?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Trichoderma
is a saprophytic, soil-borne fungus with a worldwide distribution that has been extensively studied due to their capacity to synthesize secondary metabolites with antimicrobial activity, parasitize other fungi and directly interact with plant roots, inducing resistance to disease and tolerance to abiotic stresses. Fusarium wilt caused by the soil-inhabiting fungus Fusarium oxysporum is considered one of the most important diseases that affect banana cultivars. Currently, more environmentally friendly alternatives to control this disease are being proposed, these strategies include the application of low doses of synthetic fungicides and the use of biocontrol agents such as Trichoderma or Xylaria. Thus, this study aimed to evaluate under in vitro conditions the synergistic effect of the biological control agent T. reesei C2A combined with low doses of mancozeb to inhibit the mycelial growth of F. oxysporum F1. To perform the synergistic essays, 0.1 mg/mL of mancozeb was suspended in PDA plates, then plugs of T. ressei C2A were placed at the center of the Petri dishes, the plates were incubated for 7 days at 28°C. Results showed that the mycoparasitic capacity of the biocontrol strain to inhibit the mycelial growth of F. oxysporum F1 was enhanced approximately 36% compared to the control plates. Although these results are promising, future studies under greenhouse and field conditions are necessary to corroborate the effectiveness of this approach.
1. Introduction
Banana is a tropical fruit that grows in more than 130 countries, it ranks amongst the world’s most valuable primary agricultural commodity due to its nutritional properties [Citation1,Citation2]. In the sixties, banana industry experienced dramatic losses due to Fusarium wilt caused by the soil-borne fungus Fusarium oxysporum [Citation3,Citation4]. Fusarium wilt was detected in Ecuador in 1936 at the United Fruit plantations in Tenguel [Citation5]. F. oxysporum penetrates the plant through the tertiary roots, then passes into the rhizome vascular system and pseudostem and invades the xylem vessels. The fungus produces conidia, which is carried along the vascular bundles where they start new areas of infection, causing their obstruction preventing water transport and reducing nutrients uptake [Citation6]. It also secretes toxins that induce wilting by altering cell metabolism [Citation7], withering by F. oxysporum remains a latent concern as it represents a threat to the production of banana in Ecuador, causing considerable manufacturing and exporting losses.
The main agricultural method to control this pathogen is based on the application of synthetic fungicides or sterilants, including mancozeb, methyl bromide and quaternary ammonium, which can be applied as powdered, emulsions, granulated, or solutions [Citation8]. Mancozeb is a dithiocarbamate, non-systemic agricultural fungicide with high spectrum of biological activities against a wide range of pathogenic fungi including ascomycetes, oomycetes and basidiomycetes [Citation9,Citation10]. Although the application of synthetic fungicides to control the growth of phytopathogens has been satisfactory in most cases, evidence suggests that synthetic compounds including benomyl, chlorothalonil, captan, mancozeb, maneb and propiconazole can cause adverse health effects on humans and the environment [Citation11,Citation12]. Thus, more environmentally friendly alternatives to control the growth of phytopathogens are currently being proposed. These strategies include the application of low doses of synthetic fungicides combined with the inoculation of biological agents such as nonpathogenic fungi (Trichoderma, Clonostachys and Xylaria) or bacteria (Bacillus, Lysinibacillus and Solibacillus) [Citation13,Citation14]. It has been documented that these microorganisms exhibit strong antagonistic interactions (i.e. antibiosis, parasitism and competition for space and nutrients) with plant pathogens to maintain they growth at a lower density that would occur in the absence of biological competitors [Citation14,Citation15].
Trichoderma is considered as a potential ecological alternative to transform agricultural systems highly dependent on synthetic inputs into sustainable and more productive systems [Citation16]. Under in vitro and greenhouse conditions, several strains of Trichoderma were able to inhibit the growth of a wide range of phytopathogens [Citation17–20]. Trichoderma species are also considered important plant growth-promoting fungi (PGPF) as they significantly facilitate plant growth and development by increasing solubilization of organic compounds increasing nutrient efficacy, releasing plant-growth stimulatory agents and inducing systemic resistance [Citation21–25]. This study aimed to establish under in vitro conditions an environmentally friendly alternative to control the mycelial growth of the phytopathogen causing banana Fusarium wilt. This strategy consisted of evaluating in solid media the synergistic interaction of the biological control agent T. reesei C2A combined with low doses of mancozeb to inhibit the mycelial growth of F. oxysporum F1.
