ABSTRACT
RNA interference (RNAi) by oral delivery of dsRNA in insects has great potential as a tool for integrated pest management (IPM), especially with respect to addressing the need to reduce off-target effect and slow down resistance development to chemical insecticides. Employing the natural association existing between insect and yeast, we developed a novel method to enable the knock down of vital genes in the pest insect Drosophila suzukii through oral delivery of species-specific dsRNA using genetically modified Saccharomyces cerevisae. D. suzukii that were fed with our “yeast biopesticide” showed a significant decrease in fitness. In this perspective article, we postulate that this approach could be adapted to a large number of species, given the great diversity of symbiotic interactions involving microorganisms and host species. Furthermore, we speculate that beyond its application as biopesticide, dsRNA delivery by genetically modified microbes can also serve to facilitate reverse genetic applications, specifically in non-model organisms.
Toward a diversification of dsRNA delivery methods
Taking advantage of the widespread symbiotic interactions pre-existing between yeast and Drosophila,Citation1 we have recently developed an oral delivery method to induce RNA interference (RNAi) in a pest insect by knocking down essential genes expressed in its digestive tractCitation2 (). By feeding D. suzukii with Saccharomyces cerevisae transformed with a plasmid vector expressing double-stranded RNA (dsRNA) targeting y-tubulin23C (y-tub23C), which is known to be lethal when mutated in the closely related D. melanogaster, we observed a significant decrease of y-tub23C mRNA level in larvae and adult midgut of D. suzukii.Citation2 More importantly, we observed that the reduced expression of y-tub23C mRNA correlated with a significant reduction in both larval survivorship and adult reproductive fitness in a species-specific manner. Our results are exciting because it showed that oral delivery of dsRNA expressed in yeast can induce sufficient RNAi knock down of a target gene to reduce fitness of an insect pest. The fact that the target insect for our study, D. suzukii, is a species without systemic RNAi mechanism suggests that our approach may even be more effective in species with systemic RNAi, in which the silencing signal can be more efficiently disseminated beyond the intial target cells, i.e. cells in the digestive tract. In addition, it remains to be tested in future experiments whether the delivery of dsRNA by microbes, rather than naked dsRNAs, could enhance the propagation of the RNAi silencing signal.
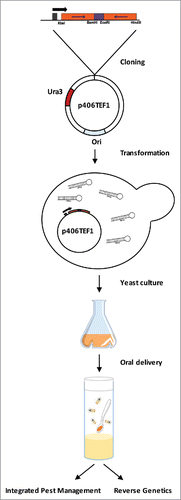
Figure 1. Schematic summarizing the protocol for production and oral delivery of genetically modified yeast expressing dsRNA to insect targets. Inverted repeats of target sequence are cloned into expression vector p406TEF1 and transformed into S. cerevisae. Transformants are selected on minimal media without Uracil. Previously expanded yeast culture is then pelleted and could be use for further applications. For more details, see ref. 2.
Since the first report of gene silencing using antisense single-stranded RNA (ssRNA) in plants at the end of the 1980sCitation3 and a few years later in C. elegans,Citation4 RNAi efficiency have been notably improved with the discovery made by Fire and MelloCitation5 highlighting the role of dsRNA in this phenomenon. The use of dsRNA molecules as a tool mediating sequence specific suppression of gene of interest (GOI) was then extensively developed and utilized in many other organisms.Citation6 Despite the fact that RNAi pathways are well conserved among eukaryotes, knockdown efficiency often varies depending on target species, target genes, and delivery methods.Citation7,8 One of the major issue limiting the success of RNAi strategies remains to be our incomplete understanding of mechanisms leading to the transport and amplification of dsRNA molecules from one cell to another. The improvement of delivery methods also constitutes a critical step to effectively mediate knockdown phenotype.Citation9 In a large number of insect taxa, oral administration has been reported to show similar efficiency on target gene expression when compared to injection methods.Citation10,11 The obvious advantages of oral delivery include reduced stress for organisms and ease of use for small insects. Nonetheless oral delivery of dsRNA is still restricted to a few number of administration mode and to date, most RNAi experiments using oral delivery are based on feeding dsRNA either synthesized in vitro or produced in bacteria.Citation12
Among emerging applications, our use of genetically modified yeast as biopesticide presents a novel approach to extend the toolbox of integrated pest management (IPM).Citation2 Studies on symbiotic interactions have indicated that yeast are not only restricted to Drosophila but broadly spread across different insect taxa.Citation13-15 In the mosquito Aedes aegypti, recent finding has shown that live yeast cells are efficiently ingested and hydrolyzed by larvae.Citation16 With the recent advances in sequencing technology, the characterization of insect gut microbiotaCitation17 will lead to the identification of novel symbiotic microorganisms amenable to be genetically modified and used as dsRNA delivering vector. In the context of pest management, the finding of new species-specific interactions between insect and microbe will considerably increase the specificity of the treatment against targeted insect.
