Abstract
Age-0 red snapper Lutjanus campechanus from the 2005–2007 year-classes were sampled in six regions across the Gulf of Mexico (Gulf) to develop nursery signatures from otolith element: Ca ratios (Ba:Ca, Mg:Ca, Mn:Ca, Sr:Ca, and Li:Ca) and stable isotope delta values (δ13C and δ18O). Element: Ca ratios were analyzed with sector field inductively coupled plasma mass spectrometry on dissolved right sagittae; isotope ratio mass spectrometry was employed to analyze pulverized left otoliths for δ13C and δ18O. Otolith chemical signatures were significantly different among regions in each year. Year-class-specific quadratic discriminant function analysis (QDFA) distinguished nursery regions with an accuracy of 82% for 2005, 70% for 2006, and 72% for 2007. However, samples were not obtained from all six study regions in 2005 and 2006. A QDFA of all year-classes combined produced an overall classification accuracy of 70%, thus indicating that region-specific otolith chemical signatures from adjacent sampling years could be used as surrogates for regions where samples were not obtained in a given year.
Received February 7, 2012; accepted June 5, 2012
The U.S. Gulf of Mexico (Gulf) stock of red snapper Lutjanus campechanus has been estimated as overexploited since the 1980s, with the major sources of fishing mortality being commercial and recreational fisheries as well as bycatch mortality caused by Gulf shrimp trawling (SEDAR Citation2005). Although it is estimated that the red snapper stock in the U.S. Gulf is no longer undergoing overfishing (SEDAR Citation2009), fishery managers are still tasked with balancing these three sources of fishing mortality and rebuilding the stock by 2032 (GMFMC Citation2010). Genetic evidence has failed to reject the null hypothesis that Gulf red snapper constitute a single panmictic stock (Camper et al. Citation1993; Pruett et al. Citation2005; Gold and Saillant Citation2007); however, demographic differences in red snapper size at age, maturation rates, and genetic effective population size occur across the northern Gulf (Fischer et al. Citation2004; Saillant and Gold Citation2006; Jackson et al. Citation2007). In fact, catch statistics suggest that there are two centers of stock abundance: one in the northwestern Gulf off the coast of Louisiana and a smaller one off the coasts of Alabama and northwest Florida (Goodyear Citation1995). Based upon these findings, the red snapper population has been categorized into eastern and western substocks, which are divided by the Mississippi River (SEDAR Citation2005). However, the information is recombined to estimate a Gulf-wide annual catch limit, and little is known about patterns of mixing between substocks.
An understanding of larval exchange rates and population connectivity of marine organisms is crucial to the analysis of marine population dynamics and management of fishery stocks (Cowen et al. Citation2000). Larval dispersal can be difficult to study, although based upon shelf currents, Johnson et al. (2009) estimated that red snapper larvae could be carried 480 km during the 4-week planktonic stage. However, only a small percentage of the western substock larvae were projected to cross the Mississippi River plume; in such an event, the larvae would most likely be transported away from the continental shelf and would have a low probability of survival. Conditions might be more favorable for western transport of larvae produced by the eastern substock (Johnson et al. Citation2009).
Conventional tagging has been used to examine postsettlement movement of juvenile and adult red snapper. Estimates of red snapper site fidelity range from 25% to 60% per year (Patterson and Cowan Citation2003; Schroepfer and Szedlmayer Citation2006; Strelcheck et al. Citation2007); several tagged fish have been recovered more than 100 km from the original tagging location, but only one fish (out of >1,000 recaptures) tagged in the eastern Gulf has been recaptured west of the Mississippi River mouth (Patterson et al. Citation2001; Strelcheck et al. Citation2007; Addis et al. Citation2008). However, problems associated with conventional tagging, such as external tag loss and low reporting rates, may result in the underestimation of red snapper movement (see Patterson Citation2007 for a review).
The use of otoliths as natural tags has become a popular tool among fishery scientists to distinguish fish from distinct nursery areas and to examine movement patterns of adults (Gillanders and Kingsford Citation1996; Thorrold et al. Citation1997). Otoliths are calcium carbonate structures located within the acoustico-lateralis system of teleost fish. Otoliths are acellular, are metabolically inert, and precipitate as the fish grows, which allows elements and stable isotopes that are accreted onto the growing surface to be permanently retained (see Campana Citation1999 for a review). Therefore, elements that are deposited during the juvenile phase can act as natural markers for the nursery area of origin. The ability of otoliths to act as a natural tag has permitted the development of a more efficient technique for studying natal origin and population connectivity than conventional tagging methods as a result of the large number of fish that must be tagged to produce a useful number of tag returns. However, chemical signatures in otoliths can differ among years due to temporal variability in water mass characteristics and elemental composition (Gillanders and Kingsford Citation2000; Rooker et al. Citation2001), thus requiring cohort-specific signatures to be identified.
