Abstract
After centuries of disturbance, environmental professionals now recognize the need to restore coastal watersheds for native fish and protect the larger ecosystems on which fish and other aquatic biota depend. Anadromous fish species are an important component of coastal ecosystems that are often adversely affected by human activities. Restoring native anadromous fish species is a common focus of both fish and coastal watershed restoration. Yet restoration efforts have met with uneven success, often due to lack of knowledge about habitat availability and use. Using habitat surveys and radio tracking of adult anadromous alewives Alosa pseudoharengus during their spring spawning migration, we illustrate a method for quantifying habitat using multiple approaches and for relating mobile fish distribution to habitat. In the Ipswich River, Massachusetts, measuring habitat units and physical conditions at transects (width, depth, and velocity) provided an ecological basis for the interpretation of landscape patterns of fish distribution. Mapping habitat units allowed us to efficiently census habitat relevant to alewives for the entire 20.6 river kilometers of interest. Our transect data reinforced the results of the habitat unit survey and provided useful, high-resolution ecological data for restoration efforts. Tagged alewives spent little time in riffle–run habitats and substantial time in pools, although the locations of pool occupancy varied. The insights we provide here can be used to (1) identify preferred habitats into which anadromous fish can be reintroduced in order to maximize fish survival and reproduction and (2) pinpoint habitat types in urgent need of protection or restoration.
Received April 3, 2011; accepted January 9, 2012
Anadromous fish species are an important focus of fish and watershed restoration. These species, which move between the ocean and freshwater, are highly valued (Frank et al. Citation2009), are susceptible to human activities in freshwater (Maes et al. Citation2008), and, in many cases, are declining throughout their range (Rulifson et al. Citation1982; Rulifson Citation1994; McDowall Citation1999; Schmidt et al. Citation2003; Saunders et al. Citation2006). Anadromous alosines, in particular, are at record low levels (Limburg and Waldman Citation2009). In fact, in the eastern United States, river herring (two closely related taxa of the genus Alosa [alewife A. pseudoharengus and blueback herring A. aestivalis]) are currently under review to be listed as threatened under the Endangered Species Act (NOAA NMFS 2011), and a recently filed lawsuit has charged management organizations (the Atlantic States Marine Fisheries Commission and the National Marine Fisheries Service) with failing to take adequate measures to stem fish declines (Earthjustice Citation2010). Knowing where spawning fish spend their time and what habitats are available to them are essential first steps to restoring these fish. A complication with assessing the prespawning and spawning distributions and habitat use of river herring is that these highly mobile anadromous fish move over large areas but also respond to local conditions (e.g., for spawning). The difficulty of obtaining high-resolution habitat data over the entire spatial range of these migratory fish has made it challenging to assess habitat availability and use in freshwater.
TABLE 1 Summary of adult alewife spawning habitat use reported in the literature since 1982. Type includes review (R) and original data (O). Although our study focused on alewives, the literature often included information on both alewives (A) and blueback herring (B). Studies that only examined blueback herring habitat use are not included here. The review of spawning habitat was generally limited to the adult (A) life stage, but early life history (ELH) citations were also included if they identified where spawning occurred.
Anadromous alewives (hereafter referred to as alewives) spawn in freshwater when they mature at ages 3–6 years (Collette and Klein-MacPhee Citation2002). In spring, often in response to water temperature, adults move from the ocean through the river corridor to access freshwater spawning habitats before returning to the ocean. Upstream migration is reported to begin at temperatures between 5°C and 10°C (Loesch Citation1987), little instream movement occurs below 8°C or over 18°C (Collette and Klein-MacPhee Citation2002), and spawning ceases at water temperatures exceeding 27°C (Kissil Citation1974). Appropriate spawning temperatures broadly fall between 10°C and 22°C (Tyus Citation1974; Pardue Citation1983; Collette and Klein-MacPhee Citation2002), although optimal spawning temperatures probably vary by region and the temperatures reported in the literature may not be ideal for all populations of river herring (O’Connell and Angermeier Citation1999). Schools of adult alewives are frequently observed moving upriver in spring (Loesch Citation1987; Collette and Klein-MacPhee Citation2002), but relatively little is known about the gender composition and group size of spawners, their spawning frequency within a season, and the amount of time that spawning fish spend in the river.
Relatively few recent empirical field studies quantify freshwater habitat use by prespawning and spawning adult anadromous alewives. Alewives are frequently counted as the adults return to spawn each spring or as juveniles egress to the ocean in summer and fall (e.g., Jessop and Harvie Citation1990; Kosa and Mather Citation2001; Yako et al. Citation2002; Rulifson and Wall Citation2006; Davis and Shultz Citation2009; Gahagan et al. Citation2010). These observations, however, typically occur at artificial checkpoints (e.g., fishways), so they reveal little about habitat use. From a landscape habitat perspective, alewives clearly require connectivity between the ocean and spawning sites because of their migratory life history (Rounsefell and Stringer Citation1945; Hall et al. Citation2011). Blockage by dams or low water can be either minimal or devastating depending on the size, quality, and spatial configuration of the primary spawning sites relative to the blockage. The larger impacts of potential fragmentation are often unknown.
The specific criteria that describe the physical habitat used by adult anadromous alewives in freshwater vary, making available information sparse and syntheses across systems and years difficult (). Many of the recent (post 1982) references for physical habitat used by adult alewives in freshwater are reviews (Pardue Citation1983; Mullen et al. Citation1986; Loesch Citation1987; Collette and Klein-MacPhee Citation2002; ). In both reviews and original research reports, adult alewives spawn in ponds associated with coastal systems or in pond-like areas within coastal rivers and streams (Pardue Citation1983; Mullen et al. Citation1986; Loesch Citation1987; Collette and Klein-MacPhee Citation2002; Walsh et al. Citation2005). Within these systems, the freshwater habitats that adult alewives use include ponds, oxbows, eddies, backwaters, stream pools, and flooded swamps (Pardue Citation1983; Mullen et al. Citation1986; Loesch Citation1987; Collette and Klein-MacPhee Citation2002; Walsh et al. Citation2005). These potential spawning habitats are typically deep and slow (Pardue Citation1983; Mullen et al. Citation1986; Loesch Citation1987; Collette and Klein-MacPhee Citation2002; O’Connell and Angermeier Citation1997; Walsh et al. Citation2005). Spawning areas are associated with a variety of substrates (gravel, sand, detritus, submerged vegetation, clay, and silt; Pardue Citation1983; O’Connell and Angermeier Citation1997). Adult alewife freshwater habitat use also may be affected by water quality (e.g., salinity, dissolved oxygen, pH, high flows, or suspended sediments; O’Connell and Angermeier Citation1997). The limited number of recent original field investigations of alewife habitat in freshwater and the very general insights they provide, despite consistent and intense recent interest in these fish, partially reflect the logistical difficulty of studying habitat availability and use by these highly mobile fish.