2. Materials and methods
2.1. Fungal strains and fungicide used
This study was conducted at the Centro de Investigaciones Biotecnológicas del Ecuador (CIBE) from the Escuela Superior Politécnica del Litoral (ESPOL). The fungal strains T. reesei C2A (biological control) and F. oxysporum F1, F2 and F3 (phytopathogens) were obtained from CIBE’s Microbial Culture Collection. Before carrying out the sensitivity and synergistic essays, the strains were reactivated three times in Potato Dextrose Agar (PDA) and grown for 7 days at 28°C, or until the mycelium sporulated. The selection of the pathogenic strain used in this study was conducted quantitatively using a Kruskal–Wallis test with multiple comparisons (p < 0.05) finding that F1 diameter growth was statistically different that F2 and F3 while confronted to T. reesei C2A for 2 days (Results not shown). Mancozeb was purchased from a certified commercial house and was used to perform the sensitivity and synergistic assays.
2.2. Macroscopic and microscopic characterization of the pathogenic and beneficial fungi
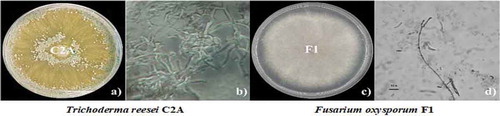
The identity of the fungal strains was confirmed by macroscopic and microscopic characteristics such as the presence of conidia, mycelium coloration and presence of hyphae. Three Petri dishes containing PDA were inoculated with 8 mm plugs of both fungi, the plates were incubated for 7 days at 28°C before performing the macroscopic and microscopic identification.
2.3. In vitro experimental design
2.3.1. Dual culture plate assay
The mycoparasitic capacity of T. reesei C2A to control the growth of F. oxysporum F1 was evaluated by the dual culture plate essay as described elsewhere [Citation26,Citation27]. The tests consisted of the inoculation of 8 mm plugs containing agar and mycelium of T. reesei C2A on one side of a Petri dish containing PDA, on the other side of the plate, a mycelial plug of F. oxysporum F1 was inoculated maintaining a distance of 6 cm between each disc. Plates were then incubated for 7 days at 28°C. T. reesei C2A mycoparasitism was measured daily using the antagonistic capacity index described in .
Table 1. Scale used to measure the antagonistic capacity of T reesei C2A
2.3.2. Minimum inhibitory concentration assays
The application of different doses of mancozeb against the beneficial strain T. reesei C2A and the pathogenic strain F. oxysporum F1 was evaluated by establishing the minimum inhibitory concentration. Mancozeb was suspended in sterile-distilled water and added to Petri dishes containing molten PDA to achieve final concentrations of 0.1, 0.01, 0.001 and 0.0001 mg/mL, respectively. Once the agar solidified, 8 mm plugs containing agar and mycelium of each fungus were transferred to the center of the plates. The plates were incubated for 7 days at 28°C, mycelial growth of the colonies were measured in two directions (vertically and horizontally) and reported as the average value of the two measurements [Citation19].The experiment was performed in triplicate and carried out three times to ensure the reproducibility of the results.
2.3.3. Determination of the percentage inhibition of diameter growth (PIDG)
The percentage inhibition of diameter growth (PIDG) was used to determine the ability of mancozeb to inhibit the mycelial growth of both the pathogen F. oxysporum F1 and the beneficial fungus T. reesei C2A [Citation29]. The PIDG was estimated by measuring the diameter growth of the fungal strains inoculated in Petri dishes containing 15 mL of PDA supplemented with 4 different concentrations of mancozeb (0.1, 0.01, 0.001 and 0.0001 mg/mL) comparing to the growth of the positive controls. Cultures were incubated for 7 days at 28°C and mycelia measuring growth was performed every 24 hours.
2.3.4. Synergistic assays using mancozeb
Synergistic inhibition of the mycelial growth of the pathogen F. oxysporum F1, was assessed based on the protocol described by [Citation30]. In vitro tests were performed in PDA Petri dishes supplemented with 0.1 mg/mL of mancozeb. 15 mL of molten PDA were transferred to a sterile 15 mL Falcon tube, an aliquot of mancozeb from a stock solution (3 mg/mL) was added to the tube to obtain a final concentration of 0.1 mg/mL (v/v), the agar was then poured into the Petri dish and gently shaken to get a homogenous mixture. Once the agar solidified, 8 mm plugs containing agar and mycelia of 5-day fungi cultures of T. reesei C2A and F. oxysporum F1 were inoculated at each side of the Petri dish at 1.5 cm from the edge and maintaining 6 cm of distance between each disc. Plates were then incubated for 7 days at 28°C. The percentage of inhibition of F. oxysporum F1 was calculated using the equation described by [Citation30,Citation31]. All experiments were performed in triplicate and independently replicated three times.
where %I = inhibition percentage (%), A1 = area of the Petri dish in mm2 covered by Fusarium oxysporum F1, control plate that was not added the commercial fungicide (mancozeb), A2 = area of the Petri dish in mm2 covered by Fusarium oxysporum F1 co-inoculated with T. reesei C2A and 0.1 mg/mL of mancozeb.
