Abstract
Critical habitats for fish and wildlife are often small patches in landscapes, e.g., aquatic vegetation beds, reefs, isolated ponds and wetlands, remnant old-growth forests, etc., yet the same animal populations that depend on these patches for reproduction or survival can be extensive, ranging over large regions, even continents or major ocean basins. Whereas the ecological production functions that support these populations can be measured only at fine geographic scales and over brief periods of time, the ecosystem services (benefits that ecosystems convey to humans by supporting food production, water and air purification, recreational, esthetic, and cultural amenities, etc.) are delivered over extensive scales of space and time. These scale mismatches are particularly important for quantifying the economic values of ecosystem services. Examples can be seen in fish, shellfish, game, and bird populations. Moreover, there can be wide-scale mismatches in management regimes, e.g., coastal fisheries management versus habitat management in the coastal zone. We present concepts and case studies linking the production functions (contributions to recruitment) of critical habitats to commercial and recreational fishery values by combining site-specific research data with spatial analysis and population models. We present examples illustrating various spatial scales of analysis, with indicators of economic value, for recreational Chinook Oncorhynchus tshawytscha salmon fisheries in the U.S. Pacific Northwest (Washington and Oregon) and commercial blue crab Callinectes sapidus and penaeid shrimp fisheries in the Gulf of Mexico.
Received June 30, 2011; accepted June 4, 2012
 “The problem of relating phenomena across scales is the central problem in biology and in all of science.” (Levin Citation1992)
“The problem of relating phenomena across scales is the central problem in biology and in all of science.” (Levin Citation1992)
Nowhere is Levin's “central problem” more apparent than at the intersection of ecological research and ecological economics. Indeed, scaling is such an important matter in ecological economics that it has been suggested as one basis for defining ecosystem service typologies (Costanza Citation2008). Ecosystem-based management (EBM) seeks to integrate the ecosystem and the services it provides with the social system (e.g., Leslie and McLeod Citation2007; Levin et al. Citation2009). Therefore, issues of scale mismatches between natural and social systems (Young Citation2002; Galaz et al. Citation2008) are of critical importance to the practice of EBM. The issue of scaling-up biological resources and their economic use is common to the fields of biology and economics. Understanding how habitat affects stocks of commercially and recreationally important species on multiple spatial scales also can inform the relevant level of social aggregation at which a particular fishery must be managed to achieve management objectives. Just as natural resource considerations are spread over spatial scales of organization, so too are management structures; the boundaries of social systems may not be the same as those of ecological systems. Biological observations and modeling can help determine which spatial scales are most relevant to management actions and whether local (land owner or county), state, national, or international levels of organization are most relevant to management and conservation.
We present examples from coastal fisheries to illustrate how landscape ecology can be used to create a bridge from fine-scale ecosystem properties and functions to ecosystem services that are delivered over extended scales of space and time. For migratory and widely distributed species, the spatial and temporal scales of production functions can be finer by several orders of magnitude than the scales of the ecological services supplied to society (e.g., commercial and recreational fishing, hunting, bird watching). Although our examples are all migratory species, these species exhibit very different patterns of migration. In the case of Gulf of Mexico (GOM) species, we examine how changes in juvenile habitat may affect overall harvestable population size and value for penaeid shrimp (brown shrimp Farfantepenaeus aztecus, pink shrimp F. duorarum, and white shrimp Litopenaeus setiferus) and blue crabs Callinectes sapidus. Conversely, in the Pacific Northwest (PNW, i.e., the coasts of Oregon and Washington), we examine how the migration of Chinook salmon Oncorhynchus tshawytscha from Yaquina Bay, Oregon, affects the relevant scales for economic valuation of the recreational fishery and how these scales have implications for the management and conservation of habitats. In the realm of governance, our case studies contrast the scales of fishery harvest and management regimes with the scales upon which essential nursery habitats are managed. Salmon in the PNW and commercially harvested shrimp and crabs in the GOM share the attributes of (1) extensive coastal or oceanic fisheries and supporting biological populations managed at state and federal levels and (2) critical in-shore habitats that typically depend on local management regimes, where state and federal entities generally do not have direct regulatory authority. We outline some methods for multiscale modeling and estimation of these phenomena. Results are presented from (1) an economically oriented model for Pacific salmon fisheries and (2) an ecologically based fishery production model for GOM blue crabs and penaeid shrimp. Both models use landscape ecology and geographic scaling to link spatially explicit habitat values for a particular locality to the values of large regional fisheries. In the sense of Cury (Citation2004), we demonstrate linkage of mesoscale process-oriented observations to macroscale pattern-oriented analyses.
BACKGROUND
In the PNW of the United States and Canada, several species and subspecies of Pacific salmon Oncorhynchus spp. support fisheries of great commercial, recreational, and cultural value. Pacific coast salmon fisheries and their recent declines have been the subject of intense scientific scrutiny and a considerable body of both scientific (reviewed in Knudsen et al. Citation1999) and economic, cultural, and institutional literature (Hanna et al. Citation2006; Hanna Citation2008). Salmon have been culturally important in the PNW for millennia (Meengs and Lackey Citation2005), but the arrival of European settlers led to major losses and modifications to salmon habitats as well as an increase in the demand for salmon as food. Today the principal threats to salmon are considered to be overharvesting, habitat loss, dams, and hatcheries (Bottom et al. Citation2009). Hatchery-reared fish comprise the majority of salmon in many systems, leading to reduced genetic diversity and reproductive fitness (Levin and Schiewe Citation2001). Despite measures aimed at improving stocks, 29% of the almost 1,400 historical salmonid populations are now extinct, and 27 stock groups are listed as threatened or endangered (Bottom et al. Citation2009). shows the combined commercial landings of Chinook salmon in Oregon and Washington since 1950. Although fluctuations in annual catches (e.g., 1987 and 1988) are driven by climatic cycles (Mantua et al. Citation1997) and the resulting variations in the availability of prey (Levin Citation2003; Hooff and Peterson Citation2006), there has been a general decline over the past 60 years.
FIGURE 1 Annual landings (metric tons [mt]) of Chinook salmon in Washington and Oregon, 1950–2007 (source: http://www.st.nmfs.noaa.gov/st1//commercial/landings/annual_landings.html).
