ABSTRACT
Stressor-induced memory enhancement has previously been shown to involve DNA methylation in the mollusc Lymnaea stagnalis. Specifically, injection of the DNA methylation inhibitor 5-AZA one hour before exposure to a memory-enhancing stressor obstructs memory augmentation. However, the duration of the influence of 5-AZA on this memory enhancement has not yet been examined. In this study, 2 memory-enhancing stressors (a thermal stress and exposure to the scent of a predator) were used to examine whether injection of the DNA methylation inhibitor 5-AZA 24 hours before stress exposure would still impair memory enhancement. Indeed, it was observed that memory is still obstructed when 5-AZA is injected 24 hours before exposure to either of these stressors in Lymnaea. Understanding that 5-AZA still effectively impairs memory enhancement after a period of 24 hours is valuable because it indicates that experimental manipulations do not need to be made within one hour after the injection of this DNA methylation inhibitor and can instead be made within one day (i.e. 24 hours). These results will allow for a future examination of the possible involvement of DNA methylation in memory enhancement related to longer-term stressors or environmental changes. This study further elucidates the involvement of epigenetic changes in memory enhancement in Lymnaea, providing insight into the process of memory formation in this mollusc.
KEYWORDS:
Introduction
Learning and memory involve complex neurobiological processes which allow organisms to adapt to ever-changing environmental conditions. The aerial respiratory behavior of the mollusc Lymnaea stagnalis can be operantly conditioned, which makes it a model organism for studying associative learning and memory.Citation1,2,3 Since this model was first established in 1996,Citation1 many studies have defined various parameters of the conditioned change in behavior. For instance, the memory formed following operant conditioning can be reconsolidatedCitation4 as well as extinguished.Citation5 Additionally, the influence of various environmental stressors on memory formation has been investigated. Specifically, exposure to the scent of a predator (crayfish)Citation6 or to a thermal stress (i.e., an aquatic environment heated to 30°C)Citation7 have both been demonstrated to enhance long-term memory (LTM) formation in this mollusc.
Recently, epigenetic mechanisms such as DNA methylation have been shown to regulate memory formation across species including the mollusc Lymnaea stagnalis.Citation8,9,10 For instance, following fear conditioning in rats, there is a corresponding upregulation of DNA methyltransferase gene expression in the hippocampus.Citation11 In invertebrates, impairing DNA methylation significantly reduces the discriminatory power of olfactory LTM in honeybees.Citation12 DNA methylation is also involved in molluscan memory formation and synaptic plasticity. For instance, application of a DNA methyltransferase inhibitor blocks serotonin-induced long-term potentiation at sensory-motor synapses in Aplysia.Citation13 Additionally, DNA methylation is essential for the enhancement of LTM following operant conditioning in the pond snail Lymnaea stagnalis.Citation9,10 In Lymnaea, LTM formation is enhanced following exposure to the scent of a predator (crayfish)Citation6 or a thermal stress.Citation7 However, when Lymnaea are treated with a DNA methyltransferase inhibitor (5-Aza-2’-deoxycytidine (5-AZA)) one hour before exposure to either of these stressors, memory is no longer enhanced.Citation9,10 Interestingly, 5-AZA does not perturb the formation of ‘normal’ (i.e., non-enhanced) memory in this mollusc.Citation9
A persistent influence of impaired DNA methylation has previously been demonstrated in various forms of learning and memory. In rats, impairing DNA methyltransferase activity immediately following a memory retrieval test blocks the reconsolidation of morphine-associated withdrawal memory 24 hours later.Citation14 Additionally, inhibiting DNA methyltransferase activity 24 hours before acquisition training in honeybees alters extinction learning in an olfactory learning task. Interestingly, neither extinction memory retention, acquisition learning, nor acquisition memory retention were altered in these honeybees when methyltransferase activity was inhibited 24 hours before acquisition training.Citation15 However, the duration of the memory-obstructing effect of the DNA methyltransferase inhibitor 5-AZA on stressor-induced memory enhancement has not previously been examined in Lymnaea. The requirement of initiating an experimental procedure within one hour of 5-AZA application limits the stressors and environmental conditions which can be studied. Thus, to further characterize the influence of 5-AZA in Lymnaea, we aimed to determine whether administrating 5-AZA 24 hours before stress exposure would still result in the impairment of stress-induced memory enhancement.
