ABSTRACT
In animals, when semen is discharged into the uterus, the seminal plasma carries the sperm to the egg. In plants, the function of pollen tube contents (PTC) is analogous to that of the seminal plasma in animals, i.e., carrying sperm cells to the ovules for fertilization. Because the function of the seminal plasma is essential for fertilization in animals, we propose that the function of PTC must be important for plant fertilization. To understand the function of PTC, we examined the transcriptional variation after the release of PTC into the embryo sac. The phenotypic analysis revealed that ovules were enlarged without fertilization when the PTC was released into the ovule, entirely consistent with the transcriptome analysis. We identified a new plant phenomenon, pollen tube-dependent ovule enlargement morphology (POEM) phenomenon that occurs only when the ovule accepts PTC, irrespective of fertilization. POEM is a new phase between the pollen tube guidance and fertilization phases as a reproductive step. Here we established the in vitro POEM assay, which effectively measures POEM activity. Using this assay, we identified that a simple dose of plant hormone(s) cannot induce POEM. We also showed that this assay could be a powerful tool for identifying POEM factor(s).
In angiosperms, the pollen tube, upon insertion into ovules, releases its contents containing sperm cells into the embryo sac and completes double fertilization. Recently, we reported that when ovules failed to be fertilized following pollen tube insertion, they expanded and initiated seed coat formation as if they had undergone fertilization.Citation1 We termed this phenomenon as pollen tube-dependent ovule enlargement morphology (POEM), and it occurs only when the ovule accepts the pollen tube content (PTC). This was the first report in plants concerning the paternal function of PTC in facilitating the maternal development of the ovule without fertilization.
In animals, when semen is discharged into the uterus, the seminal plasma carries the sperm to the egg.Citation2,3 In plants, the function of PTC is analogous to that of the seminal plasma in animals, which is carrying sperm cells to the ovules for fertilization. The seminal vesicle secretory protein 2, which is localized only in the seminal plasma, is required for fertilization in mice.Citation4 The function of the seminal plasma is essential for fertilization in animals; therefore, we propose that the function of PTC must be important for plant fertilization. To understand the function of PTC, we examined transcriptional variation after the release of PTC into the embryo sac using a gcs1 mutantCitation5 that fails to accomplish fertilization even though it releases PTC. We compared the transcriptomes between 2 types of ovule RNAs, one after normal fertilization and the other after the release of PTC without fertilization. At 12 and 24 hours after pollination (HAP), the expression profiles of both RNAs were similar. This result was unexpected because early events after pollen tube insertion were considered to be dependent on fertilization, but these events are dependent on PTC instead. Notably, at 24 and 48 HAP, multiple genes associated with cell expansion, cell division, and seed coat formation were upregulated regardless of fertilization. These results suggested that PTC could affect the shape of the ovules. Hence, we checked the phenotype of the ovules. Interestingly, when ovules accepted PTC, they expanded without fertilization. This expansion resulted from cell expansion and division of ovules and produced a partial seed coat, which was in coherence with the results of our transcriptome analysis. By using the information from the successful transcriptome analysis, we identified a new plant phenomenon POEM that occurs only when the ovule accepts PTC, irrespective of fertilization. In angiosperms, pollination is the first step toward fertilization. Once pollen reaches the stigma, pollen grains elongate to form pollen tubes and move toward synergid cells found within the female gametophyte. Fertilization occurs when pollen tubes pierce the female gametophyte, terminating their growth, and burst inside the female gametophyte to fertilize 2 sperm cells. POEM phenomenon is a new phase between pollen tube guidance and fertilization phases as a reproductive step because PTC discharge itself could induce POEM.Citation1
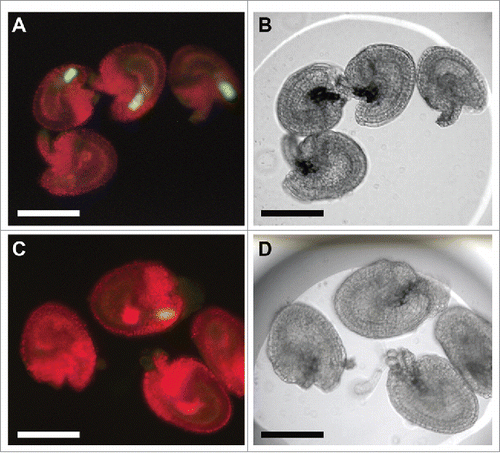
