ABSTRACT
Cells are powerful miniature electrophoresis chambers, at least during part of their life cycle. They die at the moment the voltage gradient over their plasma membrane, and their ability to drive a self-generated electric current carried by inorganic ions through themselves irreversibly collapses. Senescence is likely due to the progressive, multifactorial damage to the cell's electrical system. This is the essence of the “Fading electricity theory of aging” (De Loof et al., Aging Res. Rev. 2013;12:58–66). “Biologic electric current” is not carried by electrons, but by inorganic ions. The major ones are H+, Na+, K+, Ca2+, Mg2+, Cl− and HCO3−. Ca2+ and H+ in particular are toxic to cells. At rising concentrations, they can alter the 3D-conformation of chromatin and some (e.g. cytoskeletal) proteins: Calcitox and Protontox. This paper only focuses on Calcitox and endogenous sesquiterpenoids. pH-control and Ca2+-homeostasis have been shaped to near perfection during billions of years of evolution. The role of Ca2+ in some aspects of aging, e.g., as causal to neurodegenerative diseases is still debated. The main anti-Calcitox mechanism is to keep free cytoplasmic Ca2+ as low as possible. This can be achieved by restricting the passive influx of Ca2+ through channels in the plasma membrane, and by maximizing the active extrusion of excess Ca2+ e.g., by means of different types of Ca2+-ATPases. Like there are mechanisms that antagonize the toxic effects of Reactive Oxygen Species (ROS), there must also exist endogenous tools to counteract Calcitox. During a re-evaluation of which mechanism(s) exactly initiates the fast aging that accompanies induction of metamorphosis in insects, a causal relationship between absence of an endogenous sesquiterpenoid, namely the farnesol ester named “juvenile hormone,” and disturbed Ca2+-homeostasis was suggested. In this paper, this line of thinking is further explored and extended to vertebrate physiology. A novel concept emerges: horseshoe-shaped sesquiterpenoids seem to act as “inbrome” agonists with the function of a “chemical valve” or “spring” in some types of multi-helix transmembrane proteins (intramolecular prenylation), from bacterial rhodopsins to some types of GPCRs and ion pumps, in particular the SERCA-Ca2+-pump. This further underpins the Fading Electricity Theory of Aging.
Introduction
In most animal species the typical morphological and physiologic aging features do not start manifesting themselves right after birth, but after a species-specific delay, usually at reproductive maturity.Citation1,2 Yet, instant aging soon after birth is a possibility as illustrated in Hutchinson-Gilford Progeria, a rare fast-aging syndrome in humans. Which mechanism is responsible for the delay? None of the numerous possible driving mechanisms of aging,Citation3 neither alone nor in combination, adequately explain this phenomenon in full. In insects, the partial answer is: a high titer of an ester of the sesquiterpenoid farnesol, which acts as a “juvenile, anti-aging hormone” (JH). Vertebrates also pass through a juvenile/larval stage, just like their common ancestor with the invertebrates, but they do not use some (known) “juvenilizing hormone.” Is this evolutionary divergence due to the loss in an ancient (in)vertebrate ancestor of some essential gene in the biosynthesis of farnesol or some endogenous farnesol-like substance (FLS)? Or do vertebrates use FLS in some other way to obtain a similar juvenilizing effect? Or do we have to search for a still evolutionarily older juvenilizing/aging mechanism that was already present in (some) prokaryotic ancestors of eukaryotes? How to proceed to answer such questions?
In search for the still unidentified membrane receptor for insect JH, a fortuitous flash of new insight emerged when it was realized that farnesol (and related substances (FLS)), the plant toxin thapsigargin (a potent blocker of the SERCA-calcium pump), as well as retinol/retinoids4 all belong to the family of sesquiterpenoids. Retinal is the key cofactor in the proton-pump rhodopsin of prokaryotes (Archaea and Bacteria) and eukaryotes.Citation5 The cis-trans isomerization of retinal under the influence of light energy is essential for visual perception. It is often overlooked that retinoids not only structurally resemble the isoprenoid farnesol (both are isoprenoids),Citation7 but that some retinoids, e.g., retinoic acid (a metabolite of vitamin A (retinol)) also have “juvenile hormone” activity when tested in appropriate insect bioassays.Citation8,4 Apparently, animals lost the capacity to synthesize retinoids by themselves, but they continued to need them in some signaling pathways. This is well documented from studies in developmental biology. Animals solved this problem by ingesting vitamin A with their diet followed by its metabolization into retinal/retinoids. In insects, the onset of metamorphosis when aging phenomena start manifesting themselves is not caused by (absence of) some retinoid, but by the sudden drop of the titre of the endogenous sesquiterpenoid “juvenile hormone.” Despite 50 y of research, the mode of action of JHs, in particular at the level of cell membranes, is not yet fully understood. In contrast, substantial progress has been made in the search for the protein signaling cascade and nuclear receptors/transcription factors (Methoprene-tolerant and its paralog Gce in Drosophila).Citation9-14
In animals endogenous sesquiterpenoids are synthesized in the mevalonate pathway. In vertebrates, this pathway is best known for its role in the biosynthesis of cholesterol and steroids. In insects it is best known for its role in the biosynthesis of juvenile hormone. Because some ancestor of the arthropods seems to have lost the gene coding for the enzyme squalene synthase, the direct precursor of cholesterol in vertebrates (see later), insects cannot synthesize cholesterol by themselves. They have to ingest it as a vitamin. Hitherto, a key role of the mevalonate pathway in Ca2+-homeostasis has not been emphasized in either vertebrates or invertebrates, but exactly this may, in my opinion, be the key functional role of this pathway (this paper).Citation15
The elucidation in 201116 of both the structure of the binding pocket and the mode of action of thapsigargin, the sesquiterpenoid that is the best documented blocker of the SERCA-Ca2+ pump in all eukaryotesCitation16 was, in my opinion, a landmark in thinking about control of Ca2+-homeostasis. It suggested an overlooked causal link between endogenous sesquiterpenoids and control of some aspects of Ca2+-homeostasis. This mechanism seems to have been well conserved in evolution in (all?) eukaryotes. It triggered the formulation of the “Calcitox-aging” concept as outlined in this paper. This (novel) term denotes (accumulating) damage to cells due to rises (“explosions,” “puffs,” “sparks”) in intracellular Ca2+ ([Ca2+]i), in particular long-lasting ones. A serendipitous side aspect of the Calcitox concept is that 50 y after the chemical identification of insect juvenile hormone,Citation17 the (fast) mode of action at the membrane level of this and other endogenous sesquiterpenoids seems to get a plausible explanation.
Because some readers may not be familiar with the basic principles of the divergent topics that are needed to construct the argument, a few concise reminders (probably superfluous for specialists) highlighting the important issues will be given first.
Some introductory principles about Ca2+-homeostasis
To understand why the Ca2+ concentration in cells can drastically and abruptly change, one has to have an idea about the magnitude of the concentration gradient outside-inside cells, as well about the ways that Ca2+ can be compartmentalized in cells.
How steep is the trans-plasma membrane Ca2+-gradient? Where can Ca2+ be stored in the eukaryotic cell?
[Ca2+]i can quickly rise from very low to very high because: 1. there is a huge trans-plasma membrane calcium concentration gradient; 2. Ca2+- channels with various gating mechanisms are present; 3. a cell can store Ca2+ in high concentrations in some intracellular membrane compartments (in particular the endoplasmic reticulum and the mitochondria) from where part of the Ca2+ can be (temporarily) released upon proper stimulation. [Ca2+]i in unstimulated ( = resting) cells amounts to about 100 nM (nM). In such low concentrations Ca2+ is not toxic. [Ca2+] in the extracellular environment (blood e.g.,) is orders of magnitude higher than in the cytoplasm, from around 2 mM in blood to even 50 mM in milk (). [Ca2+]i = 100 nM versus [Ca2+]e = 1 mM represents a gradient of 10,000 times.
Figure 1. A. Schematic representation of the Ca2+ gradient (adapted from ref. Citation15). The different shades of green are not meant to give an exact representation of differences in Ca2+-concentration. B. Ca2+-induced apoptosis resulting in cell death (adapted from Wikipedia: Apoptosis).L: lysosome, N = nucleus, M = mitochondrion, RER = rough endoplasmic reticulum, SER = smooth endoplasmic reticulum. The red dots with 1, 2, and 3 correspond to the mechanisms 1–3 for keeping [Ca2+]i low (See later).
![Figure 1. A. Schematic representation of the Ca2+ gradient (adapted from ref. Citation15). The different shades of green are not meant to give an exact representation of differences in Ca2+-concentration. B. Ca2+-induced apoptosis resulting in cell death (adapted from Wikipedia: Apoptosis).L: lysosome, N = nucleus, M = mitochondrion, RER = rough endoplasmic reticulum, SER = smooth endoplasmic reticulum. The red dots with 1, 2, and 3 correspond to the mechanisms 1–3 for keeping [Ca2+]i low (See later).](/cms/asset/2d76cc45-2447-47db-a7c7-f5fdc72401e9/kcib_a_1341024_f0001_oc.gif)
The steepness of this gradient can be illustrated as follows. Imagine that you are 1.7 meters tall and that you are standing at the bottom of a canyon of which the walls on both sides are about twice as high as the Himalaya mountains, thus about 17,500 m high. Debris and dust are continuously falling down from the walls. If you do not shovel them away, you will be buried by the debris in a short time. This example (1.7 vs. 17,500 m) corresponds to an altitude gradient of 10,000 times. Such a concentration gradient would not pose a problem if the plasma membrane would be impermeable to Ca2+. However, due to the presence in all cell types of Ca2+-channels with different gating mechanisms that is not the case. As a result, Ca2+ enters the cell downhill the Ca2+-gradient ( = passive transport), and the resulting excess Ca2+ has to be dealt with one way or another.
Ca2+ can become toxic when its concentration rises above [Ca2+]i of resting cells for longer time intervals. Cells can cope with increasing [Ca2+]i in various ways. For the transport of Ca2+ against the concentration gradient ( = also called uphill transport), the major proteins involved are the plasma membrane Ca2+- ATPases or PMCAs and the Ca2+-ATPases present in the 2 types of endoplasmic reticulum ATPases (smooth (SER) and Rough (RER)), the (SERCAs). For the passive transport of Ca2+, thus down the Ca2+-gradient ( = also called the downhill transport) the various types of Ca2+-channels are responsible (). As will be dealt with in some more detail later, there are 4 gross mechanisms for keeping [Ca2+]i low.
Figure 2. Classical view of the major players in the system that regulates intracellular Ca2+ compartmentalization. Cellular Ca2+ import through the plasma membrane occurs largely by receptor-operated (for example, glutamate receptors), voltage-sensitive and store-operated channels. Once inside the cell, Ca2+ can either interact with Ca2+-binding proteins or become sequestered into the endoplasmic reticulum (ER) or mitochondria. The largest Ca2+ store in cells is found in the ER or sarcoplasmic reticulum, with local Ca2+ concentrations reaching millimolar levels. Ca2+ levels in the ER are affected by the relative distribution of sarco(endo)plasmatic reticulum Ca2+-ATPase (SERCA) pumps and of inositol-1, 4, 5-triphosphate (Ins(1, 4, 5)P3) receptors (Ins(1, 4, 5)P3Rs) and ryanodine receptors (RYRs), as well as by the relative abundance of Ca2+-binding proteins (calreticulin, calsequestrin) in the ER or sarcoplasmic reticulum. The cytosolic Ca2+ concentration in unstimulated cells is kept at approximately 100 nM by both uptake into the ER and Ca2+ extrusion into the extracellular space by the plasma-membrane Ca2+-ATPase (PMCA). ER Ca2+ release is triggered by agonist stimulation through the generation of Ins(1, 4, 5)P3 through hydrolysis of phosphatidylinositol-4,5-biphosphate (PtdIns(4,5)P2) operated by a phospholipase C (PLCγ). The mitochondria take up Ca2+ electrophoretically through a uniport transporter and can release it again through 3 different pathways: reversal of the uniporter, Na+/H+-dependent Ca2+ exchange, or as a consequence of permeability transition pore (PTP) opening. The PTP can also flicker to release small amounts of Ca2+. Ca2+ efflux from cells is regulated primarily by the PMCA, which binds calmodulin and has a high affinity for Ca2+. Ca2+ efflux might also be mediated by the Na+/Ca2+ exchanger (NCX). [Ca2+], calcium concentration; DAG, diacylglyceride. This figure was kindly provided by Prof. S. Orrenius. It served as the template for a figure in: Orrenius, S., Zhivotovsky, B., Nicotera, P. (2003). Nature Reviews of Cell Biology 4, 552–556.Citation18 The figure was slightly modified by adding the red dots with FLS, suggesting a role for endogenous sesquiterpenoids (FLS) as agonists for Ca2+-ATPases. Such role is more probable for SERCAs than for PMCAs (hence the question mark).With copyright permission for both the figure and the legend from the publisher and from Prof. S. Orrenius.