Materials and methods
Animals
Lymnaea stagnalis were originally obtained from the Vrije University in Amsterdam and subsequently bred in the laboratory environment. As in previous studies, animals were maintained at room temperature in artificial pond water (0.25 g/L Instant Ocean; 0.34 g/L CaSO4) and fed romaine lettuce daily.Citation16 All animals used in this study were adults and ranged in shell length from 21 to 27 mm. Colored markings were applied to the shells of all snails at least 24 hours before the initiation of any experimental procedures for identification purposes.
Operant conditioning of the aerial respiratory behavior
The ability to operantly condition the aerial respiratory behavior of Lymnaea stagnalis was first reported in 1996Citation1 and, thus, this approach has long been used as a means of assessing associative learning and memory formation.Citation2,3 In this study, operant conditioning was conducted as described previously.Citation2,3,9,16 Briefly, snails were placed in 500 mL of hypoxic pond water (unless otherwise specified) and permitted to acclimate for 10 minutes. A tactile stimulus was then applied to the snail's pneumostome (respiratory orifice) each time the animal attempted to open it and perform aerial respiration. Thus, the animal can associate the performance of this aerial respiratory behavior with the application of a tactile stimulus. The application of this stimulus resulted in the immediate closure of the pneumostome, but was not sufficient to elicit the whole body withdrawal response. Snails received one 30 minute training session (TS) and a 30 minute memory test (MT) was administered 24 hours later to assess LTM. The number of attempted pneumostome openings performed by each animal was recorded during the TS and MT for analysis.
Inhibition of DNA methylation
Five-Aza-2’-deoxycytidine (5-AZA) alters epigenetic activity by inhibiting DNA methyltransferase activity and subsequently blocking DNA methylation. In this study, the DNA methyltransferase inhibitor 5-AZA (Sigma-Aldrich) was dissolved in Lymnaea saline to a final concentration of 87 µM.Citation9,10,17 This concentration has previously been demonstrated to block memory enhancement when injected one hour before stress exposure in Lymnaea.Citation9,10 Lymnaea were anesthetized on ice and injected in the foot with 100 µL of either 5-AZA or saline (vehicle control). All animals were then placed in eumoxic home tanks to recover for 24 hours. Following this period of recovery, Lymnaea were exposed to either a) a thermal stress or b) the scent of a predator (crayfish effluent (CE)) and operantly conditioned as described above (refer to and ).
Figure 1. Inhibiting DNA methylation 24 hours before exposure to a thermal stress obstructs memory enhancement. i) Lymnaea were injected with either saline (ii) or 5-AZA (iii) 24 hours before exposure to a thermal stress. After a one hour period of recovery, all snails were operantly conditioned and LTM was assessed 24 hours later. ii) Animals injected with saline demonstrated memory enhancement, with LTM present 24 hours after training (**p < 0.01 relative to the TS). iii) Treatment with the DNA methylation inhibitor 5-AZA impaired this stress-induced memory enhancement.