2.4. Data analysis
Macroscopic and microscopic identification was performed by examining the morphological characteristics of the mycelial growth. Qualitative data obtained from the dual culture plate essays was analyzed using the observational ranking reported by [Citation32]. Quantitative measurements from the in vitro essays were recorded in internal records in the laboratory and then imported to Excel. Measurements of the growth of the mycelial diameter were reported in cm and included the 8 mm agar disc. Initial data entry of the mycelial growth from the macroscopic essays showed that 12 values were missing due to random contamination of the Petri dishes. The missing values were imputed using the means substitution method [Citation33].
Measurements of the mycelial growth from the dual culture plate essays were compared to the growth of the positive controls for the first 3 days because as of day 4, the mycelia of both fungi were overlapped. The mycelial growth reduction was reported as the percentage of growth inhibition and compared to the positive control. The percentage of inhibition of F. oxysporum F1 was calculated by comparing the mycelial growth of the positive controls to the growth of F. oxysporum F1 when is co-cultured in the same Petri dish with the biocontrol strain T. reesei.
he minimum inhibitory concentration of mancozeb was determined by measuring the mycelial growth of each fungal strain separately for 7 days, comparison of the mycelial growth in cm was performed using an ANOVA test in R Studio [Citation34]. This analysis was conducted independently for the four concentrations of mancozeb (0.1, 0.01, 0.001, 0.0001 mg/mL). All mean values were reported with standard deviation in centimeters (cm), and mean plots were used to illustrate the mycelial growth over time. The variables tested were the mean diameter growth for 7 days using the Tukey method with 95% confidence. Different letters were used to show a significant difference over the days, and the more distant letters were used to select the concentration that was chosen to carry out the synergy test.
To study the evolution over time of the two co-cultured fungal strains in Petri dishes supplemented with 0.1 mg/mL de mancozeb, the PIDG of T. reesei against F. oxysporum was calculated by an experimental factorial design with repetitions using multiple comparisons with the Tukey method. All statistical tests were done with a significance of 5%, using R Studio, P-values lower than 0.05 were considered to show significant differences. Finally, growth over time was tested by comparing the diameter growth from day one to day three, and the time in hours from day 1 to day 3, using the equation described by [Citation31]:
Where: TC = growth rate (cm/h); C1 = initial growth (cm); C2 = final growth (cm); T1 = initial time (h); T2 = final time (h)
3. Results
3.1. Macroscopic and microscopic characterization of the pathogenic and beneficial fungal strains
Results showed that both fungi had well-defined macroscopic and microscopic characteristics. T. reesei exhibited a rapid growth in PDA and after about 60 to 72 hours of being incubated at 28°C, the mycelia covered the entire diameter of the 9 cm Petri dish (). Typical growth characteristics of T. reesei C2A included white cottony hyphae without aerial mycelium, yellowish-greenish conidia and change of the medium coloration due to the production of a yellowish pigment. Micromorphology characteristics such as hyaline conidiophores, long primary branch and a short secondary branch were observed. T. reesei C2A exhibited whitish mycelia which grew approximately at a rate of 3.41 cm a day. After 2 days of incubation, mycelia color changed from whitish to yellowish and covered the entire diameter of the Petri dish as of day 3, mycelia color turned greenish-grayish with a smooth appearance exhibiting greenish ellipsoidal conidia. Macroscopic characteristics of F. oxysporum F1 started with an initial growth rate of 1.50 cm at day 1, with a steady increase up to 8.69 cm on day 7, it did not produce diffusible pigments, its vegetative and aerial mycelia were whitish and pinkish, respectively, macroconidia and microconidia were analyzed to identify the species of the pathogenic strain.
3.2. Dual culture plate assay
The antagonistic effect of T. reesei C2A against F. oxysporum F1 was observed after 3 of incubation as fungal colonies started competing for space and nutrients and the mycelia of the biocontrol strain grew and sporulated over the pathogen, T. reesei C2A fully covered the Petri dish including ¼ of F. oxysporum F1 (). Trichoderma mycelia changed color from greenish to grayish and invaded 50 to 100% of the Fusarium colony. T. reesei C2A experienced an exponential mycelial growth from 3.41 cm at day 1 to 9 cm at day 2. In contrast, F. oxysporum F1 showed a steady mycelial growth (1.50-day 1, 2.92-day 2, 4.24-day 3, 5.37-day 4, 6.90-day 5, 7.83-day 6 and 8.69-day 7). Growth inhibition was calculated using radial growth measurements of days 1, 2 and 3, radial growth of days 4 to 7 could not be measured as Trichoderma mycelia overgrown in the plates. Inhibition increased over time, from 59% on day 1 to 62% on days 2 and 3 (). These results suggest that T. reesei C2A exhibited strong antagonistic activity against F. oxysporum F1, inhibiting mycelial growth and preventing sporulation. Thus, T. reesei C2A could be used as a potential biological control to prevent Fusarium wilt in banana plantations.