![Figure 2. Classical view of the major players in the system that regulates intracellular Ca2+ compartmentalization. Cellular Ca2+ import through the plasma membrane occurs largely by receptor-operated (for example, glutamate receptors), voltage-sensitive and store-operated channels. Once inside the cell, Ca2+ can either interact with Ca2+-binding proteins or become sequestered into the endoplasmic reticulum (ER) or mitochondria. The largest Ca2+ store in cells is found in the ER or sarcoplasmic reticulum, with local Ca2+ concentrations reaching millimolar levels. Ca2+ levels in the ER are affected by the relative distribution of sarco(endo)plasmatic reticulum Ca2+-ATPase (SERCA) pumps and of inositol-1, 4, 5-triphosphate (Ins(1, 4, 5)P3) receptors (Ins(1, 4, 5)P3Rs) and ryanodine receptors (RYRs), as well as by the relative abundance of Ca2+-binding proteins (calreticulin, calsequestrin) in the ER or sarcoplasmic reticulum. The cytosolic Ca2+ concentration in unstimulated cells is kept at approximately 100 nM by both uptake into the ER and Ca2+ extrusion into the extracellular space by the plasma-membrane Ca2+-ATPase (PMCA). ER Ca2+ release is triggered by agonist stimulation through the generation of Ins(1, 4, 5)P3 through hydrolysis of phosphatidylinositol-4,5-biphosphate (PtdIns(4,5)P2) operated by a phospholipase C (PLCγ). The mitochondria take up Ca2+ electrophoretically through a uniport transporter and can release it again through 3 different pathways: reversal of the uniporter, Na+/H+-dependent Ca2+ exchange, or as a consequence of permeability transition pore (PTP) opening. The PTP can also flicker to release small amounts of Ca2+. Ca2+ efflux from cells is regulated primarily by the PMCA, which binds calmodulin and has a high affinity for Ca2+. Ca2+ efflux might also be mediated by the Na+/Ca2+ exchanger (NCX). [Ca2+], calcium concentration; DAG, diacylglyceride. This figure was kindly provided by Prof. S. Orrenius. It served as the template for a figure in: Orrenius, S., Zhivotovsky, B., Nicotera, P. (2003). Nature Reviews of Cell Biology 4, 552–556.Citation18 The figure was slightly modified by adding the red dots with FLS, suggesting a role for endogenous sesquiterpenoids (FLS) as agonists for Ca2+-ATPases. Such role is more probable for SERCAs than for PMCAs (hence the question mark).With copyright permission for both the figure and the legend from the publisher and from Prof. S. Orrenius.](/cms/asset/63a20881-9c63-4445-9898-b36aab3a5a76/kcib_a_1341024_f0002_oc.gif)
Ca2+-explosions, puffs, sparks, and waves: A lifelong phenomenon. Life begins and ends with a Ca2+-explosion.
Ridgway et al.Citation19 were the first to report that upon fertilization free calcium increases explosively in activating medaka eggs, hence the name “Ca2+-explosion.” Later it became documented that Ca2+ is the universal signal for egg activation in all sexually reproducing species. It is a finely tuned system. In Xenopus oocytes, the rise in [Ca2+]i lasts several minutes,Citation20 in some other species it even lasts longer. In Xenopus, PMCAs, which are attached to elements of the cytoskeleton in the egg cortex, are internalized during egg maturation because the cytoskeleton contracts due to the rise in [Ca2+]i.Citation21 Therefore they are unable to extrude Ca2+ out of the ooplasm. One could think of this Ca2+-rise as a near death experience for the egg. Sensitivity of the cytoskeleton to Ca2+ is a ubiquitous phenomenon in cell physiology. The most obvious example is muscle contraction. The cytoskeleton may also be of crucial importance for storing “cognitive information.”Citation22 At death, the Ca2+ gradient over the different membrane systems of cells collapses. This is the final Ca2+-explosion cells experience. In between the 2 key explosions, there are numerous smaller rises in [Ca2+]i.
According toCitation23,24 Ca2+-puffs are localized Ca2+ signals mediated by Ca2+ release from the endoplasmic reticulum (ER) through clusters of inositol triphosphate receptor (IP3R) channels. Ca2+-sparks result from the opening of a single, or the coordinated opening of many, tightly clustered ryanodine receptor (RyR) channels in the sarcoplasmic reticulum.Citation25 They can be frequent.Citation26 Normal sparks usually last 10 to 100 milliseconds, but long lasting sparks lasting several hundred milliseconds are also widely observed.Citation27 The recruitment of IP3R channels during puffs depends on Ca2+-induced Ca2+ release, a regenerative process that must be terminated to maintain control of cell signaling and prevent Ca2+ cytotoxicity. Physiological activation is triggered by binding of agonists to G-protein-coupled receptors (GPCRs) on the cell surface, leading to cleavage of phosphatidyl inositol biphosphate and release of IP3 into the cytosol. Many different GPCRs have already been described or predicted.Citation28,29 The human genome harbours about 800 different GPCR-coding genes. In the worm C. elegans the prediction is that there are about 128 GPCRs for neuropeptides alone. Because many GPCRs use Ca2+-puffs in their signaling system, such high figures indicate that Ca2+-puffing occurs very frequently in all cell types.
Lionel JaffeCitation30 measured the speed of Ca2+ waves, which are usually Ca2+-induced but sometimes also H+-induced in a variety of cell types.Citation31 Fast calcium waves (10–30 microns/s) can be propagated through all nucleated eukaryotic cells.
Ca2+ blinks (the corresponding depletion of Ca2+ in the sarcoplasmic reticulum during a spark, and the small Ca2+ release events called quarky SR Ca2+ release (QCR) are also terms used in the literature.Citation32
Why and when can Ca2+ be toxic, and even deadly?
H+ and Ca2+ probably were primordial regulators of the conformation of some macromolecules that were/are instrumental to control of gene expression in the very first cell on earth. Since then they continued performing key functions in cell physiology. Making the conformation of chromatin and of selected proteins in key signaling pathways change substantially, can be considered as a toxic effect. Undoubtedly, the statement that a Ca2+ puff can act as a secondary messenger, a beneficial effect at the (sub)cellular level, because it is toxic is counterintuitive. Whether Ca2+-puffs cause damage mainly depends upon the duration of the puffs ( = the speed of clearance of excess free Ca2+ from the cytoplasm), as well as upon the properties of various cell types with their specific Ca2+-homeostasis systems in which the puffs occur. The frequency of the heartbeat gives an estimate of what is “short” at the organismal level, namely a few seconds maximally. This is the time needed to restore a low [Ca2+]i. In cardiac myocytes the Ca2+-sparks themselves have a typical duration of 20–40 ms.Citation33 The opposite extreme is the long lasting Ca2+ sparks/puffs which induce apoptosis, an extreme form of cell damage and very rapid aging.Citation18,34-36
Control of gene expression by inorganic ions
In addition to changes in the conformation of macromolecules in the cytoplasm, some inorganic ions also have an effect on control of gene expression in both prokaryotes and eukaryotes as known since the 1960s.Citation37-40 In particular the experiments of Kroeger and of LezziCitation41-46 pointed to the importance of the ionic environment for the coarse regulation of gene expression while transcription factors are needed for fine tuning. These authors used induction of puffs (here puff has another meaning than in Ca2+-puffs) in the polytene chromosomes of some dipteran insects as a model. Their experiments urged for caution when interpreting results on control of gene expression by e.g., hormones, in particular when these experiments are executed with cell-tissue homogenates. Indeed, homogenization results in the release of Ca2+ from its subcellular storage sites. This influences the activity of some enzyme systems. The dilemma: is an observed effect of a given treatment on gene expression due to the altered inorganic ion environment itself, or to the interaction of e.g., the hormone under study, with a nuclear receptor? As an example: it has been reported that the change in concentration in nuclear Ca2+ itself controls the T cell fate decision between a proliferative immune response and tolerance.Citation47
Furthermore, one should also keep in mind that hydrophobic signaling molecules (e.g., some steroid and farnesol-like hormones) diffuse through all intracellular membranes () and nestle themselves in suitable binding pockets (see later). This means that such molecules not only act through nuclear receptors/transcription factors (e.g., Methoprene-tolerant and Gce for juvenile hormone), but as well through membrane receptors with a role in Ca2+-homeostasis, some of which can be located in intracellular membranes. To date, the controversy whether lipophilic signaling molecules like JH and some steroids only act through nuclear receptors/transcription factors ( = the classical Karlson hypothesis) or also through membrane receptors as already suggested by Wigglesworth in 1969,Citation48 has not yet been resolved.
Figure 3. Schematic representation of the 2 major models on the mode of action of some lipophilic signaling substances, e.g., Juvenile hormone/FLS and (some) steroids. The classical “Karlson hypothesis” says that such molecules can pass the plasma membrane unhindered, and somehow end up in the nucleus where they act through nuclear receptors/transcription factors. The inbrome modelCitation15 says that lipophilic signaling molecules first enter the whole membrane system and, if they encounter a binding pocket in some of the membrane proteins, they may exert a function. In addition, a second mode of action through nuclear receptors is also a possibility.

Proteins responsible for trans-membrane transport of Ca2+
Structure and main function of Ca2+-ATPases: PMCA and SERCA
The molecular structure of Ca2+ pumps ( and ) and Ca2+ channels is complex. A variety of agents can interfere with their activity. It is the concerted action of all controlling agents that results in keeping [Ca2+]i below the toxic limit by eliminating excess Ca2+ in various ways. The aa-sequence of PMCAs and SERCAs is very similar, suggesting a common evolutionary origin.Citation49
Figure 4. Cartoon depicting the main structural features of the PMCA at resting [Ca2+]i (A) and elevated [Ca2+]i (B). The PMCA consists of an N-terminal domain, 10 transmembrane domains, 4 cytosolic loops and a regulatory C-terminal domain. When [Ca2+]i is elevated, the Ca/CaM binds to the autoinhibitory CaM-binding domain causing a conformational change which exposes the catalytic site which consists the ATP-binding sites and the aspartate residue that is phosphorylated during the reaction cycle. This increases the Ca2+-transporting activity of the PMCA. Reprinted under the Creative Commons Attribution-NonCommercial License from: Bruce J (2013) with thanks.Citation53
![Figure 4. Cartoon depicting the main structural features of the PMCA at resting [Ca2+]i (A) and elevated [Ca2+]i (B). The PMCA consists of an N-terminal domain, 10 transmembrane domains, 4 cytosolic loops and a regulatory C-terminal domain. When [Ca2+]i is elevated, the Ca/CaM binds to the autoinhibitory CaM-binding domain causing a conformational change which exposes the catalytic site which consists the ATP-binding sites and the aspartate residue that is phosphorylated during the reaction cycle. This increases the Ca2+-transporting activity of the PMCA. Reprinted under the Creative Commons Attribution-NonCommercial License from: Bruce J (2013) with thanks.Citation53](/cms/asset/d898825c-0b5b-47ec-b80c-8a6462cb07f2/kcib_a_1341024_f0004_oc.gif)
Figure 5. Schematic representation of the structure of the SERCA2a (10 transmembrane helices) and SERCA2b (11 transmembrane helices) calcium pumps. Slightly modified (by adding a horseshoe-shaped string (in yellow) representing the binding of (endo- or exogenous) sesquiterpenoids linking the TM loops 3, 5 and 7): after Dhitavit et al. in Br J Derm 2004, Vol 150, page 823.Citation55 With permission from the publisher.
