 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pantoea ananatis is a bacterium associated with other microorganisms on Abutilon theophrasti Medik. roots. It converts 6-hydroxybenzoxazolin-2(3H)-one (BOA-6-OH), a hydroxylated derivative of the allelochemical benzoxazolin-2(3H)-one, into 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one. The compound was identified by NMR and mass spectrometric methods. In vitro synthesis succeeded with Pantoea protein, with isolated proteins from the Abutilon root surface or with horseradish peroxidase in the presence of nitrite and H2O2. Nitro-BOA-6-OH is completely degraded further by Pantoea ananatis and Abutilon root surface proteins. Under laboratory conditions, 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one inhibits Lepidium sativum seedling growth whereas Abutilon theophrasti is much less affected. Although biodegradable, an agricultural use of 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one is undesirable because of the high toxicity of nitro aromatic compounds to mammals.
6-Hydroxybenzoxazolin-2(3H)-one is produced by many plants as an intermediate for subsequent glycosylation when exposed to the allelochemical benzoxazolin-2(3H)-one.Citation1,2 The latter presents the first transformation/degradation product of the bioactive secondary metabolite 2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one (DIBOA) occurring in rye and other grasses as glucosides and as an aglycone after root exudation. Bioactive benzoxazinoids are presently investigated for their use as natural insecticides, but the compounds can cause also allelopathic suppression of neighboring plants. However, many plants counter allelopathic suppression by sophisticated detoxification strategies. Abutilon theophrasti, a widespread weed, is rather insensitive to benzoxazolin-2(3H)-one. Root-associated microorganisms support the plant in coping with the allelochemical.Citation3–5 In this context Pantoea ananatis—an Enterobacteriaceae that exists in many aquatic and terrestrial environments as well as in association with plants, insects and humansCitation6 —was identified as one member of a microbial micro-community that can colonize Abutilon theophrasti roots when growing in soils rich in organic matter. Incubation of the bacterium Pantoea ananatis with 6-hydroxybenzoxazolin-2(3H)-one (BOA-6-OH) led first to a bright yellow and then to an orange colored medium coincident with the disappearance of the added BOA-6-OH. To isolate compounds presumably responsible for the colors, the media were immediately used after occurrence of the bright yellow color. TLC of the EtOAc extracts of the media resulted in a major yellow band, traces of an orange and another wine red band which show increasing hydrophilicity. Treatment of the yellow material with KOH or NaOH led to an orange and then to the wine-red colored solution whereas NaCl and KCl addition had no effect. This indicates that the shift to alkaline pH is responsible for the color changes, accompanied by alterations in the solubility. It was therefore concluded, that only one compound is responsible for the different colors. Nevertheless, the bright yellow and the orange preparations were both used for structure identification.
Structure identification
Structural investigation was made using different spectroscopic methods (details and spectra can be found in the supplemental online material). The analyses started with UPLC-ESI-MS measurements, revealing a molecular weight of 196 for the unknown substance. The UPLC-ESI-HR-MS data are coincident with further mass spectrometric analyses. The NMR assignment was performed using standard 2D techniques (HSQC, HMBC). Characteristic shift differences in 1H and 13C were found between a neutral and ionic form of the compound. Additionally, line broadening of C-5, C-6 and C-7 was observed in the carbon spectrum of the salt. A bathochromic shift in the spectrum from 383 nm to 418 nm occurred as a result of salt formation. According to these data, the colored compound produced by Pantoea ananatis was identified as 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one. It exists in the medium first in a yellow neutral and than in an orange anionic form due to the increasing pH during continued culturing ().
Figure 1. The color of 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one depends on pH: pH 3 greenish; pH 7 yellow; pH 8 orange; pH 9–10 wine-red. Pantoea ananatis cultures, producing the compound from BOA-6-OH, change the color from yellow to orange due to the pH value which increases over time. The anionic, orange form of the compound is degraded, as shown by the HPLC chromatograms (left: analysis of the yellow medium immediately after adding synthetic nitro-BOA-6-OH (black points) before the medium turned to orange. After 3 h (right), only traces of the compound are left.
