ABSTRACT
Diabetes is a chronic and progressing disease, the number of patients increasing exponentially, especially in industrialized countries. Regenerating lost insulin-producing cells would represent a promising therapeutic alternative for most diabetic patients. To this end, using the mouse as a model, we reported that GABA, a food supplement, could induce insulin-producing beta-like cell neogenesis offering an attractive and innovative approach for diabetes therapeutics.
KEYWORDS:
The pancreas is an abdominal gland located behind the stomach and connected to the duodenum. It consists of two functionally and morphologically distinct compartments. The exocrine compartment (representing 98% of the total organ mass), mainly involved in nutrient digestion, is composed of acinar and ductal cells. Acinar cells produce digestive enzymes that catalyze the breakdown of proteins, carbohydrates and lipids. Drainage of digestive enzymes is carried out by a ductal system, which conveys the pancreatic juice to the duodenum.Citation1,2 The endocrine compartment is organized into highly vascularized and innervated cell clusters termed islets of Langerhans. They include five different hormone-secreting cell subtypes: α-, β-, δ-, ϵ-, and PP-cells secreting respectively glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide. The intimate interaction between islets and vascular cells allows a tight control of the glucose homeostasis.Citation1
Diabetes is a chronic and progressing disease characterized by elevated blood glucose levels. It represents a major threat for public health as it affects 422 million people worldwide according to the latest WHO's report (http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1). Diabetes can be subdivided into two main conditions: type I diabetes, which is mainly caused by an autoimmune-mediated destruction of insulin-producing pancreatic β-cells and type II diabetes, resulting from the resistance to insulin action in peripheral tissues and eventual β-cell failure. Both forms of diabetes result in chronic hyperglycemia.Citation3 Therefore, developing novel strategies aiming at regenerating β-cells would represent a promising therapeutic alternative for a majority of diabetic patients.
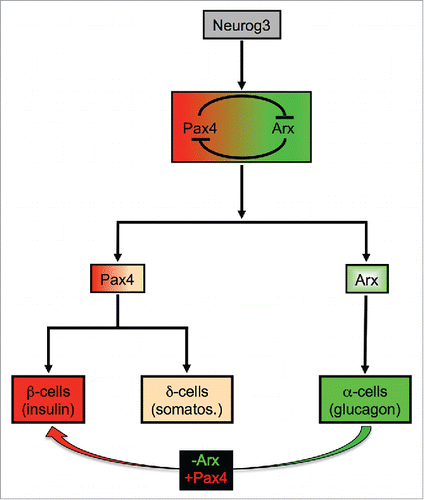
Toward this goal, several researches have been focused on deciphering the molecular mechanisms underlying β-cell genesis during embryonic development and throughout adulthood.Citation4-11 Mainly using the mouse as a model system, lineage tracing analyses combined to loss-of-function demonstrated that all pancreatic endocrine cells derive from progenitor cells expressing the basic helix-loop-helix (bHLH) transcription factor neurogenin3 (neurog3).Citation12 Subsequently, during the course of embryonic development, neurog3+ endocrine precursor cells further differentiate into specific endocrine cells, such process being driven by a complex network of transcription factors.Citation2 Among these, Arx and Pax4 play a major role in cell fate allocation as their activities were found to be required for the differentiation of glucagon-expressing α-cells and insulin-secreting β-cells, respectivelyCitation13 (). Importantly, recent studies demonstrated that, upon the misexpression of Pax4 or the inactivation of Arx,Citation14,15 terminally differentiated α-cells can be regenerated and converted into functional β-cells, either during in-utero development or adulthood (). These latter results clearly show that exploiting α-cell plasticity and regeneration capabilities could represent a possible strategy to counteract β-cell loss in diabetic patients.
Figure 1. Arx and Pax4 roles for endocrine cell specification and reprogramming. During the course of pancreas morphogenesis, the activation of Neurog3 specifies pancreatic precursor cells toward an endocrine cell fate. The subsequent activation of Pax4 or Arx (both mutually inhibiting each other's at the transcriptional level) will further drive endocrine precursor cells toward a β-/δ-cell lineage or an α-cell fate, respectively. Importantly, the misexpression of Pax4 or the loss of Arx expression in α-cells induces their neogenesis and conversion into β-like cells in vivo.
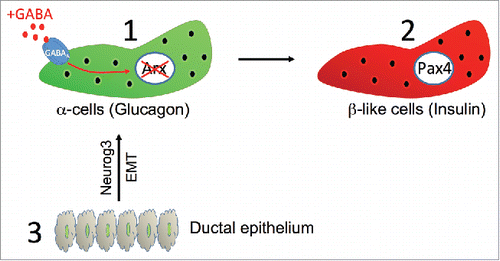
Encouraged by this exciting perspective, several screens were performed aiming to putatively identify compounds able to mimic the consequences of Pax4 misexpression / Arx inhibition and the ensuing conversion of α-cells into β-like cells. We thus identified the γ-aminobutyric acid (GABA). GABA is the main inhibitory neurotransmitter in the central nervous system.Citation16 In the pancreas, GABA is released by β-cells and acts on α-cells to decrease glucagon secretion.Citation17 Interestingly, upon daily intraperitoneal injections of GABA (GABA does not cross the blood-brain barrier) in wild-type non-diabetic mice, a dramatic β-like cell hyperplasia, leading to an increase in the number and size of the islets of Langerhans, was documented.Citation18 Of note, the β-like cell mass expansion was found to be proportional to the duration of GABA treatment and independent of the age of animals. Further analyses, combining lineage tracing, immunohistochemistry, electron microscopy, and functional tests, demonstrated that GABA acts on α-cells through the GABAA receptor in order to down-regulate Arx activities,Citation18,19 such inactivation leading to their conversion into β-like cells (). Importantly, the ensuing loss of α-cells (and resulting glucagon shortage) triggers compensatory mechanisms involving the mobilization of ductal precursor cells that differentiate into α-cells by reactivating the embryonic endocrine differentiation program in such adult context. However, upon maintained GABA exposure, the neo-generated α-cells are yet again converted into β-like cells, such cycle of neogenesis and conversion finally resulting in the expansion of β-like cell mass.