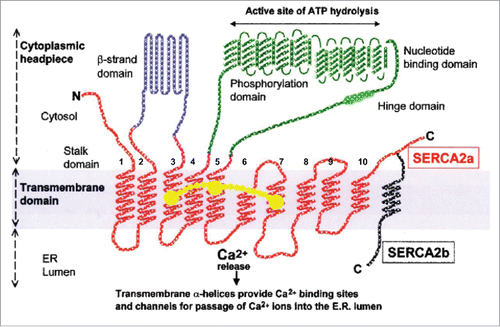
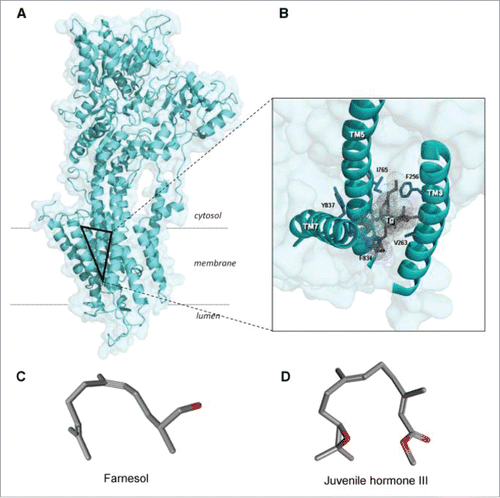
Figure 6. 3-Dimensional view of the SERCA1a and its thapsigargin-binding site (the triangle in A). Thapsigargin (Tg) is a sesquiterpenoid. B. Tilted and enlarged view of the Tg-binding site. From Vandecaetsbeek et al. (2011)Citation16 (Open access). C and D: both farnesol and its ester Juvenile Hormone III are horseshoe-shaped endogenous sesquiterpenoids (From Pubchem: https://pubchem.ncbi.nlm.nih.gov/compound database With thanks).
Plasma Membrane Ca2+ ATPase (PMCA) resides in the plasma membrane and extrudes Ca2+ from the cytoplasm of all eukaryotic cells
PMCAs reside in the plasma membrane of cells and function to remove Ca2+ from the cytosol of cells. In mammals they are encoded by 4 separate genes, and additional isoform variants are generated via alternative RNA splicing of the primary gene transcripts.Citation50,51 They have 10 transmembrane hydrophobic domains (), similar to SERCA2a (). The pump is powered by the hydrolysis of ATP. Another important player for keeping [Ca2+]i very low (at about 100 nmol) is the Na+-Ca2+ exchanger (NCX). Various isoforms of PMCA are expressed in different tissues. PMCAs are regulated by several agents, the most important being calmodulin.Citation52
Sarcoplasmic Reticulum Ca2+ ATPases (SERCAs) pump Ca2+ from the cytoplasm into the sarcoplasmic/endoplasmic reticulum
In higher vertebrates there are 3 SERCA- and 2 SPCA-pump genes which encode P-type ATPases of the P(2A)-group which are respectively found in membranes of the endoplasmic reticulum and the secretory pathway. The SERCA-pump paralogs are named SERCA1, SERCA2, and SERCA3. They are expressed at various levels in different cell types. Various splice variants are known, and the number of proteins reported to interact with SERCA is rapidly growing.Citation16,54
Ca2+-homeostasis: some control mechanisms of the activity of SERCAs and Ca2+-channels
Binding sites on SERCA for inhibitors/activators, e.g., of thapsigargin
A variety of inhibitors of the SERCA Ca2+-pump are known.Citation56,57 We only elaborate on the plant toxin thapsigargin because it is a sesquiterpenoid that, in my opinion, likely acts by displacing a natural endogenous sesquiterpenoid ligand(s) from its binding site on the SERCA pump ().Citation15,58 That ligand could be farnesol itself or a farnesol-like sesquiterpenoid (FLS). The importance of FLS will be outlined later.
Thapsigargin is a sesquiterpene lactone originally extracted for the first time from the plant Thapsia garganica.Citation59,60 Upon binding to its receptor on the SERCA-pump, it causes the release of Ca2+ from an internal store by a mechanism independent of the hydrolysis of phosphoinositides and concomitant activation of protein kinase C.Citation61 It acts in the subnanomolar range. It binds with high affinity. Inhibition leads to elevated [Ca2+]i, which, in its turn, induces apoptosis.Citation18,62-64 It binds to SERCA in a hydrophobic funnel-like cavity open to the cytosol formed by TM3, TM5 and TM7 and prevents the movement of the helices relative to each other.Citation16,65,66 Nine out of the 10 transmembrane loops of SERCA undergo rearrangements during a pumping cycle, enabling the release of Ca2+ into the lumen of the SR, and, on the cytoplasmic side, to create a pathway for entry of new Ca2+-ions (Toyoshima and Nomura (2002).Citation65 This is thought to explain the inhibitory effect of the pump (Takahashi et al. 2007). The amino acids lining the binding pocket for thapsigargin in SERCA1a are fully conserved in SERCA2b (, ).Citation16 Because thapsigargin acts as a SERCA blocker in various eukaryotes, and because SERCAs are very well conserved in evolution, it can be assumed that the binding is well conserved as well. However, this awaits experimental validation.
Does thapsigargin displace an endogenous ligand? Its nature? Its mode of action in inbrome signaling?
To date, it remains unknown whether thapsigargin binds at a pocket that is normally occupied by an endogenous ligand, or that it just binds haphazardly to that site in the SERCA-pump.Citation62 It has been suggested that the binding pocket may be sensitive to cholesterol.Citation62 An alternative hypothesis was advanced namely that thapsigargin, being a sesquiterpenoid, displaces an ubiquitous (in eukaryotic cells) sesquiterpenoid that is formed in the mevalonate biosynthetic pathway, namely farnesol or farnesol-like sesquiterpenoids (FLS).Citation15,58 Thapsigargin can displace an endogenous ligand from its receptor if it binds with a greater affinity.
The possible link with aging becomes apparent if one realizes that in insects, the hormone which keeps larval insects in their juvenile/larval stage, namely the well documented juvenile hormone, is nothing other than an ester of farnesol.Citation67 Furthermore, farnesol that occurs in all eukaryotic organisms, tissues of mammals and Homo inclusive, is also active in bioassays that visualize anti-aging (juvenilizing) effects in insects.Citation68 For figure see ref. 15. This raises the question whether, perhaps, farnesol-like endogenous sesquiterpenoids might also be causal to realize and maintain the juvenile state in vertebrates, and why such compounds are not known as hormone(s) in vertebrates? The answer is: they were not sought for. There are several reasons. One reason is that in vertebrates, unlike in insects, one cannot realize a viable situation in which farnesol/FLS are totally absent. Another reason is that no bioassays have been established in vertebrates allowing the induction or prolongation of typical juvenilizing effects in a relatively short time. The third reason is that physiologists did not consider the possibility that farnesol/FLS, in addition to serving as precursor for the synthesis of squalene and cholesterol, might have another function on their own, namely as a key adjuvant of the SERCA-pump (see later). Textbooks of biochemistry, physiology and endocrinology do not list this possibility.
Ca2+-channels: Various families
Such channels represent the passive aspect of the Ca2+-homeostasis system. They facilitate the influx of Ca2+ from the external environment into the cytoplasm. Sometimes Ca2+-channel is synonymous for voltage-dependent calcium channel. There are also receptor-gated calcium channels and store-operated ones (). In recent years a relatively large family of TRPs, Transient Receptor Potential channels has been described.Citation69 Some of these ion channels are non-selectively permeable to cations, including Na+, Ca2+ and Mg2+.Citation70 Some chemicals act as channel openers, others as blockers. Several synthetic blockers are of important medicinal use. In some tissues, a particular class of Ca2+-channels is sensitive to farnesol.Citation71-75 Free oxygen radicals (ROS) open some types of plasma membrane Ca2+- and K+-permeable channels in plant root cells.Citation76 The role of ROS in Ca2+-signaling and disease is well documented.Citation77
Gross mechanisms for coping with excess Ca2+ in the cytoplasm
Four key mechanisms
The well-known fact that different cell types age at a different pace likely depends, in part, on differences in their Ca2+-homeostasis systems. Such differences are inherent to the principles of differentiation during development. Cells have an array of strategies for coping with rising [Ca2+]i.
The 4 key mechanisms are:
| 1. | If little Ca2+ enters the cell, thus if the plasma membrane is only weakly permeable to Ca2+, the Ca2+-ATPases present in the plasma membrane (PMCAs and the sodium-calcium exchanger (NCX)) will pump the excess Ca2+ out of the cell. Such cells do not have abundant internal membrane systems (RER and SER) (red dot with number 1 in ). | ||||
| 2. | If more Ca2+ enters, some excess can be stored in intracellular storage sites, in particular the sarco/endoplasmic reticulum (SR) and mitochondria. Muscle cells are typical examples (red dot with number 2 in ). | ||||
| 3. | If both mechanisms do not suffice for extruding Ca2+, a third mechanism can be activated, namely the synthesis of proteins for secretion in the rough endoplasmic reticulum (RER)-Golgi system. This system removes excess Ca2+ along with the secreted proteins. A typical example is removal of Ca2+ by the secretion of milk proteins in mammary gland cells (red dot with number 3 in ). | ||||
| 4. | If even this is insufficient, the Ca2+-dependent apoptosis systemCitation18 is activated, and the cell dies. (). | ||||
Indirect effects of [Ca2+] on activity of some enzyme systems
The Ca2+ concentration inside the SER is about 10,000x higher than in the cytoplasm. This explains why in cells at rest, some enzymes that are anchored in the SR-membranes are inhibited and why, upon release of Ca2+, these enzymes can be temporarily activated. This is e.g., the case for some enzymes with a role in lipid- or steroid biosynthesis. When a lipid- or steroid producing cell is in rest, the SER is fully uploaded with Ca2+. The activity of the cited enzymes is very low. Apparently, the high Ca2+-concentration in the SER inhibits their activity. When the cells are stimulated by e.g., a releasing hormone from the brain, a Ca2+ puff will induce a Ca2+-induced release of stored Ca2+ from the SER. As a result of the decreasing Ca2+ concentration in the SER-membranes, some enzyme systems are no longer inhibited. For short while, lipids or steroids will be synthesized. When the released Ca2+- is taken up again in the SER, the enzyme activity will be halted. This system may explain why in vertebrates the release of e.g., pituitary hormones happens in pulses. A similar system has also been observed in the mosquito Aedes aegypti.Citation78 These authors reported that Ca2+-influx enhances neuropeptide activation of ecdysteroids hormone production by mosquito ovaries.