Knowing that the trigger for POEM was PTC, we needed to identify compounds that were required for POEM. To identify the compounds, we set up a new in vitro POEM assay (). To ensure that the method was appropriate for assaying POEM activity, we tested whether POEM is induced by PTC in vitro. We prepared 1 μl droplet of ovule culture medium and sank ovules to the solution. shows that ovules without crossing stayed the same size over 24 hours with the MYB98::GFPCitation6 synergid signal on. As we reported previously, when the PTC was released into the female gametophyte, the synergid cells were disrupted at each pollen tube insertion.Citation7,8 However, ovules crossed by gcs1/gcs1 pollenCitation9 induced POEM without fertilization in the 24 hours after pollination. We compared the area of the female gametophyte both in vivo and in vitro. When we compared the area before POEM with that measured after POEM in vivo, ovules after POEM were found to be 2.5 ± 0.8 times (mean ± SD; n = 100 ovules) larger than those before POEM. Conversely, when we compared these areas in vitro, ovules after POEM were found to be 2.3 ± 0.6 times (n = 10 ovules) larger than those before POEM. These results indicated that the sizes of the female gametophyte after POEM are similar in vivo and in vitro, suggesting that the in vitro POEM assay can measure POEM activity. In our previous transcriptome data,Citation1 one auxin synthesis gene, YUCCA6Citation10, and gibberellin synthesis genes GA20ox1, GA20ox211, GA3ox1, and GA3ox4Citation12 were upregulated by the pollination of WT or gcs1. These suggest that the synthesis of auxins and gibberellins is triggered by PTC somewhere in the harvested sample parts, including ovules and pedicels. Because auxins and gibberellins affect cell division or growth in other tissues, we investigated whether plant hormones can induce POEM. We applied 10 mM, 1 mM, and 0.1 mM 2,4-D or 10 mM, 1 mM, and 0.1 mM GA3 as a single dose, or a mixed dose of 2,4-D and GA3, to the ovule culture medium. No ovule was enlarged by these plant hormones, indicating that applying a plant hormone is not sufficient to induce POEM. These results suggest that a simple dose of plant hormone(s) cannot induce POEM, and compounds in the PTC are essential to induce POEM.
Figure 1. The ovule enlargement phenomenon by in-vitro POEM assay. (A) Ovules with MYB98::GFP synergid cell marker before pollen tube insertion. These ovules were not enlarged 2 d after incubation because no PTC was released into ovules. MYB98::GFP expression is evidence that the ovules have no PTC. One ovule is lacking the GFP signal because the ovule was damaged in the synergid cells due to a dissection error. (B) Bright field image of (A). (C) Ovules after pollen tube insertion. These ovules were enlarged 2 d after incubation. No MYB98::GFP expression is evidence that the ovules accept pollen tubes and PTC into the female gametophyte. (D) Bright field image of (C). Bars, 100 μm. The liquid medium for in vitro ovule culture contained the Nitsch basal salt mixture, 5% trehalose dihydrate, 0.05% MES-KOH, and 1 × Gamborg's vitamin solution.Citation20
It was also found that PTC was able to initiate central cell/endosperm nuclei division without fertilization when the PTC was released to an autonomous endosperm mutant, mea.Citation13,14 Since 1910, it has been known that in animals, segmentation can be induced by physical stimuli, independent of fertilization, causing some eggs to develop into normal tadpoles.Citation15,16 In plants, we discovered that PTC can increase central cell/endosperm nuclei division without fertilization, suggesting functioning parallel to that in animals in which germ cells divide in response to external stimuli, independent of fertilization. By inducing endosperm nuclei division, PTC facilitates apomixisCitation17 in important crops when POEM phenomenon is combined with autonomous endosperm and embryo mutants. In plants, seed formation without fertilization is called apomixis and is valuable for agriculture because the important genetic traits can be easily fixed in apomictic crops, which then propagate without interference from unfavorable environmental conditions. POEM could be categorized as “pseudogamy” defined as any reproductive process requiring pollination but no inheritance from male gametophyte.Citation18 Although FockeCitation19 first defined pseudogamy in 1881, as a part of apomixis, its cellular or molecular mechanisms have remained obscure. POEM may be a key to understanding pseudogamy because of concept similarity, particularly regarding pollen and PTC stimuli.
The prominent analogies between the functions of PTC that we have identified, and those of the seminal plasma blur the boundaries between plants and animals, and advanced research may provide additional clues approaching principles of hidden identity in the male fluid. Furthermore, in plants, these functions could have a great potential for producing apomictic crops.
Data and materials availability
All data needed to evaluate the conclusions in the paper are present in the paper. Additional data will be made available by the authors upon request.
Disclosure of potential conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
We thank E. Matsumoto and N. Iwata for technical assistance. We thank T. Higashiyama for the critical discussion for this project.