Application of modified yeast expressing dsRNA in reverse genetics
Beyond pest control strategies, the expansion of this technique constitutes a promising strategy to address the limits of RNAi treatment, specifically in organisms where other delivery methods such as injection and classical oral dsRNA administration remain unsuccessful or too expensive. Recent advances in genetic and molecular biology offer a broad range of powerful technologies to manipulate expression and function of specific genes. With the development of genome engineering methods like ZNFs, TALENs and more recently CRISPR/Cas9 system, our ability to generate genomic changes is bringing about a revolution in scientific discoveries.Citation18-20 In model organisms such as Drosophila, UAS/Gal4 system and its extensions constitute one of the more widespread techniques to targeted genetic manipulation.Citation21 This tool is particularly efficient to drive tissue specific and ectopic gene or dsRNA expression, using promoter restricted to certain cell populations or developmental stage. Although the development of these tools constitutes incontestable advances in terms of specificity and efficiency, there is still some exception where the use of exogenously delivered dsRNA constitutes valuable alternative. Indeed, genome engineering relies on the establishment of transgenic lines by injection of genetic constructs into embryonic germline cells, which could remain challenging in non-model insect systems. In addition, functional studies of genes playing distinct roles during development and adult life could be challenging since the loss of function of these GOI generally leads to lethal phenotype.Citation22 As shown in studies addressing the characterization of hormonal pathways for example, the same set of genes could be involved in both developmental processes and regulation of physiological state in an age-dependent manner.Citation23,24 In such scenario, the temperature dependent UAS/Gal80Citation25 or other inducible systemsCitation21 can be used in model species. Alternatively, the use of transient suppression of gene expression using RNAi should also be considered. In fact, in experiments that require temperature manipulation in the experimental design, orally administrated or injected dsRNA represent a helpful substitute to the Gal80 temperature-dependent system.
As high-throughput sequencing for genome and transcriptome acquisition is becoming more and more accessible, the opportunities to explore beyond the sphere of model organisms are now unlimited. As a consequence, the number of genes with unknown function continues to rise inexorably. In this context, the improvement of dsRNA delivery has great potential in helping researchers tackle the genome to phenome challenge. We propose that microbial delivery of dsRNA for gene silencing may be less time-consuming and labor-intensive than genome engineering and could be potentially applied to a broad range of organisms for reverse genetics study as well as integrated pest management.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Chandler JA, Eisen JA, Kopp A. Yeast communities of diverse Drosophila species: comparison of two symbiont groups in the same hosts. Appl Environ Microbiol 2012; 78:7327-36; PMID:22885750; http://dx.doi.org/10.1128/AEM.01741-12
- Murphy KA, Tabuloc CA, Cervantes KR, Chiu JC. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci Rep 2016; 6:22587; PMID:26931800; http://dx.doi.org/10.1038/srep22587
- Napoli C, Lemieux C, Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell 1990; 2:279-89; PMID:12354959; http://dx.doi.org/10.1105/tpc.2.4.279
- Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 1995; 81:611-20; PMID:7758115; http://dx.doi.org/10.1016/0092-8674(95)90082-9
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806-11; PMID:9486653; http://dx.doi.org/10.1038/35888
- Bellés X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annual review of entomology 2010; 55:111-28; http://dx.doi.org/10.1146/annurev-ento-112408-085301
- Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, Albrechtsen M, An C, Aymeric JL, Barthel A, et al. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol 2011; 57:231-45; PMID:21078327; http://dx.doi.org/10.1016/j.jinsphys.2010.11.006
- Wynant N, Santos D, Vanden Broeck J. Biological mechanisms determining the success of RNA interference in insects. Int Rev Cell Mol Biol 2014; 312:139-67; PMID:25262241; http://dx.doi.org/10.1016/B978-0-12-800178-3.00005-1
- Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J Insect Physiol 2010; 56:227-35; PMID:19837076; http://dx.doi.org/10.1016/j.jinsphys.2009.10.004
- Scott JG, Michel K, Bartholomay LC, Siegfried BD, Hunter WB, Smagghe G, Zhu KY, Douglas AE. Towards the elements of successful insect RNAi. J Insect Physiol 2013; 59:1212-21; PMID:24041495; http://dx.doi.org/10.1016/j.jinsphys.2013.08.014
- Li J, Wang XP, Wang MQ, Ma WH, Hua H. Advances in the use of the RNA interference technique in Hemiptera. Insect Sci 2013; 20:31-9; PMID:23955823; http://dx.doi.org/10.1111/j.1744-7917.2012.01550.x
- Whitten MM, Facey PD, Del Sol R, Fernández-Martínez LT, Evans MC, Mitchell JJ, Bodger OG, Dyson PJ. Symbiont-mediated RNA interference in insects. Proc Biol Sci 2016; 283:20160042; http://dx.doi.org/10.1098/rspb.2016.0042
- Hughes GL, Allsopp PG, Webb RI, Yamada R, Iturbe-Ormaetxe I, Brumbley SM, O'Neill SL. Identification of yeast associated with the planthopper, Perkinsiella saccharicida: potential applications for Fiji leaf gall control. Curr Microbiol 2011; 63:392-401; PMID:21850475; http://dx.doi.org/10.1007/s00284-011-9990-5
- Bauwens J, Millet C, Tarayre C, Brasseur C, Destain J, Vandenbol M, Thonart P, Portetelle D, De Pauw E, Haubruge E, et al. Symbiont diversity in Reticulitermes santonensis (Isoptera: Rhinotermitidae): investigation strategy through proteomics. Environ Entomol 2013; 42:882-7; PMID:24331601; http://dx.doi.org/10.1603/EN13112
- Davis TS. The ecology of yeasts in the bark beetle holobiont: a century of research revisited. Microbial ecology 2015; 69:723-32; PMID:25117532; http://dx.doi.org/10.1007/s00248-014-0479-1
- Souza RS, Diaz-Albiter HM, Dillon VM, Dillon RJ, Genta FA. Digestion of Yeasts and Beta-1,3-Glucanases in Mosquito Larvae: Physiological and Biochemical Considerations. PLoS One 2016; 11:e0151403
- Krishnan M, Bharathiraja C, Pandiarajan J, Prasanna VA, Rajendhran J, Gunasekaran P. Insect gut microbiome - An unexploited reserve for biotechnological application. Asian Pac J Trop Biomed 2014; 4:21; http://dx.doi.org/10.12980/APJTB.4.2014C95
- Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods 2013; 10(10):957-63; PMID:24076990; http://dx.doi.org/10.1038/nmeth.2649
- Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014; 346(6213):1258096; PMID:25430774; http://dx.doi.org/10.1126/science.1258096
- Byrne SM, Mali P, Church GM. Genome editing in human stem cells. Methods Enzymol 2014; 546:119-38; PMID:25398338; http://dx.doi.org/10.1016/B978-0-12-801185-0.00006-4
- Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature 1988; 332:853-6; PMID:3128741; http://dx.doi.org/10.1038/332853a0
- Lin X, Yao Y, Jin M, Li Q. Characterization of the Distal-less gene homologue, NlDll, in the brown planthopper, Nilaparvata lugens (Stål). Gene 2014; 535:112-8; PMID:24321689; http://dx.doi.org/10.1016/j.gene.2013.11.056
- Bozzolan F, Duportets L, Limousin D, Wycke MA, Demondion E, François A, Abrieux A, Debernard S. Synaptotagmin I, a molecular target for steroid hormone signaling controlling the maturation of sexual behavior in an insect. FEBS J 2015; 282:1432-44; PMID:25683246; http://dx.doi.org/10.1111/febs.13231
- Lenaerts C, Van Wielendaele P, Peeters P, Vanden Broeck J, Marchal E. Ecdysteroid signalling components in metamorphosis and development of the desert locust, Schistocerca gregaria. Insect biochemistry and molecular biology 2016; 75:10-23; PMID:27180725; http://dx.doi.org/10.1016/j.ibmb.2016.05.003
- McGuire SE, Phuong TL, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 2003; 302:1765-8; PMID:14657498; http://dx.doi.org/10.1126/science.1089035
