ABSTRACT
Dodders (Cuscuta spp.) are holoparasitic plants that enwind stems of host plants and penetrate those by haustoria to connect to the vascular bundles. Having a broad host plant spectrum, Cuscuta spp infect nearly all dicot plants – only cultivated tomato as one exception is mounting an active defense specifically against C. reflexa. In a recent work we identified a pattern recognition receptor of tomato, “Cuscuta Receptor 1“ (CuRe1), which is critical to detect a “Cuscuta factor” (CuF) and initiate defense responses such as the production of ethylene or the generation of reactive oxygen species. CuRe1 also contributes to the tomato resistance against C. reflexa. Here we point to the fact that CuRe1 is not the only relevant component for full tomato resistance but it requires additional defense mechanisms, or receptors, respectively, to totally fend off the parasite.
Metazoans and plants possess an innate immune system to mount active defense against pathogen attacks. Most plant pathogens are microbes or herbivorous arthropods that the immune systems of plants are able to detect by sensing microbe- or herbivore-associated molecular patterns (MAMPs/HAMPs).Citation1,2 These molecular patterns, indicative for “non-self,” serve as molecular signals that trigger specific plant pattern recognition receptors (PRRs) and initiate plant defense signaling to fend off the pathogen.Citation3,4 Besides the pathogens mentioned, there exist ∼4,500 plant species that live parasitic on other plants and genera such as Striga, Orobanche or Cuscuta are known to cause tremendous crop loss.
The plant genus Cuscuta (dodder) comprises about 200 species distributed in all moderate climate zones. All Cuscuta species live as stem holoparasites with a broad host spectrum, preferentially for dicotyledonous plants. The different Cuscuta species grow as yellowish, orange or slightly greenish vines that wind around the stems of their host plants.Citation5 Most dodder species have no or only marginal amounts of chlorophyll and their photosynthesis is insufficient for surviving.Citation6-9 All Cuscuta species possess neither roots nor expanded leaves and penetrate host plants with haustoria that directly connect to the vascular bundles. Right after germination, Cuscuta seedlings sense host plant volatiles which support the finding of an appropriate host.Citation10 In the parasite, initial physical contact induces the formation of haustoria,Citation11 specific organs which are generally important for parasitic plants to penetrate the host tissue.Citation12 The penetration phase is accompanied by the expression of cell-wall modifying enzymes leading to structural rearrangements within the cell-walls of the parasiteCitation13 and the loosening of the host tissues.Citation14,15 After reaching the vascular bundles, the parasitic haustorium connects to the host xylem and phloem. This allows the parasite to withdraw water, nutrients, and carbohydrates to grow and complete its lifecycle.Citation8,16,17 Cuscuta parasites also take up macromolecules such as proteins, viruses or RNAs.Citation18-22 Recently, RNAs were shown to move between host plant and parasite in a bidirectional manner and to a much higher extent than previously expected.Citation23
Not much is known about how host plants can sense parasitic Cuscuta spp. and how they initiate cellular programs to fend off plant parasites. In our recent study,Citation24 we made use of the special case Cuscuta reflexa and its resistant host plant Solanum lycopersicum (cultivated tomato) to get insights in the early steps occurring in the plant-plant dialog. Tomato displays an active and clearly visible resistance reaction directly at the penetration sites of the parasitic haustoria a few days after the initial contact with the parasite and successfully fends off C. reflexa.Citation25-27
In this study we show that extracts of C. reflexa induce the production of reactive oxygen species (ROS) and the biosynthesis of the stress related phytohormone ethylene, plant defense responses usually known to occur during plant–microbe interaction and typically induced by pathogen-associated molecular patterns (PAMPs).Citation2,28 We could isolate and characterize the trigger of these responses from C. reflexa, a 2 kDa peptide with an o-esterified modification, and we further screened an introgression library of S. lycopersicum x S. pennelliiCitation29 to map responsiveness to this parasitic factor, since S. pennellii is insensitive to parasitic extracts and susceptible for a C. reflexa infestation.Citation13 We identified a gene encoding a plasma membrane-bound receptor, the Leucine-rich repeat receptor like protein (LRR-RLP) “Cuscuta receptor 1′ (CuRe1) which senses the parasitic “Cuscuta factor” (CuF). CuF initiates defense responses in the formerly insensitive host plant Nicotiana benthamiana after transient expression of CuRe1. Stable transformation of a CuRe1 construct into N. benthamiana lead to a drastically reduced C. reflexa growth and to an increased resistance.
