ABSTRACT
The small GTPase Rab proteins are key regulators of membrane trafficking. Rab11 is one of the best-characterized molecules among the Rab family proteins and it plays multiple roles in endocytic recycling, exocytosis, and cytokinesis. However, it remains unclear how Rab11 is activated at a precise timing and location and regulates its diverse functions. Specifically, our knowledge of the upstream regulatory factors that activate Rab11 is limited. In this regard, we have identified the RAB-11-interacting protein-1 (REI-1) as a novel guanine nucleotide exchange factor (GEF) for RAB-11 in Caenorhabditis elegans (C. elegans). REI-1 family proteins are conserved among metazoans, and its human homolog, SH3BP5, also exhibits strong GEF activity toward human Rab11. In C. elegans, REI-1 is expressed in the germline and co-localizes with RAB-11 on late-Golgi membranes. The loss of REI-1 impaired the targeting of RAB-11 to the late-Golgi compartment, as well as the recycling endosomes in embryos and further reduced the recruitment of RAB-11 to the cleavage furrow, resulting in the delay of cytokinesis. We suggest that REI-1 is the GEF responsible for regulating RAB-11 localization and function in early embryos.
Rab proteins are evolutionarily conserved, small (21–25 kDa), monomeric GTPases, which switch their conformation between a GTP-bound active state and a GDP-bound inactive state.Citation1 Guanine nucleotide exchange factors (GEFs) are upstream regulators promoting the exchange of GDP with GTP on Rab proteins, while GTPase activating proteins (GAPs) facilitate Rab GTPase activity. In their active state, Rab proteins are targeted to specific membrane compartments and act together with downstream effectors thereby exerting their biological effects.Citation2 Rab11 is a ubiquitously expressed member of the Ypt/Rab gene family. Rab11 localizes to recycling endosomes (REs), the trans-Golgi network, and post-Golgi vesicles, whereby it regulates a variety of biological processes, including endocytic recycling, secretion, cell motility, and cytokinesis.Citation3 Although Rab11 is one of the best-studied Rab GTPases, the upstream mechanisms by which Rab11 is activated remain unclear.
REI-1 family proteins are conserved GEFs for Rab11
We have previously shown that C. elegans RAB-11.1, a homolog of human Rab11a, dynamically changes its localization and performs essential functions during the oocyte-to-embryo transition.Citation4 To investigate the molecular mechanism by which RAB-11.1 is activated, we searched for RAB-11.1 binding proteins using a mutant form of RAB-11.1 (S25N), which mimics GDP-bound RAB-11.1, and successfully identified RAB-11-interacting protein-1 (REI-1) in C. elegans (). The C. elegans genome also contains a rei-1 homolog, namely rei-2, and its gene product, REI-2, was also found to interact with RAB-11.1 (). REI-1 and REI-2 also strongly interacted with a nucleotide-free mutant of RAB-11.1 (N124I). These binding patterns were reminiscent of the properties of known Rab GEF proteins.Citation2 Based on this, we hypothesized that REI-1 is a GEF for RAB-11.1. Consistent with this hypothesis, REI-1 showed a strong GDP-GTP exchange activity toward RAB-11.1 in vitro. Importantly, REI-1 possessed GEF activity only in the presence of liposomes. We also found that REI-1 has the ability to bind liposomes, suggesting that the interaction of REI-1 with membranes modulates its GEF activity.
Figure 1. (A) Domain structure of the REI-1 family of proteins. Our results show that almost full-length REI-1 (1-228 aa) is required for RAB-11.1 binding. (B) A model describing the role of REI-1 in RAB-11 activation and translocation. REI-1 activates RAB-11, by exchange of GDP with GTP, principally at late-Golgi membranes. RAB-11-positive post-Golgi vesicles or compartments are then targeted to the cell cortex and cleavage furrow to regulate cytokinesis and membrane traffic.
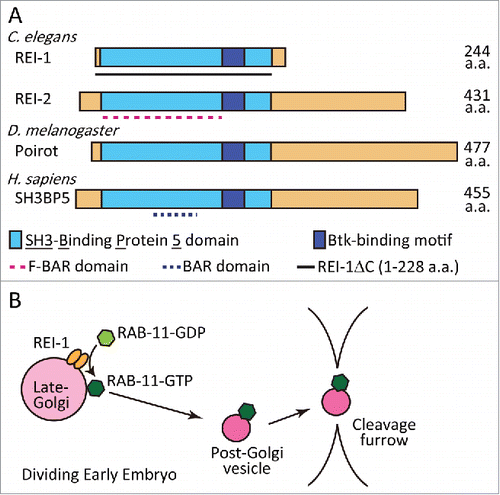
Since REI-1 and REI-2 form a protein family that is well conserved in metazoans, including DrosophilaCitation5 and humanCitation6 (), we further examined whether the molecular function of REI-1 is conserved in its mammalian homolog, namely SH3-binding protein 5 (SH3BP5). As expected, SH3BP5 interacted with the GDP-bound and nucleotide-free forms of human Rab11a and possessed a strong GEF activity toward human Rab11a. Interestingly, the REI-1 family proteins do not possess any domains with sequence similarity to known Rab-GEF domains, such as the DENN and Vps9 domains.Citation2 However, REI-2 and SH3BP5 do exhibit a sequence similarity to BAR and F-BAR domains, respectively. These domains potentially function as modules for dimerization, lipid binding, and membrane-curvature sensing.Citation7 In fact, REI-1 interacts with itself in a homophilic manner and directly binds to membranes. These observations suggest that the BAR/F-BAR domains may function to target REI-1 family proteins to a specific compartment where they activate Rab11.