![FIGURE 1 Annual landings (metric tons [mt]) of Chinook salmon in Washington and Oregon, 1950–2007 (source: http://www.st.nmfs.noaa.gov/st1//commercial/landings/annual_landings.html).](/cms/asset/36c9ba53-4ae1-4e13-ba53-59665146d4d4/umcf_a_703162_o_f0001g.gif)
The economic value of commercial and recreational fishing for salmon in the PNW has been recognized and studied for many decades, and valuation of recreational fishing for salmon in the PNW is not a new field. For example, Brown et al. (Citation1964) calculated the net economic value of salmon and steelhead in Oregon at US$3 million for 1962 ($20.6 million in 2007 dollars) based on expenditures by anglers. More recently, nonmarket valuation techniques have been used to estimate the consumer surplus for recreational salmon fisheries (Brown et al. Citation1983; Meyer et al. Citation1983; Riley Citation1988; Loomis Citation1989; Olsen et al. Citation1991; Bell et al. Citation2003). Economic methods to assess the value of recreational fisheries are well established and relatively robust. These values usually are expressed in terms of willingness to pay (WTP) for some unit of recreational use (angler-day, trip, etc.) or social aggregation (household, individual). Willingness-to-pay values subsume the worth of an aggregated bundle of various ecosystem services. For example, the preference of an individual to fish in a given location may be due to the supply of fish (a provisioning service) or the aesthetics of the location itself (cultural services), and these quantity-of-fish, quality-of-location values in turn result from the supporting and regulating services of the surrounding ecosystem. For many systems, analysis of values suffers from a lack of adequate economic valuation literature (Pendleton et al. Citation2007), but this is not particularly true for PNW salmon fisheries. Nevertheless, despite the relative abundance of available literature, the anadromous nature of salmon species means that the spatial allocation of economic values may prove to be a greater challenge than the valuation process itself.
Blue crabs and penaeid shrimp support some of the most important coastal fisheries in the U.S. GOM and Atlantic southeastern states, with a combined exvessel value of ∼$471 million for the commercial fisheries in 2008 (NMFS Citation2011). These species also support recreational and subsistence fisheries, but we limit our analysis to the larger and better-documented commercial components. Blue crabs and penaeid shrimp share three important ecological attributes: (1) unitary populations extending over large regions (Nance et al. Citation1989; McMillen-Jackson et al. Citation1994; Guillory et al. Citation2001), (2) diadromous migratory life histories (Haas et al. Citation2004; Dantin et al. Citation2005), and (3) dependence of early-stage juveniles on vegetated, shallow-water habitats in estuaries and coastal lagoons (e.g., Minello et al. Citation2003).
Chinook salmon exhibit two principal life history strategies (Taylor Citation1990). “Ocean-type” juveniles generally migrate downstream, undergo smoltification, and enter the ocean within 150 d of hatching if conditions are favorable. At this time the juvenile salmon, or parr, may make use of estuarine habitats, although there is considerable variability in the amount of time the salmon remain in the estuary. Within the estuary, emergent marsh habitats seem to play a particularly important role in the rearing of juvenile salmon (Burke Citation2004) and the amount of estuarine habitat per se also increases survival rates of salmon (Magnusson and Hilborn Citation2003). “Stream-type” Chinook salmon migrate to the ocean during their second spring. Survivorship of the estuarine and early ocean phases of the salmon life cycle is as low as 0.017 (Kareiva et al. Citation2000), and suitable habitat is critical for the survivorship of these early life stages. The salmon remain at sea for 1–6 years, though most commonly 2–4 years, before the homeward migration. The Pacific Ocean provides a vast rearing ground for Chinook salmon. Ocean-type Chinook salmon tend to utilize estuaries and coastal areas more extensively than stream-type Chinook salmon; the stream variety tends to migrate to the central North Pacific (Healey Citation1991). illustrates the results of tagging studies that have defined the range of Chinook salmon ocean-rearing habitat (Beamish et al. Citation2005). In the reproductive phase, salmon migrate from the oceans through the estuary back to their natal streams to spawn and die. Each habitat type has its own specific function in the salmon life cycle and its own set of threats relevant to the continuation of salmon fisheries.
FIGURE 2 Ocean habitat of Chinook salmon (darker area) spawned on the West Coast of North America (redrawn from Beamish et al. Citation2005 showing the location of Lincoln County, Oregon [circle]).
![FIGURE 2 Ocean habitat of Chinook salmon (darker area) spawned on the West Coast of North America (redrawn from Beamish et al. Citation2005 showing the location of Lincoln County, Oregon [circle]).](/cms/asset/dfa6079c-6ed4-4eec-b3c8-06b417037993/umcf_a_703162_o_f0002g.gif)
FIGURE 3 Schematic of blue crab and penaeid shrimp migratory life histories. Blue crabs typically are spawned near the mouths of estuaries, whereas shrimp may be spawned farther offshore.
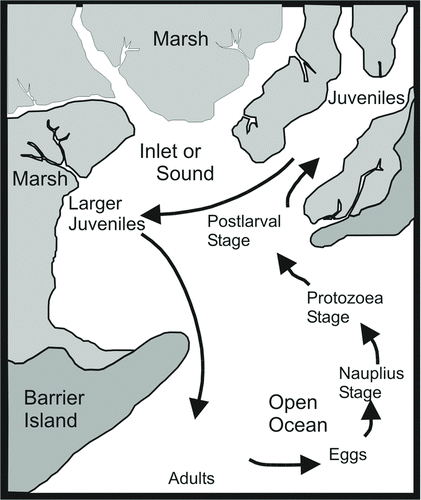
Mature penaeid shrimp migrate from estuaries into offshore coastal waters to spawn. After mating in estuarine habitats, female blue crabs migrate seaward to spawn near the mouths of estuaries. The larvae of both taxa spend a few weeks in offshore waters, where they can be distributed widely by winds and currents. As the larvae mature into postlarvae, they move into estuaries, settling preferentially in shallow-water vegetated habitats such as salt marshes (principally along the marsh–water interface, or marsh edge) and seagrass beds. These structured coastal habitats provide appropriate food sources and refuges from predation for the initial, vulnerable juveniles. As the animals mature, they disperse widely within estuaries (Dantin et al. Citation2005; ). In a pioneering landscape ecology study, Browder et al. (Citation1989) related salt marsh disintegration in Louisiana to observed and predicted changes in the brown shrimp fishery. Because the extent of marsh edge increases and then decreases in a hyperbolic pattern as marsh is lost, recruitment to the fishery would be expected to increase up to a point and then decrease. Although Browder et al. (Citation1989) modeled the functional relationship between marsh loss and habitat extent, the link between these phenomena and shrimp production was purely correlational. Our study includes three species of shrimp and blue crabs in three important habitat types—submersed aquatic vegetation (SAV), shallow nonvegetated bottom (SNB), and marsh edge (ME)—in a semideterministic model that predicts the effects of habitat loss and gain on the fisheries.