Figure 2. Assessment of antagonistic activity of T. reesei C2A against F. oxysporum F1. a) Dual plate culture essay in PDA exhibiting the antagonistic capacity of T. reesei C2A to inhibit the growth of F. oxysporum mycelia. b) Growth of positive controls in PDA. C) Antagonist capability of T. reesei C2A using a 0–4 scale as stated by [Citation28]
![Figure 2. Assessment of antagonistic activity of T. reesei C2A against F. oxysporum F1. a) Dual plate culture essay in PDA exhibiting the antagonistic capacity of T. reesei C2A to inhibit the growth of F. oxysporum mycelia. b) Growth of positive controls in PDA. C) Antagonist capability of T. reesei C2A using a 0–4 scale as stated by [Citation28]](/cms/asset/31f36ff4-b08a-4797-9c5d-35ac261b32a5/kcib_a_1829267_f0002_oc.jpg)
Table 2. Diameter growth (30 observations) of T. reesei C2A and F. oxysporum F1 in Petri dishes incubated at 28°C, data were daily recorded for 3 days
3.3. Minimum inhibitory concentration assays
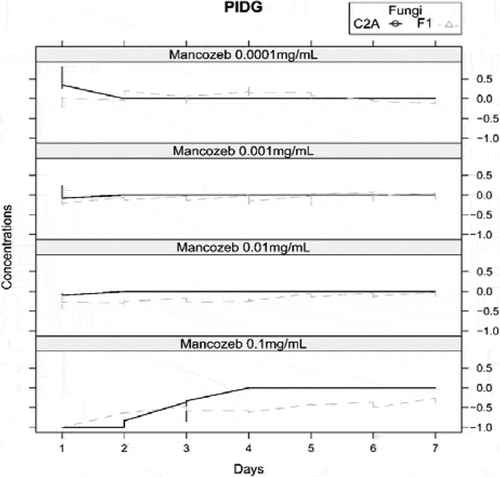
Mycelial growth was reported as the average of the vertical and horizontal measures in centimeters. Using the Tukey’s method, we determined that during the 7-day assay there were significant differences between the mycelial growth of the biocontrol agent and the phytopathogenic strain. The mycelial growth of T. reseei C2A was not significantly affected by any of the concentrations used (0.1, 0.01, 0.001 and 0.0001 mg/mL) (P < 0.0001). Although when mancozeb concentrations of (0.1, 0.01, 0.001 and 0.0001 mg/mL) were added to the culture medium, the mycelial growth of F. oxysporum F1 was reduced by 33.2, 8.3, 6.8 and 7.4%, respectively, compared to the positive control (). The growth of the phytopathogen was significantly reduced (33.2%, P < 0.0001) only when 0.1 mg/mL of the fungicide were suspended in the medium. As the growth of F. oxysporum F1 was significantly affected at 0.1 mg/mL, this concentration was selected to perform the synergistic assays ().
Figure 3. a) Mycelial growth of T. reesei C2A and F. oxysporum F1 in 9 cm Petri dishes supplemented with 4 different concentrations of mancozeb (0.1, 0.01, 0.001 and 0.0001 mg/mL) after 7 days of incubation at 28°C. b) Plot of means of the mycelial growth of T. reesei C2A and F. oxysporum F1 after 7 days of incubation at 28°C
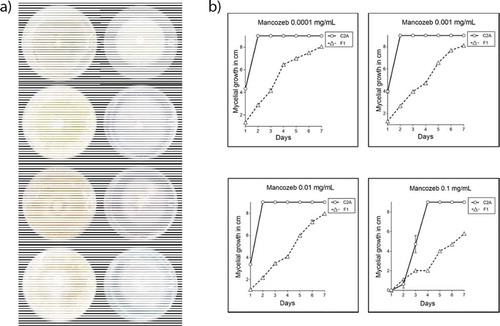
Table 3. Growth diameter in cm (SD) of T. reesei C2A and F. oxysporum F1 in experiments with four different concentrations of mancozeb
3.4. Percentage inhibition of diameter growth (PIDG)
The interaction between both fungi was measured by PIDG in PDA with 4 different concentrations of mancozeb. , shows that over time the biocontrol agent grew faster than the pathogen strain in presence of 0.1 mg/mL of mancozeb (). The Figure indicates that only at 0.1 mg/mL of mancozeb, the percentage of inhibition of Fusarium had negative values from day 1 till day 7, meaning that the mycelial growth of F. oxysporum F1 is significantly inhibited compared to the positive control, whereas since day 4, the growth of T. reesei C2A was not affected by the presence of the fungicide.
3.5. Synergistic tests between T. reesei C2A and 0.1 mg/mL of mancozeb against F. oxysporum F1
The synergistic tests of T. reesei C2A against F. oxysporum F1 in PDA plates supplemented with 0.1 mg/mL of mancozeb, showed that the first day of the essay, the mycelial growth of the phytopathogen was completely inhibited (100%). As of day 2, growth inhibition of F. oxysporum F1 decreased to 51% and T. reesei C2A mycelia started overgrowing toward the pathogenic strain (). By day 3, F. oxysporum F1 mycelial growth inhibition was 36% compared to the positive control and growth inhibition of T. reesei C2A was reduced only 10% compared to the control. The following days, the biocontrol strain continued overgrowing toward F. oxysporum F1 till day 7, when the Petri dish was totally covered by Trichoderma ().