When sampling habitat, researchers seek to balance the trade-offs in grain (the size of the areas actually sampled), extent (the collective area covered by the sampling regime), and resolution (the detail in each sample) (Wiens Citation1989). Collecting and analyzing high-resolution habitat data at a large grain that covers a large extent is extremely challenging and labor-intensive, yet this is the information that researchers and managers need to conserve anadromous fish. Here we seek to use multiple approaches, which differ in grain and resolution, to understand the patterns of freshwater habitat availability for and use by alewives. This information is needed for highly mobile fish species that both traverse long distances and use habitats defined by local conditions to complete their life history. Specifically, we first quantified the habitat conditions available to prespawning adult alewives within our 20.6–river kilometer (rkm) study area of the Ipswich River (an urbanizing watershed in coastal Massachusetts) by assessing habitat units and then quantifying the physical conditions at select transects. Second, we measured the habitat characteristics of the areas used by tagged alewives within the discrete range of radio receivers. The methods and results we provide here can facilitate science-based decision making about habitat conservation and restoration for anadromous alewives, a species that is declining throughout much of its native range.
STUDY SYSTEM
The Ipswich River is a 72-km, low-gradient, meandering coastal river that drains a 401-km2 watershed in northeastern Massachusetts (, b). This watershed, located in the third most densely populated state in the United States (U.S. Census Bureau Citation2006), is affected by the degradation associated with an increasing human population. Specifically, much land is in suburban and urban uses at the expense of forest. Substantial water withdrawals have been permitted, and the more than 70 dams and 500 culverts associated with road crossings potentially fragment the watershed (http://ipswichriver.org/).
FIGURE 1 Maps of the study area indicating (a) the locations of the Ipswich and Nemasket rivers; (b) the area of the Ipswich River in which we tracked alewives, showing the Atlantic Ocean, three dams, and a historical spawning site (Great Wenham Swamp); (c) the distribution of the 10 river areas used for the habitat unit survey within the lower 20.6-rkm study area (there were slight variations in the size of these areas from upstream to downstream because of the presence of dams and other landmarks that acted as natural divisions); (d) the locations of the 180 transects within 18 clusters at which depth, velocity, and width were measured for the lower 20.6-rkm study area; and (e) the locations of the nine radiotelemetry receivers with the upstream and downstream fish release sites denoted by stars. Receiver 1 was in area 1 and was only encountered as tagged fish left the system. Receivers 2–3 were in area 2, which was bounded upstream by Ipswich Mills dam. Receivers 4–7 were in areas 3–6. Willowdale Dam was the boundary between areas 4 and 5. Receiver 8 was in area 9. Receiver 9 was excluded from the analysis because no fish used this area. Areas 7, 8, and 10 had no receivers. The mean discharge recorded at the U.S. Geological Survey gauge at Willowdale Dam (station 01102000) for the study period was 1.48 m3/s. MassGIS was used to make these and other maps.
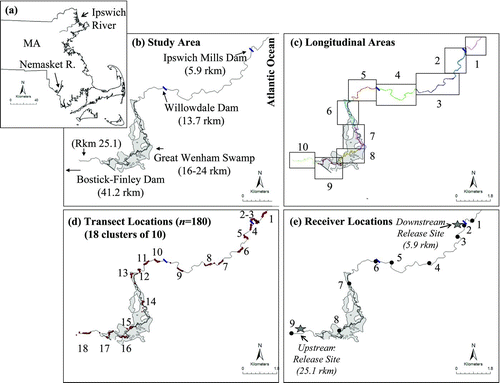
Anthropogenic stressors also adversely affect migratory fish. In 1912, the size of the Ipswich River alewife harvest exceeded 157,000 fish (Belding Citation1920), while the estimated and actual counts from 1999 to 2008 averaged only 230 fish annually (range = 98–420 adults per year; Massachusetts Division of Marine Fisheries, personal communication). This dramatic decline occurred in spite of stocking over 46,000 adult alewives between 1990 and 2007. Except for the Great Wenham Swamp, an extensive wetland covering 6.47 km2 between rkm 16 and 24 (), historical spawning sites are no longer accessible because of dams on tributaries or use of river ponds as municipal water supplies. Fish passage varies at the three low-head main-stem dams (). Ipswich Mills Dam at rkm 5.9 had adequate fish passage through a Denil fish ladder. Willowdale Dam, located at rkm 13.7, provided adequate fish passage through a notched weir–pool fishway during high discharge (such as 2007, the year of this study). Bostik-Finley Dam, at rkm 41.2, had no fish passage.
METHODS
We used multiple approaches to assess habitat throughout the 20.6-rkm study area within which a restoration effort exists for anadromous alewives. We quantified (1) habitat units within 10 river areas throughout the entire study area (), (2) local physical conditions at select transects throughout the entire study area (), (3) the locations (based on cumulative tracking time) of tagged stocked and native alewives within the ranges of the lower eight radiotelemetry receivers (), and (4) habitat units and the local physical conditions at transects adjacent to radiotelemetry receivers (<0.5 km radius) (). Below we describe how each type of data was collected and summarized. For our study, native alewives were adult anadromous alewives captured as they returned to the Ipswich River during the spring spawning season. Stocked alewives were adult alewives from the Nemasket River, Massachusetts (), that were captured, transported, and released (i.e., stocked) into the Ipswich River for fisheries restoration.
To census habitat units, on 1–2 August 2007 we kayaked the entire 20.6-rkm study area from upstream to downstream (rkm 25.1–4.5; ). Using a Global Positioning System (GPS), we created tracks and waypoints to identify and quantify the boundaries and locations of 19 potential habitat units with a modification of the classification system described by McCain et al. (Citation1990) that we created for the Ipswich River (). For reliability, all habitat unit classifications were agreed upon by two people based on flow, depth, and unique habitat features. We used the GPS tracks and waypoints and their associated habitat unit descriptions from the field to map the boundaries of each identified habitat unit. In the laboratory, these GPS boundaries were used to create polygons for each habitat unit. We used the resulting geographical information systems (GIS) maps to compare trends graphically within 10 areas of nearly equal size (mean length per area = 2.1 km; ). In addition, we compared the habitat units quantitatively with a Pearson's chi-square test to determine whether or not habitat units had the same or different composition in these 10 areas.
TABLE 2 Major categories, characteristics, and habitat characteristics of the 19 habitat units used in the larger-scale survey (modified from McCain et al. Citation1990).
