ABSTRACT
Catalases are ubiquitous enzymes that detoxify H2O2 in virtually all organisms exposed to oxygen. The filamentous, nitrogen-fixing cyanobacterium, Anabaena PCC 7120, shows the presence of 2 genes (katA and katB) that encode Mn-catalases. We have recently shown that pre-treatment of Anabaena with NaCl causes substantial induction of the KatB protein, which consequently leads to increased oxidative stress resistance in that cyanobacterium. Interestingly, when compared to the wild-type, the katB mutant shows decreased growth and impaired photosynthetic activity in the presence of NaCl. Furthermore, the NaCl-treated katB mutant is extremely sensitive to H2O2. In this study, the ultrastructural changes occurring in the katB mutant and the wild-type Anabaena cells are analyzed to understand the cellular basis of the above-mentioned protective phenomena. Other data show that a wide variety of osmolytes induce katB expression in Anabaena, indicating that katB is a genuine osmo-inducible gene. These results have important biotechnological implications for the development of novel cyanobacterial biofertilzers and transgenic plants with improved resistance to salinity.
The presence of oxygen is a double-edged sword for aerobes. The transfer of electrons through the respiratory chain to oxygen, that drives the production of ATP, also results in the concomitant generation of the deleterious reactive oxygen species (ROS) such as the superoxide radical (O2·), hydrogen peroxide (H2O2) or the hydroxyl radical (·OH).Citation1 In living systems, H2O2 forms the veritable connection between O2· and ·OH. Although, H2O2 is produced directly by several oxidases, the dismutation of O2· by superoxide dismutase (SOD) is the major source of H2O2 production in cells.Citation2 However, though not very reactive by itself, H2O2 can very quickly become the key ROS that imperils cell's survival. In the presence of iron, an essential micronutrient, H2O2 reacts with Fe2+ to generate the ·OH, which can damage all the cellular macromolecules at diffusion-controlled rates.Citation3 Thus, decomposition of H2O2 is very pertinent for cellular existence.
Detoxification of H2O2 is primarily brought about by peroxidases and catalases. Peroxidases (e.g. ascorbate peroxidase, peroxiredoxins, etc.) require electron-donating reducing agents as accessories to decompose H2O2; whereas catalases directly detoxify H2O2 by a dismutation reaction, forming H2O and molecular oxygen in the process.Citation4 Catalases, essentially are of 2 types viz; heme catalases or manganese (Mn)-catalases. The typical monofunctional catalase and the catalase-peroxidase (i.e. KatG) contain heme whereas Mn-catalases lack heme, but contain Mn at their active site.Citation5,6 The heme catalases are widespread in both eukaryotes and prokaryotes while Mn-catalases are found exclusively among prokaryotes and archea.Citation5
Cyanobacteria, widely regarded as the progenitors of plant chloroplasts, were responsible for the early oxygenation of Earth's atmosphere.Citation7 Due to their close association with O2, they are likely to have developed multiple stratagems to surmount the toxic effects of ROS. Moreover, the nitrogen-fixing strains of cyanobacteria remain the only form life that can harvest solar energy to fix atmospheric nitrogen.Citation8 Incidentally, naturally occurring, filamentous, nitrogen-fixing cyanobacteria (e.g., Anabaena) are widely used as biofertlizers in the paddy fields of Southeast Asia.