Besides CuRe1, there are 3 genes encoding for CuRe1 homologs (CuRe1-likes; Solyc04g0014400; Solyc08g016210; Solyc08g016310) within the tomato genome, sharing 64 – 81 % amino acid sequence identity (). Receptors with up to 80 % aa-sequence identity to CuRe1 seem exclusively present in Solanaceaus plants. Only receptors with less than 45 % aa-sequence can be found outside the Solanaceae. We cloned all CuRe1-like genes from tomatoCitation24 and expressed them heterologously in N. benthamiana. However, in contrast to CuRe1 none of these receptors was able to trigger defense-related responses like ethylene induction when treated with the CuF or crude C. reflexa extract ().
Figure 1. Functionality of CuRe1-like receptors. (A) Tree shows relationship of CuRe1 and CuRe1-like genes; Eix2: receptor for fungal XylanaseCitation33 served as reference. (B) Ethylene response of N. benthamiana leaves expressing receptor CuRe1-like constructs and treated with C. reflexa extract or controls (mock = 0.01 mg/ml BSA in water; Penicillium extract = positive control); values represent means of n = 3 replicates plus stdv.
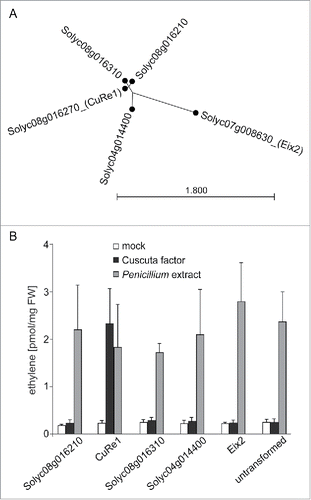
The recognition of the parasitic cell wall associated CuF or related other Cuscuta factors by these receptors could be supposable. Nonetheless, the initiated cellular signaling program must be distinct from the defense related responses induced by CuRe1 as we could not measure the emission of ethylene () after treatment with CuF.
During a susceptible interaction the parasite has to hook up the host plant's developmental processes to establish a connection to the vascular system. Therefore, the parasite has to (ab-)use existing host mechanisms including the signals and perception systems to succeed in infecting other plants. If the CuRe1-like receptors are critical to recognize and process any molecular cues of Cuscuta spp is possible but remains to be demonstrated. The roles of CuRe1-likes for the harbouring host plant e.g. as receptors for endogenous signals involved in developmental processes or as receptors to detect MAMPs is still unclear and up to date no function could be assigned to any receptor of this clade.
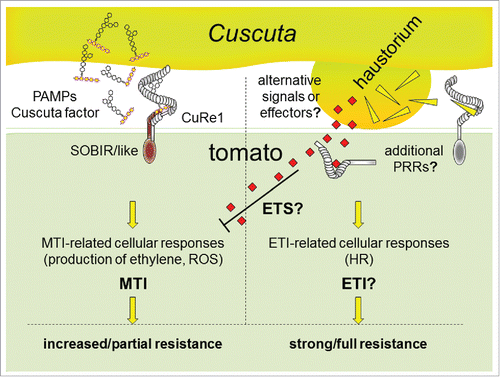
In fact, the specific recognition of the Cuscuta factor by tomato CuRe1Citation24 and the induction of the plant defense system seems unique and has probably evolved by incident exclusively in tomato. As far as tested, the Cuscuta factor seems present in other Cuscuta species as well but seems absent from plant species outside this genus.Citation24 The full resistance toward parasitic C. reflexa, however, seems not to depend on CuRe1 alone but requires additional mechanisms maybe related to those known for Effector triggered immunity (ETI) occurring during plant–microbe interaction (overview in ).Citation24,30,31 An nucleotide binding site leucine-rich repeat (NBS-LRR) protein, as part of a second layer of immunity and as a potential element of ETI, has been found to be relevant for resistance during the plant-plant interaction of cowpea against witch-weed (Striga spp.).Citation32 In case of the C. reflexa interaction with tomato additional components of resistance still have to be identified. If the CuRe1-like or other receptors are involved in such tomato-specific defense—maybe in a long term process—has to be further studied.