At 180 transects throughout the 20.6-rkm study area we measured local habitat metrics, including stream depth, velocity, and width. At each of 18 locations (), we collected data from 10 transects that were 1 mean stream width apart (about 30 m; Simonson et al. Citation1994). These groups of transects were not evenly distributed throughout the study area because of logistics associated with access. At each transect, we recorded the wetted channel width, took 10 evenly spaced depth measurements, and described riparian land use (forested, developed, commercial, or agricultural). At 1 of every 10 transects, velocity was measured at one or two locations in the thalweg (0.6 × the depth if the stream was ≤0.5 m deep or 0.2 and 0.8 × the depth if the stream was >0.5 m deep; Gordon et al. Citation2004). These local data on width, depth, and velocity were summarized for (1) the entire study site (20.6 rkm), (2) each of the 10 areas described above (), and (3) the three major categories of habitat unit (riffle, run, and pool). We used Kruskal–Wallis tests to determine whether the depth, velocity, and width measurements were different among the major categories of habitat units. If a Kruskal–Wallis test was significant, we used Wilcoxon signed-rank tests to determine which habitat categories differed. The Kruskal–Wallis and Wilcoxon tests were performed using the functions kruskal.test and wilcox.test (R Development Core Team Citation2009). We also ran Moran's I-tests to assess spatial autocorrelation (i.e., the correlation among spatially adjacent locations) for all habitat variables. We tracked alewives from two different origins (native versus stocked) that were released at two different locations (upstream versus downstream). To observe the natural migration behavior of native Ipswich River alewives, we released native fish downstream at rkm 5.9, where native migrants were captured as they naturally returned to the river (). We also released stocked Nemasket River fish and native Ipswich River alewives upstream at rkm 25.1, a location above two dams and near the Great Wenham Swamp, where state managers have historically stocked alewives.
In all three treatments, alewives were similarly sized and treated alike as much as possible except for the specific treatment effects being tested (origin and location of release). In the native–downstream treatment (N–down), on 23–27 April 2007 alewives (n = 21, mean total length [TL] = 267 mm, SE = 3.57) were captured in a wire mesh trap (122 cm long, 61 cm wide, and 61 cm deep) set at the top of the first Ipswich River dam fishway, tagged (as described below), then released where they were captured (rkm 5.9) so they could continue their upstream migration. In the stocked–upstream treatment (S–up), we simulated the translocation procedures used by local fisheries managers. Specifically, on 30 April 2007 alewives (n = 39, mean TL = 268 mm, SE = 1.78) were captured with a dip net in the Nemasket River fishway as they actively migrated upstream, tagged, transported by stocking truck to the Ipswich River, and then released upstream in the Ipswich River at rkm 25.1 through a 1-m-diameter cylindrical chute attached to the stocking truck. By observing fish in the native–upstream treatment (N–up), on 27 April 2007 we quantified how native Ipswich River fish (n = 15, mean TL = 273 mm, SE = 5.07) behaved when they were captured downstream (rkm 5.9) in the trap described above, transported upstream by stocking truck, then released into the Ipswich River at rkm 25.1, near their historical spawning habitats. Additional details of the capture, tagging, transport, release, and tracking are described in Frank et al. (Citation2011). The mean daily river temperatures during tagging and tracking fell within the range proposed for spawning (10.29–22.31°C).
To track fish distribution within the Ipswich River, we gastrically inserted Lotek Nanotags NTC-6-1 transmitters (9.1 mm in diameter; 22.4 mm long; weight in air, 2.8 g) and tracked them using nine stationary Lotek SRX_400 receivers. Briefly, a radio tag about 1.6% of the mean fish weight was inserted into the intestinal tracts of unanesthesized alewives using a hollow plastic tube (12.3 cm long, 8 mm in diameter tapering to 5 mm) following the procedures developed and tested by Smith et al. (Citation2009). Fish were tagged within 15 min of the time they were captured in the fishway. The tagging procedure lasted less than 30 s per fish. The time from initial contact to the completion of tagging was less than 60 s. Fish were kept for observation until they were able to swim upright (<5 min), then released into the river or put into a tank for transport. Smith et al. (Citation2009) demonstrated that, using these tagging protocols, alewives retained their tags and survived the tagging process. During tagging and transport, we observed no mortality or noticeable scale loss.
Receivers were installed throughout the lower Ipswich River on 28 March 2007 and removed on 5 June 2007 (). Receiver range was determined by documenting the distance that an active tag could be heard by a receiver at two times: prior to the release of any tagged fish (31 March to 1 April 2007) and again before removing the receivers from the field (5–6 June 2007). The receivers monitored different, widely separated sections of the river. Fish only encountered receiver 1 as they exited the river. No fish were observed at receiver 9 (upstream of the upstream release location), so this receiver was removed from the analysis. We defined our 20.6-rkm study area as the extent from just downstream of the first dam (rkm 4.5) to the upstream stocking site (rkm 25.1) because no fish were detected outside that area. Instead of using autocorrelated trajectory data, we calculated the duration of time that tagged fish spent in each of the eight receiver range areas. Specifically, we summed the times when tagged fish were within each receiver range area. These duration data are presented as the proportion of the total freshwater residence of each fish in each receiver area. As fish move up and down during transit, exploration, and spawning, they spend variable amounts of time in different areas of the river, such that the total time spent in a particular receiver area did not depend on the amount of time spent in adjacent areas.
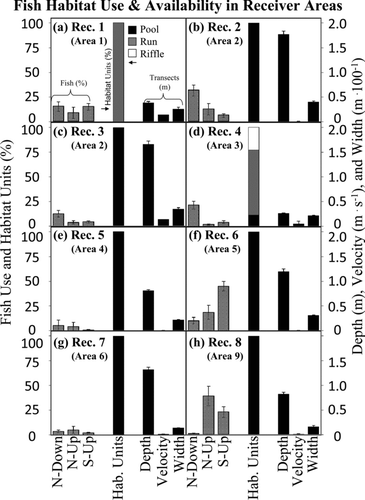
We related fish distribution (the amount of time they spent in each receiver area) to the habitat metrics (habitat units and transects) measured adjacent to the telemetry receivers. To calculate habitat availability in those parts of the river for which we had fish distribution data, we quantified the composition of the three major habitat units (riffle, run, and pool) and the average width, depth, and velocity adjacent to each of the eight radiotelemetry receivers (within about 0.50 km; ). We graphically compared the percent of time fish spent in each receiver area with the habitat characteristics of those areas. To test whether fish in each of the three treatments spent more time at specific receivers, we conducted a chi-square test for each treatment in which we compared the percent of time fish spent at receivers 1–8 to the percent of time that would be expected if they were distributed evenly (8/100 or 12.5%; chisq.test function; R Development Core Team Citation2009).