The characteristic scale of the processes determining year-class strength in shrimp and blue crab populations, based on habitat-dependent late larval and early-juvenile growth and survival, is on the order of centimeters to meters. Ontogenetic migrations scale with estuaries (tens to hundreds of kilometers), whereas dispersal by larvae and errant adults maintains panmictic populations over thousands of kilometers. Therefore, an analysis of the ecosystem services produced by these populations, how the services vary, and how they might change in future scenarios involves spatial scales ranging over 6–8 orders of magnitude.
METHODS
Blue crab and shrimp spatial–population–fishery models.—For blue crabs and shrimp, we linked models over a wide range of spatial and temporal scales, based on the approach of Jordan et al. (Citation2009), to estimate the functions of preferred habitat types in producing recruits to the fishable (adult) stocks. At the largest, regional scale (the U.S. GOM, which has a coastline ∼27,000 km in length, including bays and estuaries), fishery population models were constructed from fishery landings and effort data compiled by the National Marine Fisheries Service (NMFS Citation2011) from 1950 to 2004 (blue crabs) and 1961–2008 (shrimp), in combination with fishery-independent data collected by state fishery programs. At the landscape scale, we employed shallow-water and shoreline habitat GIS coverages for the Mobile Bay, Alabama, estuarine area, one of the Gulf's larger estuarine systems (∼1,000 km2), in a spatially-tiled framework (cells of 55.2 km2) based on the National Coastal Assessment (USEPA Citation2008). At the finest (patch) scale, we used primary data in combination with information from the scientific literature to assign densities and survival rates for three physical habitat types (SAV, SNB, and ME) and salinity zones (oligo-, meso-, and polyhaline); these data typically have been generated at a scale of ≤1 m2. The results were three-stage models (early juveniles, prerecruits, and adults) in which areal habitat coverage could be manipulated to predict the long-term effects of habitat changes on the fisheries. provides a conceptual overview of the multiscale modeling approach. In summary, to generate the numbers of recruits the models employed an accounting method, multiplying the densities of juveniles by habitat areas and survival rates. The contribution of recruits to the fishery was estimated by (1) converting the numbers of recruits to biomass using published length–weight relationships and (2) linking the recruitment models to dynamic population models that estimated recruitment to the Gulfwide fisheries. The linkage was achieved by converting the mean instantaneous rate of population change (net recruitment per year; r) from the historical time series to absolute biomass so that it could be increased or decreased as a function of predicted recruitment biomass for any scenario of habitat change.
For this paper, we have adapted the blue crab and shrimp models to simulate the effects of changes in habitat based on loss or restoration of SAV. We applied the habitat geographic coverages from Mobile Bay, as described in Jordan et al. (Citation2009), modifying the areal extent of SAV to predict the effects of loss or gain on the GOM blue crab and shrimp fisheries. The loss scenario involved decreasing SAV coverage by a total of 20%, distributed over three hexagonal grid cells, as described in Jordan et al. (Citation2009). The “no change” scenario simply reflected the recent trends in recruitment, with no change in habitat extent. For the three restoration scenarios, we added 50 ha of SAV to each of 10, 20, or 31 previously unvegetated cells (31 was the total number of unvegetated cells), reducing the area of SNB by the same amount. In this version of the blue crab model, we added a biomass carrying capacity parameter (K) derived from long-term fishery data and reformulated the model using the following logistic population equation rather than the exponential equation described by Jordan et al. (Citation2009):
TABLE 1 Parameters and sources of information for Gulf of Mexico blue crab and shrimp models; SAV = submersed aquatic vegetation, SNB = shallow, nonvegetated bottom.
The addition of carrying capacity to the model maintained reasonable predictions for habitat restoration scenarios, as opposed to the habitat loss scenarios simulated in our previous research. The K parameter was set at the maximum estimated harvestable stock biomass over the period of record, 1950–2004 (). To achieve the scale of the entire U.S. GOM, we assumed that the changes simulated for Mobile Bay would have occurred Gulfwide (Jordan et al. Citation2009).
The shrimp model was constructed in the same manner as the blue crab model. Our original intent was to produce a model to examine habitat scenarios for the Tampa Bay, Florida, estuarine system as part of an effort to quantify ecosystem services for the Tampa region (http://www.epa.gov/ged/tbes/index.html). It was clear from preliminary work, however, that we could not match or apply landings and effort data at this (county) scale because (1) the fishery operates at a much larger spatial scale and (2) reported effort data (number of fishing trips) could not be adjusted to unit effort (e.g., days fished). Therefore, as in the blue crab model, the shrimp model was parameterized at the Gulfwide scale. The blue crab model employed an annual time step, consistent with life history and recruitment traits. The shorter life cycle of shrimp, with great intra-annual variation in harvest and recruitment, required a monthly time step, illustrated by the hindcast shown in . Model parameters and values are listed in . Both models were validated partially by testing them against historical data, although this method demonstrates only that the model reproduces the landings data used in the parameterization. Additional lines of validation for the blue crab model were discussed by Jordan et al. (Citation2009). Comparisons of model-dependent and model-independent methods of estimating recruitment from Mobile Bay to the Gulfwide fisheries were used as further validation that the models produced reasonable estimates of production.
The large-regional modeling approach supplies information that can be used to assign ecologically based monetary and nonmonetary values to specific habitat types in the context of regional fisheries. From the perspectives of policy, management, and ecological restoration, it is also useful to quantify ecosystem services and values at finer scales. Thus, at an intermediate scale, we estimated the contributions of a large estuary (Mobile Bay) to the GOM blue crab and shrimp fisheries using two independent methods. The first method simply used the area of the estuary divided by the total estuarine area in the U.S. GOM, assuming that recruitment to the fishery was proportional to the estuarine area. The second method employed the areal extent of specific habitat types in combination with biological information (observed densities and survival rates by habitat type for early-stage juveniles plus estimated survival rates of later-stage prerecruits) to estimate recruitment to the fishery from the estuary. A comparison of the two methods supplies information about the robustness and accuracy of the spatial scaling models. We also estimated the total exvessel value of shrimp and crab production from the Mobile Bay system and the values per hectare of SAV for each scenario. The blue crab model has been applied at a finer, subestuary scale (O’Higgins et al. Citation2010).