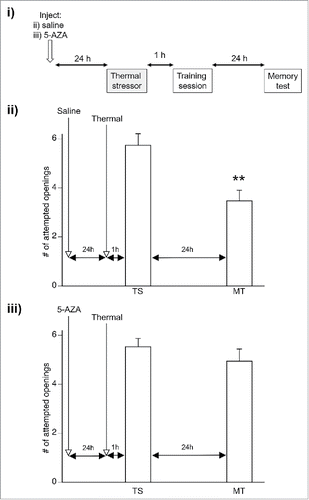
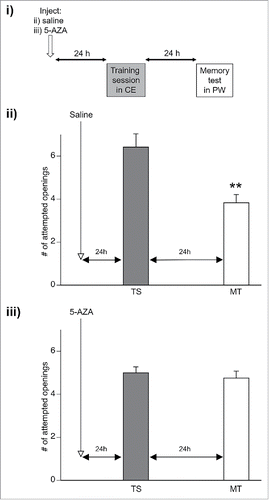
Figure 2. Memory is no longer enhanced by crayfish effluent 24 hours after 5-AZA injection. i) Either saline (ii) or 5-AZA (iii) was injected 24 hours before Lymnaea were operantly conditioned in crayfish effluent (TS; gray bar on graphs). Memory was assessed 24 hours after the TS in normal pond water (PW) ii) Memory was enhanced in animals injected with saline and trained in crayfish effluent (CE). (**p < 0.01 relative to the TS) iii) Animals injected with the DNA methylation inhibitor 5-AZA no longer demonstrated enhanced memory persistence when trained in CE.
Stress exposure
In this study, Lymnaea were exposed to one of 2 stressors known to enhance LTM: a) a thermal stressCitation7 or b) the scent of a predator (crayfish effluent (CE)).Citation6
Thermal stress
The thermal stress was created as described previously.Citation7 A water bath was first heated to 30°C and a 1 L beaker containing 500 mL of clean artificial pond water was then placed in this water bath and allowed to heat to 30°C. Snails were placed in the beaker for one hour and were then permitted to recover in their home tanks (containing room temperature artificial pond water) for one hour. Following this recovery period, all snails were operantly conditioned as described above (refer to ).
Crayfish effluent
Crayfish are a predator of Lymnaea in the wild.Citation18 In this study, exposure to the scent of a predator (Cherax quadricarinatus) was achieved by training Lymnaea in hypoxic crayfish effluentCitation6 (CE; pond water from an aquarium housing a single crayfish fed a diet of snails in the laboratory setting). Following the 30-minute training session in CE, memory was assessed 24 hours later in normal hypoxic pond water (refer to ).
Statistical analysis
Long-term memory (LTM) was defined as a significant reduction in the number of attempted pneumostome openings performed during the memory test (MT) compared with the number observed in the training session (TS).Citation2,3 All data passed the D'Agostino and Pearson omnibus normality test. A 2 way repeated measures ANOVA was used for statistical analysis. Comparisons were made using a Bonferroni post-hoc test and differences were considered significant when p < 0.05. All values are presented as mean ± SEM.
Results
Exposure to 5-AZA 24 hours before a thermal stress impairs memory enhancement
It has previously been demonstrated that impairing DNA methylation one hour before exposure to a thermal stress, obstructs memory enhancement in Lymnaea.Citation10 In this study, we aimed to examine whether memory enhancement would still be impaired when DNA methylation was blocked 24 hours before this same stress exposure. Thus, animals were injected with either saline (vehicle control) or 5-AZA 24 hours before exposure to the thermal stress and were subsequently operantly conditioned (). A 2 way repeated measures ANOVA revealed a significant interaction (F(1, 30) = 4.71, p = 0.038). As expected, animals injected with saline (n = 15; vehicle control group) demonstrated LTM lasting 24 hours (p < 0.01; ), as the number of attempted pneumostome openings in the MT was significantly less than that observed in the TS. However, animals injected with 5-AZA showed no significant change in the number of attempted pneumostome openings between the TS and MT, indicating a lack of LTM formation (p > 0.05; n = 17; . Thus, inhibition of DNA methylation 24 hours before exposure to the thermal stress successfully obstructed the enhancement of LTM persistence in Lymnaea.