We also used redundancy analysis (RDA) to examine the relationship between habitat variables and the time that the alewives in the three treatments spent in the range area of each receiver. Redundancy analysis is a constrained ordination technique that can be used to identify species’ responses to environmental gradients (Legendre and Legendre Citation1998). It is similar to principal components analysis except that the latter is an unconstrained ordination. Redundancy analysis assumes that Euclidean distance is appropriate to describe the relationships among objects (Legendre and Legendre Citation1998). It was used instead of canonical correspondence analysis because the first axis gradient length of the detrended correspondence analysis was less than 4 standard deviations (ter Braak and Prentice Citation1988). The explanatory variables included three uncorrelated standardized variables: percent pool, depth, and width. The dependent variables, the average percent of time that fish in the three treatments spent at each of eight receivers, were log10-transformed. River kilometer was used as a conditioning variable (i.e., the variation explained by this variable was removed before determining the variation explained by the habitat variables). Monte Carlo permutation tests (permutations = 99) were used to determine the significance of the observed relationships between alewife distribution and physical habitat variables. Statistical tests were performed using the vegan package (Oksanen et al. Citation2009), which provides multivariate tools for ecological data sets using R (R Development Core Team Citation2009). By including river kilometer, this constrained ordination accounted for the spatial relationship across the 10 areas and 80 transects.
RESULTS
Landscape patterns varied by habitat unit type and size, the spatial configuration of the habitat units, and the amount of connectivity among habitat units. Sixteen of 19 possible habitat units were observed in the Ipswich River (). Except for area 1, all 10 areas of the low-gradient Ipswich River were dominated by pool-type habitat units (–k), especially nontrench pools (36.36%), shallow-water pools (19.73%), and trench-chute pools (18.51%). Area 1, which was closest to the ocean, differed from the other areas of the river in that it was tidal and consisted primarily of run habitat units (). The area upstream of the Ipswich Mills Dam formed a series of large-pool habitat units (Area 2; ). Areas 3–4 included runs and riffles interspersed with smaller pool habitat units (–e). Much of the river upstream of Willowdale Dam (Areas 5, 6, 9, and 10; –g, j–k) was predominantly pool uninterrupted by runs or riffles. Additionally, much of the upper study area (Areas 5–9; –j) was within an Audubon sanctuary and a state preserve with forested uplands (55.45%).
FIGURE 2 For (a) the 10 areas in the 20.6-rkm study area, panels (b)–(k) show the amounts and locations of 19 habitat units with (l) the codes for habitat units. The descriptions of the habitat units were adapted from McCain et al. (Citation1990) and are shown in . Also indicated in each panel are the area number, the total length of each area in river kilometers, and the radiotelemetry receivers located in each area. The dominant habitat units were nontrench pools (0.22 km2), shallow-water pools (0.12 km2), and trench–chute pools (0.11 km2).
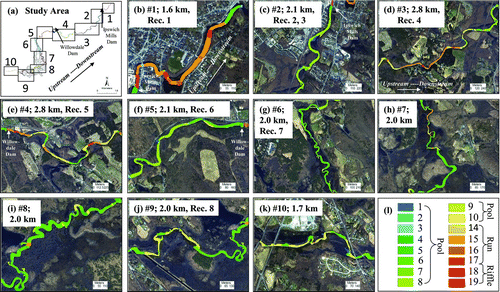
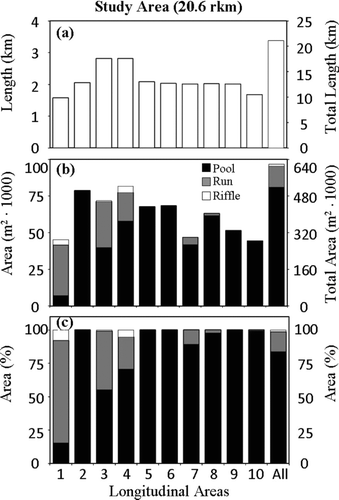
Comparing the three major habitat categories (pool, run, and riffle) across all of the 10 areas of nearly equal length (), the predominant habitat category was pool, covering approximately 0.52 km2 (83.7%; , c). The least common habitat category across all areas was riffle, covering approximately 0.01 km2 (1.4%). Except for Area 1, all 10 areas were more than 50% pool. Areas 2, 5, 6, and 9 were 100% pool, and areas 7, 8, and 10 had more than 89% pool (). Run habitat was quite common in downstream areas 1, 3, and 4, but in upstream areas 7, 8, and 10 run habitat only occurred below beaver dams (). Riffle habitat was limited to the areas downstream of Willowdale Dam and near the estuary (areas 1, 3, and 4; ). The 10 areas differed in habitat composition (χ2 = 517.44, P ≤ 0.0001); Areas 1, 3, and 4 had less pool than expected based on the study area average; in Areas 2 and 5–10, pool habitat exceeded that expected.
FIGURE 3 For each of the 10 areas in the lower 20.6-rkm study area shown are (a) the length, (b) the areal size, and (c) the percent area of combined pool, run, and riffle habitat units. The units for areas 1–10 are shown on the left y-axis; those for the length and size of the entire study area (in the “all” column) are shown on the right y-axis.
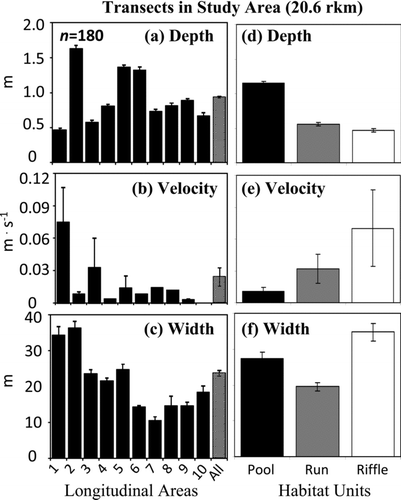
For transects (n = 180) across all 10 areas, the average depth for the 20.6-rkm study area was 0.93 m, the average velocity was 0.02 m/s, and the average width was 23.7 m (–c). Area 1 at the mouth of the river was shallow and fast. Areas 2, 5, and 6 were deep and slow (, b). Area 3 was moderately shallow and slightly faster than the study area average (, b). Areas 4 and 7–10 were of intermediate depth and slow (, b). Because pools were deep and slow, riffles were shallow and fast, and runs were intermediate ( d, e), the smaller-scale transect data confirmed the patterns detected in the larger-scale habitat unit survey. Depth was significantly different between habitat types (Kruskal–Wallis test; P ≤ 0.0001). Velocity was different between pool and riffle habitat (Kruskal–Wallis test: P = 0.0230; Wilcoxon rank-sum test: P = 0.0173). Width generally increased from upstream to downstream () and was not predictably different across habitat units ().
