Glickman MH. Proteasomal AAA-ATPases: structure and function. Biochim Biophys Acta 2012; 1823:67-82; PMID:21820014; http://dx.doi.org/10.1016/j.bbamcr.2011.07.009
- Tian G, Park S, Lee MJ, Huck B, McAllister F, Hill CP, Gygi SP, Finley D. An asymmetric interface between the regulatory and core particles of the proteasome. Nat Struct Mol Biol 2011; 18:1259-67; PMID:22037170; http://dx.doi.org/10.1038/nsmb.2147
- Gonzalez F, Delahodde A, Kodadek T, Johnston SA. Recruitment of a 19S proteasome subcomplex to an activated promoter. Science 2002; 296:548-50; PMID:11964484; http://dx.doi.org/10.1126/science.1069490
- Sun L, Johnston SA, Kodadek T. Physical association of the APIS complex and general transcription factors. Biochem Biophys Res Commun 2002; 296:991-9; PMID:12200147; http://dx.doi.org/10.1016/S0006-291X(02)02026-0
- Moon BS, Kim HY, Kim MY, Yang DH, Lee JM, Cho KW, Jung HS, Choi KY. Sur8/Shoc2 involves both inhibition of differentiation and maintenance of self-renewal of neural progenitor cells via modulation of extracellular signal-regulated kinase signaling. Stem cells 2011; 29:320-31; PMID:21732489; http://dx.doi.org/10.1002/stem.586
- Kaduwal S, Jeong WJ, Park JC, Lee KH, Lee YM, Jeon SH, Lim YB, Min do S, Choi KY. Sur8/Shoc2 promotes cell motility and metastasis through activation of Ras-PI3K signaling. Oncotarget 2015; 6(32):33091-105; PMID:26384305.
- Jeoung M, Jang ER, Liu J, Wang C, Rouchka EC, Li X, Galperin E. Shoc2-tranduced ERK1/2 motility signals - Novel insights from functional genomics. Cell Signal 2016; 28:448-59; PMID:26876614; http://dx.doi.org/10.1016/j.cellsig.2016.02.005
- Kaplan FM, Kugel CH, 3rd, Dadpey N, Shao Y, Abel EV, Aplin AE. SHOC2 and CRAF mediate ERK1/2 reactivation in mutant NRAS-mediated resistance to RAF inhibitor. J Biol Chem 2012; 287:41797-807; PMID:23076151; http://dx.doi.org/10.1074/jbc.M112.390906
- Rouchka EC, Jeoung M, Jang ER, Liu J, Wang C, Li X, Galperin E. Data set for transcriptional response to depletion of the Shoc2 scaffolding protein. Data Brief 2016; 7:770-8; PMID:27077079; http://dx.doi.org/10.1016/j.dib.2016.03.012
- Traini S, Piccolo E, Tinari N, Rossi C, La Sorda R, Spinella F, Bagnato A, Lattanzio R, D'Egidio M, Di Risio A, et al. Inhibition of tumor growth and angiogenesis by SP-2, an anti-lectin, galactoside-binding soluble 3 binding protein (LGALS3BP) antibody. Mol Cancer Ther 2014; 13:916-25; PMID:24552775; http://dx.doi.org/10.1158/1535-7163.MCT-12-1117
- Piccolo E, Tinari N, D'Addario D, Rossi C, Iacobelli V, La Sorda R, Lattanzio R, D'Egidio M, Di Risio A, Piantelli M, et al. Prognostic relevance of LGALS3BP in human colorectal carcinoma. J Transl Med 2015; 13:248; PMID:26219351; http://dx.doi.org/10.1186/s12967-015-0606-x
- Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet 2013; 14:355-69; PMID:23875798; http://dx.doi.org/10.1146/annurev-genom-091212-153523
- Tidyman WE, Rauen KA. Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway. Expert Rev Mol Med 2008; 10:e37; PMID:19063751; http://dx.doi.org/10.1017/S1462399408000902
- Baldassarre G, Mussa A, Banaudi E, Rossi C, Tartaglia M, Silengo M, Ferrero GB. Phenotypic variability associated with the invariant SHOC2 c.4A>G (p.Ser2Gly) missense mutation. Am J Med Genet A 2014; 164A:3120-5; PMID:25331583; http://dx.doi.org/10.1002/ajmg.a.36697
- Gripp KW, Zand DJ, Demmer L, Anderson CE, Dobyns WB, Zackai EH, Denenberg E, Jenny K, Stabley DL, Sol-Church K. Expanding the SHOC2 mutation associated phenotype of Noonan syndrome with loose anagen hair: structural brain anomalies and myelofibrosis. Am J Med Genet A 2013; 161:2420-30.