The mevalonate biosynthetic pathway for farnesyl-PP, farnesol, juvenile hormone, cholesterol and steroid hormones: Similarities and differences in invertebrates-vertebrates
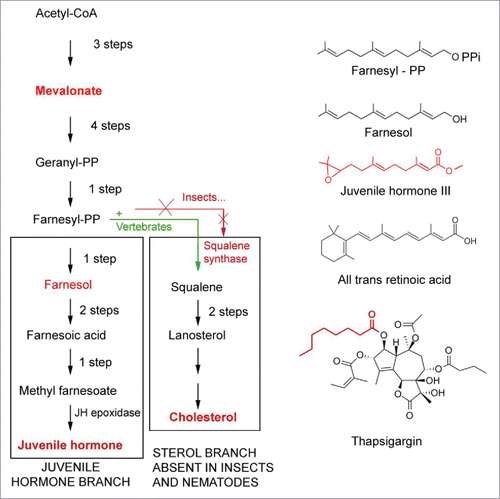
It may not be superfluous to refresh one's insight into the nature of the mevalonate biosynthetic pathway with an emphasis on a key difference between the pathway in vertebrates vs. in insects. Vertebrates and arthropods share the same early steps in the biosynthetic pathway of the endogenous isoprenoid farnesylpyrophosphate ().Citation79 In vertebrates, farnesyl-PP is converted into squalene by squalene synthase. Squalene in its turn is converted into cholesterol that, in addition to other roles, can act as the precursor of steroid hormones. Arthropods and plants cannot synthesize squalene and cholesterol. The widely accepted explanation says that, in insects, this is due to a silencing mutation of the gene coding for squalene synthase in an ancient ancestor of arthropods. Another possibility is that the ancient ancestors of arthropods never had a gene coding for squalene synthase, and that such gene was a specific acquisition in the evolution of chordates/vertebrates. Whatever the right explanation is, the result is that for insects cholesterol is a vitamin that has to be ingested with the food, or that has to be metabolized from particular steroid precursors present in the food (e.g., plant sterols). This food-borne cholesterol can be enzymatically converted into steroids, e.g., ecdysteroids. If the loss of the squalene synthase gene is the correct explanation, the inability to convert farnesyl-PP into squalene must have prompted the mutant ancestor and its progeny to come up with a solution for the problem as to how to cope with the excess farnesyl-PP. The solution seems to have been to transform this compound into farnesol that was next esterified. The addition of an epoxy group made it into a compound with juvenile hormone activity (in bioassays) that is much more potent than farnesol in the same bioassays.Citation48 That explains why, in insects, the corpora allata (CA), very tiny glands connected to the brain, which are the only site of JH synthesis in larvae, can produce enough hormone activity to serve the physiological role of farnesol in vertebrates where e.g., the liver, a large organ, is the major site of synthesis of FLS and cholesterol.
Figure 7. Comparison of the mevalonate biosynthetic pathways in insects and vertebrates. Modified after.Citation15,58,79 Insects do not have the gene coding for squalene synthase. As a result, they cannot synthesize cholesterol by themselves. Some chemical structures of endogenous sesquiterpenoids, retinoic acid and the SERCA-pump blocker thapsigargin (which is also a sesquiterpenoid) are also represented.
Recently, Schenk et al.Citation80 reported that the brain of the annelid worm Nereis virens produces methylfarnesoate of which the major function is the suppression of reproduction. This is achieved by inhibiting the release of stored reserves for the production of yolk and eggs in females committed to reproduction. This is probably a Ca2+-mediated process. This strengthens the hypothesis that sesquiterpenoids are ancient animal hormones that were already active in the marine ancestors of arthropods.Citation80
Farnesol, prenylation, retinoic acid
Farnesol: A noble unknown in vertebrate physiology
Farnesol is derived from the name of the Farnese acacia tree Vachellia farnesiana, from which the compound was isolated for the first time. Vachellia is one of the plant species that is used for extraction of compounds of interest to the perfume industry.Citation81 Farnesol-related sesquiterpenoids are hydrophobic, thus immiscible with water. Upon contact with membranes, which are invariably very rich in lipids, they insert themselves into such membranes and diffuse rapidly all over the interconnected membrane systems of cells: plasma membrane, SER, RER and nuclear envelope, to name the major ones (). Although farnesol is a ubiquitous isoprenoid compound in animals, plants and fungi, little is known about its possible functions except its role as a precursor of squalene or juvenile hormone.
Retinal: An ancient functional precursor of farnesol/FLS? Vitamin A
To better understand the mode of action of farnesol/FLS, some data from the physiological archeology of farnesol may be helpful. The structural- and functional similarities between retinoids (Pubchem: 11-cis-retinal) and FLS/sesquiterpenoids (Pubchem: Farnesol, Juvenile Hormone) are striking.Citation67
Retinoic acid is a metabolite of vitamin A.Citation82 Structurally it is related to insect juvenile hormone.Citation4 (). It has functions in control of growth, regeneration and development (Hox-genes). It affects calcium signaling, e.g., in adult molluscan neuronsCitation83-84 and in mouse ovarian granulosa cells.Citation85 Various retinoids are active in increasing adhesive properties of some cell types.Citation86
Retinal (), is a cofactor in bacterial and eukaryotic rhodopsins.Citation87 Bacterial protorhodopsins were probably H+-pumps. Some evolved into Na+-rhodopsins and Cl−-pumping rhodopsins.Citation6 The ur-proton pump is also thought to be ancestral to the various inorganic ion transporting ATPases present in eukaryotes.Citation88
Figure 8. Left: Schematic representation of the structure of bovine rhodopsin (Wikipedia: Rhodopsin).Citation87 It consists of a protein moiety called scotopsin. Like all opsins it has 7 transmembrane domains, and it belongs to the 7TM G protein-coupled receptor family. The 7 TMs form a pocket where a photoreactive chromophore called retinal covalently binds in a very well-defined and evolutionarily well conserved region, namely to a lysine residue in the seventh transmembrane domain. Isomerization of 11-cis-retinal into all-trans-retinal by light induces a conformational change (bleaching) in opsin. Right: 3D-structure of 11-cis-retinal (from Pubchem). For visualization of the flexibility of the molecule, use the “Animate” function in the Pubchem 2–3 D Conformer tool.
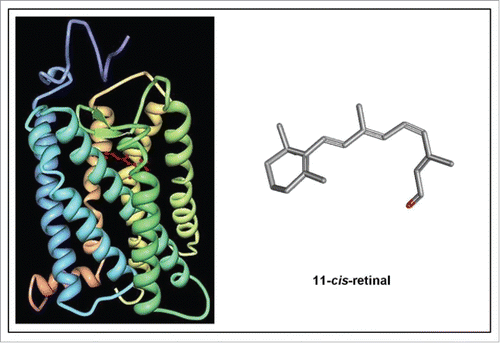
To animal physiologists rhodopsin is best known as a light-sensitive receptor protein involved in visual phototransduction. It is a biological pigment found in the rods of the retina. It is a G-protein coupled receptor (GPCR). When rhodopsin is exposed to light, it immediately photobleaches. In animals this is due to the isomerization of the covalently bound chromophore 11-cis-retinal into all-trans-retinal by light which induces a conformational change (bleaching) in opsin, the protein moiety of rhodopsin that has 7 transmembrane α helices.Citation87 The protein subsequently undergoes a series of relaxations to accommodate the altered shape of the isomerized cofactor. The way retinal is positioned inside rhodopsin and the way H+ is transported is well documented.Citation6
Animal rhodopsins are thought to be derived from ancestral prokaryotic rhodopsins which functioned as proton pumps. In microbial rhodopsins, it is all-trans retinal that photo-isomerizes into the 13-cis form, thus the opposite from the situation in animals. In evolution, other types of ion-pumping microbial rhodopsins appeared: chloride pump, sodium pump, cation channel, sensor (membrane transducer) and sensor (soluble transducer).Citation6 Many animal GPCRs have a rhodopsin-like structure and are therefore thought to be possibly evolutionarily related to prokaryotic rhodopsins.Citation29
Rhodopsins and GPCRs
G protein-coupled receptors (GPCRs), also known as 7TM receptors represent a large protein family of receptors, to which molecules outside the cell selectively bind. They activate internal signal transduction pathways and, ultimately, cellular responses.Citation29 They are only found in eukaryotes. One of the classification systems group the numerous GPCRs in 6 classes. The largest class that codes for about 85% of all the GPRC genes comprises the Rhodopsin-like GPCRs. Such GPCRs still harbour some of the properties of the ancient rhodopsins, the involvement of isoprenoids inclusive.
The GPCR arranges itself into a tertiary structure resembling a barrel, with 7 transmembrane helices forming a cavity within the plasma membrane that serves a ligand domain. The eventual effect of the binding of an agonist is a change in the relative orientation of the TM helices (likened to a twisting motion). It causes activation of a G protein. G proteins cannot diffuse far away due to the palmitoylation of Gα and the presence of an isoprenoid moiety that has been covalently added to the C-termini of Gγ. The primary effectors of Gβγ are various ion channels, some types of (voltage-gated) Ca2+-channels inclusive. Inverse agonists and antagonists may also bind to several different sites, but the eventual effect must be prevention of this TM helix reorientation.
Mode of action of farnesol/FLS? Rotatable bond count. FLS: Important inbromes in control of Ca2+-homeostasis?
One of the chemical properties of farnesol that is seldom focused on in studies of its role in endocrinology is its “rotatable bond count.” Rotatable bond is defined as any single non-ring bond, bounded to terminal heavy (i.e., non-hydrogen) atom. Amide C-N bonds are not considered because of their high rotational energy barrier (from Molinspiration).Citation89 The number of rotatable bonds (nrotb) is a measure of molecular flexibility, an important issue in the functioning of molecules that are cofactors in e.g., multi-helix transmembrane proteins. With its alternation of single and double bonds and a nrotb-value of 5, farnesol is indeed moderately flexible, as are retinal (value 5), retinoic acid (value 5) and JH III (value 8). Thus all these molecules have, in principle at least, the right flexibility to function as “molecular glues” to keep several (usually 3) helices of complex transmembrane proteins well aligned, and bring them back into their “relaxed position” after an activity cycle, e.g., pumping an ion across the membrane. Exactly this property may explain its key function as an inbrome. Inbrome stands for intramembrane signaling molecule, a term introduced for the first time by De Loof et al.Citation15,58 This mode of action of some lipophilic signaling molecules, hormones inclusive, is undervalued in physiology and endocrinology.
With respect to the mode of action of FLS at the level of the membrane, antagonism to the mode of action of thapsigargin is, in my opinion, a plausible explanation. As cited before, thapsigargin binds to SERCA in a hydrophobic funnel-like cavity open to the cytosol formed by the transmembrane loops TM3, TM5 and TM7, and prevents the movement of the helices relative to each other. This is thought to explain the inhibitory effect of the pump.Citation16,66
The likely opposite effect brought about by the natural ligand which, in my opinion, is an endogenous farnesol-like sesquiterpenoid, is gluing together the 3 TMs in a moderately flexible way. This is visualized by the yellow string in . Thus, the basic function of FLS is, in my opinion, to allow temporary movements of transmembrane helices of the SERCA pump during its pumping cycle: stretching followed by narrowing. In other words, farnesol may act as a spring inbrome with the function of a “chemical valve” that makes that the TM3, TM5, TM7 transmembrane loops of SERCAs are brought back in their original position after each Ca2+-pumping cycle. To function this way, the sesquiterpenoid must be firmly anchored (covalently like retinal?) to at least one of the helices. It should be noted that the sidechain of thapsigargin does not have double bonds, making it more flexible than farnesol. Neither does it have methyl-groups. This part of the molecule may not be of prime importance for binding of thapsigargin to SERCA and blocking it.
Farnesol has also been reported to inhibit L-type Ca2+ channels in vascular smooth muscle cells without affecting membrane fluidity or Ca2+-ATPase activities (in vitro).Citation90
Prenylation
Because of the importance of prenylation in the fast-aging Hutchinson-Gilford Progeria syndrome, a short explanation may not be superfluous. Prenylation, a post-translational modification of proteins, involves the enzymatic attachment of a farnesyl-PP or geranylgeranyl-PP moiety to a CAAX motif at the C terminus of a protein () (Wikipedia: Prenylation).Citation91 Such moieties are lipophilic. The enzymes involved are farnesyl transferase or geranylgeranyl transferases.
Figure 9. The 2 major molecules instrumental to Prenylation. See also for the horseshoe-shaped configuration of farnesol.