FIGURE 4 Depth, velocity, and width (a)–(c) for transects within the 10 areas and (d)–(f) for the three major habitat categories (pool, run, and riffle) in the lower 20.6-rkm study area. Data are means ± SEs.
Alewives did not spend equal amounts of time in all areas of the river. Prior to their exit from the river during the downstream phase of their migration, fish in all three treatments spent 10–20% of their in-river freshwater residence near the estuary in Area 1 (receiver 1; ), probably for physiological and behavioral reasons (e.g., postspawning rest, adapting to changes in salinity, or preparation for ocean entry). Except for this egress from the river, alewives generally spent more time in areas that were primarily pool habitat and less time in the transitional riffle and run habitats. However, the pools in which they spent time depended on where they were released. Fish in the native N–down treatment did not spend an equal amount of time at each of the eight receivers (χ2 = 57.78, P ≤ 0.0001); rather, they spent much more time than expected at receivers 2 and 4 (, d), the expected amount of time at receiver 3 (), and less time than expected at receivers 5–8 (–h). The N–up and S–up fish also did not spend an equal amount of time at each of the eight receivers (χ2 = 98.67, P ≤ 0.0001; χ2 = 128.47, P ≤ 0.0001, respectively). Instead, both groups spent more time than expected in the deep, slow pool habitat near receivers 6 and 8 (, h).
FIGURE 5 Fish habitat use (percent of time spent in a particular habitat; left y-axis), major habitat unit categories (percentage of pool, run, or riffle area; left y-axis), and habitat characteristics (depth, velocity, and width; right y-axis), with respect to (a)–(h) the eight receivers at which alewives were detected. The scales are standardized across all receiver sites so that habitat use and availability can be compared across panels. The code N–down indicates native alewives that were tagged and released downstream; the codes N–up and S–up indicate native and stocked alewives that were released upstream. Habitat units and transect data were measured for the 0.5 km nearest each receiver. Also shown (in parentheses) is the area in which each radiotelemetry receiver was found.
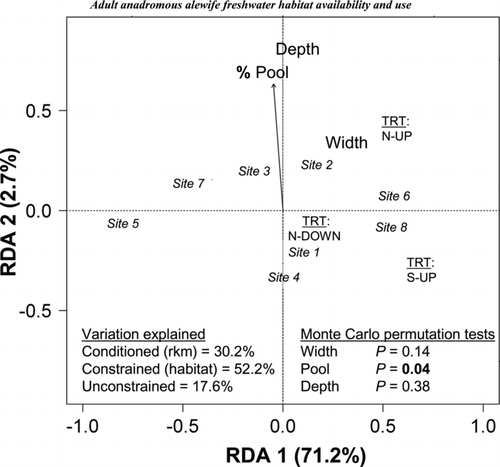
The multivariate RDA analysis confirmed the univariate trends and added synthetic insights gained by examining all variables together. After the variation related to river kilometer (30.2%) was removed, the RDA explained 52.2% of the overall variation when constrained by the three habitat variables (percent pool, width, and depth; ). The first RDA axis explained 71.2% of the variation within the constrained ordination, whereas the second axis only explained 2.7%. Based on the results from Monte Carlo permutation tests, percent pool was the only significant habitat variable (P = 0.04). Examining the constrained ordination plot, the N–down alewives spent the most time at receiver 2 (100% pool, a deep, wide site), some time at receiver 1 on their exit only, and some time at receiver 4. The N–up and S–up alewives spent more time at receivers 6 and 8, both of which were located near 100% deep pool habitat. In no treatment did alewives spend much time near receivers 5 or 7. No spatial autocorrelation in habitat variables was observed across the 10 river areas, the 8 receiver ranges, or the transects (Moran's I-test, P > 0.05)
FIGURE 6 Constrained ordination plot showing the habitat use associated with the distribution of three alewife treatments (N–down, N–up, and S–up; see ) at eight receiver sites using redundancy analysis (RDA). The plot was first conditioned by river kilometer, then constrained by the habitat variables (percent pool, depth, and width). Alewife treatment centroids are indicated by capital letters. The numbers in italics are receiver sites. Depth and width are represented as centroids because they were not significant. Percent pool is represented by the vector arrow. Shown are the percentages of variation explained by the conditioned, constrained, and unconstrained analyses and the P-values from Monte Carlo permutation tests. The percent variation explained is indicated on the axis labels.
DISCUSSION
Habitat units and transects (at which width, depth, and velocity were measured) provided an ecological basis for the interpretation of the landscape patterns of fish distribution. As native alewives released downstream made their natural trajectory upstream and then back downstream to the ocean, they spent a substantial amount of time in the deep and slow downstream pools above the first dam. Both native and stocked fish that were released upstream spent the most time in the deep and slow upstream pool habitat near Great Wenham Swamp, a historically important spawning site. Related research found a statistically different fish distribution across receiver locations (Frank et al. Citation2011). This flexibility in the use of upstream and downstream pools has implications for fisheries and watershed restoration, especially if fish are stocked for restoration purposes. Specifically, adults placed near specific spawning areas may stay there to spawn. For native fish moving from the ocean upstream through the river, pool habitat units in the lower part of our study site required less travel over fewer dams but they were smaller in size and more fragmented by riffles and runs than were pool habitat units in the upper study site.
Mapping habitat units allowed us to efficiently census habitat relevant to alewives for the entire 20.6 rkm of interest. The consideration of a large spatial area is a goal that is probably shared by many fisheries and watershed restoration practitioners. Because of the variety of habitat provided by the 19 habitat units, our larger-scale survey captured the diversity in habitat availability and fish habitat use. Without the survey of habitat units, we would not have had a comprehensive picture of all the habitats available to highly mobile fish throughout the restoration area. Our larger-scale survey of habitat units mirrored some elements of basinwide assessments and multistage approaches employed by others (Dolloff and Jennings Citation1997; Toepfer et al. Citation2000). In other systems, GIS provides a useful summary of habitat when the metrics being mapped are relevant to fish (Kocovsky et al. Citation2008). Our transect data reinforced the results of the habitat unit survey while providing useful high-resolution ecological data for restoration efforts. Data from 180 transects spaced throughout the lower 20.6 rkm of river confirmed that the pool habitat units were deeper and slower, the riffle habitat units were shallower and faster, and the run habitat units were intermediate. Width did not follow a predictable trend because of the variations associated with the riffles and runs in the wider, lower part of the river. Detailed information on depth, velocity, and other physical habitat attributes allows researchers and managers to assess how fish and other organisms respond to local conditions (McMahon et al. Citation1996), making transects a common method for assessing fish habitat (Simonson et al. Citation1994). The interpretation and synthesis of transect data alone, however, can be difficult for a large spatial area. Although the collection of detailed transect data was time consuming, it provided an essential quality control check for the larger-scale habitat surveys.