- Hoban R, Roberts AE, Demmer L, Jethva R, Shephard B. Noonan syndrome due to a SHOC2 mutation presenting with fetal distress and fatal hypertrophic cardiomyopathy in a premature infant. Am J Medical Genetics. Part A 2012; 158A:1411-3; http://dx.doi.org/10.1002/ajmg.a.35318
- Gargano G, Guidotti I, Balestri E, Vagnarelli F, Rosato S, Comitini G, Wischmeijer A, La Sala GB, Iughetti L, Cordeddu V, et al. Hydrops fetalis in a preterm newborn heterozygous for the c.4A>G SHOC2 mutation. Am J Med Genet A 2014; 164A(4):1015-20; PMID:24458587; http://dx.doi.org/10.1002/ajmg.a.36376
- Capalbo D, Scala MG, Melis D, Minopoli G, Improda N, Palamaro L, Pignata C, Salerno M. Clinical heterogeneity in two patients with noonanlike syndrome associated with the same SHOC2 mutation. Ital J Pediatr 2012; 38:48; PMID:22995099; http://dx.doi.org/10.1186/1824-7288-38-48
- Choi JH, Oh MY, Yum MS, Lee BH, Kim GH, Yoo HW. Moyamoya syndrome in a patient with Noonan-like syndrome with loose anagen hair. Pediatr Neurol 2015; 52:352-5; PMID:25563136; http://dx.doi.org/10.1016/j.pediatrneurol.2014.11.017
- Lo FS, Wang CJ, Wong MC, Lee NC. Moyamoya disease in two patients with Noonan-like syndrome with loose anagen hair. Am J Med Genet A 2015; 167:1285-8; PMID:25858597; http://dx.doi.org/10.1002/ajmg.a.37053
- Ferrero GB, Picco G, Baldassarre G, Flex E, Isella C, Cantarella D, Corà D, Chiesa N, Crescenzio N, Timeus F, et al. Transcriptional hallmarks of Noonan syndrome and Noonan-like syndrome with loose anagen hair. Hum Mutat 2012; 33:703-9; PMID:22253195; http://dx.doi.org/10.1002/humu.22026
- Hannig V, Jeoung M, Jang ER, Phillips JA 3rd, Galperin E. A Novel SHOC2 Variant in Rasopathy. Hum Mutat 2014; 35(11):1290-4; PMID:25137548.
- RE(ACT)®: International congress on research on rare and orphan diseases. Mol Syndromol 2012; 2:262-288; PMID:22670144
- Digilio MC, Romana Lepri F, Dentici ML, Henderson A, Baban A, Roberti MC, Capolino R, Versacci P, Surace C, Angioni A, et al. Atrioventricular canal defect in patients with RASopathies. Eur J Hum Genet 2013; 21:200-4; PMID:22781091; http://dx.doi.org/10.1038/ejhg.2012.145
- Langeberg LK, Scott JD. Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol 2015; 16:232-44; PMID:25785716; http://dx.doi.org/10.1038/nrm3966
- Garbett D, Bretscher A. The surprising dynamics of scaffolding proteins. Mol Biol Cell 2014; 25:2315-9; PMID:25122925; http://dx.doi.org/10.1091/mbc.E14-04-0878