![Figure 1. The color of 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one depends on pH: pH 3 greenish; pH 7 yellow; pH 8 orange; pH 9–10 wine-red. Pantoea ananatis cultures, producing the compound from BOA-6-OH, change the color from yellow to orange due to the pH value which increases over time. The anionic, orange form of the compound is degraded, as shown by the HPLC chromatograms (left: analysis of the yellow medium immediately after adding synthetic nitro-BOA-6-OH (black points) before the medium turned to orange. After 3 h (right), only traces of the compound are left.](/cms/asset/6969a81e-f460-4f42-b9d7-9a5b7b5cf360/kcib_a_1302633_f0001_oc.gif)
In vitro synthesis of 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one
BOA-6-OH was converted to 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one by proteins isolated from P. ananatis in presence of nitrite and H2O2. Replacing nitrite with nitrate decreased product formation (). Thus, we concluded that nitrate present in the Czapek medium is reduced by bacterial nitrate reductase and the resulting nitrite is used for the nitration of BOA-6-OH by a peroxidase. Secretion of peroxidase and periplasmic nitrate reductase by certain bacteria is known.Citation7,8 Pantoea ananatis possesses genes for nitrate reductase (UniProtKB-A0A0H3KVM0 (A0A0H3KVM0_PA-NAA) and catalase/hydroperoxidase (NCBI Reference Sequence: WP_014592998.1).Citation9,10 Nitrate reductase may be located within the periplasmic space of the Gram-negative Pantoea ananatis.
Figure 2. Enzymatic nitration by Pantoea ananatis (P.a.) exuded protein, horseradish peroxidase (HRP) and Abutilon (Ab) root surface proteins in presence of nitrite; P.a. and HRP also in the presence of nitrate. No enz. = no enzyme was added to the assay. Ab/B-6-OH: root surface proteins from seedlings pre-incubated with BOA-6-OH. Means ± SD are shown; asterisk(s) indicate significant differences (t-test, * = p < 0.05; *** = p < 0.0001).
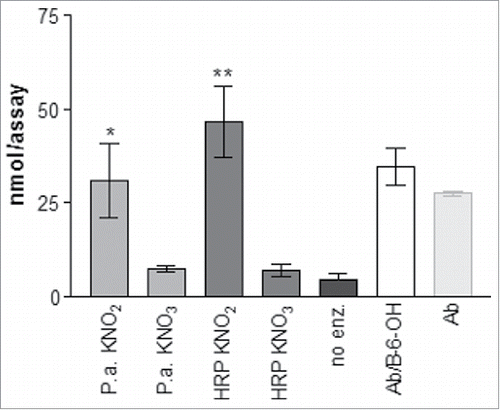
Figure 3. 6-Hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one (NO2-BOA-6-OH) inhibits the growth of Lepidium sativum (I50 values: lowest at pH 6.0 with 20 µM for root growth and 10 µM for the shoot growth). As expected, A. theophrasti is rather insensitive. The I50 values for nitrated BOA-6-OH increased with pH from averaged 300 µM at pH 4.0 and 5.0 to 1.7 mM at pH 6.0 and 2 mM at pH 7.0 for root growth. The averaged I50 value of 0.7 mM for Abutilon shoot growth was approximately the same for all tested pH values. Means ± SD are shown; different letters indicate significant differences (t-test, a = p<0.05; b = <0.005; c = p<0.0001).