Figure 2. Model for defense and resistance of tomato to Cuscuta spp infestation. (Left): The Cuscuta factor is detected as a parasite-associated molecular pattern (PAMP) by the plasma membrane-bound PRR CuRe1 and initiates MTI-type responses in tomato, including the production of ethylene and ROS. MTI, apart from increasing resistance against various microbial pathogens, leads to increased resistance of tomato to Cuscuta attacks. (Right): Hypothesized ETS (effector triggered susceptibility), ETI (effector-triggered immunity) or alternative principles in tomato might, synergistically with or independently from MTI, confer full resistance of tomato to Cuscuta infestation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Work of the M.A. lab was funded by the DFG (AL 1426/1-1 and AL 1426/1-2).
References
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Ann Rev Genet 2010; 44:1-24; PMID:20649414; http://dx.doi.org/10.1146/annurev-genet-102209-163500
- Boller T, Felix G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu Rev Plant Biol 2009; 60:379-406; PMID:19400727; http://dx.doi.org/10.1146/annurev.arplant.57.032905.105346
- Böhm H, Albert I, Fan L, Reinhard A, Nurnberger T. Immune receptor complexes at the plant cell surface. Curr Opin Plant Biol 2014; 20C:47-54; http://dx.doi.org/10.1016/j.pbi.2014.04.007
- Macho AP, Zipfel C. Plant PRRs and the activation of innate immune signaling. Mol Cell 2014; 54:263-272; PMID:24766890; http://dx.doi.org/10.1016/j.molcel.2014.03.028
- Yuncker TG. The genus Cuscuta. Memoirs of the Torrey Botanical Club 1932; 18:109-331
- Funk HT, Berg S, Krupinska K, Maier UG, Krause K. Complete DNA sequences of the plastid genomes of two parasitic flowering plant species, Cuscuta reflexa and Cuscuta gronovii. BMC Plant Biol 2007; 7:1-12; PMID:17714582; http://dx.doi.org/10.1186/1471-2229-7-45
- Garcia MA, Costea M, Kuzmina M, Stefanovic S. Phylogeny, character evolution, and biogeography of Cuscuta (dodders; Convolvulaceae) inferred from coding plastid and nuclear sequences. Am J Bot 2014; 101:670-90; PMID:24688058; http://dx.doi.org/10.3732/ajb.1300449
- Hibberd JM, et al. Localization of photosynthetic metabolism in the parasitic angiosperm Cuscuta reflexa. Planta 1998; 205:506-13; http://dx.doi.org/10.1007/s004250050349
- McNeal JR, Arumugunathan K, Kuehl JV, Boore JL, Depamphilis CW. Systematics and plastid genome evolution of the cryptically photosynthetic parasitic plant genus Cuscuta (Convolvulaceae). BMC Biol 2007; 5:55; PMID:18078516; http://dx.doi.org/10.1186/1741-7007-5-55
- Runyon JB, Mescher MC, De Moraes CM. Volatile chemical cues guide host location and host selection by parasitic plants. Science 2006; 313:1964-7; PMID: 17008532; http://dx.doi.org/10.1126/science.1131371
- Dawson JHM, LJ, Wolswinkel JP, Dörr I. Biology and Control of Cuscuta. Weed Sci 1994; 6:265-317
- Yoshida S, Cui S, Ichihashi Y, Shirasu K. The haustorium, a specialized invasive organ in parasitic plants. Annu Rev Plant Biol 2016; 67:643-67; PMID:27128469; http://dx.doi.org/10.1146/annurev-arplant-043015-111702
- Johnsen HR, Striberny B, Olsen S, Vidal-Melgosa S, Fangel JU, Willats WG, Rose JK, Krause K. Cell wall composition profiling of parasitic giant dodder (Cuscuta reflexa) and its hosts: a priori differences and induced changes. New Phytologist 2015; 207:805-16; PMID:25808919; http://dx.doi.org/10.1111/nph.13378
- Vaughn KC. Attachment of the parasitic weed dodder to the host. Protoplasma 2002; 219:227-37; PMID:12099223; http://dx.doi.org/10.1007/s007090200024
