ABSTRACT
Natural killer (NK) cells are essential for killing transformed and virally infected cells. To prevent auto-reactivity, NK cell activation is inhibited by inhibitory receptors that activate the tyrosine phosphatase SHP-1, which dephosphorylates signaling molecules crucial for NK cell activation. Initially, only a single SHP-1 substrate was identified in NK cells, the GEF VAV1. We recently demonstrated that under inhibitory conditions, LAT, PLCγ1 and PLCγ2 serve as novel SHP-1 substrates in NK cells. Furthermore, we showed that during NK cell inhibition, LAT is ubiquitylated by c-Cbl and Cbl-b, leading to its proteasomal degradation, abolishing NK cell cytotoxicity. Here, we address the mechanism through which the Cbl proteins are activated following inhibitory receptor engagement. We demonstrate that during NK cell inhibition, the expression level of the Cbl proteins significantly increases. These data suggest that inhibitory KIR receptors regulate the stability of the Cbl proteins, thereby enabling Cbl-mediated inhibition of NK cell cytotoxicity.
Natural killer (NK) cells are key components of the immune system, crucial for killing cancerous and virally infected cells. NK cells mediate their effects through their cytotoxicity toward the target cells, and production of cytokines that activate the adaptive immune response. To prevent auto-reactivity, NK cell activation is regulated by a dynamic balance between activating and inhibitory signals, transduced upon engagement of activating and inhibitory receptors expressed on the NK cell surface.Citation1
NK cell activation is dependent on the activity of the phospholipase Cγ (PLCγ) family of proteins, including PLCγ1 and PLCγ2.Citation2-4 PLCγ isoforms are responsible for the cleavage of membrane-associated phosphatidylinositol 4,5-bisphosphate into inositoltriphosphate and diacylglycerol, which induce intracellular Ca2+ mobilization and activation of signaling pathways essential for NK cell effector functions.Citation5-7
To perform their activity, PLCγ1/2 must be recruited to the contact site of the NK cell with its target, known as the NK immunological synapse (NKIS). In T cells, PLCγ1 recruitment is mediated by the transmembrane adaptor protein, the linker for activation of T cells (LAT).Citation8 LAT contains several tyrosine residues, which upon phosphorylation, serve as docking sites for the formation and integration of signaling complexes crucial for cellular activation.Citation5,9,Citation10 We recently showed that following NK cell activation, PLCγ1/2 are recruited to the NKIS via LAT tyrosine 132, where they undergo tyrosine phosphorylation that regulates their activity. We showed that the formation of these LAT:PLCγ complexes is crucial for NK cell cytotoxicity.Citation11
The inhibition of NK cell activity is regulated by several mechanisms that ensure self-tolerance to the host. These mechanisms comprise the activity of inhibitory receptors specific to MHC class I molecules, including the killer-cell immunoglobulin-like receptors (KIR). Inhibitory receptors block NK cell activation by promoting dephosphorylation of signaling proteins via the recruitment and activation of the protein tyrosine phosphatases, SH2-domain-containing protein tyrosine phosphatase-1 (SHP-1) and SHP-2.Citation12-15 SHP-1 activity is a key inhibitory mechanism attenuating NK cell activation. However, VAV1 was the only known direct substrate of SHP-1.Citation16 We recently revealed that LAT, PLCγ1 and PLCγ2 serve as additional substrates of SHP-1 during NK cell inhibition. We demonstrated that SHP-1 dephosphorylates LAT tyrosine 132, thereby blocking the recruitment of PLCγ1/2 to the inhibitory NKIS, resulting in diminished cancer cell killing.Citation11
Lymphocyte activity is also regulated by signaling molecule ubiquitylation, which results in their internalization or degradation. This modification is mediated by the E3 ubiquitin ligases c-Cbl and Cbl-b, which negatively regulate immune-cell activation.Citation17-23 In T cells, we extensively explored the role of the Cbls in terminating T cell activity by controlling the stability of signaling proteins, including WASp,Citation18 NckCitation19 and LAT.Citation22 This ubiquitylation dependent degradation serves as a mechanism for blocking signal propagation delivered following activating receptor engagement.Citation17-23 However, until recently no studies described the role of ubiquitylation as a mechanism for NK cell inhibition via inhibitory receptor engagement. We revealed that at the inhibitory NKIS, LAT is ubiquitylated by c-Cbl and Cbl-b on lysines 52 and 204, leading to its proteasomal degradation, and thereby abolishing NK cell activation and cytotoxicity. Expression of the ubiquitylation resistant LAT significantly increased NK cell mediated killing of inhibitory target cells, converting the inhibitory NK cell response into an activating one.Citation11
We found that LAT ubiquitylation is dependent on its phosphorylation,Citation11 suggesting that only LAT molecules that escape SHP-1-mediated dephosphorylation are targeted to proteasomal degradation. These data imply that NK cell inhibition relies on cooperative activity of SHP-1 and the Cbls to ensure immune tolerance to the host. However, while the mechanism through which inhibitory receptors recruit and activate SHP-1 was previously explored, the mechanisms responsible for promoting Cbl-mediated inhibition of NK cell cytotoxicity are still unknown.