FIGURE 4 Conceptual overview of multiscale modeling for coastal migratory fish species. At the patch scale, data from sampling and experiments (original or reported in the scientific and technical literature) are used to estimate the production of juveniles per unit area (m2). At the landscape scale, geographical information on the extent, distribution, and quality of critical habitats is used to expand unit production to a larger area. At the regional scale (e.g., the U.S. GOM), harvest and economic data are used in models to estimate habitat contributions to major fisheries.
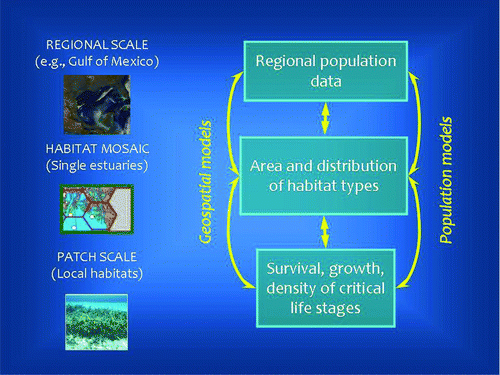
FIGURE 5 Combined monthly landings of brown, pink, and white shrimp (metric tons [mt]) in the U.S. Gulf of Mexico, 1961–2007, as reported by the National Marine Fisheries Service (observed) and estimated by a fishery population model (predicted).
![FIGURE 5 Combined monthly landings of brown, pink, and white shrimp (metric tons [mt]) in the U.S. Gulf of Mexico, 1961–2007, as reported by the National Marine Fisheries Service (observed) and estimated by a fishery population model (predicted).](/cms/asset/d7a9b94b-e77b-42b7-85ea-848a57c64184/umcf_a_703162_o_f0005g.jpg)
Economic geography of salmon fisheries.—Economic valuation of the Yaquina Bay recreational salmon fishery was carried out using two different methods. In one method, published estimates of WTP per angler-day of salmon fishing in Oregon and Washington were combined with estimates of the number of recreational fishery trips within Yaquina Bay based on salmon harvest in the Yaquina River and angler effort (Kroeger and McMurray Citation2008). The WTP estimates () were based on the travel cost method in which WTP represents a minimum value to recreational fishers based on the distance they are willing to travel to avail themselves of the recreational fishing resource. For comparison, in the second method the economic values of the within-estuary recreational fishery were calculated on a per-household basis based on a published WTP value of $134 per year per household to avoid the loss of salmon in Yaquina Bay specifically (Bell et al. Citation2003). The value estimate was based on information solicited from the public using survey techniques.
TABLE 2 Salmon values per angler-day in Oregon using the travel cost method adapted from Hanna et al. (Citation2006).
Analysis of the spatial scales relevant to the Chinook salmon population in an individual estuary (Yaquina Bay) was conducted using ArcGIS 9.3. Primary data for coverages of emergent marsh were taken from the National Wetland Inventory (http://www.fws.gov/wetlands/); watershed data were from the U.S. Environmental Protection Agency (unpublished data); and salmon migration data were georeferenced from published literature (Beamish et al. Citation2005).
RESULTS
Pacific Northwest: Economic Value of Chinook Salmon in Yaquina Bay
The total annual value of recreational salmon fishing in the Yaquina estuary, estimated as the product of mean value per angler-day () and angler effort, was $540,817.
The results of estimates based on household WTP and different levels of social organization for recreational fisheries are shown in . The values of the Yaquina Bay recreational fishery estimated by the angler-effort method ($540,817) and by the household method when confined to Newport, Oregon ($551,008) are in good agreement. However, at wider social scales of aggregation (county, state, and region) the values are considerably higher, depending on the number of households included in the analysis. In our example, the estimate of recreational WTP presented for the Pacific Northwest (), assuming that every household throughout Washington and Oregon values the Yaquina estuary equally, is three orders of magnitude greater than the estimate based on the Newport population.
TABLE 3 Value in terms of willingness to pay (WTP) to avoid loss in salmon catch for Yaquina Bay aggregated at different spatial scales. Household data are from the 2000 U.S. Census; those for the PNW are aggregates of those for Oregon and Washington. The WTP value for the Yaquina Bay estuary is from Bell et al. (Citation2003).
The GIS analysis demonstrates the multiple spatial scales of the broad major habitat types for Chinook salmon and the ecological role of those habitats in the salmon life cycle (). The habitat types required for the completion of the Chinook salmon life cycle span at least 7 orders of spatial magnitude. Each habitat type has its own specific function in the salmon life cycle and its own set of threats and survival rates; each type is also subject to different legislative and administrative jurisdictions with respect to the management and sustainability of salmon fisheries. shows the estimated WTP per unit area of habitat type, where all of the value (WTP per household at a given spatial level of aggregation, i.e., county, state, etc.) is attributed entirely to a single habitat type. As the spatial scale increases, values per unit area diminish rapidly. Conversely, as the geographical scale of economic benefit increases (i.e., taking into account the number of households at the state and regional scale), the benefits per unit area from the estuary or marshes within the estuary increase by three orders of magnitude.
TABLE 4 Various scales of habitats for Chinook salmon in the Yaquina Bay estuary and the roles they play in the salmon life cycle.
TABLE 5 Estimates of willingness to pay (WTP; dollars per hectare per year except for total [dollars/year]) for each of the major habitat types in the Chinook salmon life cycle. Each estimate assumes that all WTP is assigned to one habitat type rather than being partitioned among habitat types. Subestuary = Yaquina Bay salt marsh habitat.
Gulf of Mexico Blue Crab and Shrimp Fisheries
Three of the model scenarios predicted long-term declines in commercial landings of blue crabs (). Simulated restoration of 500 ha of SAV reduced, but did not reverse, the recent negative trend in recruitment. This negative trend may be merely an artifact of the modeling process, because the mean rate of population change calculated by the hindcasting model was slightly less than zero; conversely, it may be a real downward trend in recruitment. Fishery landings stopped increasing about 1990, with slight evidence for a decline thereafter. Restoration of larger areas of SAV—1,000 and 1,550 ha— led to predictions of positive trends in recruitment and simulated landings. In the most positive scenario, the effect of carrying capacity can be seen in the shape of the uppermost curve in ; the limit on landings is about 32,650 mt.