- Vaughn KC. Dodder hyphae invade the host: a structural and immunocytochemical characterization. Protoplasma 2003; 220:189-200; PMID:12664283; http://dx.doi.org/10.1007/s00709-002-0038-3
- Hibberd JM, Quick WP, Press MC, Scholes JD, Jeschke WD. Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant Cell Environ 1999; 22:937-47; http://dx.doi.org/10.1046/j.1365-3040.1999.00462.x
- Jeschke WD, Hilpert A. Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: Nitrogen and carbon relations of the parasitic association Cuscuta reflexa-Ricinus communis. Plant Cell Environ 1997; 20:47-56; http://dx.doi.org/10.1046/j.1365-3040.1997.d01-2.x
- Haupt S, Oparka KJ, Sauer N, Neumann S. Macromolecular trafficking between Nicotiana tabacum and the holoparasite Cuscuta reflexa. J Exp Botany 2001; 52:173-77; PMID:11181727; http://dx.doi.org/10.1093/jexbot/52.354.173
- Kim G, Westwood JH. Macromolecule exchange in Cuscuta-host plant interactions. Curr Opin Plant Biol 2015; 26:20-25; PMID:26051214; http://dx.doi.org/10.1016/j.pbi.2015.05.012
- Alakonya A, Kumar R, Koenig D, Kimura S, Townsley B, Runo S, Garces HM, Kang J, Yanez A, David-Schwartz R, et al. Interspecific RNA interference of SHOOT MERISTEMLESS-like disrupts cuscuta pentagona plant parasitism. Plant Cell 2012; 24:3153-66; PMID:22822208; http://dx.doi.org/10.1105/tpc.112.099994
- David-Schwartz R, Runo S, Townsley B, Machuka J, Sinha N. Long-distance transport of mRNA via parenchyma cells and phloem across the host-parasite junction in Cuscuta. New Phytologist 2008; 179:1133-41; PMID:18631294; http://dx.doi.org/10.1111/j.1469-8137.2008.02540.x
- Roney JK, Khatibi PA, Westwood JH. Cross-species translocation of mRNA from host plants into the parasitic plant dodder. Plant Physiol 2007; 143:1037-43; PMID:17189329; http://dx.doi.org/10.1104/pp.106.088369
- Kim G, LeBlanc ML, Wafula EK, dePamphilis CW, Westwood JH. Plant science. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 2014; 345:808-11; PMID:25124438; http://dx.doi.org/10.1126/science.1253122
- Hegenauer V, Fürst U, Kaiser B, Smoker M, Zipfel C, Felix G, Stahl M, Albert M. Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 2016; 353:478-81; PMID:27471302; http://dx.doi.org/10.1126/science.aaf3919
- Albert M, Belastegui-Macadam X, Kaldenhoff R. An attack of the plant parasite Cuscuta reflexa induces the expression of attAGP, an attachment protein of the host tomato. Plant J 2006; 48:548-56; PMID:17076801; http://dx.doi.org/10.1111/j.1365-313X.2006.02897.x
- Ihl B, Tutakhil N, Hagen A, Jacob F. Studies on Cuscuta-Reflexa Roxb.7. Defense-Mechanisms of Lycopersicon-Esculentum Mill. Flora 1988; 181:383-93
- Kaiser B, Vogg G, Furst UB, Albert M. Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front Plant Sci 2015; 6:45; PMID:25699071; http://dx.doi.org/10.3389/fpls.2015.00045
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 1999; 18:265-76; PMID:10377992; http://dx.doi.org/10.1046/j.1365-313X.1999.00265.x
- Eshed Y, Zamir D. An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 1995; 141:1147-62; PMID:8582620
- Ntoukakis V, Gimenez-Ibanez S. PLANT BIOLOGY. Parasitic plants–A CuRe for what ails thee. Science 2016; 353:442-3; PMID:27471291; http://dx.doi.org/10.1126/science.aag3111
- Jones JD, Dangl JL. The plant immune system. Nature 2006; 444:323-9; PMID:17108957; http://dx.doi.org/10.1038/nature05286
- Li J, Timko MP. Gene-for-gene resistance in Striga-cowpea associations. Science 2009; 325:1094; PMID:19713520; http://dx.doi.org/10.1126/science.1174754
- Ron M, Avni A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 2004; 16:1604-15; PMID:15155877; http://dx.doi.org/10.1105/tpc.022475