REI-1 regulates RAB-11.1 localization and function in C. elegans embryos
We further addressed the mechanism by which REI-1 regulates the diverse functions of RAB-11.1 in vivo. In growing oocytes, RAB-11.1 is found to localize on REs as well as on the Golgi and it regulates yolk receptor recycling.Citation4,8 When oocytes mature, RAB-11.1 is targeted to the cortical granules (CGs) and regulates CG exocytosis after fertilization, contributing to proper egg shell formation.Citation4,9 In embryos, RAB-11.1 redistributes to the Golgi and RE.Citation10 Our results show that REI-1 is expressed in germline cells and co-localizes with RAB-11.1 on late-Golgi membranes. The deletion of the rei-1 and rei-2 genes did not strongly affect RAB-11.1 localization in oocytes, yolk uptake by oocytes or CG exocytosis in zygotes. In contrast, in early embryos, targeting of RAB-11.1 to the late-Golgi compartment and recycling endosomes was significantly impaired in rei-1 mutants and this defect was enhanced by rei-2 mutation. These results suggest that REI-1 and REI-2 specifically regulate RAB-11.1 localization in early embryos.
In C. elegans embryos, RAB-11.1 also localizes to the cleavage furrow during cell division, and plays an essential role in cytokinesis, a phenomenon that has also been previously shown in mammals.Citation11 RNAi-treated rab-11.1 embryos fail to complete cytokinesis.Citation10 In rei-1 mutant embryos, ingression occurred but RAB-11.1 was not targeted to the late-Golgi or the cleavage furrow, which resulted in delayed cytokinesis, and this defect was enhanced by rei-2 mutation. In wild-type embryos, REI-1 itself and a late-Golgi marker SYN-16 were not recruited to the cleavage furrow. Based on this, we propose a model whereby REI-1-dependent activation of RAB-11.1 on the late-Golgi compartment is a prerequisite for the targeting of RAB-11.1 to the cleavage furrow thereby facilitating its involvement in cytokinesis ().
Our results also indicate that RAB-11.1 is able to bind membranes even in the absence of its GEFs, REI-1 and REI-2. This is consistent with a previous report on a yeast Rab-GEF mutant.Citation12 This observation could potentially explain why the phenotypes of rei-1 and rei-2 mutants are milder than that of the rab-11.1 RNAi.Citation10 Alternatively, another unidentified potential GEF could partially activate RAB-11.1 even in rei-1 and rei-2 mutants. Additional analysis using a deletion series of the REI-1 protein suggests that almost full-length of REI-1 is required for its Rab11-binding ability ().
It has been reported that human Rab11 is involved in a variety of disease settings including cancer progression.Citation3,13 Therefore, as REI-1 family proteins regulate the activation of Rab11, the actions of these GEFs may present novel targets for therapeutic invention. More extensive studies of the REI/SH3BP5 family proteins could uncover molecular mechanisms of tissue-specific and spatiotemporal regulation of Rab11 and will likely prompt further research in the field of membrane trafficking.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Ann Rev Biochem 2012; 81:637-59; PMID:22463690; http://dx.doi.org/10.1146/annurev-biochem-052810-093700
- Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol 2010; 22:461-70; PMID:20466531; http://dx.doi.org/10.1016/j.ceb.2010.04.007
- Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol 2014; 24:407-15; PMID:24675420; http://dx.doi.org/10.1016/j.tcb.2014.02.004
- Sato M, Grant BD, Harada A, Sato K. Rab11 is required for synchronous secretion of chondroitin proteoglycans after fertilization in Caenorhabditis elegans. J Cell Sci 2008; 121:3177-86; PMID:18765566; http://dx.doi.org/10.1242/jcs.034678
- Sinka R, Jankovics F, Somogyi K, Szlanka T, Lukacsovich T, Erdelyi M. poirot, a new regulatory gene of Drosophila oskar acts at the level of the short Oskar protein isoform. Development 2002; 129:3469-78; PMID:12091316
- Matsushita M, Yamadori T, Kato S, Takemoto Y, Inazawa J, Baba Y, et al. Identification and characterization of a novel SH3-domain binding protein, Sab, which preferentially associates with Bruton's tyrosine kinase (BtK). Biochem Biophys Res Commun 1998; 245:337-43; http://dx.doi.org/10.1006/bbrc.1998.8420
- Qualmann B, Koch D, Kessels MM. Let's go bananas: revisiting the endocytic BAR code. EMBO J 2011; 30:3501-15; PMID:21878992; http://dx.doi.org/10.1038/emboj.2011.266
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell 1999; 10:4311-26; PMID:10588660; http://dx.doi.org/10.1091/mbc.10.12.4311
- Sato K, Sato M, Audhya A, Oegema K, Schweinsberg P, Grant BD. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol Biol Cell 2006; 17:3085-94; PMID:16672374; http://dx.doi.org/10.1091/mbc.E06-03-0211
- Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol 2001; 11:735-46; PMID:11378383; http://dx.doi.org/10.1016/S0960-9822(01)00231-7
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, et al. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell 2005; 16:849-60; PMID:15601896; http://dx.doi.org/10.1091/mbc.E04-10-0927
- Cabrera M, Ungermann C. Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases. J Biol Chem 2013; 288:28704-12; http://dx.doi.org/10.1074/jbc.M113.488213
- Bhuin T, Roy JK. Rab11 in disease progression. Int J Mol Cell Med 2015; 4:1-8; PMID:25815277