FIGURE 6 Simulated effects of changes in the amount of submersed aquatic vegetation (SAV) habitat on (A) blue crab and (B) penaeid shrimp fisheries in the GOM. Geographic information was combined with data from studies of habitat dependence in early juvenile blue crabs and long-term fishery information to model recruitment (Jordan et al. Citation2009).
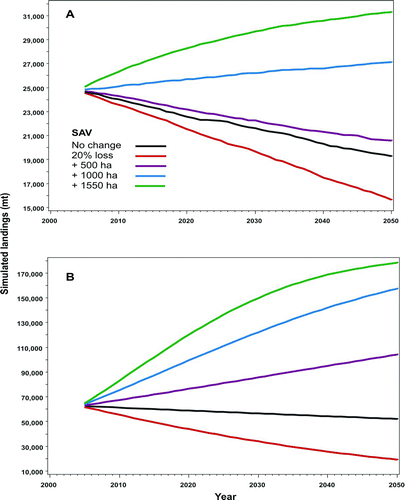
Projections of shrimp harvest based on the same scenarios described above for blue crabs resulted in similar, but overall more positive trends. Long-term shrimp harvests declined with a 20% SAV loss, remained virtually unchanged for the no-change scenario, and increased for each of the SAV restoration scenarios ().
The blue crab model indicates that under the baseline scenario (no change in habitat) the Mobile Bay estuary system would produce about 17% of the blue crab recruited biomass in the Gulfwide fishery, whereas the shrimp model for the same area generates 8.6% of total recruited biomass. The area of the Mobile Bay estuarine system (including the Alabama portion of Mississippi Sound and Perdido Bay in Alabama and Florida) is 8.8% of the total estuarine area in the U.S. GOM, so the model generates approximately 1.9 times the biomass of blue crab recruits and 1.0 times the biomass of shrimp recruits predicted by area-proportioning of the fishery stock. The discrepancy for blue crabs may be explained partially by our omission of the soft-shell crab, peeler crab, and recreational fisheries, along with unreported landings, which would be included in model estimates but not in the landings data. The shrimp harvest, in contrast, is dominated by the commercial sector. These factors could account for the closer correspondence of the area-proportioned and modeled biomass estimates for shrimp.
The simulations of SAV restoration provide a means of estimating the gross value and value per hectare of restoration for the blue crab and shrimp fisheries. In 2008, live hard-shell blue crabs sold for an average of $1,761/mt exvessel; shrimp averaged $7,847/mt (heads off). The model was used to estimate the numbers and biomass of recruits from the Mobile Bay system, the fraction that would be captured by the fishery, and the exvessel values for each scenario. The value of SAV restoration per hectare increased as the magnitude of restored habitat increased ().
TABLE 6 Differential gross exvessel values of blue crabs and shrimp associated with habitat change scenarios for Mobile Bay, assuming 2008 prices of $1,761/mt for crabs and $7,845/mt for shrimp (heads off); the values per hectare of submersed aquatic vegetation (SAV [existing and restored combined]; last column) are for shrimp and crab production combined.
DISCUSSION
We have demonstrated some aspects of the scaling problem in relating ecosystem functions to ecosystem services and economic values in the context of coastal fisheries, employing contrasting species, regions, and analytical methods. The principal problems addressed are (1) that some ecosystem services are delivered to society at spatial scales much more extensive than those at which they are produced and (2) that the ecosystem properties essential to the sustainability of fisheries (e.g., tidal wetlands and undeveloped shorelines) are managed by entities entirely different from those that manage fisheries, with the entities operating at very different spatial scales and managing toward different ends.
In the case of Pacific salmon, the scale of delivery to recreational fisheries is poorly defined, so we have expressed potential values at several scales. In the case of GOM shrimp and crabs, the scale of delivery to commercial fisheries is fairly well defined, as are the habitat areas and qualities that contribute to production. For the latter species, more explicit modeling of relationships between habitat conditions, habitat conservation, and commercial fisheries is feasible (the considerable uncertainties are discussed below).
These analyses require data at all scales: ecological data at the finest scales, landscape (seascape) data at intermediate to regional scales, and biological monitoring and economic data over large expanses of space and time. We have emphasized scaling in the spatial domain, but the time domain is equally important. Science and policy would, perhaps, show far less interest in the PNW salmon fishery were it not for the well-documented long-term decline in the resources and the services they supply to society. The blue crab model was constructed from a 54-year time series of fishery data at the Gulfwide scale and then used to simulate 50-year future scenarios. A 47-year time series of data was available for the shrimp fishery. Although we have expressed gross economic values for fishery resources on an annual basis, these resources have cumulative values over periods of time that are limited (if sustainably managed) only by the discount rate one chooses to apply or (if not sustainably managed) by economic extinction of the resources. Beyond fishery management, economic extinction could also be the outcome of failures to conserve coastal nursery habitats of sufficient extent and quality.
In the temporal context, it is of paramount importance to resolve long-term trends (whether past or future) from the considerable noise of temporal variability ( and ). Habitat loss is an insidious threat to the types of populations we discuss here (Peterson and Lowe Citation2009) because it is generally a slow process, with only marginal effects on annual recruitment () that can be overlooked given the multiple climatic and anthropogenic signals affecting the baseline, and habitat change can interact with other positive and negative forces. Decades of direct observations may be necessary for confident quantification of a downward population trend that has been occurring all along, obscured by stochastic variation. Therefore, models at the appropriate scales are essential, even though they may have large uncertainties. For example, point predictions from the blue crab model for various scenarios could not be distinguished on the basis of 95% confidence intervals but had distinctly different probabilities (Jordan et al. Citation2009). In these situations, ecologists, modelers, economists, and society need to address questions of sustainability in terms of probabilities rather than unattainable certainties. The blue crab model was used previously in Monte Carlo simulations to estimate the probabilities of sustaining the fishery under scenarios of habitat loss (Jordan et al. Citation2009). These simulations showed large uncertainty for predicted point values (i.e., annual stock biomass and harvest), dominated by unexplained variations in annual recruitment. Although we show model results as mean predicted trends in , for purposes of management decisions Monte Carlo simulations would be used to generate probabilities of meeting selected targets, thereby incorporating major sources of uncertainty (see Jordan and Coakley Citation2004 for examples of this approach in a management context).