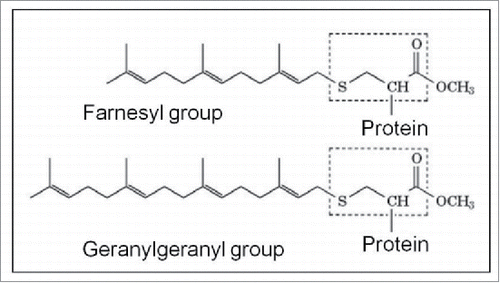
The best known prenylated proteins include the oncogene product p21ras and other low molecular weight proteins GTP-binding proteins, the nuclear lamins, and the γ-subunit of the heterotrimeric G proteins many of which play a role in GPCR-functioning.Citation91,92
Protein prenylation facilitates the anchoring of proteins into the cell membrane and mediates protein-protein interactions.Citation93 The alternation of single and double bonds suggests a moderate degree of flexibility in such adhesion system. Isoprenylated proteins have also key roles in cytoskeletal function, vesicle trafficking and protein functionality, protein maturation, cell growth and in signal transduction via some GPCRs, this list not being exhaustive.Citation93 In a medicinal context, they seem to have a central role in some types of cancer, and they are likely involved in the pathogenesis of and progression of atherosclerosis, Alzheimer disease,Citation94,95 and in the Hutchinson-Gilford Progeria syndrome. In the Discussion, the idea is forwarded to include the gluing of several helices of a multihelix transmembrane protein in prenylation, under the name “intramolecular prenylation,” thereby complementing the classical “intermolecular prenylation.”
The Hutchinson-Gilford Progeria fast aging syndrome in humans: a role for prenylation
Classical analysis of the syndrome
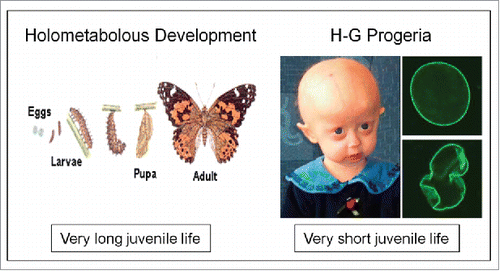
As cited in the introduction, Progeria is an extremely rare (estimated at 1 case in 4 million births) disorder in which symptoms resembling aspects of aging are manifested at a very early age (). The first symptoms develop during the patient's first few months, indicating that such mutants do not have a normal juvenile stage in their development. Apparently, the juvenilizing factor or mechanism, whatever its nature, is missing.
Figure 10. Extremes in longevity: insects vs. patients with the Hutchinson-Gilford Progeria syndrome (modified afterref. 15).
The cause of the syndrome resides in a mutation in a lamin gene, more specifically at position 1824 of the LMNA gene, thus in a protein that contributes in the nucleus to the formation of the nuclear lamina.Citation96 Lamins not only give support to the nucleus (), they also help to organize RNA and DNA synthesis.Citation97
Figure 11. Nuclear lamina (from Wikipedia: Nuclear lamina).Citation97 The localization of lamins just underneath the nuclear envelope is shown in an electron-micrograph and in a schematic representation. For full details, see Wikipedia.
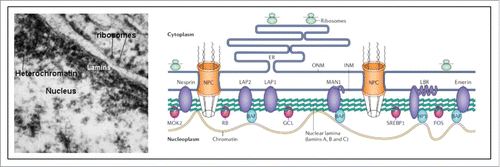
A-type lamins also have a key role in the maintenance of telomere structure, length and function and they also play a role in the DNA damage response pathway.Citation98,99 In normal, non-mutated individuals, the LMNA gene codes for a structural protein called prelamin A. Like all other proteins it is made in the cytoplasm. Here it undergoes a series of processing steps before ending up in its final form, called lamin A, inside the nucleus. One of the steps involves prenylation of prelamin A. This means that the protein undergoes a modification by which a farnesyl- or a geranylgeranyl group is attached. Such lipohilic group helps to associate/attach the protein to membranes. It makes it possible, in proper conditions, to influence other proteins that already reside in the membrane. In the case of prelamin A, the prenylation allows prelamin to temporarily attach to the nuclear rim. Once the protein is attached, it is cleaved by a protease thereby removing the farnesyl group along with a few adjacent amino acids. The truncated protein is now named Lamin A. Lamin A, along with 2 other members of this protein family namely lamin B and lamin C, make up the nuclear rim.
In progeria a mutation generates a cryptic splice site within exon 11. This results in the formation of an abnormal variant of prelamin A from which the farnesyl group cannot be removed. It has been named progerin. It remains permanently attached to the nuclear rim and does not become part of the nuclear lamina. That is thought to be the cause of the abnormal shape of the nuclei (). Progerin production is activated in typical human senescent cells.
When the nuclear lamina is deficient, normal chromatin organization and mitosis are likely compromised.Citation96 To date, it is not known whether or not lamins play a role in enabling the nucleus to control its own ionic environment.Citation100,101
Something overlooked in the lamin A mutation-explanation in Progeria? A thapsigargin-like effect on Ca2+-homeostasis?
Because farnesyl-groups are lipophilic, prenylation helps prelamin A to bind to the nuclear membrane. Like all membranes of the endoplasmic reticulum, the nuclear membrane also contains Ca2+-ATPases. It is thus theoretically possible that the prenyl-group comes in contact with the binding site for thapsigargin on the SERCA-pump, and who knows, also inhibits or overstimulates to some extent the SERCA pumps present in the nuclear envelope.
In my opinion, the misshapen abnormal form of the nucleus may by itself be insufficient for accounting for the numerous symptoms in Progeria. The fact that in Progeria patients mitosis goes on for some years indicates that other mechanisms are playing a role. Furthermore, lamin A does not seem to be necessary for life: it is rather the build-up of prelamin A in the wrong place that matters.Citation102
Maybe one of the prenylated forms of prelamin causes thapsigargin-like effects. There may be other as yet unknown endogenous metabolites that might compete with endogenous sesquiterpenoids for binding to their receptor site. If that indeed occurs, treatment with highly active farnesol-esters, like juvenile hormone and its thousands of synthetic analogs,Citation103 might be tried in rescuing experiments. Treatments with drugs that target some aspects of prenylation, e.g., statins, have been tried, some with positive results.Citation104 However, no treatment has as yet been proven fully effective.
The opposite of Progeria: Extra-long larval/juvenile life in insects
With respect to aging, insects have 2 features that make them into better models than mammals. First, insects usually have a long lasting juvenile life (as larvae) as compared with the duration of their adult-reproductive life. Passing through a series of larval stages with intermittent moults, enables the larvae to stockpile substantial amounts of reserves. This is beneficial for producing either many eggs, or one or a few very big eggs or a larva (e.g., in tsetse flies). By contrast, the juvenile state of mammals, thus the period before gametes start being shed, is short as compared with their adult-reproductive life. In humans, larval life ends around the age of 12 y (puberty).
The extraordinarily long larval-juvenile life of insects is enabled by the ongoing production of juvenile hormone (JH). The rule is that as long as the JH titre remains high, a larva will moult into another larval instar. It has been argued before that the key role of high titres of farnesol/FLS in maintaining the juvenile state is to keep [Ca2+]i low enough to prevent the induction of Ca2+-induced programmed cell death/apoptosis.Citation15 At a given moment in their larval development, they stop producing farnesol and juvenile hormone, they degrade the remaining JH that circulates in the haemolymph, thereby creating a situation of complete absence of farnesol and JH, thus of endogenous sesquiterpenoids. That drop to zero initiates metamorphosis, the onset of fast aging in insects. This is a major argument in favor of a crucial role of farnesol/FLS in promoting some aging effects.Citation58 Such a drop to zero of farnesol/FLS is unknown in vertebrates. This means that in vertebrates FLS are thought to be always present and to be devoid of any hormonal function. However, that does not exclude that they act as “inbromes.”
Chemical nature of arthropod Juvenile hormone
The structure of the first JH was elucidated by Röller and Dahm ( and ).Citation17 Later it was discovered that, depending upon the species, JH occurs in 6 different isoforms.Citation67 All natural JHs are esters of the endogenous sesquiterpenoid farnesol.Citation105 Like farnesol, they are horseshoe-shaped,Citation106 and they are moderately flexible (rotatable bond count of JH III is 8). WigglesworthCitation48 assayed 42 compounds for juvenile hormone activity in a typical insect bioassay. He found that the natural hormone of cecropia ( = Juvenile hormone I or the DL-trans, trans, cis isomer) was the most active one. He also emphasized the importance of the trans, trans configuration at C-2 and C-6 in all the compounds, as well as the importance of a suitable balance between apolar and polar properties. This resembles the situation of retinal in some rhodopsins as cited before. In addition to the natural JHs, many JH-analogues have been synthesized during the past decades. The main purpose was to find novel insecticides (acting as growth regulators). This effort yielded a few commercial insecticides.
JH-receptors: A still ongoing search
Since 1968, numerous studies aiming at unravelling the mode of action of JH have been undertaken, with moderate success. Interesting results have been obtained with respect to some proteinaceous (nuclear) receptors (Methoprene-tolerant, Gce).Citation9-14 However, it is known from electrophysiological studies that JH can exert very fast effects (in seconds).Citation108,109 Such effects can only be generated via membrane receptors. Despite numerous efforts, the isolation of the membrane receptor(s) for juvenile hormone (JH) of insects) and retinoic acid by chromatographic techniques has as yet not been successful.
The mode of application, injection or topical application, matters
One of the reasons of the failure is that JH “sticks to everything,” even to glass, a common saying in the 1960–80ties. But, sticking to everything thereby gluing together transmembrane protein loops may be its overlooked key function. In my opinion, WigglesworthCitation48 was very forethoughtful when he wrote that the juvenile hormone influences the gene-controlled enzyme system within the cells by regulating permeability reactions in cellular membranes. More specifically, he hypothesized that “lipoprotein membranes could be the basic structure concerned, and that the effectiveness of substances with JH activity may rest upon their capacity for gaining access to membranes of oriented lipid.” In an evolutionary perspective, if farnesol substitutes for retinal of prokaryotes as hypothesized in this paper, the binding of FLS in a pocket in a complex multi-helix transmembrane protein may, perhaps, be of the same type as that of retinal in rhodopsins.
When testing JH- and juvenoids for their physiological effects, researchers have to keep in mind that to be physiologically active, the way of application of farnesol/FLS is very important. In insects, injection of farnesol and some other JH-analogs (emulsions prepared with a variety of wetting agents) often gave negative results, while topical application ( = to the surface of the (abraded) cuticle) was very effective.Citation48 The best results were obtained if FLS are administered as a slow release formula, e.g., by mixing them with some oil, e.g., arachis oil. Apparently, a relatively long exposure to the active compound is needed for a physiological response, but it is unclear what the underlying principle might be. My hypothesis is that to be active, JH must be able to operate in a well-defined cyto-architecture, e.g., in an epithelium with internal membranes containing the JH-receptor(s). It may take time (days) to realize such structure. To the contrary, the effects of total absence of JH as is typical at the onset of metamorphosis (e.g., on the protein composition of the haemolymph) usually become visible in less than a day.
Is the thapsigargin receptor also the membrane receptor for farnesol/FLS/juvenile hormone? The missing link with Ca2+-homeostasis?
A positive answer to these questions has already been suggested before.Citation15 In short: as already cited before thapsigargin is, like JH, also a sesquiterpenoid. In my opinion, the most logical explanation why thapsigargin binds to the SERCA pump is that it probably displaces an endogenous farnesol-like sesquiterpenoid from its natural binding site on the SERCA-pump. Both absence of JH and presence of the SERCA Ca2+-blocker thapsigargin induce apoptosis, probably according to the principle of Ca2+-induced apoptosis (in selected tissues).Citation18 However, experimental evidence that FLS bind to the thapsigargin pocket still needs to be forwarded. Kenneth Davey,Citation109,110 using a completely different approach, also came to the conclusion that the membrane receptor for JH (and triiodothyronine as well), may be a Ca2+-ATPase. The membrane receptor for JH is probably a promiscuous receptor, given the fact that about 4,000 synthetic bio analogues of JH are active in various JH-bioassays.Citation15,103
Discussion
As so often in science, serendipity may open novel ways of thinking. My interest in the role of Ca2+ in cellular aging was triggered by the study of how, during insect metamorphosis, the absence of juvenile hormone, an endogenous sesquiterpenoid, that is an ester of farnesol, induces apoptosis in some tissues. Such effect can also be induced by the SERCA pump blocker thapsigargin, which is also a sesquiterpene. Because Primordial Signaling Pathways in cell physiology tend to be very well conserved in evolution, comparing data from prokaryotes, invertebrates and vertebrates in an evolutionary perspective may yield novel insights.