While the majority of marine studies utilizing otolith chemistry to estimate natal origin have focused on estuarine and nearshore nursery habitats (Thorrold et al. Citation1998; Gillanders and Kingsford Citation2000; Dorval et al. Citation2005), this technique also has been successfully used to identify nursery origins of highly migratory pelagic species (Rooker et al. Citation2001). In fact, otolith chemistry has been utilized previously to examine temporal and spatial variability in otolith elemental signatures of northern Gulf red snapper. Patterson et al. (Citation2008) were able to distinguish among three nursery regions of the northern Gulf by using elemental signatures, with mean classification accuracy of 80% for four of the five cohorts examined. Elemental variability in otolith chemical signatures was attributed to hydrologic and oceanographic differences among regions. However, these same oceanographic processes may have been the source of poor discrimination in one of the cohorts examined. Thorrold et al. (Citation1998) reported an improvement in accuracy of weakfish Cynoscion regalis classification to estuarine nurseries along the Atlantic coast when stable isotope (δ13C and δ18O) ratios were combined with trace elemental data. Furthermore, Rooker et al. (Citation2008) were able to determine the nursery of origin for Atlantic bluefin tuna Thunnus thynnus over a larger spatial scale by using only otolith δ13C and δ18O ratios. Thus, the addition of stable isotope ratios to otolith elemental signatures may increase the accuracy of nursery origin classifications for Gulf red snapper.
FIGURE 1 Nursery regions along the continental shelf of the Gulf of Mexico (Gulf), where age-0 red snapper representing the 2005–2007 year-classes were sampled. The 200-m depth contour indicates the continental shelf edge (regions: EG = eastern Gulf; NCG = north-central Gulf; NWG = northwestern Gulf; SWG = southwestern Gulf; MEX1 = Mexico region 1; MEX2 = Mexico region 2).
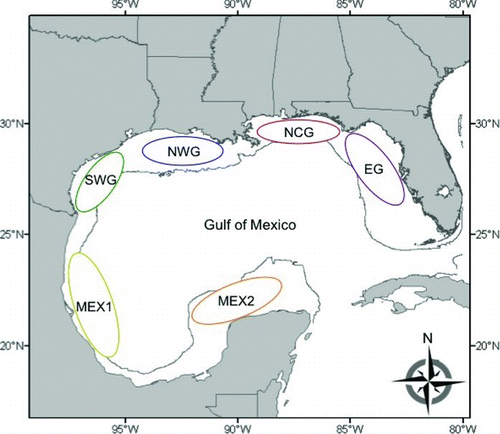
The purpose of this study was to examine otolith chemical signatures in age-0 red snapper from six regions within the Gulf. Specifically, natural tags derived from otolith element: Ca ratios and stable isotope ratios (collectively, “constituents”) were used to discriminate red snapper nursery regions on the continental shelf of U.S. and Mexican portions of the Gulf. Region-specific nursery signatures were developed for three consecutive year-classes to determine whether discriminant classifications were strong enough to validate the use of nursery signatures for estimating the source of recruits to Gulf regions.
METHODS
Sample collection.—Age-0 red snapper representing the 2005–2007 year-classes were sampled from six regions in the Gulf (): the eastern Gulf along the west Florida shelf (EG); north-central Gulf (NCG); northwestern Gulf (NWG); southwestern Gulf (SWG); southern Gulf shelf between Tampico and Veracruz, Mexico (MEX1); and Campeche Banks (MEX2). Boundary lines between NCG, NWG, and SWG follow the delineation detailed by Patterson et al. (Citation2008). The objective was to collect 30 juveniles from each region for each year-class (n = 540). Samples from NCG, NWG, and SWG were collected in the fall (October and November) during the National Marine Fisheries Service (NMFS) Fall Groundfish Survey using otter trawls deployed from either the R/V Oregon II or the R/V Gordon Gunter. Juvenile fish were subsampled from a trawl catch with systematic random sampling, which targeted fish smaller than 150 mm TL. Immediately after selection, the fish were placed in plastic bags and frozen. Upon arrival at the dock, fish were transferred to the Fisheries Laboratory at the University of West Florida (UWF) for processing.
The collection of samples from EG, MEX1, and MEX2 was opportunistic and difficult to achieve. Juvenile red snapper from EG were collected in the fall of 2005 and 2007 from observers employed by the Gulf and South Atlantic Fisheries Foundation and the NMFS Galveston Laboratory, respectively. Red snapper were stored in plastic bags, frozen, and transported to UWF. Juvenile red snapper from the MEX1 and MEX2 regions were collected in the winter (December–March) of 2007 and 2008 from shrimp trawl bycatch. Fish TL was measured and otoliths were extracted before samples were shipped to Louisiana State University (LSU) for further processing. Unfortunately, samples were not obtained from the MEX1 or MEX2 regions for the 2005 year-class or from the EG region for the 2006 year-class ().