Tracking fish provided a useful example of how habitat data collected using multiple approaches can contribute to fisheries and watershed restoration. It was only through the use of telemetry that we were able to document the movement of fish up and down the river punctuated by the prolonged use of specific habitats. If electrofishing had been used to sample fish, these patterns may not have been observed because of the brief temporal and spatial snapshot that electrofishing provides. Even with telemetry, the sampling of detailed movements and complex distributions within and across specific habitats over a large area was challenging. Blanketing the entire study area with stationary receivers would be a very effective way to get definitive future information on habitat use by these and other migratory fish species.
Many fisheries and watershed restoration projects in urban areas address fragmentation. Quantifying habitat use and fish distribution concurrently provided insights about the importance of specific habitats and the connections among them. In our study, alewives forayed up and down the Ipswich River past the two lower dams in both directions, so their use of specific habitats was not determined by an inability to move past these dams. Furthermore, pools above dams created habitats that match those conditions considered suitable (in the literature) for spawning by adult river herring. However, even when fish can pass dams, detrimental delays may occur (Marschall et al. Citation2011). The trade-offs associated with dams that both create potential upstream spawning habitat but introduce potential delays illustrate why restoration projects within a watershed need to be addressed from a holistic perspective.
For restoration to be successful in an urbanizing environment where most of the stressors are human induced, integrating human dimensions and the biophysical aspects of restoration is essential. For example, in the Ipswich River, removing dams that have no fish passage to reestablish access to original tributary spawning ponds might benefit alewives. However, restoring these connections may be challenging because dam-created ponds provide a variety of benefits, ranging from drinking water to esthetically pleasing settings for local, politically influential stakeholders. Interdisciplinary approaches are needed for ecological problem solving and restoration (Ziemer Citation1997; USEPA Citation2000; Grimm et al. Citation2000) as well as for fish restoration (Mather et al. Citation1998). Clearly, information provided by the social and biophysical sciences needs to be integrated (Redman Citation1999; Carpenter et al. Citation2009), injected into the political agenda (Baron et al. Citation2002), and incorporated into restoration decisions. Operationally, however, these are not easy tasks (Alberti et al. Citation2003). Because humans both value (Frank et al. Citation2009) and impact anadromous fish, the approaches identified here can guide biophysical–social science integration.
There are other empirical, analytical, and conceptual challenges to quantifying freshwater habitat use by anadromous alewives. First, anadromous herring returning to freshwater to spawn must go through one section of the river to get to another, making an evaluation of the independence of sample data necessary. Our telemetry receivers were placed at considerable distances from each other and generally monitored different river locations. As a result, habitat variables in the 10 river sections and 8 receiver areas were not spatially autocorrelated (Moran's I-test, P > 0.05). In our study, not all anadromous herring went to all areas of the river, not all fish spent the same amount of time in all receiver areas, and many fish spent more time in specific sections of the river than in others. We chose to quantify these distributional patterns by summing the duration of time each fish spent in each receiver area regardless of the sequence of movements. Although trajectories (e.g., distributions through time) are, by definition, autocorrelated, the response variable that we used, the amount of time spent in each part of the river, was not.
Quantifying the distribution, movement, and habitat use of small, nearly extirpated populations of anadromous fish is an increasingly common goal for fish and watershed restoration. To determine the spatial distribution of these fish, a statistically defensible number of fish must be trapped and tagged. Within a large system, manually tracking multiple fish that are too small for satellite tags is extremely labor-intensive and requires a large team of trackers with multiple mobile receivers and many worker hours of labor, which most research teams do not have available to them. Using a series of stationary receivers along the migration pathway, as we have done here, solves the problem of locating highly mobile fish as they return to freshwater to spawn. Using stationary receivers to quantify mobile fish distributions also introduces additional challenges for identifying the habitats used by returning fish. First, although these receivers can be used as gates, the areas of the river not covered by receivers are often large. Second, most traditional habitat assessment and analysis methods use a large number of independent, often regularly spaced observations of fish location and habitat use (McMahon et al. Citation1996; Rogers and White Citation2007). Using stationary receivers to track highly mobile animals necessarily limits habitat use data to a few fixed locations. Although multivariate tools like RDA can still be used, the habitat data set associated with stationary receivers has a limited number of points for which fish and habitat data exist. Even though the stationary receiver methodology reported here provides previously unavailable information about the distribution of migratory fish, innovative analytical tools are still needed to analyze these types of habitat relationships.
Our conclusions may seem intuitive, and the results of our habitat assessment might have been more surprising had our study system been more physically diverse. Nevertheless, a better understanding of habitat use by anadromous herring demands that the patterns and drivers of habitat relationships in freshwater be tested with a standardized methodology. Although many researchers and managers speculate widely about the distribution of anadromous alewives (e.g., movement patterns, categories of use, habitat needs, distribution across pools and riffles, and the carrying capacity of each habitat type), few studies provide quantitative tests (). If researchers and managers are to restore anadromous alewives, empirical data on habitat availability and use in a variety of systems using a standardized methodology, such as the one we propose here, will be needed.
Determining the appropriate grain size, extent, and resolution needed to quantify freshwater habitat availability and use by anadromous fish is methodologically challenging. Adult river herring move across substantial distances in freshwater during transit and exploration but also use local habitats for specific life history functions (e.g., spawning). The general time and place of the migration are predictable but the specific distribution and timing of the movements are not. What is needed are data collected across large distances that identify where river herring go, combined with the high-resolution quantification of local habitat conditions to identify what cues fish use. Here, we propose a hybrid method that quantifies habitat availability and use with multiple approaches including methods that have (1) large-grain, large-extent, and moderate-resolution samples (from censusing a diverse number of habitat units across the entire restoration area), (2) large-grain, large-extent, and low-resolution samples (from reducing habitat units into major categories like pools, riffles, and runs), and (3) small-grain, large-extent, and high-resolution samples (from quantifying specific habitat conditions at limited transects). Detailed habitat units illustrated the big picture, and the 16 types of habitat units that we measured, although difficult to analyze, provided more resolution than just quantifying pools, riffles, and runs. However, summarizing patterns into the major types of habitat units (pools, riffles, and runs) was also insightful. Transects provided a high-resolution view of select locations within the river as well as a way to verify the accuracy of the habitat unit classifications. Using these multiple approaches to habitat availability and use by tagged fish will facilitate the development of a conceptual framework that can guide both watershed and anadromous fish restoration.