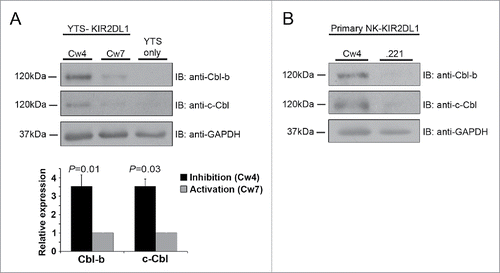
To address the Cbl activation mechanisms, we investigated whether Cbl expression levels differ following NK cell inhibition versus activation. To this end, primary NK cells isolated from donor blood, and the YTS NK cell line, expressing the inhibitory KIR2DL1 receptor, were incubated with either inhibitory 721.221 target cells that express the cognate HLA- Cw4 molecule (i.e. KIR2DL1 ligand), or with activating 721.221 target cells expressing no HLA or the HLA-Cw7 molecule. Using western blot analysis we demonstrated that the expression levels of both c-Cbl and Cbl-b are upregulated in NK cells during the establishment of an inhibitory interaction, as compared to an activating one (). Potentially, accumulation of the Cbl proteins enables their ubiquitylation of LAT, thereby inhibiting NK cell cytotoxicity.
Figure 1. Increased expression of Cbl-b and c-Cbl during NK cell inhibition versus activation. (A) YTS NK cells expressing the inhibitory KIR2DL1 receptor (YTS-KIR2DL1 cells) were incubated with either inhibitory 721.221-Cw4 (Cw4), or activating 721.221-Cw7 (Cw7) target cells at 37°C for 20 min, followed by cell lysis. Cell lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane that was immunoblotted using the indicated antibodies. Cbl-b and c-Cbl expression levels were measured by densitometric analysis, relative to the loading control, using ImageJ. (B) Primary NK-KIR2DL1 cells were incubated with inhibitory 721.221-Cw4, or activating 721.221 (.221) target cells at 37°C for 20 min, and the cells were lysed. Cell lysates were subjected to Immunobloting (IB) with anti-Cbl-b and anti-c-Cbl antibodies. Data are representative of at least 3 independent experiments.
Several mechanisms might be responsible for the elevated expression of Cbl-b and c-Cbl following NK cell inhibition vs. activation. These include the increased expression of the Cbl protein at the transcriptional level following NK cell inhibition, or protein degradation following cellular activation. However, it is not known whether the expression of the Cbls is regulated by inhibitory or activating signals, and therefore further investigation is required to determine the precise mechanism through which inhibitory receptors promote the E3 ligase activity of Cbl-b and c-Cbl. The identification of these mechanisms is critical for the full understanding of how the Cbl proteins mediate ubiquitylation dependent degradation of key signaling molecules that regulate NK cell function.
Abbreviations
| Cbl | = | Casitas-B-lineage lymphoma |
| CD3 | = | Cluster of differentiation 3 |
| DAG | = | Diacylglycerol |
| GEF | = | Guanine nucleotide exchange factor |
| IP3 | = | Inositoltriphosphate |
| KIR | = | Killer-cell immunoglobulin-like receptor |
| LAT | = | Linker for activation of T cells |
| MHC | = | Major histocompatibility complex |
| NK | = | Natural killer |
| NKIS | = | Natural killer immunological synapse |
| PIP2 | = | Phosphatidylinositol 4,5-bisphosphate |
| PLCγ | = | phospholipase Cγ |
| SHP-1 | = | SH2-domain-containing protein tyrosine phosphatase-1 |
| TCR | = | T-cell receptor |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was funded by the Israel Science Foundation grants no. 1503/08 and 747/13, grant no. 3-10151 from the Chief Scientist Office of the Ministry of Health, and a Taubenblatt Family Foundation Bio-Medicine excellence grant.