![Figure 3. 6-Hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one (NO2-BOA-6-OH) inhibits the growth of Lepidium sativum (I50 values: lowest at pH 6.0 with 20 µM for root growth and 10 µM for the shoot growth). As expected, A. theophrasti is rather insensitive. The I50 values for nitrated BOA-6-OH increased with pH from averaged 300 µM at pH 4.0 and 5.0 to 1.7 mM at pH 6.0 and 2 mM at pH 7.0 for root growth. The averaged I50 value of 0.7 mM for Abutilon shoot growth was approximately the same for all tested pH values. Means ± SD are shown; different letters indicate significant differences (t-test, a = p<0.05; b = <0.005; c = p<0.0001).](/cms/asset/144e14b4-3f54-4105-b1a4-90398198bf6a/kcib_a_1302633_f0003_oc.gif)
To determine whether peroxidase activity is able to perform the nitration step, commercial horseradish peroxidase (HRP) was investigated. This enzyme was successfully used by Sakihama et al.Citation11 for nitration of p-coumaric acid and cinnamic acids. Similar to the observations of Sakihama et al.Citation11 with other acceptor molecules, nitration of BOA-6-OH in position 5 was almost immediately accomplished after application of horseradish peroxidase () resulting in a product identical to the previously identified compound. The OH group at C-6 might increase electron density at C-5 and C-7 due to its own donor effect, which facilitates nitration. For steric reasons, C-5 should be preferred for electrophilic attacks and is therefore favored for nitration of BOA-6-OH. Since benzoxazolin-2(3H)-one (BOA) lacks the OH group, nitration with horseradish peroxidase failed when BOA was used as a substrate in the assay. Aromatic nitro compounds are rare in natureCitation12 and natural nitrated benzoxazolinones have not been previously reported. Zikmundova et al.Citation13 who identified N-(2-hydroxy-5-nitrophenyl)acetamide and N-(2-hydroxy-3-nitrophenyl)acetamide as fungal detoxification products of benzoxazolinone-derived 2-aminophenol, assumed an enzymatic reduction of nitrate, a constituent of the culture medium, and use of the resulting nitrite for nitration. This mechanism was also suggested by Rousseau et al.Citation14 for tocopherol nitration by Streptomyces catenulae.
A question was whether the compound would be formed at the root surface. Therefore, the root surface proteins were collected and assayed for peroxidase-dependent synthesis in the presence of nitrite. After 3 min, approximately 30 nmol of nitro compound could be extracted from the assay mixture; when seedlings were pre-incubated with 2 mM BOA-6-OH () more product was formed.
Degradation of 6-Hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one
Since the compound disappeared during prolonged cultivation of P. ananatis, biological degradation was considered because the isolated, purified compound did not show any indication of instability. To elucidate whether P. ananatis can metabolize 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one when existing in the anionic form, the bacterium was taken from a previous culture grown in the presence of BOA-6-OH and incubated with the purified, in vitro synthesized compound. Aliquots of the media revealed that anionic 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one is almost completely metabolized within 3 h (). The compound is also degraded by proteins collected from the Abutilon root surface colonized by the microbial community (shown in the supplemental online material). Thus, 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one presents an intermediate of a catabolic sequence.
The nitration of 6-hydroxybenzoxazolin-2(3H)-one and its subsequent degradation presents a hitherto unknown possibility for the elimination of benzoxazolinone by a plant-bacterium cooperation. For BOA-6-OH, nitration seems to be a necessary step to initiate degradation. Electron-withdrawing NO2-group substitution at the aromatic ring position 5 may facilitate enzymatic attacks by destabilizing the heterocyclic molecule and starting the decomposition. It is known that nitro aromatic compounds can be completely degraded by several bacteria such as Pseudomonas, Ralstonia and Comamonas species resulting in small biooxidizable molecules that can enter the TCA cycle.Citation15
Bioassays
The purified compound was tested for its effects on germinating seeds of Abutilon theophrasti and Lepidium sativum using the bioassay procedure of Macias and coworkers.Citation16 Since the water solubility of the compound changes with pH, the bioassays were performed at pH 4, 5, 6 and 7.0. Abutilon root growth was, however, reduced with increasing pH, the growth of Lepidium roots only at pH 7.0. L. sativum exhibits a higher sensitivity to nitrated-BOA-6-OH than Abutilon theophrasti at all pH values (). Exposure to low amounts of nitro-BOA-6-OH results in shoot growth stimulation of Abutilon (significant with 86 µM at pH 7.0.).Taken together, nitro-BOA-6-OH is less toxic for Abutilon than for Lepidium. The results of the bioassays obtained under laboratory conditions indicate that only sensitive plant species such as L. sativum may be considerably inhibited, but it is questionable whether the compound has any phytotoxic effects under field conditions when bacteria able to degrade the compound are present. An agricultural use of nitrated BOA-6-OH is undesirable because of the known toxicity of nitro aromatic compounds against animals due to their mutagenic, carcinogenic and teratogenic properties. Citation17,18 The results are in line with the formerly observed insensitivity of A. theophrasti against benzoxazinoids.Citation3–5 It is assumed that root associated microorganisms can trigger effects of benzoxazolinone derived allelochemicals by molecule modifications and by shortening the time of exposure due to degradation processes.