Figure 5. Synergistic effect of T. reesei C2A against F. oxysporum F1 in PDA plates supplemented with 0.1 mg/mL of mancozeb
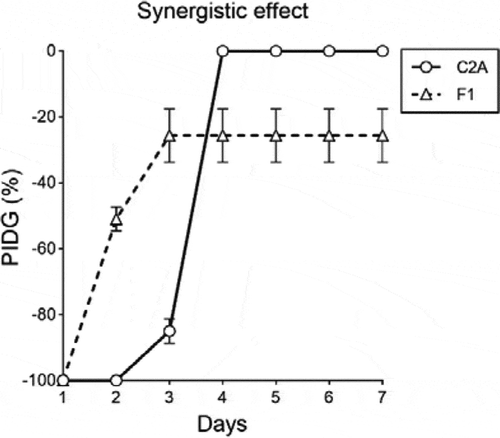
Figure 6. Synergistic effect between the biocontrol strain T. reesei C2A and 0,1 mg/mL of mancozeb to inhibit the growth of F. oxysporum F1. a) Picture was taken after 3 days of incubation at 28°C. b) Picture was taken after 7 days of incubation at 28°C
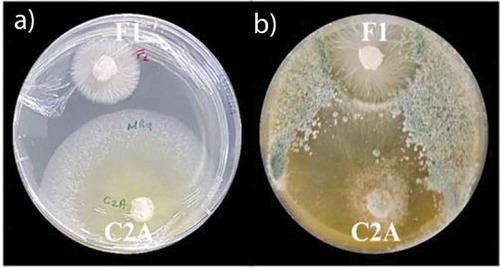
These results suggested that T. reesei C2A could be used in combination with low doses of the fungicide as an environmentally friendly alternative to inhibit under in vitro conditions the growth of banana phytopathogens.
4. Discussion
Trichoderma species are soilborne organisms associated with the roots of plants that have been widely used in agriculture for their potential to control plant diseases. Species of Trichoderma are recognized for their mycoparasitic and antibiosis capability to inhibit the mycelial growth of various pathogens including F. oxysporum, F. solani, Alternaria alternata, Botrytis cinerea and Rhizoctonia solani [Citation35,Citation36]. In this study, the biocontrol strain T. reesei C2A and the pathogenic strain F. oxysporum F1 were characterized based on their macroscopic and microscopic features as reported elsewhere [Citation37–40]. T. reesei C2A showed a typical whitish cottony mycelium that changed to a photosensitive greenish-grayish color after sporulation (i.e. after 3 or 4 days of incubation). The production of diffusible pigments, which are characteristic of T. reesei isolates, were detected at the bottom of the plates. Furthermore, distinguishing conidiophores, conidia and intercalary phialides were observed too [Citation41]. F. oxysporum F1 on the other hand, exhibited concentric rings, hyaline conidiophores with pyramidal ramifications and fixed solitary accessories as the ones reported by [Citation38,Citation42]. Finally, ITS (Internal transcribed spacer) molecular markers were used to completed the taxonomic identity of the fungal strains (data not shown). The dual culture plate assays and synergy tests allowed us to monitor for 7 days under in vitro conditions the antagonistic and synergistic capacity of T. reesei C2A to inhibit the mycelial growth of F. oxysporum F1. The evolution over time of the percentage of inhibition in the confrontational essays shown during the first 3 days, confirmed the ability of T. reesei C2A to inhibit the growth of F. oxysporum F1. On the first day, the PIDG of F. oxysporum F1 was 59% compared to the positive control, inhibition then increased to 61% and 62% on the second and third day, respectively, demonstrating the mycoparasitic mechanism of the biocontrol strain overgrowing toward the phytopathogen. Similar results were reported by [Citation43–45], who using four different strains (T29, T1, T2 and T3) of T. reesei demonstrated that they were able to inhibit in a high percentage the mycelial growth of F. oxysporum f. sp. cubense, F. oxysporum f. sp. cicero and F. oxysporum f. sp. melongenae. T. reesei isolates are known to activate a large battery of secondary metabolites and/or enzymes including plant cell wall degrading enzymes (CWDEs) which degrade the cell wall of soil-plant pathogens [Citation46,Citation47].