Funding
This work was supported by PRESTO (no. 13416724 to R. D. K; Kasahara Sakigake Project), Japan Science and Technology Agency (JST). This work was also supported by grants-in-aid (nos. 25840106 to R. D. K) from the Japanese Society for the Promotion of Science. A part of this work was supported by the Japan Science and Technology Agency (START no. 15657559 and PRESTO no. 15665754 to M.N.).
References
- Kasahara RD, Notaguchi M, Nagahara S, Suzuki T, Susaki D, HonmaY Maruyama D, Higashiyama T. Pollen tube contents initiate ovule enlargement and enhance seed coat development without fertilization. Sci Adv 2016; 2:e1600554; PMID:27819041; https://doi.org/10.1126/sciadv.1600554
- Sasanami T, Izumi S, Sakurai N, Hirata NT, Mizushima S, Matsuzaki M, Hiyama G, Yorinaga E, Yoshimura T, Ukena K, Tsutsui K. A unique mechanism of successful fertilization in a domestic bird. Sci Rep 2015; 9:7700; https://doi.org/10.1038/srep07700
- Sasanami T, Sugiura K, Tokumoto T, Yoshizaki N, Dohra H, Nishio S, Mizushima S, Hiyama G, Matsuda T. Sperm proteasome degrades egg envelope glycoprotein ZP1 during fertilization of Japanese quail (Coturnix japonica). Reproduction 2012; 144:423-31; PMID:22859519; https://doi.org/10.1530/REP-12-0165
- Kawano N, Araki N, Yoshida K, Hibino T, Ohnami N, Makino M, Kanai S, Hasuwa H, Yoshida M, Miyado K, Umezawa A. Seminal vesicle protein SVS2 is required for sperm survival in the uterus. Proc Natl Acad Sci USA 2014; 18:4145-50; https://doi.org/10.1073/pnas.1320715111
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 2006; 8:64-71; PMID:16378100; https://doi.org/10.1038/ncb1345
- Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN. MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 2005; 17:2981-92; PMID:16214903; https://doi.org/10.1105/tpc.105.034603
- Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol 2012; 22:1084-9; PMID:22608509; https://doi.org/10.1016/j.cub.2012.03.069
- Kasahara RD, Maruyama D, Higashiyama T. Fertilization recovery system is dependent on the number of pollen grains for efficient reproduction in plants. Plant Signal Behav 2013; 8:e23690; PMID:23425849; https://doi.org/10.4161/psb.23690
- Nagahara S, Takeuchi H, Higashiyama T. Generation of a homozygous fertilization-defective gcs1 mutant by heat-inducible removal of a rescue gene. Plant Reprod 2015; 28:33-46; PMID:25673573; https://doi.org/10.1007/s00497-015-0256-4
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001; 291:306-9; PMID:11209081; https://doi.org/10.1126/science.291.5502.306
- Plackett ARG, Powers SJ, Fernandez-Garcia N, Urbanova T, Takebayashi Y, Seo M, Jikumaru Y, Benlloch R, Nilsson O, Ruiz-Rivero O, Phillips AL, Wilson ZA, Thomas SG, Hedden O. Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 2012; 24:941-60; PMID:22427334; https://doi.org/10.1105/tpc.111.095109
- Mitchum MG, Yamaguchi S Hanada A, Kuwahara A, Yoshioka Y, Kato T, Tabata S, Kamiya Y, Sun T. Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J 2006; 45:804-18; PMID:16460513; https://doi.org/10.1111/j.1365-313X.2005.02642.x
- Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science 1998; 280:446-50; PMID:9545225; https://doi.org/10.1126/science.280.5362.446
- Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 1999; 96:296-301; PMID:9874812; https://doi.org/10.1073/pnas.96.1.296
- Bataillon E. ‘L'embryogénèse complète provoquèe chez les Amphibiens par figure de l′œuf vierge, larves parthénogénétiques de Rana fusca.’ CR Acad Sci Paris 1910; 150.
- Bataillon E. ‘La parthénogénèse experimentale chez Bufo vulgaria.’ CR Acad Sci Paris 1910; 152.
- Koltunow AM, Grossniklaus U. Apomixis: a developmental perspective. Annu Rev Plant Biol 2003; 54:547-74; PMID:14503003; https://doi.org/10.1146/annurev.arplant.54.110901.160842
- Nogler GA. Gametophytic apomixis. In: Embryology of angiosperms 1984; 475-518.
- Focke WO. Die Pflanzen–mischlinge, ein Beitrag zur Biologie der Gewächse. Borntraeger Berlin 1881.
- Gooh K, Ueda M, Aruga K, Park J, Arata H, Higashiyama T, Kurihara D. Live-cell imaging and optical manipulation of Arabidopsis early embryogenesis. Dev Cell 2015; 34:242-51; PMID:26166301; https://doi.org/10.1016/j.devcel.2015.06.008