ACKNOWLEDGMENTS
This project was administered through the Massachusetts Cooperative Fish and Wildlife Research Unit, a cooperation among the University of Massachusetts, the U.S. Geological Survey, the Massachusetts Division of Marine Fisheries, the Massachusetts Division of Fisheries and Wildlife, and the Wildlife Management Institute. The Massachusetts Division of Marine Fisheries, Ebsco Publishing, New England BioLabs, Ipswich Bay Fly Fishing Derby, numerous alewife adopters, Northeast Utilities, and the U.S. Fish and Wildlife Service are acknowledged for support. A. Silberzweig, S. Turner, and M. Burak provided field assistance. Special thanks to the Plum Island Long Term Ecological Research (Ocean Sciences 9726921) for generously providing lodging. This research was conducted under the approved University of Massachusetts IACUC 27-02-03. Use of brand names does not imply endorsement by the U.S. government. Comments from M. Armstrong, A. Haro, D. Beauchamp, P. Budy, R. Rulifson, and one anonymous reviewer improved the manuscript.
Subject editor: Anthony Overton, East Carolina University, Greenville, North Carolina, USA
REFERENCES
- Alberti , M. , Marzluff , J. M. , Shulenberger , E. , Bradley , G. , Ryan , C. and Zumbrunnen , C. 2003 . Integrating humans into ecology: opportunities and challenges for studying urban ecosystems . Bioscience , 53 : 1169 – 1179 .
- Baron , J. S. , Poff , N. L. , Angermeier , P. L. , Dahm , C. M. , Gleick , P. H. , Hairston , N. G. , Jackson , R. B. , Johnston , C. A. , Richter , B. D. and Steinman , A. D. 2002 . Meeting ecological and societal needs for freshwater . Ecological Applications , 12 : 1247 – 1260 .
- Belding , D. L. 1920 . A report upon the alewife fisheries of Massachusetts , Boston : Commonwealth of Massachusetts Division of Fisheries and Game, Department of Conservation .
- Carpenter , S. R. , Mooney , H. A. , Agard , J. , Capistrano , D. , Defries , R. S. , Diaz , S. , Dietz , T. , Duraiappah , A. K. , Oteng-Yeboah , A. , Pereira , H. M. , Perrings , C. , Reid , W. V. , Sarukhan , J. , Scholes , R. J. and Whyte , A. 2009 . Science for managing ecosystem services: beyond the millennium ecosystem assessment . Proceedings of the National Academy of Sciences of the USA , 106 : 1305 – 1312 .
- Collette , B. B. and Klein-MacPhee , G. 2002 . Fishes of the Gulf of Maine, 3rd edition , Washington , D.C. : Smithsonian Institution Press .
- Davis , J. P. and Shultz , E. T. 2009 . Temporal shifts in demography and life history of an anadromous alewife population in Connecticut . Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science [online serial] , 1 : 90 – 106 .
- Dolloff , C. A. and Jennings , H. E. 1997 . A comparison of basinwide and representative reach habitat survey techniques in three southern Appalachian watersheds . North American Journal of Fisheries Management , 17 : 339 – 347 .
- Earthjustice . 2010 . Lawsuit filed to protect river herring and shad . Available: http://earthjustice.org/news/press/2010/lawsuit-filed-to-protect-river-herring-and-shad. (February 2012)
- Frank , H. J. , Mather , M. E. , Smith , J. M. , Muth , R. M. and Finn , J. T. 2011 . Role of origin and release location in pre-spawning movements of anadromous alewives . Fisheries Management and Ecology , 18 : 12 – 24 .
- Frank , H. J. , Mather , M. E. , Muth , R. M. , Pautzke , S. M. , Smith , J. M. and Finn , J. T. 2009 . The adopt-a-herring program as a fisheries conservation tool . Fisheries , 34 : 496 – 507 .
- Gahagan , B. I. , Gherard , K. E. and Schultz , E. T. 2010 . Environmental and endogenous factors influencing emigration in juvenile anadromous alewives . Transactions of the American Fisheries Society , 139 : 1069 – 1082 .
- Gordon , N. , McMahon , T. A. , Finlayson , B. L. , Gippel , C. J. and Nathan , R. J. 2004 . Stream hydrology: an introduction for ecologists, 2nd edition , Hoboken , New Jersey : Wiley .
- Grimm , N. B. , Grove , J. M. , Pickett , S. T. A. and Redman , C. L. 2000 . Integrated approaches to long-term studies of urban ecological systems . Bioscience , 50 : 571 – 584 .
- Hall , C. J. , Jordaan , A. and Frisk , M. G. 2011 . The historic influence of dams on diadromous fish habitat with a focus on river herring and hydrologic longitudinal connectivity . Landscape Ecology , 26 : 95 – 107 .
- Jessop , B. M. and Harvie , C. J. 1990 . Evaluation of designs of periodic count surveys for the estimation of escapement at a fishway . North American Journal of Fisheries Management , 10 : 39 – 45 .
- Kissil , G. W. 1974 . Spawning of anadromous alewife, Alosa pseudoharengus, in Bride Lake, Connecticut . Transactions of the American Fisheries Society , 103 : 312 – 317 .
- Kocovsky , P. M. , Ross , R. M. and Dropkin , D. S. 2008 . Linking landscapes and habitat suitability scores for diadromous fish restoration in the Susquehanna River basin . North American Journal of Fisheries Management , 28 : 906 – 918 .
- Kosa , J. T. and Mather , M. E. 2001 . Processes contributing to variability in regional patterns of juvenile river herring abundance across small coastal systems . Transactions of the American Fisheries Society , 130 : 600 – 619 .
- Legendre , P. and Legendre , L. 1998 . Numerical ecology, 2nd edition , Amsterdam : Elsevier .
- Limburg , K. E. and Waldman , J. R. 2009 . Dramatic declines in North Atlantic diadromous fishes . BioScience , 59 : 955 – 965 .
- Loesch , J. G. 1987 . “ Overview of life history of anadromous alewife and blueback herring in freshwater habitats ” . In Common strategies of anadromous and catadromous fishes , Edited by: Dadswell , M. , Klauda , R. J. , Moffitt , C. M. and Saunders , R. L. 89 – 103 . Bethesda , Maryland : American Fisheries Society . Symposium 1
- Maes , J. , Stevens , M. and Breine , J. 2008 . Poor water quality constrains the distribution and movements of twaite shad Alosa fallax fallax (Lacepede, 1803) in the watershed of river Scheldt . Hydrobiologia , 602 : 129 – 143 .
- Mather , M. E. , Parrish , D. L. , Folt , C. L. and DeGraaf , D. M. 1998 . Integrating across scales: effectively applying science for the successful conservation of Atlantic salmon (Salmo salar) . Canadian Journal of Fisheries and Aquatic Sciences , 55 : 1 – 8 .
- Marschall , E. A. , Mather , M. E. , Parrish , D. L. , Allison , G. W. and McMenemy , J. R. 2011 . Migration delays caused by anthropogenic barriers: modeling dams, temperature, and success of migrating salmon smolts . Ecological Applications , 21 : 3014 – 3031 .