Although the current methods to control Fusarium wilt are based on the application of synthetic fungicides such as mancozeb, methyl bromide and quaternary ammonium, the pathogen is not always eliminated and might continue sporulating in necrotic tissues due to its saprophytic capacity [Citation8,Citation48,Citation49]. The use of these fungicides is not an economically and environmentally sustainable practice because their overuse have led to serious environmental pollution problems, generating pathogen resistance and leaving toxic residues on fruits [Citation50]. Thus, more environmentally friendly alternatives to control the development of phytopathogenic fungi are currently being proposed to reduce the potentially harmful effects of the continuous application of synthetic pesticides and fertilizers on agricultural lands [Citation51,Citation52]. These strategies involve the synergistic application of low doses of synthetic fungicides combined with biocontrol agents such as the nonpathogenic fungi Trichoderma, Clonostachys and Xylaria [Citation13,Citation14]. Recent studies under in vitro conditions have shown that when mancozeb is added to the culture medium in concentrations lower than 5 mg/mL, the mycelial growth of Trichoderma is not significantly inhibited [Citation53,Citation54]. Furthermore [Citation55], tested the tolerance of 26 Trichoderma isolates against 4 pesticides and evaluated their antagonistic capacity against the sheath blight pathogen of rice Rhizoctonia solani. The authors reported that the pesticide pyrethroid significantly enhanced the growth of all Trichoderma isolates and the strains T. reesei and T. longibrachiatum were the most effective in inhibiting the growth and the sclerotial formation of R. solani. Furthermore [Citation30], were able to quantify under in vitro conditions the synergistic interaction of the synthetic fungicide Captan 50® and Trichoderma asperelleum T8a, their results showed that by using this integrated alternative, the mycelial growth of Colletotrichum gloeosporioides, the causal agent of anthracnose in mango, was inhibited 99% compared to the positive control. The authors suggested that this approach could help reduce economic and environmental problems in the field as lower amounts of Captan 50® might be used to control the growth of C. gloeosporioides. The synergistic essays performed in this study using 0.1 mg/mL of mancozeb combined with the fungal strain T. ressei C2A, demonstrated that the mycoparasitic capacity of the biocontrol strain was enhanced when lower concentrations of the fungicide were applied to the culture medium. As depicted in , T. ressei C2A grew faster than F. oxysporum F1 as of the second day of incubation and continued growing faster till the third day. As of day 3, T. ressei C2A overgrowth toward the pathogen inhibiting 36% of the mycelial growth of F. oxysporum F1 compared to the control plates. Our results strongly suggest that the biocontrol agent T. ressei C2A could be used in combination with the synthetic fungicide mancozeb to promote a synergistic effect that inhibits in a higher percentage of the growth of the phytopathogens. Although these results are promising, future studies under greenhouse and field conditions are necessary to corroborate the effectiveness of this approach.
5. Conclusion
The biocontrol strain Trichoderma reesei C2A obtained from the Microbial Culture Collection held at the Centro de Investigaciones Biotecnológicas del Ecuador, exhibited under in vitro conditions mycoparasitic activity, reducing 62% the mycelial growth of F. oxysporum F1after 3 days of incubation at 28°C. The synergistic essays demonstrated that when the growth medium is suspended with low concentrations of mancozeb (0.1 mg/mL) combined with plugs of the biocontrol strain T. ressei C2A, the mycoparasitic capacity of the biocontrol strain was enhanced approximately by 36% compared to the positive control. These results strongly suggest that T. ressei C2A could be used in combination with mancozeb as an environmentally friendly alternative to control the growth of F. oxysporum, the causal agent of Fusarium wilt.
Disclosure of potential conflicts of interest
The authors of this manuscript declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Koberl M , Dita M , Martinuz A , et al. Members of Gammaproteobacteria as indicator species of healthy banana plants on Fusarium wilt-infested fields in Central America. Sci Rep. 2017;27(7):45318.
- Perez J. Estudio de factibilidad para el establecimiento de una exportadora de banano en Guayaquil, Ecuador, para su comercialización en Alemania. Escuela Agrícola Panamericana. Universidad Zamorano. Tegucigalpa, Honduras. 2018. p.1–8.
- Bubici G , Estudio de factibilidad para el establecimiento de una exportadora de banano en Guayaquil, Ecuador, para su comercialización en Alemania. Escuela Agrícola Panamericana. Front Microbiol. 2019;10:616.
- Jeger MJ , Waller JM , Johanson A , et al. Monitoring in banana pest management. Crop Prot. 1996;15(4):391–397.
- Gondard P. Cambios históricos en el aprovechamiento del medio natural ecuatoriano. Cultura. 1986;24:567–577.
- Davis R . Fusarium wilt (Panama disease) of banana. Pest Advis Leaflet. 2004;42:1–4.
- Revelo J . El hongo Fusarium oxysprorum. INIAP-Estación Experimental Santa Catalina. Quito, EC: INIAP, Estación Experimental Santa Catalina, Departamento de Protección Vegetal; 1991. p. 1–8.
- Nel B , Steinberg C , Labuschagne N , et al. Evaluation of fungicides and sterilants for potential application in the management of Fusarium wilt of banana. Crop Prot. 2007;26(4):697–705.