What characterizes the “juvenile state”?
The juvenile state precedes the reproductive state at which aging is usually thought to intensify. What changes at the transition? It is difficult to answer this question univocally in organisms in which this transition is smooth and slow, like in vertebrates. In insects, in particular in species which undergo a complete metamorphosis, this transition is abrupt. It starts early in the last larval instar. In many species it is completed in a few days or weeks. In insects, the juvenile state is characterized by a low [Ca2+]i.Citation15 There is no reason to assume that this should not be the very essence of being a juvenile in vertebrates as well.
Why vertebrates do not need a “juvenilizing hormone”
From an evolutionary perspective, it is intriguing that in contrast to annelids,Citation80 arthropods/insects and probably other less well studied invertebrates as well, vertebrates do not seem to need a specific hormone for realizing a juvenile stage in their development. This is indeed intriguing because they both descend from common prokaryotic and eukaryotic ancestors. As already outlined in the Introduction, in my opinion the sequence of events was: first some prokaryotes started to use retinal as a necessary cofactor in proto-rhodopsin, not as a means to perceive light as such, but as means for harvesting solar energy for their metabolism. Later, some early eukaryotes lost the capacity for synthesizing retinoids, but they could overcome this loss by synthesizing sesquiterpenoids in the mevalonate biosynthetic pathway. Archaeal microbial rhodopsins were probably proton pumps, and had a 7 transmembrane helix configuration.Citation6 Some of such rhodopsins mutated into bacterial rhodopsins that transport other inorganic ions (e.g., Na+, Cl−)6, into the common ion pumps of contemporary eukaryotes (Ca2+-ATPase, Na++K+-ATPases etc.),Citation88 and, last but not least into a huge variety of GPCRs. Thus, many different proteins share a common origin and common structural and physiological features. Hence the possibility exists that a given controlling agent concurrently acts on several functionally related proteins, e.g., actors in Ca2+-homeostasis.
Farnesol extracted from mammalian tissues has juvenile hormone activity.Citation68 The reason why vertebrates do not have to use farnesol/FLS as a hormone is that they can synthesize farnesol/FLS in all their tissues, not necessarily in similar (relative) amounts. Thus, farnesol/FLS can end up in the membranes of the cells in which they are synthesized: no need for transport as hormone. This does not exclude that farnesol that is present in bloodCitation58 can be taken up in some cell types. Experimental data are missing. In larval insects, only the corpora allata, which are tiny glands produce JH. It is transported via the haemolymph to other tissues. Thus, the difference between insects and vertebrates with respect to the role of farnesol/FLS in realizing the juvenile state does not seem to reside in major differences in the chemical nature of the family(ies) of controlling agents, but in the way these compounds are delivered to the membrane system of target cells.
Calcitox: A counterintuitive idea
The emphasis that in teaching and in vulgarizing advertising is given to the beneficial effects of calcium (calcareous skeleton, egg shell, milk, sexual differentiation, Calcigender,Citation111 as secondary messenger etc.) strengthens the perception that it is unlikely that Ca2+ might be toxic and/or causal to major diseases and to aging. To non-specialists in Ca2+-homeostasis, it may require a drastic switch in thinking to accept that Ca2+ is the most ubiquitous toxic pollutant on earth, and that it can serve as a second messenger in cell signaling, undoubtedly a beneficial activity, because it is toxic. In fact, its beneficial effects (at the organismal level) are means to get rid of excess toxic Ca2+.Citation111-113 It is only at very low cytoplasmic concentrations, up to about 100 nM, that Ca2+ is not toxic. Toxicity starts playing a role when [Ca2+]i rises too frequently too high.
It is difficult to unequivocally answer the question from which Ca2+-concentration on damage occurs. It depends upon the situation: which cell/tissue types in which environment and in which stage of development? Indeed, the Ca2+-homeostasis system of any cell is subject to change lifelong. The toxic effects likely occur when there is a Ca2+-explosion or a serious of puffs. Because puffs do not last long, it will be rather the frequency of the puffs that is more important for the accumulation of damage, e.g., to the cytoskeleton. For example, the fall to zero of the FLS titer at the onset of metamorphosis of holometabolous insects induces (Ca2+-induced) programmed cell death in all tissues that actively secrete proteins.Citation15 Meanwhile, tissues that are less active in the production of proteins for secretion such as nervous tissue are not affected. The nervous system continues to further develop normally during metamorphosis. Thus the question to which degree Ca2+ is causal to aging and more specifically to neurodegenerative diseases, needs to be answered with caution.
The cytoskeleton: A key target of Ca2+
Changing concentrations of Ca2+ influence a lot of cellular processes, ranging from the synthesis of proteins, lipids, and steroids, over contributing to controlling chromatin structure and the shape of the cytoskeleton, to induction of Ca2+-induced apoptosis, this list being far from exhaustive.Citation34 The most obvious effect of rising Ca2+ concentrations is muscle contraction. However, not only the cytoskeleton of muscle cells but of all cell types reacts to changes in [Ca2+]i. Many transmembrane proteins are somehow anchored to elements of the cytoskeleton. If an activity cycle of such a protein results in the local release of Ca2+ from an internal storage site, local contraction of the peripheral cytoskeleton may result, with possible displacement of attached proteins as a result. This is well documented in egg activation.Citation21 In internalization of membrane receptors, a similar mechanism may operate. In a recent paper, it has been pointed out that there are good arguments to attribute a major role to the plasma membrane-cytoskeletal complex, and in particular to the electric phenomena that continuously take place in that system, for storing and retrieving cognitive information in the as yet enigmatic cognitive memory system.Citation22 Problems with the cognitive memory system are one of the aspects of aging.
Prenylation: inter- and intramolecular prenylation? A widespread mechanism in multi-helix solute-transporting membrane proteins?
In endocrine studies on the mode of action of juvenile hormone, farnesol and some GPCRs, little attention is given to the importance of prenylation. Yet, it may be an important issue not only for connecting different proteins, e.g., a γ-type G-protein to an ion channel. Indeed, such a connecting/gluing function may also be at work in selected multi-helix transmembrane proteins, in particular to keep the transmembrane helices that form the core of an ion-pumping unit (e.g., TM3, TM5, TM7 in the SERCA pump) or the helices involved in H+-pumping in rhodopsinsCitation6 back into the original relaxed position after each pumping cycle. In such cycle the helices of the active core move away from each other for a short while and distance. They have to be brought back into their “relaxed positions” to prevent uncontrolled flow of ions through the membrane. It is striking that the physiologically active stereoisomers of farnesol and juvenile hormone all have a horseshoe-shape and a moderate flexibility (rotatable count number around 5). It is also striking that trans, trans, cis farnesol is more active in specific JH-bioassays than other stereo-configurations.Citation48 Acting as a hydrophobic molecular spring in a multi-helix transmembrane protein is a new idea in Ca2+-homeostasis and in endocrinology. Perhaps, the term intramolecular prenylation may apply to such activity.
Are FLS with their link to Ca2+-homeostasis causally relevant, directly or indirectly, for some neurological and other disorders? Alzheimer, Progeria, Parkinson, Obesity
Progeria (Hutchinson-Gilford) and Alzheimer are diseases for which there is not yet a (pharmacological) cure. The situation is less dramatic for Parkinson (at least temporarily) and obesity. For diseases in which dysfunctional Ca2+-homeostasis is thought to play a role, e.g., Alzheimer disease (AD),Citation114-119 or Parkinson,Citation120 farnesol/FLS attracted only minor attention. The reason may be that up to a few years ago the hypothesis that endogenous Farnesol/FLS sesquiterpenoids likely act as necessary adjuvants of the SERCA-pump that help to keep the cytoplasmic Ca2+ concentration low,Citation15 these compounds were not considered as key players in Ca2+-homeostasis.
With respect to AD, Liang et al.Citation116 reported that: “Dysregulation of intracellular Ca2+-signaling has been observed as an early event before the presence of clinical symptoms of AD and is believed to be a crucial factor contributing to its pathogenesis. The progressive and sustaining increase in the resting level of cytosolic Ca2+ will affect downstream activities and neural functions. In AD patients there is an increasing Ca2+ release from the endoplasmic reticulum. Hitherto the cause is sought in mutations in the presinilin genes and amyloid-β oligomers.” I suggest that one should consider the possibility that the early cause of AD may, perhaps, be due to a decrease in the production of endogenous farnesol/FLS sesquiterpenoids, or in the displacement of farnesol/FLS from their natural binding pockets on the SERCA pump by lipoic competitors that can bind to the same pocket with higher affinity. Why not give a chance to think about AD as an FLS-deficiency syndrome or as a sort of thapsigargin-like poisoning by some as yet unknown metabolite?
Excessive lipid deposition in some tissues with obesity as a result, is a normal physiological process in insects and other animals that prepare for hibernation, diapause and metamorphosis. In some insects, a major causal agent is the disappearance of juvenile hormone from the blood. The explanation may be that obesity is a normal result of a diminishing Ca2+-gradient over the membranes of the SER. As long as the SER is fully loaded with Ca2+, the enzymes needed for lipid and steroid biosynthesis are inhibited. When Ca2+-release is triggered by e.g., some hormones, the inhibition is temporarily lifted and synthesis of some lipids and steroids can proceed.