- McCain , M. , Fuller , D. , Decker , L. and Overton , K. 1990 . Stream habitat classification and inventory procedures for Northern California , Eureka , , California : U.S. Forest Service, Region 5 Fish Habitat Relationships Technical Bulletin 1 .
- McDowall , R. M. 1999 . Different kinds of diadromy: different kinds of conservation problems . ICES Journal of Marine Science , 56 : 410 – 413 .
- McMahon , T. E. , Zale , A. V. and Orth , D. J. 1996 . “ Aquatic habitat measurements ” . In Fisheries Techniques, 2nd edition , Edited by: Murphy , B. R. and Willis , D. W. 83 – 120 . Bethesda , Maryland : American Fisheries Society .
- Mullen , D. M. , Fay , C. W. and Moring , J. R. 1986 . Alewife/blueback herring. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (North Atlantic series) . U.S. Fish and Wildlife Service Biological Report 82 ,
- NOAA NMFS (National Oceanic and Atmospheric Administration National Marine Fisheries Service) . 2011 . Listing endangered and threatened wildlife and plants; 90-day finding on a petition to list alewife and blueback herring as threatened under the Endangered Species Act . Federal Register , 76 : 67652 – 67656 . 212(2 November 2011):
- O’Connell , A. M. and Angermeier , P. L. 1997 . Spawning location and distribution of early life stages of alewife and blueback herring in a Virginia stream . Estuaries , 20 : 779 – 791 .
- O’Connell , A. M. and Angermeier , P. L. 1999 . Habitat relationships for alewife and blueback herring spawning in a Virginia stream . Journal of Freshwater Ecology , 14 : 357 – 370 .
- Oksanen , J. , Kindt , R. , Legendre , P. , O’Hara , B. , Simpson , G. L. , Solymos , P. , Stevens , M. H. H. and Wagner , H. 2009 . Vegan: community ecology package, R package version 1.15-4 . Available: http://CRAN.R-project.org/package= vegan. (February 2012)
- Pardue , G. B. 1983 . Habitat suitability index models: alewife and blueback herring . U.S. Fish and Wildlife Service FWS-OBS-82/10.58
- R Development Core Team . 2009 . R: a language and environment for statistical computing , Vienna : R Foundation for Statistical Computing . Available: http://www.R-project.org(February 2012)
- Redman , C. L. 1999 . Human dimensions of ecosystem studies . Ecosystems , 2 : 296 – 298 .
- Rogers , K. B. and White , G. C. 2007 . “ Analysis of movement and habitat use from telemetry data ” . In Analysis and interpretation of freshwater fisheries data , Edited by: Guy , C. S. and Brown , M. L. 625 – 676 . Bethesda , Maryland : American Fisheries Society .
- Rounsefell , G. A. and Stringer , L. D. 1945 . Restoration and management of the New England alewife fisheries with special reference to Maine . Transactions of the American Fisheries Society , 73 : 394 – 424 .
- Rulifson , R. A. 1994 . “ Status of anadromous Alosa along the east coast of North America ” . In Anadromous Alosa symposium , 134 – 158 . Virginia Beach , Virginia : American Fisheries Society . in J. E. Cooper, R. T. Eades, R. J. Klauda, and J. G. Loesch, editors., Tidewater Chapter
- Rulifson , R. A. , Huish , M. T. and Thoesen , R. W. 1982 . “ Status of anadromous fishes in southeastern U.S. estuaries ” . In Estuarine comparisons , Edited by: Kennedy , V. S. 413 – 425 . New York : Academic Press .
- Rulifson , R. A. and Wall , B. L. 2006 . Fish and blue crab passage through water control structures in a coastal bay lake . North American Journal of Fisheries Management , 26 : 317 – 326 .
- Saunders , R. , Hachey , M. A. and Fay , C. W. 2006 . Maine's diadromous fish community: past, present, and implications for Atlantic salmon recovery . Fisheries , 31 : 537 – 545 .
- Schmidt , R. E. , Jessop , B. M. and Hightower , J. E. 2003 . “ Status of river herring stocks in large rivers ” . In Biodiversity, status, and conservation of the world's shads , Edited by: Limburg , K. E. and Waldman , J. R. 171 – 182 . Bethesda , Maryland : American Fisheries Society . Symposium 35
- Simonson , T. D. , Lyons , J. and Kanehl , P. D. 1994 . Quantifying fish habitat in streams: transect spacing, sample size, and a proposed framework . North American Journal of Fisheries Management , 14 : 607 – 615 .
- Smith , J. M. , Mather , M. E. , Frank , H. J. , Muth , R. M. , Finn , J. T. and McCormick , S. D. 2009 . Evaluation of a gastric radio tag insertion technique for anadromous river herring . North American Journal of Fisheries Management , 29 : 367 – 377 .
- ter Braak , C. J. F. and Prentice , I. C. 1988 . A theory of gradient analysis . Advances in Ecological Research , 18 : 271 – 317 .
- Toepfer , C. S. , Fisher , W. L. and Warde , W. D. 2000 . A multistage approach to estimate fish abundance in streams using geographic information systems . North American Journal of Fisheries Management , 20 : 634 – 645 .
- Tyus , H. M. 1974 . Movements and spawning of anadromous alewives at Lake Mattamuskeet, North Carolina . Transactions of the American Fisheries Society , 103 : 392 – 396 .
- U.S. Census Bureau . 2006 . Section 1: population. U.S , Washington , D.C. : Census Bureau . Available: http://www.census.gov/prod/2005pubs/06statab/pop.pdf (February 2012)
- USEPA (U.S. Environmental Protection Agency) . 2000 . Principles for the ecological restoration of aquatic resources , Washington , D.C. : USEPA, Office of Water . EPA841-F-00-003, Available: http://water.epa.gov/type/wetlands/restore/principles.cfm. (February 2012)
- Walsh , H. J. , Settle , L. R. and Peters , D. S. 2005 . Early life history of blueback herring and alewife in the lower Roanoke River, North Carolina . Transactions of the American Fisheries Society , 134 : 910 – 926 .
- Wiens , J. A. 1989 . Spatial scaling in ecology . Functional Ecology , 3 : 385 – 397 .
- Yako , L. A. , Mather , M. E. and Juanes , F. 2002 . Mechanisms for migration of anadromous river herring: an ecological basis for effective conservation . Ecological Applications , 12 : 521 – 534 .
- Ziemer , R. R. 1997 . “ Temporal and spatial scales ” . In Watershed restoration: principles and practices , Edited by: Williams , J. E. , Wood , C. A. and Dombeck , M. P. 80 – 95 . Bethesda , Maryland : American Fisheries Society .