Over the last few years, our laboratory has focused on dissecting the mechanisms responsible for overcoming oxidative stresses in the filamentous, heterocystous, nitrogen-fixing cyanobacterium, Anabaena PCC 7120.Citation9-13 This cyanobacterium contains an arsenal of ROS detoxifying enzymes, which include SODs, peroxiredoxins (Prxs), catalases etc.Citation14 Along with the presence of several genes encoding peroxiredoxins (Prxs), this Anabaena has 2 genes that encode Mn-catalase (alr0998 i.e., katA, and alr3090 i.e. katB). In spite of the presence of genes encoding Mn-catalases, no detectable catalase activity is observed in the control (i.e., unstressed) or H2O2-treated Anabaena.Citation10 In comparison, Prxs like all541 (encoding All1541) and alr4641 (encoding Alr4641) are found to be transcriptionally induced in response to H2O2 or methyl viologen. Additionally, enhanced synthesis of the All1541 and Alr4641 proteins is observed when Anabaena is subjected to oxidative stress.Citation9,11 These observations apparently suggest that, rather than catalases, Prxs may be the primary proteins that detoxify H2O2 in Anabaena. However, despite this distinct induction, endogenous production of Prxs is unable to rescue Anabaena from the deleterious effects brought about by exposure to H2O2. In fact, treatment with just 0.5 – 1 mM H2O2 leads to cell lysis/death in Anabaena.Citation13
Surprising results with wide ramifications for oxidative stress resistance were obtained while performing cross-protection experiments with Anabaena PCC 7120. Anabaena cells that were pre-treated with NaCl showed high degree of resistance to H2O2 and could withstand exposure to as high as 3 mM H2O2.Citation13 Keeping in mind the presence of katA/B genes in Anabaena and catalase being the known ‘classical’ enzyme that protects organisms from H2O2, catalase activity of the above-mentioned cultures was assessed on zymograms. Interestingly, the salt-treated Anabaena cells showed the presence of a distinct catalase activity. When probed with the KatA or KatB antiserum (on native Western blots), the zone of catalase activity matched exactly with the signal obtained from the KatB antiserum, demonstrating that KatB was the catalase induced in response to salt stress in Anabaena PCC 7120.Citation13
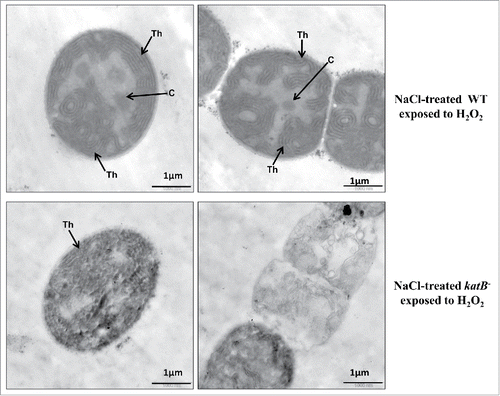
Unlike the wild-type Anabaena PCC 7120, the NaCl-treated katB mutant was found to be particularly sensitive to H2O2, and exposure to H2O2 caused increased formation of lipid peroxides, oxidized proteins and total peroxides in the katB mutant.Citation13 In fact, a considerably higher level of oxidized proteins was found in the culture medium of the mutant at the end of 24 h (). Under the transmission electron microscope, distinct structural changes were observed between the wild-type cells and the katB mutant (). The wild-type cells appeared to be robust and showed proper thylakoid integrity even after exposure to the oxidizing agent. In contrast, the katB mutant lost the thylakoid ultrastructure, and in some cells, loss of cellular contents (indicative of lysis) was also observed. Carboxysomes were observed in the wild-type cells whereas no such structures were seen in the katB mutant. In filamentous cyanobacteria, the content of chlorophyll a is a good indicator of growth/cellular integrity and this parameter is routinely used to assess resistance to various stresses. Hence, to further substantiate the TEM data, the chlorophyll a content of the NaCl-treated wild-type or the katB mutant was monitored. After 24 h, drastic reduction (∼10-fold) in the chlorophyll a content was observed in the katB mutant whereas only around 10% decrease was observed in the corresponding wild-type. Clearly, the induced KatB protein detoxified H2O2, consequently ameliorating its toxic effects to a large extent in the wild–type strain, whereas the deleterious effects of H2O2 were obvious in the katB mutant.