Crayfish effluent no longer enhances memory persistence 24 hours after DNA methylation is impaired
The memory-enhancing influence of crayfish effluent (CE) is no longer effective when Lymnaea are injected with the DNA methylation inhibitor 5-AZA one hour before exposure to this stressor.Citation9 Here, we examined whether memory enhancement could occur if 5-AZA was injected 24 hours before stress exposure and Lymnaea were injected with either saline (n = 12; vehicle control) or 5-AZA (n = 12) 24 hours before being trained in hypoxic CE. Memory was assessed 24 hours later in hypoxic pond water (). A 2 way repeated measures ANOVA revealed a significant interaction between condition and session (F(1, 22) = 7.25, p = 0.013). As expected, animals receiving saline injections demonstrated LTM at 24 hours (p < 0.01; ), indicating that CE effectively enhanced memory formation. However, once again, animals injected with 5-AZA did not form LTM, as the number of attempted pneumostome openings performed during the MT was not significantly different than that observed during the TS (p > 0.05; ). Thus, CE-induced memory enhancement was obstructed when DNA methylation was impaired 24 hours before stress exposure and subsequent training.
Discussion
Epigenetic changes are involved in learning and memory formation in many vertebrate and invertebrate species.Citation8 Previous studies indicate that memory enhancement in Lymnaea is impaired when the DNA methylation inhibitor 5-AZA is injected one hour before the animal is exposed to a memory-promoting stressor.Citation9,10 In this study, we extend these findings to further characterize the role of DNA methylation in memory enhancement in this mollusc. Specifically, we demonstrate that impairing DNA methylation 24 hours before exposure to memory-enhancing stressors still obstructs memory augmentation. This provides further insight into the role DNA methylation has in enhancing memory persistence in Lymnaea.
This finding is valuable for designing future experiments because it indicates that the role of DNA methylation in memory enhancement can still be investigated if various experimental manipulations are conducted up to 24 hours after the administration of 5-AZA. In previous studies using Lymnaea short or acute stressors were used to examine the enhancement of memory persistence.Citation9,10 However, it is now possible to examine whether DNA methylation is also involved in the effects of longer-term stressors or environmental changes (up to 24 hours), such as food deprivation or a change in environmental light (to constant light or constant darkness). Additionally, it will be possible to investigate the influence of combinations of stressors which may need to be applied in succession over a period of several hours. It also must be remembered that administration of 5-AZA one hour before training does not perturb the formation of ‘normal’ (i.e., non-enhanced) memory in Lymnaea.Citation9 Thus, the role of DNA methylation in memory enhancement can now be further investigated using several different environmental stimuli over a prolonged period of time.
The observation that 5-AZA is still effective 24 hours post-injection may suggest that its influence is activity dependent. It does not appear to have been broken down or used up within the 24 hour period preceding stress exposure in this study. It remains to be elucidated whether 5-AZA can obstruct memory enhancement if administered earlier than 24 hours before the initiation of an experimental procedure.
Epigenetic changes, such as DNA methylation, are emerging as a common mechanism in memory formation across species.Citation8 Several studies have implicated DNA methylation in various forms of learning and memory formation, as well as synaptic plasticity, in invertebrates. In particular, DNA methylation has been shown to be involved in olfactory LTM in honeybees,Citation12 as well as long-term potentiation at sensory-motor synapses in Aplysia.Citation13 The work using Lymnaea adds to this growing area of research and further highlights the common mechanisms of memory formation between vertebrate and invertebrate species.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada awarded to KL. CMR was supported by an Alberta Innovates – Health Solutions Postdoctoral Fellowship.