Despite the relative abundance of data regarding the value of the recreational salmon fishery in the PNW and specific information relevant to the Yaquina Bay estuary in particular, the true value of recreational salmon fisheries in Yaquina Bay remains unknown. The estimated value for recreational WTP depends largely on the geographical level at which recreational benefits are aggregated. In our example, at the scale of Newport, Oregon, the close correspondence between the WTP values assessed by two separate methods suggests that the estimates (at a local level of geographical aggregation) are relatively robust. Applying the same WTP to wider geographic scales (state, regional) and the correspondingly larger numbers of households undoubtedly results in overestimation of the recreational WTP. As the distance from the estuary increases, the assumption that households might hold the same values for Yaquina Bay weakens. Nonetheless, households outside of the immediate local area (Newport and Lincoln County) undoubtedly have some recreational WTP for Yaquina estuary salmon fisheries (the bay attracts recreational fishermen from around the United States) and these nonlocal values are also crucial in determining total recreational value. Therefore, determining the appropriate level of social aggregation for analysis (i.e. the boundaries of the social element of the social–ecological system) is not trivial but crucial to understanding the values associated with Yaquina Bay and similar systems. If ecosystem services are to be included in management decisions, understanding the extent of both the social and ecological systems is essential. While these social boundaries of the system are unknown, it will remain impossible to find a precise value for the Yaquina Bay recreational estuarine fishery. Nevertheless, an examination of scales and attribution of economic values can be useful in informing cost–benefit decisions for combined social–ecological systems.
indicates that no matter what level of social aggregation is considered, the recreational values per unit area of the open ocean for Yaquina Bay salmon are vanishingly small. The higher unit values associated with other salmon habitats offer a more reasonable opportunity for investment in conservation.
At the finest spatial scale examined, the emergent wetlands in Yaquina Bay have the most value per unit area and thus offer the most attractive management investment option. The combined recreational WTP for emergent marsh habitats within Yaquina Bay aggregated at the county level is on the order of $3,700/ha. On a cost–benefit basis, therefore, investments to protect emergent marsh habitat based solely on local interests should not exceed such a threshold. The Wetlands Conservancy recently purchased conservation easements for emergent marsh in the Yaquina estuary (Pacific Coast Joint Venture Citation2008) at the price of $2,400/acre ($5,928/ha). This cost is higher than the associated benefit to recreational fisheries when aggregated at either the local or county level. Therefore, the justification for such a purchase either must include additional benefits of the habitat (other provisioning, regulating, or cultural services) or imply that the benefits accrue over a wider social scale. We use emergent marsh as our smallest habitat unit, but in reality channel subhabitat within a marsh is more critical to salmon than the total amount of vegetated marsh habitat (Simenstad and Cordell Citation2000). However, the granularity of salmon habitat is not the same as that of land ownership, and although individual subhabitats of an emergent marsh may have more value to the salmon, parcels of wetland are bought and sold in units of acres (hectares are not used in U.S. markets). From a cost–benefit perspective, it is therefore sensible to consider habitats at the level of granularity of the market.
Just as understanding the upper size of the social boundaries of the system is problematic, the vast scale of the oceanic migration of Chinook salmon also results in great uncertainty. Juvenile Chinook salmon from the Yaquina system do not uniformly cover the entire area of the Pacific Ocean delineated in , but their migration patterns are not known precisely; in the absence of such information, apportioning value equally throughout the region is the simplest, most logical approach.
We have expressed values for the GOM commercial blue crab and shrimp fisheries only in terms of the price paid to fishers for their catch and have not attempted any deeper economic analysis (nor have we considered the relatively minor soft-shell and peeler crab fisheries or the poorly documented recreational fisheries). To obtain the actual monetary value (rent) to the fishers, we would have to subtract the costs of harvesting from the price paid for the catch. The real value of commercial fisheries, though, involves cultural and social dimensions such as family and community fishing traditions, the needs of fishers for immediate cash even at the cost of sustainable income, the public health benefits of seafood consumption, and the pleasure people take in eating seafood. Moreover, the trade-offs between commercial fishing and the availability of coastal fishery resources to recreational fishers should be considered in a comprehensive economic analysis. A complete valuation of habitats such as SAV and marsh edge also would include values for additional species such as the speckled seatrout Cynosion nebulosus. For these reasons, the monetary values that we express for habitat contributions to blue crab and shrimp production should be understood as indices of value rather than absolute values. Even so, these value indices can be used to evaluate and compare alternatives for conserving and restoring coastal habitats. Restoration and protection of SAV is a priority for several National Estuary Programs, including that in Mobile Bay (MBNEP Citation2001).
Ecologists are beginning to develop models of how ecosystems produce services at the scales necessary to examine regional, national, and global outcomes and the quantitative benefits of habitat restoration (Jordan and Peterson Citation2012). For ecologists, ascertaining the relative contribution of different habitats to the stocks is confounded by the broad spatial scales associated with the life cycles of important recreational and commercial species. For economists, the boundaries of the social system and the relative values of the individuals within it present other challenging scaling issues. In the management realm, it needs to be understood clearly and more widely that the best fishery management (in the traditional sense) will fail if coastal habitats are not managed with full respect for the ecosystem services they support. Understanding and matching the relevant scales of social and ecological analysis therefore remains a major challenge in the implementation of effective management strategies to protect ecosystem services. Despite the prevailing uncertainties, examination of the biological and social scales associated with the supply of ecosystem services can illuminate management possibilities. The studies reported here are more than illustrations of the problem; although not ecologically or economically definitive, they advance our understanding and point the way toward sturdier solutions. If the services of coastal habitats are to be quantified and valued properly, collaborations between economists, fishery scientists, landscape ecologists, and process-oriented ecologists will be essential.
CONCLUSION
Ecological production functions generally are observed at fine spatial scales for brief spans of time, whereas the resulting ecosystem services and their economic values may be delivered over broad geographic and temporal scales. Likewise, prevailing governance structures are not conducive to coordinated—much less integrated—management of fisheries and the habitats that support them. Our studies demonstrate methods of modeling and estimation that link fishery production and its associated economic indicators to the distributions and attributes of coastal habitats across scales ranging from habitat patches to large ocean basins. Although there are substantial knowledge gaps and uncertainties, these methods of analysis can be applied to assess the probable costs of habitat loss and the benefits of habitat restoration for coastal fisheries at multiple geographic scales.