Figure 2. GABA induces α-cell-mediated β-like cell neogenesis. GABA acts via the GABAA receptor located on α-cells (1), leading to the inactivation of Arx and the subsequent conversion of α-cells into Pax4+ insulin-producing β-like cells (2). The ensuing shortage of glucagon induces compensatory mechanisms involving the mobilization of ductal precursor cells and their differentiation into α-like cells (3). This process involves the reactivation of the embryonic endocrine differentiation program with the re-expression of the developmental gene Neurog3, such Neurog3+ ductal cells undergoing epithelium-to-mesenchymal transition (3).
Impressively, GABA treatment alone appeared sufficient to rescue mice from streptozotocin-induced β-cell ablation and subsequent diabetes, even once the animals were hyperglycemic. Equally important was the finding that mice rendered diabetic repeatedly could regenerate several times their β-cell mass upon GABA administration. However, when GABA exposure was halted, a prompt arrest of β-cell neogenesis was observed, suggesting that these regeneration processes can be tightly controlled.
Aiming to determine whether GABA could also convert human α-cells into β-like cells, we performed in vitro and ex-vivo analyses.Citation18 Interestingly, in both instances, a clear decrease in the number of α-cells and a concomitant augmentation in the insulin+ counts were observed following GABA treatment. These results therefore suggest that GABA can reprogram human α-cells into cells expressing the insulin hormone. While much work remains to be done, the identification of a food supplement able to restore the β-cell mass using regenerating α-cells in such an efficient and tightly controlled way raises hopes toward an alternative treatment of diabetes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Napolitano T, Avolio F, Courtney M, Vieira A, Druelle N, Ben-Othman N, Hadzic B, Navarro S, Collombat P. Pax4 acts as a key player in pancreas development and plasticity. Sem Cell Dev Biol 2015; 44:107-14; https://doi.org/10.1016/j.semcdb.2015.08.013
- Avolio F, Pfeifer A, Courtney M, Gjernes E, Ben-Othman N, Vieira A, Druelle N, Faurite B, Collombat P. From pancreas morphogenesis to beta-cell regeneration. Curr Top Dev Biol 2013; 106:217-38; PMID:24290351
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005; 54(Suppl 2):S97-107; PMID:16306347; https://doi.org/10.2337/diabetes.54.suppl_2.S97
- Collombat P, Hecksher-Sorensen J, Serup P, Mansouri A. Specifying pancreatic endocrine cell fates. Mech Dev 2006; 123:501-12; PMID:16822656; https://doi.org/10.1016/j.mod.2006.05.006
- Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest 2007; 117:961-70; PMID:17404619; https://doi.org/10.1172/JCI29115
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009; 138:449-62; PMID:19665969; https://doi.org/10.1016/j.cell.2009.05.035
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002; 129:2447-57; PMID:11973276
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 1993; 12:4251-9; PMID:7901001
- Smith SB, Gasa R, Watada H, Wang J, Griffen SC, German MS. Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem 2003; 278:38254-9; PMID:12837760; https://doi.org/10.1074/jbc.M302229200
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000; 127:2317-22; PMID:10804174
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 1997; 386:399-402; PMID:9121556; https://doi.org/10.1038/386399a0
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Nat Acad Sci USA 2000; 97:1607-11; PMID:10677506; https://doi.org/10.1073/pnas.97.4.1607
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003; 17:2591-603; PMID:14561778; https://doi.org/10.1101/gad.269003
- Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, Druelle N, Avolio F, Ravassard P, Leuckx G, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell 2013; 26:86-100; PMID:23810513;https://doi.org/10.1016/j.devcel.2013.05.018
- Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben-Othman N, Pfeifer A, Avolio F, Leuckx G, Lacas-Gervais S, et al. The inactivation of Arx in pancreatic alpha-cells triggers their neogenesis and conversion into functional beta-like cells. PLoS Genet 2013; 9:e1003934; PMID:24204325; https://doi.org/10.1371/journal.pgen.1003934
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol 2003; 13:341-7; PMID:12850219; https://doi.org/10.1016/S0959-4388(03)00064-3
- Franklin IK, Wollheim CB. GABA in the endocrine pancreas: its putative role as an islet cell paracrine-signalling molecule. J Gen Physiol 2004; 123:185-90; PMID:14769848;https://doi.org/10.1085/jgp.200409016
- Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, Hadzic B, Druelle N, Napolitano T, Navarro-Sanz S, et al. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 2017; 168:73-85 e11; PMID:27916274; https://doi.org/10.1016/j.cell.2016.11.002
- Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, Huber KV, Schmitner N, Kimmel RA, Romanov RA, et al. Artemisinins target GABAA receptor signaling and impair alpha cell identity. Cell 2017; 168:86-100 e15; PMID:27916275