Figure 1. Detection of oxidized proteins. Proteins were extracted by TCA precipitation from the culture medium of NaCl-treated wild-type Anabaena (WT) or the katB mutant (katB−) cells after exposure to H2O2 (1mM). These proteins were derivatized with dinitrophenol (DNP), resolved on SDS-PAGE and transferred to nitrocellulose membrane. Subsequently, these proteins were probed with the monoclonal DNP antiserum. A Ponceau S-stained part of the blot is shown in the lower panel as loading control. The oxidized proteins were detected as mentioned in the OxyBlot oxidized protein detection kit (Thermo Scientific, 23280).
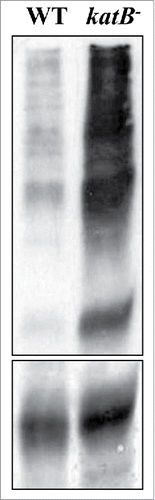
Figure 2. Ultrastructural features of the NaCl-treated wild-type Anabaena (WT, upper panel) or katB mutant (katB−, lower panel) after exposure to H2O2 (for 24 h) as seen under the transmission electron microscope. Samples were processed for transmission electron microscopy as described earlier.Citation22 Thylakoid membranes (Th) and carboxysomes (C) are indicated. Severely disintegrated thylakoid membranes and a distinct loss of ultrastructure are evident in the katB mutant filaments exposed to H2O2.
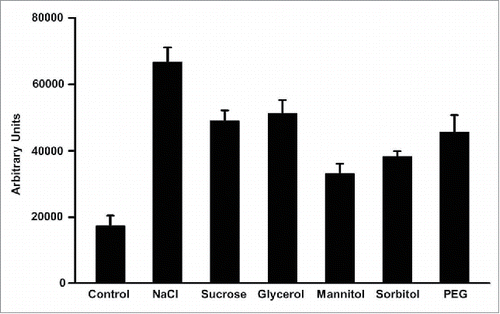
The katB promoter-gfp fusion constructCitation13 was gainfully employed to determine the various osmotic stimuli that could activate katB expression. All the osmolytes tested, i.e. NaCl, sucrose, glycerol, manitol, sorbitol, and PEG, were able to activate the katB promoter in Anabaena (). Induction of this promoter with ionic osmolytes (NaCl) as well as non ionic cell permeable osmolytes, suggests that katB is indeed an osmotically induced gene. Unexpectedly, KatB is not induced with its own substrate, hydrogen peroxide. Desiccation, an extreme form of osmotic stress, also induces synthesis of KatB in Anabaena. Also, katB expression is regulated by the nitrogen status of the medium and there is a distinct lack of katB transcription in heterocysts (cells that fix nitrogen).Citation13 Thus mechanism underlying the transcriptional activation of katB is complicated and several environmental cues are integrated in this process. The relatively long (∼450-bp) regulatory region between the katB promoter and the translational start of KatB is likely to provide ample space for various factors to bind in trans and modulate expression. In the unicellular cyanobacterium, Synechocystis PCC 6803, histidine kinases are known to regulate several osmotically induced genes.Citation15,16 The presence of these homologs in Anabaena lends support to the idea that katB too may be regulated by proteins such as histidine kinases.
Figure 3. The katB promoter-gfp fusion construct was transformed into Anabaena PCC 7120 and the katB promoter activity was monitoredCitation13 in the presence of various osmolytes such as NaCl (150 mM), sucrose (300 mM), glycerol (300 mM), manitol (300 mM), sorbitol (300 mM), and PEG (100 mM). Cells were exposed to the above-mentioned osmolytes for 18h. The green fluorescence (λex = 490 nm, λem = 520 nm) of the reporter GFP is plotted as bar diagram. Standard deviation for 5 independent experiments is shown as error bars.