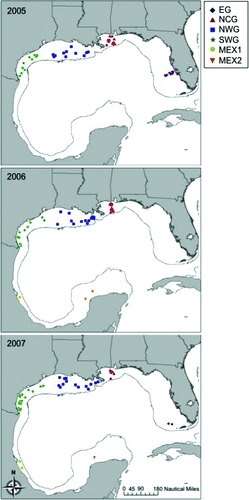
FIGURE 2 Specific locations where age-0 red snapper were sampled during fall 2005–2007. The 200-m depth contour indicates the continental shelf edge. Nursery region codes are defined in Figure 1.
Frozen age-0 fish that were collected within U.S. Gulf waters were thawed in the laboratory at UWF; weight was determined to the nearest 0.01 g, and TL was measured to the nearest millimeter. Sagittal otoliths were removed with glass probes and polyethylene tweezers; all materials that came into contact with extracted otoliths were acid-leached and triple-rinsed with double-deionized water (DDIH2O; ultrapure, 18-MΩ/cm water). Sagittae were cleaned with a synthetic-bristle brush to remove any adhering tissue, were rinsed with DDIH2O, and were placed in polyethylene vials to air-dry under a class-100 clean hood.
Otolith preparation and analysis.—Both right and left otolith samples were cleaned prior to elemental or stable isotope analysis under class-100 clean hoods in the laboratory at either UWF or LSU. Before and after cleaning, dry otoliths were weighed to the nearest 0.01 mg. Whole otoliths were immersed in a 1% solution of ultrapure nitric acid (HNO3) for 30 s to oxidize any material that was adhering to the surface; the solution was then flooded with DDIH2O to remove the acid. Otoliths were dried under a class-100 clean hood for at least 24 h.
All right otoliths were prepared for elemental analysis at UWF. Otoliths were dissolved in high-density polyethylene vials by adding 1% ultrapure HNO3 until a dilution factor of approximately 1,000-fold was achieved. Although total dissolution typically occurred within 1 h, samples were not manipulated for at least 24 h once acid digestion began. Aliquots (5 mL) of otolith solutions were sent to the University of Southern Mississippi for trace elemental analysis with a Thermo Fisher Element2 sector field (SF) inductively coupled plasma (ICP) mass spectrometer. Otolith solutions were spiked with indium at a concentration of 2 ng/mL as an internal standard and then were analyzed for 137Ba, 48Ca, 7Li, 55Mn, 25Mg, and 88Sr. Three of the elements (Li, Mn, and Ba) were determined in low resolution, while the other elements were determined in medium resolution. Blanks were prepared from 1% ultrapure HNO3 and were processed through the same stages of sample preparation as the otolith sample solutions. Blanks were analyzed concurrently with otolith sample solutions to determine instrument limits of detection, which were estimated as three SDs of mean blank values. Instrument performance and matrix effects were checked by assaying elemental concentrations of an otolith standard reference material (SRM) that was prepared from adult red snapper otoliths (Sturgeon et al. Citation2005). Solutions of the SRM were prepared and analyzed in a manner similar to that used for age-0 red snapper otolith samples.
All left otoliths were prepared for stable isotope analysis at LSU. Otoliths were pulverized with an agate mortar and pestle, and the resulting otolith powder was transferred into 2-mL microcentrifuge tubes. Subsamples (>1 mg) of homogenized, pulverized otoliths were sent to the Stable Isotope Laboratory (Department of Geology, University of California, Davis) for stable isotope (δ13C and δ18O) analysis with a Finnigan MAT 251 isotope ratio mass spectrometer. The instrument was calibrated against the International Atomic Energy Agency's carbonate standard, NBS-19. Accuracy of analytical runs was measured through routine analysis of a check standard that had been stringently calibrated against NBS-19. Method precision based on long-term monitoring of the NBS-19 standard was ±0.02‰ for δ13C and ±0.06% for δ18O. The isotopic composition of otoliths is reported in standard delta (δ) notation relative to the Vienna Pee Dee belemnite reference standard:
where R represents the ratio of heavy to light isotope (13C/12C or 18O/16O).
Statistical analysis.—There were minor differences in fish size among regions and among years (). Thus, to examine relationships between constituent concentrations and otolith weight (used as an alternative for fish size), a correlation analysis was performed to test whether significant relationships existed within and among nursery regions and years. Significant relationships were detected for Ba, Mg, Mn, and C. To ensure that differences in fish size did not confound any regional differences in otolith chemical signatures, the size effect was removed by subtracting the product of otolith weight and common within-group linear slope from each observed concentration of Ba, Mg, Mn, and C.