Materials and methods
Pantoea ananatis was isolated from a microbial micro-community colonizing Abutilon theophrasti roots. The identity of the bacterium was confirmed by the DMSZ (Leibniz-Institut - Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig). The bacterium was pre-cultured in LB medium for 2 weeks. One ml of the cultures was added to 50 ml flasks with 25 ml liquid Czapek medium supplemented with 1–5 mg BOA-6-OH (stocks from Dieter Sicker, University of Leipzig). Subsequent culturing was without shaking at 23 °C. The culture media were filtered as soon as the color of the medium had turned luminous yellow and the filtrate was extracted with ethyl acetate. The organic phase was evaporated to dryness in vacuo and the yellow residue was dissolved in ethyl acetate. The fraction was purified on TLC Silgur-25 sheets (Macherey-Nagel) with water-saturated chloroform:EtOAc 3:2 as the liquid phase. The yellow band was extracted with EtOAc, centrifuged and evaporated to dryness. The remaining solid was washed with water. Yellow crystals precipitated during washing. They were collected, and the residual liquid was evaporated. During the first extraction, a portion of the yellow compound turned to orange accompanied by a higher hydrophilicity. The orange portion could be separated by further TLC runs using the liquid phase mentioned above. The orange band was extracted with 50% methanol. The solution was centrifuged and evaporated to dryness.
Identification of 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one based on the following data:
6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one: yellow solid; UV-Vis (MeOH) λmax 223, 250, 291, 380 nm. IR (KBr): [cm−1] = 3443, 3079, 1816, 1622, 1540, 1482. 1H-NMR (DMSO-d6, 300 MHz) δ 7.54 (1 H, s, H-4), 7.04 (1H, s, H-7). 13C-NMR (DMSO-d6, 75 MHz) δ 154.6 (C, C-2), 150.9 (C, C-6), 149.1 (C, C-7a), 132.2 (C, C-5), 123.7 (C, C-3a), 105.6 (CH, C-4), 100.7 (CH, C-7). HR-MS (ESI negative): 195.00497; Calc. C7H4N2O5: 196.01202. EI-MS m/z 196 [M]+ (100), 180 (1), 179 (2), 166 (10), 150 (4), 149 (2), 138 (5), 122 (4), 121 (4), 106 (7), 93 (5), 80 (10), 69 (21), 53 (17), 52 (18), 39 (10). Salt of 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one: orange, amorphous; UV-Vis (MeOH): λmax 231, 291, 418 nm; IR (KBr):
[cm−1] = 3647, 3432, 1699, 1532, 1471, 1439; 1H-NMR (DMSO-d6, 300 MHz) δ 7.20 (1 H, s, H-4), 6.40 (1H, s, H-7). 13C-NMR (DMSO-d6, 75 MHz) δ 160.4 (C, C-2), 158.7 (C, C-6), 155.0 (C, C-7a), 131.7 (C, C-5), 129.3 (C, C-3a), 103.7 (CH, C-4), 99.8 (CH, C-7). The nature of the cation could not be detected by spectroscopy. However, the Czapek medium contains mainly potassium salts, and the orange compound is likely a K+ salt of the 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one
The synthesis of the BOA-6-OH derivative succeeded with Pantoea ananatis protein, horseradish peroxidase (HRP) and Abutilon root surface protein. Pantoea ananatis, cultured as described above with BOA-6-OH, was pelleted at 4000 g for 15 min from cultures that started to turn yellow. The proteins in the supernatant were precipitated on ice with 80% (NH4)2SO4. After centrifugation at 10.000 g, the precipitate was dissolved in 50 mM phosphate buffer pH 5.8 and desalted with PD-columns using the phosphate buffer for elution. Fractions with the highest protein content according to the Bradford method were combined and assayed for peroxidase activity. To determine the class of enzyme responsible for introduction of the NO2-group, additional assays were run with horseradish peroxidase (HRP; Sigma). The assays contained 50 µl 50 mM phosphate buffer pH 5.8, 50 µl of the protein preparation, 160 µM NaNO2, 80 µM BOA-6-OH, 140 µM H2O2, 8 µM FeSO4 and 40 µM catalase inhibitor 3-amino-1,2,4-triazole (Sigma). Assays with HRP were performed with 1 µl enzyme solution (100 mU), 98 µl 50 mM phosphate buffer pH 5.8, 160 µM NaNO2, 80 µM BOA-6-OH, and 140 µM H2O2. After rigorous vortexing with 200 µl EtOAc, the phases were separated by centrifugation for 2 min at 10,000 g, and the organic phase was analyzed for products by HPLC-DAD (Shimadzu) using a 0–100% methanol gradient as described.Citation5
Abutilon theophrasti seeds were purchased from Herbiseed (UK). They were germinated and grown hydroponically under greenhouse conditions for 10 d on cheesecloth-covered containers filled with tap water. The seedlings were transferred successively with their roots to 5 ml 50 mM phosphate buffer pH 5.8 supplemented with protease inhibitor cocktail (Sigma) and sonicated for 5 sec. The resulting protein solution was centrifuged for 10 min at 10000 g and 4 °C. The supernatant was assayed for 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one synthesis using the assay conditions described for the P. ananatis protein for 3 min. All EtOAc phases of the assays were analyzed by HPLC. Calculations of the product amounts based on standard curves established with the purified 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one.