- Gullino ML , Tinivella F , Garibaldi A , et al. Mancozeb: past, present, and future. Plant Disease. 2010;94(9):1076–1087
- Runkle J , Flocks J , Economos J , Dunlop AL . A systematic review of Mancozeb as a reproductive and developmental hazard. Environment International. 2017;99:29–42 doi:10.1016/j.envint.2016.11.006
- Chabi MC , Dassou AG , Dossou-Aminon I , et al. Banana and plantain production systems in Benin: ethnobotanical investigation, varietal diversity, pests, and implications for better production. J Ethnobiol Ethnomed. 2018;14(78). DOI:10.1186/s13002-018-0280-1
- Kleinstreuer NC , Dix DJ , Houck KA , et al. In vitro perturbations of targets in cancer hallmark processes predict rodent chemical carcinogenesis. Toxicol Sci. 2012;131(1):40–55.
- Kannan V , Sureendar R . Synergistic effect of beneficial rhizosphere microflora in biocontrol and plant growth promotion. J Basic Microbiol. 2009;49(2):158–164.
- Tirado-Gallego PA , Lopera-Álvarez A , Ríos-Osorio LA . Estrategias de control de Moniliophthora roreri y Moniliophthora perniciosa en Theobroma cacao L.: revisión sistemática. Ciencia y Tecnología Agropecuaria. 2016;17:417–430.
- Viterbo A , Horwitz BA . Mycoparasitism. In: Borkovich KA , Ebbole DJ , editors. Cellular and molecular biology of filamentus fungi. Washington (DC): ASM Press; 2010. p. pp. 666–667.
- Rivera-Méndez W . Control microbiológico como experiencia de sostenibilidad local en la agricultura centroamericana. Revista Tecnología en Marcha. 2017;30(4):31–40.
- de Marco JL , Felix CR . Characterization of a protease produced by a Trichoderma harzianum isolate which controls cocoa plant witches’ broom disease. BMC Biochem. 2002;3:1–7.
- Maymon M , Minz D , Barbul O , et al. Note: identification of Trichoderma biocontrol isolates to clades according to ap-PCR and ITS sequence analyses. Phytoparasitica. 2004;32(4):370–375.
- Pérez-Moreno L , Belmonte-Vargas JR , Núñez-Palenius HG , et al. Sensibilidad in vitro de dos especies de Sclerotinia spp. y Sclerotium cepivorum a agentes de control biológico y fungicidas. Revista mexicana de fitopatología. 2015;33(2):256–267.
- Sharma P , Sharma M , Raja M , et al. Status of Trichoderma research in India: A review. Indian Phytopathol. 2014;67(1):1–19.
- Azarmi R , Hajieghrari B , Giglou A . Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr J Biotechnol. 2011;10:5850–5855.
- Kapri A , Tewari L . Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz J Microbiol. 2010;41:787–795.
- Singh BN , Singh A , Singh GS , et al. Potential role of Trichoderma asperellum T42 strain in growth of pea plant for sustainable agriculture. J Pure Appl Microbiol. 2015;9:1069–1074.
- Yadav RL , Shukla SK , Suman A , et al. Trichoderma inoculation and trash management effects on soil microbial biomass, soil respiration, nutrient uptake and yield of ratoon sugarcane under subtropical conditions. Biol Fertili Soils. 2009;45:461–468.
- Zhang F , Xu X , Huo Y , et al. Trichoderma-inoculation and mowing synergistically altered soil available nutrients, rhizosphere chemical compounds and soil microbial community, potentially driving alfalfa growth. Front Microbiol. 2019;9:3241.
- Anith KN , Manomohandas TP . Combined application of Trichoderma harzianum and Alcaligenes sp. strain AMB 8 for controlling nursery rot disease of black pepper. Indian Phytopathol. 2001;54:335–339.
- Ashwini N , Srividya S . Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. Biotechnology. 2013;3. DOI:10.1007/s13205-013-0134-4.
- Ezziyyani M , Pérez-Sánchez C , Requena ME , et al. Biocontrol por Streptomyces rochei–Ziyani–, de la podredumbre del pimiento (Capsicum annuum L.) causada por Phytophthora capsici . Anales de Biología. 2004b;26:69–78.
- Harun W , Razak F . Antifungal susceptibility and growth inhibitory response of oral Candida species to Brucea javanica Linn. extract. BMC Complement Altern Med. 2013;13(1):1–8.
- Peláez-Álvarez A , de Los Santos-villalobos S , Yépez EA , et al. Synergistic effect of Trichoderma asperelleum T8A and captan 50® against Colletotrichum gloeosporioides (Penz.). Revista Mexicana de Ciencias Agrícolas. 2016;7(6):1401–1412.
- de Los Santos-villalobos S , Guzmán-Ortiz DA , Gómez-Lim MA , et al. Potential use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a biological control agent against anthracnose in mango (Mangifera indica L. Biol Control. 2013;64(1):37–44.