Perspectives for pharmacology
Drugs for curing the cited aging-related diseases are still missing. It is unclear how to come up with novel approaches. In the sesquiterpenoid domain of pharmacology, it is probably not widely known that during recent decades, over 4,000 synthetic juvenoids ( = analogues of insect juvenile hormone) have been synthesized.Citation103 The purpose was to find novel insecticides that act as insect growth regulators. Many of these analogues are not toxic to vertebrates. Only a few compounds made it into a commercial insecticide. The other compounds are by now largely forgotten. Perhaps, some may fit in the thapsigargin pocket and act as activators of the SERCA pump to keep [Ca2+]i low. To my knowledge, these compounds were never tested for possible beneficial medicinal effects. Given the fact that the pioneers in this domain, if they are still alive, have passed the retirement age, not much time is left to validate some of their knowledge and recover some of the synthesized compounds.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Thanks to all authors of the figures that I borrowed from Open Access documents as well as Marijke Christiaens for her help with the figures. Also thanks to Michael Gaffney for text correction.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
References
- Cannon ML. What is aging? Disease-a-Month 2015; 61:454-9; PMID:26519984; https://doi.org/10.1016/j.disamonth.2015.09.002
- MS, King M. Biological theories of aging. Disease-a-Month 2015; 61:460-66; PMID:26490576; https://doi.org/10.1016/j.disamonth.2015.09.005
- Pinto da Costa J, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T. A synopsis on aging – Theories, mechanisms and future prospects. Ageing Res Rev 2016; 29:90-112; PMID:27353257; https://doi.org/10.1016/j.arr.2016.06.005
- Adam G, Perrimon N, Noselli S. The retinoic-like juvenile hormone controls the looping of left-right asymmetric organs in Drosophila. Development 2003; 130:2397-06; PMID:12702654; https://doi.org/10.1242/dev.00460
- Palczewski K. Chemistry and biology of vision. J Biol Chem 2013; 287:1612-9; https://doi.org/10.1074/jbc.R111.301150
- Kandori H. Ion pumping microbial rhodopsins. Front Mol Biosci 2015; 2:52; PMID:26442282; https://doi.org/10.3389/fmolb.2015.00052
- Reichrath J, Lehmann B, Carlberg C, Varani J, Zouboulis CC. Vitamins as hormones. Horm Metab Res 2007; 39:71-84; PMID:17326003; https://doi.org/10.1055/s-2007-958715
- Němec V, Kodrík D, Matolín S, Laufer H. Juvenile hormone-like effects of retinoic acid in insect metamorphosis, embryogenesis and reproduction. J Insect Physiol 1993; 39:1083-93; https://doi.org/10.1016/0022-1910(93)90132-B
- Konopova B, Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. PNAS 2007; 104:10488-93; PMID:17537916; https://doi.org/10.1073/pnas.0703719104
- Konopova B, Smykal V, Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous insects. PLoS One 2011; 6(12):e28728; PMID:22174880; https://doi.org/10.1371/journal.pone.0028728
- Charles JP, Iwema T, Chandana Epa V, Takaki K, Rynes J, Jindra M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci U.S.A 2011; 108:21128-33; PMID:22167806; https://doi.org/10.1073/pnas.1116123109
- Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol 2013; 58:181-204; PMID:22994547; https://doi.org/10.1146/annurev-ento-120811-153700
- Wen D, Rivera-Perez C, Abdou M, Jia Q, He Q, Liu X, Zyaan O, Xu J, Bendena WG, Tobe SS, et al. Methyl farnesoate plays a dual role in regulating Drosophila metamorphosis. PLos Genet 2015; 11(3):e1000005038; https://doi.org/10.1371/journal.pgen.1005038
- Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLos Genet 2015; 11(7):e1005394; PMID:26161662; https://doi.org/10.1371/journal.pgen.1005394
- De Loof A, De Haes W, Janssen T, Schoofs L. The essence of insect metamorphosis and aging: electrical rewiring of cells driven by the principles of juvenile hormone-dependent Ca(2+)-homeostasis. Gen Comp Endocrinol 2014; 199:70-85; PMID:24480635; https://doi.org/10.1016/j.ygcen.2014.01.009
- Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 2011; 3(5):a0004184; https://doi.org/10.1101/cshperspect.a004184
- Röller H, Dahm KH. The chemistry and biology of juvenile hormone. Recent Prog Horm Res 1968; 24:651-80; PMID:4882329
- Orrenius S, Zhihotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature Rev Cell Biol 2003; 4:552-65; https://doi.org/10.1038/nrm1150
- Ridgway EB, Gilkey JC, Jaffe LF. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci U.S.A 1977; 74:623-27; PMID:322135; https://doi.org/10.1073/pnas.74.2.623
- El-Jouni W, Jang B, Haun S, Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Dev Biol 2005; 288:514-25; PMID:16330019; https://doi.org/10.1016/j.ydbio.2005.10.034
- El-Jouni W, Haun S, Machaca K. Internalization of plasma membrane Ca2+-ATPase during Xenopus oocyte maturation. Dev Biol 2008; 324:99-107; PMID:18823969; https://doi.org/10.1016/j.ydbio.2008.09.007
- De Loof A. 2016 The cell's self-generated “electrome:” the biophysical essence of the immaterial dimension of Life? Commun Integr Biol 2016; 9(5):e1197446. eCollection; PMID:27829975; https://doi.org/10.1080/19420889.2016.1197446
- Wiltgen SM, Dickinson GD, Swaminathan D, Parker I. Termination of calcium puffs and coupled closings of inositol triphosphate receptor channels. Cell Calcium 2014; 56:157-68; PMID:25016315; https://doi.org/10.1016/j.ceca.2014.06.005
- Lock JT, Smith IF, Parker I. Comparison of Ca2+ puffs evoked by extracellular agonists and photoreleased IP3. Cell Calcium 2017; 63:43-47; https://doi.org/10.1016/j.ceca.2016.11.006
- Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson S, Lederer WJ, et al. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+-channels: a functional unit for regulating arterial tone. Acta Physiol Scand 1996; 64(4):577-87
- Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol 1998; 274(6 Pt 1):C1755-1761; PMID:9611142
- Song Z, Kama A, Weiss JN, Qu Z. Long-lasting sparks: multi-metastability and release competition in the calcium release unit network. PLoS Comput Biol 2016; 12(1):e1004671; PMID:26730593; https://doi.org/10.1371/journal.pcbi.1004671
- Frooninckx L, Van Rompay L, Temmerman L, Van Sinay E, Beets I, Jansen T, Husson SJ, Schoofs L. Neuropeptide GPCRs in C. elegans. Front Endocrin (Lausanne) 2012;3:167 https://doi.org/10.3389/fendo.2012.00167. eCollection 2012.
- Wikipedia: G protein-coupled receptor. (http://en.wikipedia.org/wiki/G protein-coupled receptor) 11/05/2017
- Jaffe LF. Fast calcium waves. Cell Calcium 2010; 48:102-13; PMID:20883893; https://doi.org/10.1016/j.ceca.2010.08.007
- Jaffe LF. A proton-led model of fast calcium waves. Cell Calcium 2004; 36:83-7; PMID:15126059; https://doi.org/10.1016/j.ceca.2003.12.004
- Brochet DX, Yang D, Cheng H, Lederer WJ. Elementary calcium release events from the sarcoplasmic reticulum in the heart. Adv Exp Med Biol 2012; 740:499-509; PMID:22453956
- Sato D, Shannon TR, Bers DM. Sarcoplasmatic reticulum structure and functional properties that promote long-lasting calcium sparks. Biophys J 2016; 110(2):382-90; PMID:26789761; https://doi.org/10.1016/j.bpj.2015.12.009
- Nicotera P, Orrenius S. The role of calcium in apoptosis. Cell Calcium 1998; 23:173-80; PMID:9601613; https://doi.org/10.1016/S0143-4160(98)90116-6
- Nicotera P, Rossi AD. Nuclear Ca2+: physiological regulation and role in apoptosis. Mol Cell Biochem 1994; 135:89-98; PMID:7816060; https://doi.org/10.1007/BF00925964
- Verkhratsky A. Calcium and cell death. Subcell Biochem 2007; 45:465-80; PMID:18193648
- Record MT, Jr, Anderson CF, Mills P, Mossing M, Roe JH. Ions as regulators of protein-nucleic acid interactions in vitro and in vivo. Adv Biophys 1985; 20:109-35; PMID:3914831; https://doi.org/10.1016/0065-227X(85)90033-4
- De Loof A. The electrical dimension of cells: the cell as a miniature electrophoresis chamber. Int Rev Cytol 1986; 104:251-352; PMID:3531065
- Vanden Broeck J, De Loof A, Callaerts P. Electrical-ionic control of gene expression. Int J Biochem 1992; 24:1907-16; PMID:1473603; https://doi.org/10.1016/0020-711X(92)90286-A
- De Loof A, De Haes W, Boerjan B, Schoofs L. The fading electricity theory of ageing: the missing biophysical principle? Ageing Res Rev 2013; 12:58-66; PMID:22940501; https://doi.org/10.1016/j.arr.2012.08.001
- Kroeger H. Chemical nature of the system controlling gene activities in insect cells. Nature 1963; 200:1234-5; PMID:14089937; https://doi.org/10.1038/2001234a0
- Kroeger H. Insect hormones and gene activity. Endocrinol Exp 1971; 5:108-13; PMID:5317415
- Kroeger H. The control of puffing by ions: A reply. Mol Cell Endocrinol 1977; 7:105-110; PMID:863095; https://doi.org/10.1016/0303-7207(77)90059-4
- Kroeger H, Muller G. Control of puffing activity in three chromosomal segments of explanted salivary gland cells of Chironomus thummi by variation in extracellular Na+, K+ and Mg2+. Exp Cell Res 1973; 82:89-94; PMID:4127432; https://doi.org/10.1016/0014-4827(73)90248-6
- Lezzi M. Differential gene activation in isolated chromosomes. Int Rev Cytol 1970; 29:127-68; PMID:4930417
- Lezzi M, Gilbert LI. Differential effects of K+ and Na+ on specific bands of isolated polytene chromosomes of Chironomus tentans. J Cell Sci 1970; 6:615-27; PMID:5452086
- Monaco S, Jahraus B, Samstag Y, Bading H. Nuclear calcium is required for human T cell activation. J Cell Biol 2016; 215(2):231-43; PMID:27810914; https://doi.org/10.1083/jcb.201602001
- Wigglesworth VB. Chemical structure and juvenile hormone activity: comparative tests on Rhodnius prolixus. J Insect Physiol 1969; 15:73-94; https://doi.org/10.1016/0022-1910(69)90213-3
- Guarini D, Zecca-Mazza A, Carafoli E. Single amino acid mutations in transmembrane domain 5 confer to the plasma membrane Ca2+ pump properties typical of the Ca2+ pump of endo(sarco)plasmic reticulum. J Biol Chem 2000; 275:31361-8; PMID:10899160; https://doi.org/10.1074/jbc.M003474200
- Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane Calcium pumps. Physiol Rev 2001; 81:21-50; PMID:11152753
- Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med 2004; 4:323-35; PMID:15101689; https://doi.org/10.2174/1566524043360735
- Carafoli E, Garcia-Martin E, Guerini D. The plasma membrane calcium pump: recent developments and future perspectives. Experientia 1996; 52:091-1100; https://doi.org/10.1007/BF01952107
- Bruce JIE. PMCA. Pancreapedia: Exocrine Pancreas Knowledge Base 2013; https://doi.org/10.3998/panc.2013.7
- Vandecaetsbeek I, Cristensen SB, Liu H, Van Veldhoven PP, Waelkens E, Eggermont J, Raeymaekers L, Møller JV, Nissen P, Wuytack F, Vangheluwe P. Thapsigargin affinity purification of intracellular P2A-type Ca2+ ATPases. Biochim Biophys Acta 2011; 1813:1118-27; PMID:21215281; https://doi.org/10.1016/j.bbamcr.2010.12.020
- Dhitavat J, Fairclough RJ, Hovnanian A, Burge SM. Calcium pumps and keratinocytes: lessons from Darier's disease and Hailey-Hailey disease. Br J Derm 2004; 150(5):821-1; https://doi.org/10.1111/j.1365-2133.2004.05904.x
- Inesi G, Hua S, Xu C, Ma H, Seth M, Prasad AM, Sumbilla C. Studies of Ca2+ ATPase (SERCA) inhibition. J Bioenerg Biomembr 2005; 37(3):5-368
- Michelangeli F, East JM. A diversity of SERCA and East Ca2+ pump inhibitors. Biochem Soc Trans 2011; 39:789-97; PMID:21599650; https://doi.org/10.1042/BST0390789