For the degradation of 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one, BOA-6-OH primed Pantoea ananatis cultures were supplemented with 500 µl 40 µM 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one and incubated at 25 °C. Aliquots of the culture medium were taken directly after addition of the nitro-compound and then every hour over a period of 3 h and analyzed by HPLC. The Abutilon root surface protein fraction was assayed for compound degradation by adding 5 µl of 10 mM 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one to 50 µl phosphate buffer (pH 6.5).
The inhibitory activity of the nitro compound was bioassayed with Abutilon theophrasti and Lepidium sativum. Lepidium sativum seeds were purchased from a local garden supplier. Abutilon and Lepidium seedlings (Abutilon 8; Lepidium 15 germinating seeds/ dish) with emerging radicles were transferred on filter paper placed in Petri dishes. The bioassays were performed as described by Macias and coworkers,Citation16 at pH 4.0, 5.0, 6.0 (all 100 mM Mes buffers) and 7.0 (100 mM Hepes buffer) with each 0, 8.6, 86, 430 and 860 µM 6-hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one. Root and shoot lengths were measured after 1.5 d. The bioassays were repeated 5 times. IC50 values were determined as described by Baerson and coworkers.Citation19 The t-test was used for statistical analysis of all data. The error bars are based on the SD. In figures, the data are presented as the mean ± standard deviation. Each data point is based on at least 3 biologic replicates from 3 independent experiments, if not otherwise noted.
Abbreviations
| 6-Hydroxy-5-nitrobenzo[d]oxazol-2(3H)-one | = | NO2-BOA-6-OH |
| BOA-6-OH | = | 6-hydroxybenzoxazolin-2(3H)-one |
| UPLC-ESI-MS | = | Ultra Performance Liquid Chromatography- Electrospray Ionisation Mass Spectrometry |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_material.zip
Download Zip (541.6 KB)References
- Schulz M, Tabaglio V, Marocco A, Macias FA, Molinillo JMG. Benzoxazinoids in rye allelopathy – from discovery to application in sustainable weed control and organic farming. J Chem Ecol 2013; 39:154-74; PMID:23385365; https://doi.org/10.1007/s10886-013-0235
- Schulz M, Wieland I. Variations in metabolism of BOA among species in various field communities - biochemical evidence for co-evolutionary processes in plant communities? Chemoecology 1999; 9:133-41; https://doi.org/10.1007/s000490050044
- Tabaglio V, Gavazzi C, Schulz M, Marocco A. Alternative weed control using the allelopathic effect of natural benzoxazinoids from rye mulch. Agron Sustain Dev 2008; 28:397-401; https://doi.org/10.1051/agro:2008004
- Schulz M, Marocco A, Tabaglio V. BOA detoxification of four summer weeds during germination and seedling growth. J Chem Ecol 2012; 38:933-46; PMID:22614450; https://doi.org/10.1007/s10886-012-0136-4
- Haghi Kia S, Schulz M, Ayah E, Schouten A, Müllenborn C, Paetz C, Schneider B, Hofmann D, Disko U, Tabaglio V, et al. Abutilon theophrasti´s defense against the allelochemical benzoxazolin-2(3H)-one: Support by Actinomucor elegans. J Chem Ecol 2014; 40:128-98; PMID:25432667; https://doi.org/10.1007/s10886-014-0529-7