Our study has provided new insights into the enhancement of cyanobacterial salt tolerance by combined nitrogen that was reported from our laboratory over 25 y ago.Citation17 In that study, the inhibition of Na+ influx by combined nitrogen was suggested to be a major mechanism for overcoming salt stress in Anabaena. Salt is known to increase ROS (e.g. H2O2) in several organisms including Anabaena.Citation13,18 Interestingly, our recent findings show that the presence of combined nitrogen enhances production of KatB in Anabaena.Citation13 Consequently, the increased levels of KatB are likely to help Anabaena overcome the oxidative effects of salt stress. So, along with the inhibition of Na+ influx, reduction in the levels of ROS brought about by KatB may also contribute to the salinity tolerance of Anabaena observed under these conditions.
As far as the future prospects are concerned, 2 lines of research appear to be particularly appealing. One, off course, is to have a detailed understanding of how the katB gene is regulated in Anabaena. The other aspect has more practical applications i.e., can the KatB-overexpressing Anabaena be employed as a more efficient biofertilzer or can the katB gene transferred to plants in the hope of improving their stress resistance? Once synthesized, KatB persists in cells for several days even when the inducing stimuli (e.g., NaCl) is removed.Citation13 Thus, Anabaena can be exposed to NaCl, which can be washed off subsequently. These natural, non-recombinant, KatB-overproducing strains could possibly work as more efficient biofertilizers than their normal unstressed counterparts. In plants, overexpression of catalase is directly correlated with improved resistance to salinity. For example, heterologous expression of E. coli KatE (a KatG-type catalases) led to a considerable increase in the ability of rice to with stand salt stress.Citation19 However, it should be noted that many heme catalases lose their activity when the temperature rises above 40°C.Citation10,20 In comparison, KatB has been shown withstand temperatures over 80°C and remain functional over a wide range of pH or salt concentrations.Citation13,21 In our opinion, due to its more robust nature, KatB appears to be a promising candidate for transfer to crop plants in order to improve their resistance to various environmental stresses. All these aspects are currently being explored in our laboratory.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol 2003; 57:395-418; PMID:14527285; http://dx.doi.org/10.1146/annurev.micro.57.030502.090938
- Collen J, Del Rio MJ, García-Reina G, Pedersén M. Photosynthetic production of hydrogen peroxide by Ulvarigida C. Ag (Chlorophyta). Planta 1995; 196:225-30; http://dx.doi.org/10.1007/BF00201378
- Halliwell B, Gutteridge JMC. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys 1986; 246:501-14; PMID:3010861; http://dx.doi.org/10.1016/0003-9861(86)90305-X
- Bernroitner M, Zamocky M, Furtmüller PG, Peschek GA, Obinger C. Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J Exp Bot 2009; 60:423-40; PMID:19129167; http://dx.doi.org/10.1093/jxb/ern309
- Zamocky M, Furtmüller PG, Obinger C. Evolution of Catalases from Bacteria to Humans. Antioxid Redox Signal 2008; 10:1527-48; PMID:18498226; http://dx.doi.org/10.1089/ars.2008.2046
- Bihani SC, Chakravarty D, Ballal A. Purification, crystallization and preliminary crystallographic analysis of KatB, a manganese catalase from Anabaena PCC 7120. Acta Crystallogr Sect F Struct Biol Cryst Commun 2013; 69:1299-302; PMID:24192374; http://dx.doi.org/10.1107/S1744309113028017