- De Loof A, Marchal E, Rivera-Perez C, Noriega FG, Schoofs L. Farnesol-like endogenous sesquiterpenoids in vertebrates: the probable but overlooked functional inbrome anti-aging counterpart of juvenile hormone in insects? Front Endocrinol Lausanne 2015; 5:222; PMID:25610425
- Christensen SB. Thapsigargin and thapsigarcin, two histamine liberating sesquiterpene lactones from Thapsia garganica. J Org Chem 1982; 47:649-52; https://doi.org/10.1021/jo00343a009
- Quynh Doan NT, Christensen SB. Thapsigargin, origin, chemistry, structure-activity relationships and prodrug development. Curr Pharm Des 2015; 21:5501-17; PMID:26429715; https://doi.org/10.2174/1381612821666151002112824
- Jackson TR, Pattersen SI, Thastrup O, Hanley MR. A novel tumour promotor, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J 1988; 253:81-6; PMID:3138987; https://doi.org/10.1042/bj2530081
- Winther AM, Liu H, Sonntag Y, Olesen C, le Maire M, Soehoel H, Olsen CE, Christensen SB, Nissen P, Møller JV. Critical roles of hydrophobicity and orientation of side chains for inactivation of sarcoplasmatic reticulum Ca2+-ATPase with thapsigargin and thapsigargin analogs. J Biol Chem 2010; 285:28883-93; PMID:20551329; https://doi.org/10.1074/jbc.M110.136242
- Andersen TB, Lopez CQ, Manczak T, Martinez K, Simonsen HT. Thapsigargin – from Thapsia L to mipsagargin. Molecules 2015; 20:6113-27; PMID:25856061; https://doi.org/10.3390/molecules20046113
- Wang C, Li T, Tang S, Zhao D, Zhang C, Zhang S, Deng S, Zhou Y, Xiao X. Thapsigargin induces apoptosis when autophagy is inhibited in HepG2 cells and both processes are regulated by ROS-dependent pathway. Environ Toxicol Pharmacol 2016; 41:167-79; PMID:26708201; https://doi.org/10.1016/j.etap.2015.11.020
- Toyoshima C, Nomura H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 2002; 418(6898):605-61; PMID:12167852; https://doi.org/10.1038/nature00944
- Takahashi M, Kondou Y, Toyoshima C. Interdomain communication in calcium pump as revealed in the crystal structures with transmembrane inhibitors. Proc Natl Acad Sci U.S.A 2007; 104:5800-5; PMID:17389383; https://doi.org/10.1073/pnas.0700979104
- Wikipedia: Juvenile hormone (http://en.wikipedia.org/wiki/Juvenile hormone) 11/05/2017
- Williams CM, Moorhead LV, Pulis JF. Juvenile hormone in thymus, human placenta and other mammalian organs. Nature 1959; 183:405; PMID:13632741; https://doi.org/10.1038/183405a0
- Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Compr Physiol 2012; 2:563-608; PMID:23728980
- Wikipedia: Transient receptor potential (TRP) channel. (http://en.wikipedia.org/wiki/Transient receptor potential channel) 19/02/2017
- Roullet JB, Spaetgens RL, Burlingame T, Feng ZP, Zamponi GW. Modulation of voltage-gated calcium channels by farnesol. J Biol Chem 1999; 374:25439-6; https://doi.org/10.1074/jbc.274.36.25439
- Luft UC, Bychkov R, Gollasch M, Gross V, Roullet JB, McCarron DA, Ried C, Hofmann F, Yagil Y, Luft UC, et al. Farnesol blocks the L-type Ca2+ channel by targetting the alpha 1 subunit. Arterioscler Thromb Vasc Biol 1999; 19:959-66; PMID:10195923; https://doi.org/10.1161/01.ATV.19.4.959
- Beedle AM, Zamponi GW. Block of voltage-dependent calcium channels by aliphatic monoamines. Biophys J 2000; 79:260-70; PMID:10866952; https://doi.org/10.1016/S0006-3495(00)76288-6
- Bringmann A, Skatchkov SN, Faude F, Enzmann V, Reichenbach A. Farnesol modulates membrane currents in human retinal glial cell. J Neurosci Res 2000; 62:396-402; PMID:11054809; https://doi.org/10.1002/1097-4547(20001101)62:3%3c396::AID-JNR10%3e3.0.CO;2-E
- Zoccarato F, Cavallini L, Alexandre A. The adenosine inhibition of glutamate exocytosis in synaptosomes is removed by the collapse of the vesicle-cytosol deltapH plus the opening of farnesol-sensitive Ca(2+) channels. Cell Calcium 2003; 33:273-82; PMID:12618148; https://doi.org/10.1016/S0143-4160(03)00010-1
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. Free oxygen radicals regulate plasma membrane Ca2+- and K+ permeable channels in plant roots. J Cell Sc 2003; 116:81-8; https://doi.org/10.1242/jcs.00201
- Ambudchar IS, Muallem S. ROS in Ca2+ signaling and disease-part 2. Cell Calcium 2016; 60:153-4; PMID:27553171; https://doi.org/10.1016/j.ceca.2016.08.001
- McKinney DA, Eum JH, Dhara A, Strand MR, Brown MR. Calcium influx enhances neuropeptide activation of ecdysteroids hormone production by mosquito ovaries. Insect Biochem Mol Biol 2016; 70:160-9; PMID:26772671; https://doi.org/10.1016/j.ibmb.2016.01.001
- Bellés X, Martin D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol 2005; 50:181-99; PMID:15355237; https://doi.org/10.1146/annurev.ento.50.071803.130356
- Schenk S, Krauditsch C, Frühauf P, Gerner C, Raible F. Discovery of methylfarnesoate as the annelid brain hormone reveals an ancient role of sesquiterpenoids in reproduction. eLIFE 2016; 5:e17126; PMID:27894418; https://doi.org/10.7554/eLife.17126
- Lapczynski A, Bhatia SP, Letizia CS, Api AM. Fragrance material review on farnesol. Food Chem Toxicol 2008; 46(Suppl 11):S149-156
- Wikipedia: Retinoic acid (http://en.wikipedia.org/wiki/Retinoic acid) 11/05/2017
- Farrar NR, Dmetrichuk JM, Carlone RL, Spencer GE. A novel, nongenomic mechanism underlies retinoic acid-induced growth cone turning. J Neurosci 2009; 29:14136-42
- Vesprini ND, Dawson TF, Yuan Y, Bruce D, Spencer GE. Retinoic acid affects calcium signaling in adult molluscan neurons. J Neurophysiol 2015; 113:172-81; PMID:25343782
- Demczuk M, Huang H, White C, Kipp JL. Retinoic acid regulates calcium signalling to promote mouse ovarian granulosa cell proliferation. Biol Reprod 2016; 95(3):70; PMID:27488031
- De Luca LM, Sasak W, Adamo S, Bhat PV, Akalovsky I, Silverman-Jones CS, Maestri N. Retinoid metabolism and mode of action. Environ. Health Perspect 1980; 35:147-52; PMID:7408796
- Wikipedia: Rhodopsin (http://en.wikipedia.org/wiki/Retinoic acid) 11/05/2017
- Wilson TH, Maloney PC. Speculations on the evolution of ion transport mechanisms. Fed Proc 1976; 35:2174-9; PMID:133032
- Molinspiration, Calculation of molecular properties, Number of Rotatable Bonds. www.molinspiration.com/services/properties.html
- Roullet JB, Xue H, Chapman J, McDougal P, Roullet CM, McCarron DA. Farnesyl analogues inhibit vasoconstriction in animal and human arteries. J Clin Invest 1996; 97:2384-90; PMID:8636420
- Wikipedia: Prenylation (http://en.wikipedia.org/wiki/Prenylation) 19/02/2017
- Sinenski M, Lutz RJ. The prenylation of proteins. Bioassays 1992; 14:25-31
- McTaggart SJ. Isoprenylated proteins. Cell Mol Life 2006; 63:255-67; https://doi.org/10.1007/s00018-005-5298-6
- Cole SL, Vasar R. Isoprenoids and Alzheimer disease: a complex relationship. Neurobiol Dis 2006; 22:209-22; PMID:16406223; https://doi.org/10.1016/j.nbd.2005.11.007
- Hottman DA, Li L. Protein prenylation and synaptic plasticity: implications for Alzheimer's disease. Mol Neurobiol 2014; 50:177-85; PMID:24390573; https://doi.org/10.1007/s12035-013-8627-z
- Wikipedia: Progeria (http://en.wikipedia.org/wiki/Progeria) 11/05/2017
- Wikipedia: Lamin (http://en.wikipedia.org/wiki/Lamin) 111/05/2017
- Gonzalez-Suarez I, Gonzalo S. Nurturing the genome: A type lamins preserve genomic stability. Nucleus 2010; 1:129-35; PMID:21326943
- Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, Morgado-Palacin L, Gapud EJ, Sleckman BP, Sullivan T, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J 2009; 28:2414-27; PMID:19629036; https://doi.org/10.1038/emboj.2009.196
- Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev 2001; 81:1-19; PMID:11152752
- Matzke AJ, Weiger TM, Matzke M. Ion channels at the nucleus: electrophysiology meets the genome. Mol Plant 2010; 3:642-52; PMID:20410254; https://doi.org/10.1093/mp/ssq013
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 1999; 147:913-20; PMID:10579712; https://doi.org/10.1083/jcb.147.5.913
- Sláma K. Insect hormones: more than 50 years after the discovery of insect juvenile hormone analogues (JHA, juvenoids). Terrestrial Arthropod Rev 2013; 6:257-333; https://doi.org/10.1163/18749836-06041073
- Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R, Ocorr K, Williams RT, Wang Y, Ablao KP. Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. PLoS One 2012; 7(6):e39581; PMID:22737247; https://doi.org/10.1371/journal.pone.0039581
- Wikipedia: Farnesol (http://en.wikipedia.org/wiki/Farnesol) 11/05//2017
- Pubchem compound database: (https://pubchem.ncbi.nlm.nih.gov/compound data base): farnesol; JH III, retinal, thapsigargin
- Telfer WH. Intracellular exchange in vitellogenic moth follicles. XVI International Congress of Entomology, Kyoto, Japan, 1980; 3–9 August. Abstract 5S-6, 40
- Ilenchuk TT, Davey KG. Juvenile hormone increases ouabain-binding capacity of microsomal preparations from vitellogenic follicle cells. Can J Biochem Cell Biol 1983; 61:826-31; PMID:6627094; https://doi.org/10.1139/o83-105
- Davey KG. From insect ovaries to sheep red blood cells: a tale of two hormones. J Insect Physiol 2007; 53:1-10; PMID:17126363; https://doi.org/10.1016/j.jinsphys.2006.10.005
- Davey KG. Do thyroid hormones function in insects? Insect Biochem Mol Biol 2000; 30:877-44; PMID:10876133; https://doi.org/10.1016/S0965-1748(00)00061-8
- De Loof A. The essence of female-male physiological dimorphism: differential Ca2+-homeostasis enabled by the interplay between farnesol-like endogenous sesquiterpenoids and sex-steroids? The Calcigender paradigm. Gen Comp Endocrinol 2015; 211:131-46; https://doi.org/10.1016/j.ygcen.2014.12.003
- De Loof A, Vandersmissen T, Marchal E, Schoofs L. Initiation of metamorphosis and control of ecdysteroid biosynthesis in insects: The interplay of absence of juvenile hormone, PTTH, and Ca(2+)-homeostasis. Peptides 2015; 68:120-9; PMID:25102449; https://doi.org/10.1016/j.peptides.2014.07.025
- De Loof A, Schoofs L, Huybrechts R. The endocrine system controlling sexual reproduction in animals: Part of the evolutionary ancient but well conserved immune system? Gen Comp Endocrinol 2016; 226:56-71; PMID:26707056; https://doi.org/10.1016/j.ygcen.2015.12.016
- Mattson MP, Chan SL. Dysregulation of cellular calcium homeostasis in Alzheimer's disease: bad genes and bad habits. J Mol Neurosci 2001; 17:205-24; PMID:11816794; https://doi.org/10.1385/JMN:17:2:205
- Small DH. Dysregulation of calcium homeostasis in Alzheimer's disease. Neurochem Res 2009; 34:1824-9; PMID:19337829; https://doi.org/10.1007/s11064-009-9960-5
- Liang J, Kulasin D, Samarasinghe S. Ca2+ dysregulation in the endoplasmic reticulum related to Alzheimer's disease: A review on experimental progress and computational modelling. Biosystems 2015; 134:1-15; PMID:25998697; https://doi.org/10.1016/j.biosystems.2015.05.003
- Eitan E, Hutchison ER, Marosi K, Comotto J, Mustapic M, Nigam SM, Suire C, Maharana C, Jicha GA, Liu D, et al. Extracellular vesicle-associated Aβ mediates trans-neuronal bioenergetic and Ca2+-handling deficits in Alzheimer's disease models. NPJ Aging Mech Dis 2016; 2:pii: 16019; https://doi.org/10.1038/npjamd.2016.19
- Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell 2007; 6:267-73; PMID:17517038; https://doi.org/10.1111/j.1474-9726.2007.00296.x
- Camandola S, Mattson MP. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease. Biochim Biophys Acta 2011; 1813:965-73; PMID:20950656; https://doi.org/10.1016/j.bbamcr.2010.10.005
- Mattson MP. Parkinson's disease: don't mess with calcium. J Clin Invest 2012; 122:1195-8; PMID:22446181; https://doi.org/10.1172/JCI62835