- Walterson A M, Stavrinides J. Pantoea: insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMS Microbiol Rev 2015; 39:968-84; PMID:26109597; https://doi.org/10.1093/femsre/fuv027
- Barloy-Hubler F, Chéron A, Hellégouarch A, Galibert F. Smc01944, a secreted peroxidase induced by oxidative stresses in Sinorhizobium meliloti 1021. Microbiology 2004; 150:657-64; PMID:14993315; https://doi.org/10.1099/mic.0.26764-0
- Madec S, Pichereau V, Jacq A, Paillard M, Boisset C, Guérard F, Paillard C, Nicolas JL. Characterization of the secretomes of two vibrios pathogenic to mollusks. PLoS one 2014; 9:e113097; PMID:25401495; https://doi.org/10.1371/journal.pone.0113097
- De Maayer P, Chan WY, Rubagotti E, Venter SN, Toth IK, Birch PRJ, Coutinho TA. Analysis of the Pantoea ananatis pan-genome reveals factors underlying its ability to colonize and interact with plant, insect and vertebrate hosts. BMC Genomics 2014; 15:404; PMID:24884520; https://doi.org/10.1186/1471-2164-15-404
- Hara Y, Kadotani N, Izui H, Katashkina JI, Kuvaeva TM, Andreeva IG, Golubeva LI, Malko DB, Makeev VJ, Mashko SV, et al. The complete genome sequence of Pantoea ananatis AJ13355, an organism with great biotechnological potential. Appl Microbiol Biotechnol 2012; 93:331-41; PMID:22159605; https://doi.org/10.1007/s00253-011-3713-5
- Sakihamaa Y, Tamakia R, Shimojia H, Ichibac T, Fukushib Y, Taharab S, Yamasakia H. Enzymatic nitration of phytophenolics: Evidence for peroxynitrite-independent nitration of plant secondary metabolites. FEBS Letters 2003; 553:377-80; PMID:14572654; https://doi.org/10.1016/S0014-5793(03)01059-7
- Winkler R, Hertweck C. Sequential enzymatic oxidation of aminoarenes to nitroarenes via hydroxylamines. Angew Chem 2005; 4:4083-7; https://doi.org/10.1002/anie.200500365
- Zikmundova M, Drandarov K, Bigler L, Hesse M, Werner C. Biotransformation of 2-benzoxazolinone and 2-hydroxy-1,4-benzoxazin-3-one by endophytic fungi isolated from Aphelandra tetragona. Appl Environ Microbiol 2002; 68:4863-70; PMID:12324332; https://doi.org/10.1128/AEM.68.10.4863-4870.2002
- Rousseau B, Dostal L, Rosazza JPN. Biotransformations of tocopherols by Streptomyces catenulae. Lipids 1997; 32:79-84; PMID:9075197
- Ju KS, Parales RE. Nitroaromatic compounds, from synthesis to biodegradation. Microbiol Molec Biol Rev 2010; 74:250-72; PMID:20508249; https://doi.10.1128/MMBR.00006-10
- Macías FA, Marín D, Oliveros-Bastidas A, Castellano D, Simonet AM, Molinillo MGJ. Degradation studies on benzoxazinoids. Soil degradation dynamics of (2r)-2-O-β-D-glucopyranosyl-4-hydroxy-(2h)-1,4-benzoxazin-3(4h)-one (DIBOA-Glc) and its degradation products, phytotoxic allelochemicals from gramineae. Agric Food Chem 2005; 53:538-48; PMID:15686401; https://doi.10/1021/jf048702l
- Bruhn C, Lenke H, Knackmuss J. Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol 1987; 53:208-10; PMID:16347259
- Sax IR, Lewis RJ. Dangerous Properties of Industrial Material. 7th ed., vol. II; New York: Van Nostrand Reinhold; 1989.
- Baerson SR, Sanchez-Moreira A, Pedrol-Bonjoch N, Schulz M, Kagan IN, Agarwal AK, Reigosa MJ, Duke SO. Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J Biol Chem 2005; 280:21867-81; PMID:15824099; https://doi.10.1074/jbc.M500694200