ACKNOWLEDGMENTS
We thank colleagues Steve Ferraro, Pat Clinton, Lisa Smith, Janet Nestlerode, and Pete Bourgeois for contributing to the ideas and analyses reported here. Virginia Engle contributed a preliminary review, and the article has been substantially improved based on the comments of three anonymous reviewers. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. This is contribution number 1399 from the Gulf Ecology Division and a product of the U.S. Environmental Protection Agency's Ecosystem Services Research Program.
Subject editor: Syma Ebbin, University of Connecticut, Avery Point Campus
REFERENCES
- Beamish , R. J. , McFarlane , G. A. and King , J. R. 2005 . Migratory patterns of pelagic fishes and possible linkages between open ocean and coastal ecosystems off the Pacific coast of North America . Deep-Sea Research, Part II , 52 : 739 – 755 .
- Bell , K. P. , Huppert , D. and Johnson , R. L. 2003 . Willingness to pay for local coho salmon enhancement in coastal communities . Marine Resource Economics , 18 : 15 – 31 .
- Bottom , D. L. , Jones , K. K. , Simenstad , C. A. and Smith , C. L. 2009 . Reconnecting social and ecological resilience in salmon ecosystems . Ecology and Societyy [online serial] , 14 ( 1 ) article 5
- Browder , J. A. , May , L. N. Jr. , Rosenthal , A. , Gosselink , J. G. and Baumann , R. H. 1989 . Modeling future trends in wetland loss and brown shrimp production in Louisiana using thematic mapper imagery . Remote Sensing of Environment , 28 : 45 – 59 .
- Brown , W. G. , Singh , A. and Castle , E. N. 1964 . An economic evaluation of the Oregon salmon and steelhead sport fishery . Oregon Agricultural Experimental Station Technical Bulletin , 78
- Brown , W. G. , Sorhus , C. , Chou-Yang , B. and Richards , J. A. 1983 . Using individual observations to estimate recreation demand functions: a caution . American Journal of Agricultural Economics , 65 : 154 – 157 .
- Burke , J. L. 2004 . “ Life histories of juvenile Chinook salmon in the Columbia River estuary, 1916 to the present. Master's thesis ” . Corvallis : Oregon State University .
- Costanza , R. 2008 . Ecosystem services: multiple classification systems are needed . Biological Conservation , 141 : 350 – 352 .
- Cury , P. M. 2004 . Tuning the ecoscope for the ecosystem approach to fisheries . Marine Ecology Progress Series , 274 : 272 – 275 .
- Dantin , D. D. , Fisher , W. S. , Jordan , S. J. and Winstead , J. T. 2005 . “ Fishery resources and threatened coastal habitats in the Gulf of Mexico ” . Gulf Breeze , Florida : U.S. Environmental Protection Agency, National Health and Environmental Effects Research Laboratory, Report EPA/600/R-05/051 .
- Galaz , V. , Hahn , T. , Olsson , P. , Folke , C. and Svedin , U. 2008 . “ The problem of fit among biophysical systems, environmental and resource regimes, and broader governance systems: insights and emerging challenges ” . In Institutions and environmental change: principal findings, applications, and research frontiers , Edited by: Young , O. R. , Schroeder , H. and King , L. A. 147 – 182 . Cambridge , Massachusetts : MIT Press .
- Guillory , V. , Perry , H. , Steele , P. , Wagner , T. , Keithly , W. , Pellegrin , B. , Petterson , J. , Floyd , T. , Buckson , B. , Hartman , L. , Holder , E. and Moss , C. 2001 . “ The blue crab fishery of the Gulf of Mexico, United States: a regional management plan ” . Mississippi : Gulf States Marine Fisheries Commission, Publication 96, Ocean Springs . Available: www.gsmfc.org/publications/GSMFC%20Number%20096.pdf. (June 2011)
- Haas , H. L. , Rose , K. A. , Fry , B. , Minello , T. J. and Rozas , L. P. 2004 . Brown shrimp on the edge: linking habitat to survival using an individual-based simulation model . Ecological Applications , 14 : 1232 – 1247 .
- Hanna , S. S. 2008 . Institutions for managing resilient salmon (Oncorhynchus spp.) ecosystems: the role of incentives and transaction costs . Ecology and Society [online serial] , 13 ( 2 ) article 35
- Hanna , S. S. , Sylvia , G. , Harte , M. and Achterman , G. 2006 . “ Review of economic literature and recommendations for improving economic data and analysis for managing Columbia River spring Chinook ” . Corvallis : Report to Oregon Department of Fish and Wildlife, Agreement 005-4132S-Wild, Oregon State University .
- Healey , M. C. 1991 . “ Life history of Chinook salmon (Oncorhynchus tshawytscha) ” . In Pacific salmon life histories , Edited by: Groot , C. and Margolis , L. 311 – 394 . Vancouver : University of British Columbia Press .
- Hooff , R. C. and Peterson , W. T. 2006 . Copepod biodiversity as an indicator of changes in ocean and climate conditions of the northern California current ecosystem . Limnology and Oceanography , 51 : 2607 – 2620 .
- Jordan , S. J. and Coakley , J. M. 2004 . Long-term projections of eastern oyster populations under various management scenarios . Journal of Shellfish Research , 23 : 63 – 72 .
- Jordan , S. J. and Peterson , M. S. 2012 . “ Contributions of estuarine habitats to major fisheries ” . In Estuaries: classification, ecology, and human impacts , Edited by: Jordan , S. J. 75 – 92 . Hauppauge , New York : Nova Science Publishers . Available: www.novapublishers.com/catalog/product_info.php?products_id=35102. (June 2011)
- Jordan , S. J. , Smith , L. M. and Nestlerode , J. A. 2009 . Cumulative effects of coastal habitat alterations on fishery resources: toward prediction at regional scales . Ecology and Society [online serial] , 14 ( 1 ) article 16
- Kareiva , P. , Marvier , M. and McClure , M. 2000 . Recovery and management options for spring/summer Chinook salmon in the Columbia River basin . Science , 290 : 977 – 979 .
- Knudsen , E. E. , Steward , C. R. , MacDonald , D. D. , Williams , J. E. and Reiser , D. W. 1999 . Sustainable fisheries management: Pacific salmon , Boca Raton , Florida : CRC Press .
- Kroeger , T. and McMurray , A. 2008 . “ Economic benefits of conserving natural lands: case study—Yaquina Bay conservation opportunity area, Oregon ” . Washington , D.C. : Report to the Doris Duke Charitable Foundation, Defenders of Wildlife .