References
- Lukowiak K, Ringseis E, Spencer G, Wildering W, Syed N. Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalis. J Exp Biol 1996; 199:683-91; PMID:9318425
- Lukowiak K, Adatia N, Krygier D, Syed N. Operant conditioning in Lymnaea: Evidence for intermediate- and long-term memory. Learn Mem 2000; 7:140-50; PMID:10837503
- Lukowiak K, Cotter R, Westly J, Ringseis E, Spencer G, Syed N. Long-term memory of an operantly conditioned respiratory behaviour pattern in Lymnaea stagnalis. J Exp Biol 1998; 201:877-82; PMID:9464968
- Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J Neurosci 2003; 23:8034-40; PMID:12954865
- Sangha S, McComb C, Lukowiak K. Forgetting and the extension of memory in Lymnaea. J Exp Biol 2003; 206:71-7; PMID:12456698; https://doi.org/10.1242/jeb.00061
- Orr MV, Lukowiak K. Electrophysiological and behavioral evidence demonstrating that predator detection alters adaptive behaviors in the snail Lymnaea. J Neurosci 2008; 28(11):2726-34; PMID:18337402; https://doi.org/10.1523/JNEUROSCI.5132-07.2008
- Teskey ML, Lukowiak KS, Riaz H, Dalesman S, Lukowiak K. What's hot: The enhancing effects of thermal stress on long-term memory formation in Lymnaea stagnalis. J Exp Biol 2012; 215:4322-9; PMID:22972889; https://doi.org/10.1242/jeb.075960
- Zovkic IB, Guzman-Karlsson MC, Sweatt JD. Epigenetic regulation of memory formation and maintenance. Learn Mem 2013; 20:61-74; PMID:23322554; https://doi.org/10.1101/lm.026575.112
- Lukowiak K, Heckler B, Bennett TE, Schriner EK, Wyrick K, Jewett C, Todd RP, Sorg BA. Enhanced memory persistence is blocked by a DNA methyltransferase inhibitor in the snail Lymnaea stagnalis. J Exp Biol 2014; 217:2920-9; PMID:24902747; https://doi.org/10.1242/jeb.106765
- Sunada H, Riaz H, de Freitas E, Lukowiak K, Swinton C, Swinton E, Protheroe A, Shymansky T, Komatsuzaki Y, Lukowiak K. Heat stress enhances LTM formation in Lymnaea: role of HSPs and DNA methylation. J Exp Biol 2016; 219:1337-45; PMID:27208033; https://doi.org/10.1242/jeb.134296
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron 2007; 53(6):857-69; PMID:17359920; https://doi.org/10.1016/j.neuron.2007.02.022
- Biergans SD, Jones JC, Treiber N, Galizia CG, Szyszka P. DNA methylation mediates the discriminatory power of associative long-term memory in honeybees. PLoS One 2012; 7(6):e39349; PMID:22724000; https://doi.org/10.1371/journal.pone.0039349
- Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, Kandel ER. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 2012; 149:693-707; PMID:22541438; https://doi.org/10.1016/j.cell.2012.02.057
- Liu P, Zhang J, Li M, Sui N. Distinctive roles of 5-aza-2’-deoxycytidine in anterior agranular insular and basolateral amygdala in reconsolidation of aversive memory associated with morphine in rats. Front Behav Neurosci 2016; 10:50; PMID:27014010; https://doi.org/10.3389/fnbeh.2016.00050
- Gong Z, Wang C, Nieh JC, Tan K. Inhibiting DNA methylation alters olfactory extinction but not acquisition learning in Apis cerana and Apis mellifera. J Insect Physiol 2016; 90:43-8; PMID:27262427; https://doi.org/10.1016/j.jinsphys.2016.05.007
- Hughes E, Shymansky T, Sunada H, Lukowiak K. Qualitatively different memory states in Lymnaea as shown by differential responses to propranolol. Neurobiol Learn Mem 2016; 136:63-73; PMID:27670620; https://doi.org/10.1016/j.nlm.2016.09.013
- Carpenter S, Rothwell CM, Wright ML, de Hoog E, Walker S, Hudson E, Spencer GE. Extending the duration of long-term memories: Interactions between environmental darkness and retinoid signaling. Neurobiol Learn Mem 2016; 136:34-46; PMID:27646787; https://doi.org/10.1016/j.nlm.2016.09.008
- Nystrom P, Perez JR. Crayfish predation on the common pond snail (Lymnaea stagnalis): the effect of habitat complexity and snail size on foraging efficiency. Hydrobiologia 1998; 368:201-8; https://doi.org/10.1023/A:1003266603371