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev 1992; 56:340-73; PMID:1620069
- Stewart WDP. Some aspects of structure and function in nitrogen-fixing cyanobacteria. Annu Rev Microbiol 1980; 34:497-536; PMID:6108091; http://dx.doi.org/10.1146/annurev.mi.34.100180.002433
- Banerjee M, Ballal A, Apte SK. A novel glutaredoxin domain containing peroxiredoxin ‘All1541’ protects the N2-fixing cyanobacterium Anabaena PCC7120 from oxidative stress. Biochem J 2012; 442:671-80; PMID:22150556; http://dx.doi.org/10.1042/BJ20111877
- Banerjee M, Ballal A, Apte SK. Mn-catalase (Alr0998) protects the photosynthetic, nitrogen fixing cyanobacterium Anabaena PCC7120 from oxidative stress. Environ Microbiol 2012; 14:2891-900; PMID:22897147; http://dx.doi.org/10.1111/j.1462-2920.2012.02847.x
- Banerjee M, Chakravarty D, Ballal A. Redox-dependent chaperone/peroxidase function of 2-Cys-Prx from the cyanobacterium Anabaena PCC7120: role in oxidative stress tolerance. BMC Plant Biol 2015; 15:60; PMID:25849452; http://dx.doi.org/10.1186/s12870-015-0444-2
- Tailor V, Ballal A. Over-expression of Alr4642, a novel Prx-like peroxiredoxin, defends the cyanobacterium Anabaena PCC7120 from oxidative stress. J Appl Phycol 2015; 27:2261-70; http://dx.doi.org/10.1007/s10811-014-0503-3
- Chakravarty D, Banerjee M, Bihani SC, Ballal A. A salt-inducible Mn-catalase (KatB) protects cyanobacterium from oxidative stress. Plant Physiol 2016; 170:761-73; PMID:26645454; http://dx.doi.org/10.1104/pp.15.01632
- Banerjee M, Raghavan PS, Ballal A, Rajaram H, Apte SK. Oxidative stress management in the filamentous, heterocystous, diazotrophic cyanobacterium, Anabaena PCC7120. Photosynth Res 2013; 118:59-70; http://dx.doi.org/10.1007/s11120-013-9929-8
- Shoumskaya MA, Paithoonrangsarid K, Kanesaki Y, Los DA, Zinchenko VV, Tanticharoen M, Suzuki I, Murata N. Identical Hik-Rre systems are involved in perception and transduction of salt signals and hyperosmotic signals but regulate the expression of individual genes to different extents in Synechocystis. J Biol Chem 2005; 280:21531-8; PMID:15805106; http://dx.doi.org/10.1074/jbc.M412174200
- Los DA, Zorina A, Sinetova M, Kryazhov S, Mironov K, Zinchenko VV. Stress sensors and signal transducers in cyanobacteria. Sensors (Basel) 2010; 10:2386-415; PMID:22294932; http://dx.doi.org/10.3390/s100302386
- Reddy BR, Apte SK, Thomas J. Enhancement of cyanobacterial salt tolerance by combined nitrogen. Plant Physiol 1989; 89:204-10; PMID:16666516; http://dx.doi.org/10.1104/pp.89.1.204
- Choudhury S, Panda P, Sahoo L, Panda SK. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 2013; 8:e23681; PMID:23425848; http://dx.doi.org/10.4161/psb.23681
- Moriwaki T, Yamamoto Y, Aida T, Funahashi T, Shishido T, Asada M, Prodhan SH, Komamine A, Motohashi T. Overexpression of the Escherichia coli catalase gene, katE, enhances tolerance to salinity stress in the transgenic indica rice cultivar, BR5. Plant Biotechnol Rep 2008; 2:41-6; http://dx.doi.org/10.1007/s11816-008-0046-7
- Zámocký M, Furtmüller PG, Obinger C. Evolution of structure and function of Class I peroxidases. Arch Biochem Biophys 2010; 500:45-57; http://dx.doi.org/10.1016/j.abb.2010.03.024
- Bihani SC, Chakravarty D, Ballal A. KatB, a cyanobacterial Mn-catalase with unique active site configuration: implications for enzyme function. Free Radic Biol Med 2016; 93:118-29; PMID:26826576; http://dx.doi.org/10.1016/j.freeradbiomed.2016.01.022
- Kulkarni S, Ballal A, Apte SK. Bioprecipitation of uranium from alkaline waste solutions using recombinant Deinococcus radiodurans. J Hazard Mater 2013; 262:853-861; PMID:24140537; http://dx.doi.org/10.1016/j.jhazmat.2013.09.057