- Leslie , H. M. and McLeod , K. L. 2007 . Confronting the challenges of implementing marine ecosystem-based management . Frontiers in Ecology and the Environment , 5 : 540 – 548 .
- Levin , P. S. 2003 . Regional differences in responses of Chinook salmon populations to large-scale climatic patterns . Journal of Biogeography , 30 : 711 – 717 .
- Levin , P. S. , Fogarty , M. J. , Murawski , S. A. and Fluharty , D. 2009 . Integrated ecosystem assessments: developing the scientific basis for ecosystem-based management of the ocean . PLoS (Public Library of Science) Biologyy [online serial] , 7 ( 1 ) doi: 10.1371/journal.pbio.1000014
- Levin , P. S. and Schiewe , M. 2001 . Preserving salmon biodiversity . American Scientist , 89 : 220
- Levin , S. A. 1992 . The problem of pattern and scale in ecology . Ecology , 73 : 1943 – 1967 .
- Loomis , J. B. 1989 . Estimation of and variation in site specific marginal values for recreational fisheries . Journal of Environmental Management , 29 : 183 – 191 .
- Loomis , J. B. , Sorg , C. F. and Donnelly , D. M. 1986 . Evaluating regional demand models for estimating recreation use and economic benefits: a case study . Water Resources Research , 22 : 431 – 438 .
- Magnusson , A. and Hilborn , R. 2003 . Estuarine influence on survival rates of coho (Oncorhyncus kisutch) and Chinook salmon (Oncorhynchus tsawytscha) released from hatcheries on the U.S . Pacific coast. Estuaries , 26 : 1094 – 1103 .
- Mantua , N. J. , Hare , S. R. , Zhang , Y. , Wallace , J. M. and Francis , R. C. 1997 . A Pacific interdecadal climate oscillation with impacts on salmon production . Bulletin of the American Meteorological Society , 78 : 1069 – 1079 .
- MBNEP (Mobile Bay National Estuary Program) . 2001 . “ A call to action: draft comprehensive conservation and management plan. MBNEP, Mobile, Alabama ” . Available: www.mobilebaynep.com/wp-content/uploads/2010/07/CCMP-Volume-I.pdf. (February 2011)
- McMillen-Jackson , A. L. , Bert , T. M. and Steele , P. 1994 . Population genetics of the blue crab Callinectes sapidus: modest population structuring in a background of high gene flow . Marine Biology , 118 : 53 – 65 .
- Meengs , C. C. and Lackey , R. T. 2005 . Estimating the size of historical Oregon salmon runs . Reviews in Fisheries Science , 13 : 51 – 66 .
- Meyer , P. A. , Brown , W. G. and Hsiao , C. K. 1983 . “ An updating analysis of differential sport fish values for Columbia River salmon and steelhead ” . National Marine Fisheries Service . Silver Spring, Maryland
- Minello , T. J. 1999 . “ Nekton densities in shallow estuarine habitats of Texas and Louisiana and the identification of essential fish habitat ” . In Fish habitat: essential fish habitat and rehabilitation , Edited by: Benaka , L. R. 43 – 75 . Bethesda , Maryland : American Fisheries Society, Symposium 22 .
- Minello , T. J. , Able , K. W. , Weinstein , M. P. and Hays , C. G. 2003 . Salt marshes as nurseries for nekton: testing hypotheses on density, growth and survival through meta-analysis . Marine Ecology Progress Series , 246 : 39 – 59 .
- Nance , J. M. 1997 . “ Stock assessment report for the Gulf of Mexico shrimp fishery ” . In Proceedings of the 20th annual MEXUS–Gulf symposium , Edited by: Kumpf , H. E. and Jones , A. C. 11 – 14 . NOAA Technical Memorandum NMFS-SEFSC-403 .
- Nance , J. M. , Klima , E. F. and Czapla , T. E. 1989 . “ Gulf of Mexico shrimp stock assessment workshop ” . NOAA Technical Memorandum NMFS-SEFC-239
- NMFS (National Marine Fisheries Service) . 2011 . “ Commercial fisheries statistics ” . Maryland : NMFS, Fisheries Statistics Division, Silver Spring . Available: www.st.nmfs.gov/st1/commercial/index.html. (January 2011)
- O’Higgins , T. G. , Ferraro , S. P. , Dantin , D. D. , Jordan , S. J. and Chintala , M. M. 2010 . Habitat scale mapping of fisheries ecosystem service values in estuaries . Ecology and Societyy [online serial] , 15 ( 4 ) article 7
- Olsen , D. , Richards , J. and Scott , R. D. 1991 . Existence and sport values for doubling the size of Columbia River basin salmon and steelhead runs . Rivers , 2 : 44 – 56 .
- Pacific Coast Joint Venture . 2008 . Yaquina Bay estuarine marsh, coastal wetland grant. Available: http://pcjv.conservationregistry.org/projects/196839. (May 2009)
- Pendleton , L. , Atiyah , P. and Moorthy , A. 2007 . Is the non-market literature adequate to support coastal and marine management? . Ocean and Coastal Management , 50 : 363 – 378 .
- Peterson , M. S. and Lowe , M. R. 2009 . Implications of cumulative impacts to estuarine and marine habitat quality for fish and invertebrate resources . Reviews in Fisheries Science , 17 : 505 – 523 .
- Riley , P. L. 1988 . “ Economic valuation of marine recreational fishing, volume 4 ” . Washington , D.C. : National Marine Fisheries Service .
- Simenstad , C. A. and Cordell , J. R. 2000 . Ecological assessment criteria for restoring anadromous salmonid habitat in Pacific Northwest estuaries . Ecological Engineering , 15 : 283 – 302 .
- Taylor , E. B. 1990 . Phenotypic correlates of life-history variation in juvenile Chinook salmon, Oncorhynchus tshawytscha . Journal of Animal Ecology , 59 : 455 – 468 .
- USEPA (U.S. Environmental Protection Agency) . 2008 . “ National coastal condition report III ” . Washington , D.C. : USEPA, Office of Research and Development/Office of Water, EPA/842-R-08-002 . Available: www.epa.gov/owow/oceans/nccr3/downloads.html. (June 2011)
- Young , O. R. 2002 . “ The institutional dimensions of environmental change: fit, interplay, and scale ” . Cambridge , Massachusetts : MIT Press .