- Ezziyyani M , Pérez-Sánchez C , Requena ME , et al. Biocontrol por Streptomyces rochei–Ziyani–, de la podredumbre del pimiento (Capsicum annuum L.) causada por Phytophthora capsici. Anales de Biol. 2004a;26:69–78.
- Ganser G . An accurate substitution method for analyzing censored data. J Occup Environ Hyg. 2010;7(4):233–244.
- RStudio T (2020). RStudio: Integrated Development for R. Boston (MA): RStudio, PBC. Available from: http://www.rstudio.com/
- de Lima FB , Félix C , Osório N , et al. Trichoderma harzianum T1A constitutively secretes proteins involved in the biological control of Guignardia citricarpa . Biol Control. 2017. DOI:10.1016/j.biocontrol.2017.01.003
- Kumar T , Veena S , Karthikeyan S , et al. Compatibility of Trichoderma asperellum with fungicides, insecticides, inorganic fertilizers and bio-pesticides. J Root Crops. 2017;43(2):68–75.
- Aguaysol N . Marchitamiento de plantas en cultivos de garbanzo (Cicer arietinum) del norte argentino, causado por Fusarium oxysporum y Rhizoctonia sp. Sanidad Vegetal. 2013;34(4):26–27.
- Evans H , Stalpers J , Samson A , et al. On the taxonomy of Monilia roreri, an important pathogen of Theobroma cacao in South America. 1978;56(20):2528–2532.
- Papavizas G . Trichoderma and Biology, Ecology, and Potencial for Biocontrol Phytopathology. 1985;23:23–54.
- Romero-Arenas O , Huerta-Lara M , Damián-Huato M , et al. Características de Trichoderma harzianum, como agente limitante en el cultivo de hongos comestibles. Revista Colombiana De Biotecnología. 2009;XI(2):143–151.
- Siddiquee S . Morphology-based characterization of Trichoderma species. In: SiddiqueeS , editor. Practical handbook of the biology and molecular diversity of Trichoderma species from tropical regions. Cham, Switzerland: Springer International Publishing; 2017.
- Maryani N , Lombard L , Poerba YS , et al. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud Mycol. 2019;92:155–194.
- Anuragi M , Sharma T . Effect of different growth parameters on Fusarium oxysporum f. sp. ciceri (wilt causing pathogen of chickpea). Flora Fauna. 2016;22(1):11–16.
- Cherkupally C , Amballa H , Reddy BN . In vitro antagonistic activity of Trichoderma species against Fusarium oxysporum f. sp. melongenae. Int J Appl Agric Res. 2017;12(1):87–95.
- Galarza L , Akagi Y , Takao K , et al. Characterization of Trichoderma species isolated in Ecuador and their antagonistic activities against phytopathogenic fungi from Ecuador and Japan. J Gen Plant Pathol. 2015;81(3):201–210.
- Kubicek CP , Herrera-Estrella A , Seidl-Seiboth V , et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011;12:R40.
- Seiboth B , Karimi RA , Phatale PA , et al. The putative protein methyltransferase LAE1 controls cellulase gene expression in Trichoderma reesei. Mol Microbiol. 2012;84(6):1150–1164.
- Dita MA , Echegoyén P , Pérez LF . Plan de contingencia ante un brote d ela raza topical de Fusarium oxysporum f. sp. cubense en un país de la región OIRSA. San Salvador (El Salvador): Organismo Internacional Regional de Sanidad Agropecuaria; 2017.
- Martinez L (2016). Mal de Panamá Fusarium oxysporum f. sp. cubense (E.F. Sm.) W. C. Snyder & H. N. Hansen Raza 4 Tropical (Foc R4T). Available from: http://www.cesaveson.com/files/docs/campanas/vigilancia/fichas2016/MALDEPANAMA.pdf
- Wu CH , Bernard SM , Andersen GL , et al. Developing microbe-plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microb Biotechnol. 2009;2(4):428–440.
- Minh-Luan N (2018). Biostimulant effects of rhizobacteria on wheat growth and nutrient uptake under contrasted N supplies (Doctoral Degree). The University of Liège, Gembloux, Belgium.
- Xu L , Geelen D . Developing biostimulants from agro-food and industrial by-products. Front Plant Sci. 2018;9:1567.
- Bhale U , Rajkonda J . Compatibility of fungicides and antagonistic activity of Trichoderma spp. against plant pathogens. Biosci Methods. 2015;6(3):1–9.
- Wedajo B . Compatibility studies of fungicides with combination of Trichoderma species under in vitro conditions. Virology Mycology. 2015;4:149.
- Chakravarthy S , Nagamani K , Ratnakumari AR , et al. Antagonistic ability against Rhizoctonia solani and pesticide tolerance of Trichoderma strains. Adv Environ Biol. 2011;5(9):2631–2638.