References
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science 2004; 306:1517-9; PMID:15567854; http://dx.doi.org/10.1126/science.1103478
- Caraux A, Kim N, Bell SE, Zompi S, Ranson T, Lesjean-Pottier S, et al. Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood 2006; 107:994-1002; PMID:16204312; http://dx.doi.org/10.1182/blood-2005-06-2428
- Upshaw JL, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. The isoforms of phospholipase C-gamma are differentially used by distinct human NK activating receptors. J Immunol 2005; 175:213-8; PMID:15972651; http://dx.doi.org/10.4049/jimmunol.175.1.213
- Tassi I, Presti R, Kim S, Yokoyama WM, Gilfillan S, Colonna M. Phospholipase C-gamma 2 is a critical signaling mediator for murine NK cell activating receptors. J Immunol 2005; 175:749-54; PMID:16002670; http://dx.doi.org/10.4049/jimmunol.175.2.749
- Balagopalan L, Coussens NP, Sherman E, Samelson LE, Sommers CL. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol 2010; 2:a005512; PMID:20610546; http://dx.doi.org/10.1101/cshperspect.a005512
- Clapham DE. Calcium signaling. Cell 2007; 131:1047-58; PMID:18083096; http://dx.doi.org/10.1016/j.cell.2007.11.028
- Joseph N, Reicher B, Barda-Saad M. The calcium feedback loop and T cell activation: how cytoskeleton networks control intracellular calcium flux. Biochimica et biophysica acta 2014; 1838:557-68; PMID:23860253; http://dx.doi.org/10.1016/j.bbamem.2013.07.009
- Braiman A, Barda-Saad M, Sommers CL, Samelson LE. Recruitment and activation of PLCgamma1 in T cells: a new insight into old domains. Embo J 2006; 25:774-84; PMID:16467851; http://dx.doi.org/10.1038/sj.emboj.7600978
- Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Che 2000; 275:23355-61; PMID:10811803; http://dx.doi.org/10.1074/jbc.M000404200
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 1998; 92:83-92; PMID:9489702; http://dx.doi.org/10.1016/S0092-8674(00)80901-0
- Matalon O, Fried S, Ben-Shmuel A, Pauker MH, Joseph N, Keizer D, Piterburg M, Barda-Saad M. Dephosphorylation of the adaptor LAT and phospholipase C-gamma by SHP-1 inhibits natural killer cell cytotoxicity. Sci Signal 2016; 9:ra54; PMID:27221712
- Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev 2008; 224:70-84; PMID:18759921; http://dx.doi.org/10.1111/j.1600-065X.2008.00660.x
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495-502; PMID:18425106; http://dx.doi.org/10.1038/ni1581
- Lanier LL. NK cell recognition. Annu Rev Immunol 2005; 23:225-74; PMID:15771571; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115526
- Eissmann P, Davis DM. Inhibitory and regulatory immune synapses. Curr Topic Microbiol Immunol 2010; 340:63-79; PMID:19960309
- Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol 2003; 23:6291-9; PMID:12917349; http://dx.doi.org/10.1128/MCB.23.17.6291-6299.2003
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol 2005; 6:79-87; PMID:15688069; http://dx.doi.org/10.1038/nrm1552
- Reicher B, Joseph N, David A, Pauker MH, Perl O, Barda-Saad M. Ubiquitylation-dependent negative regulation of WASp is essential for actin-cytoskeleton dynamics. Mol Cell Biol 2012; 15:3153-63; http://dx.doi.org/10.1128/MCB.00161-12
- Joseph N, Reicher B, David A, Matalon O, Barda-Saad M. Ubiquitylation-dependent downregulation of Nck regulates its functional activity. FEBS letters 2014; 588:3808-15; PMID:25218436; http://dx.doi.org/10.1016/j.febslet.2014.08.033
- Matalon O, Reicher B, Barda-Saad M. Wiskott-Aldrich syndrome protein–dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes. Immunol Rev 2013; 256:10-29; PMID:24117810; http://dx.doi.org/10.1111/imr.12112
- Paolini R, Molfetta R, Piccoli M, Frati L, Santoni A. Ubiquitination and degradation of Syk and ZAP-70 protein tyrosine kinases in human NK cells upon CD16 engagement. Proc Natl Acad Sci U S A 2001; 98:9611-6; PMID:11493682; http://dx.doi.org/10.1073/pnas.161298098
- Balagopalan L, Barr VA, Sommers CL, Barda-Saad M, Goyal A, Isakowitz MS, et al. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol Cell Biol 2007; 27:8622-36; PMID:17938199; http://dx.doi.org/10.1128/MCB.00467-07
- Huang F, Gu H. Negative regulation of lymphocyte development and function by the Cbl family of proteins. Immunol Rev 2008; 224:229-38; PMID:18759930; http://dx.doi.org/10.1111/j.1600-065X.2008.00655.x