TABLE 1 Sample size and size range of age-0 red snapper (2005–2007 year-classes) collected from six nursery regions in the Gulf of Mexico (Gulf; regions: EG = eastern Gulf; NCG = north-central Gulf; NWG = northwestern Gulf; SWG = southwestern Gulf; MEX1 = Mexico region 1; MEX2 = Mexico region 2).
Multivariate ANOVA (MANOVA) and two-way ANOVA were used to test for differences in otolith elemental and stable isotope signatures among regions and year-classes. Pillai's trace was used as the test statistic because it is the most robust to violations of homogeneity of variance (Wilkinson et al. Citation1996). However, only the regions that were sampled during each year of the study (i.e., NCG, NWG, and SWG) were examined with the MANOVA and two-way ANOVA. To determine whether otolith chemical concentrations varied among year-classes within the regions surveyed, one-way ANOVAs were performed on mean elemental and stable isotope ratios for each region, with year-class as the random factor. Variance components from each ANOVA were included to evaluate the percentage of total variance in each constituent ratio that was caused by variation among year-classes within a region. Constituents that did not have homogeneous variance across year-classes were either log e transformed or subjected to reciprocal transformation, and statistical analyses were recalculated. However, the transformations did not alter the significance of ANOVA and MANOVA, and therefore only the results of the untransformed data are presented.
A stepwise discriminant analysis (SDA) was computed to determine which constituents were the most significant in discriminating among regions within year-classes. An SDA is used to find a set of the original quantitative variables that best discriminate samples among sites or groups. To test the ability of otolith chemical signatures to distinguish red snapper nursery regions, we performed year-class-specific quadratic discriminant function analyses (QDFAs) as well as a QDFA model in which all year-classes were combined. Jackknifed cross validation classification accuracies were computed to estimate the success of classification to respective regions by year-class and with all year-classes combined. A canonical discriminant analysis (CDA) was used to visualize region-specific multivariate otolith chemical signatures for each year-class and for all year-classes combined. A CDA is computed to determine (1) the best linear combination of quantitative variables for which the means of the groups are most different and (2) whether this difference varies by year-class. All statistical analyses were performed by using the Statistical Analysis System (SAS Citation2006) with a significance level α of 0.05.
RESULTS
In total, 430 age-0 red snapper collected from six nursery regions across the Gulf were analyzed for otolith chemical signatures (). For all samples, concentrations of the six elements (Ba, Ca, Li, Mn, Mg, and Sr) were at least two orders of magnitude above detection limits. The SRM samples were within 5% of certified values for elements analyzed with SF-ICP mass spectrometry. Chemical signatures differed significantly among nursery regions (MANOVA: F 14, 512 = 9.8, P < 0.001) and among year-classes (MANOVA: F 14, 512 = 11.8, P < 0.001), and the region × year-class interaction was significant (MANOVA: F 28, 1,032 = 6.4, P < 0.001). When only the NCG, NWG, and SWG regions were considered, most of the element: Ca and stable isotope ratios differed significantly (ANOVA: P ≤ 0.05) among nursery regions and among year-classes and demonstrated a significant region × year-class interaction. The exceptions were Mg:Ca for the region × year-class interaction (ANOVA: P = 0.085), Sr:Ca for the region effect (ANOVA: P = 0.356), and δ13C for the year-class effect (ANOVA: P = 0.692).
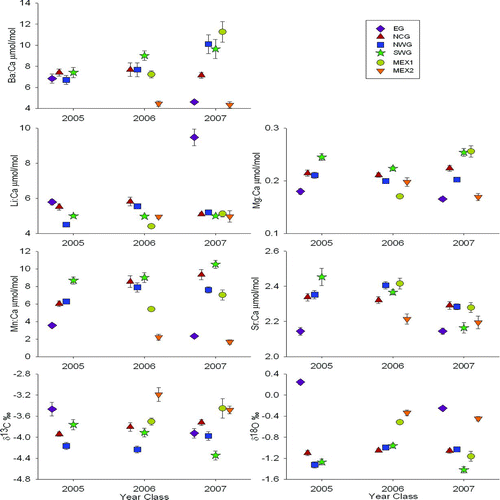
Mean concentrations of element: Ca and stable isotope ratios varied among nursery regions and among year-classes (). When present, fish sampled from EG tended to have constituent values that were either lower or higher than the values for the other regions. The same was true of fish sampled from MEX2. In fact, for the 2007 year-class, samples from EG and MEX2 had similar element: Ca and stable isotope ratios, with the only exception being Li:Ca. Fish collected from SWG tended to have higher values for Mg:Ca and Mn:Ca across all year-classes, and their Sr:Ca steadily decreased across years. Overall, NCG and NWG tended to have similar constituent values for each year-class (). Significant differences in otolith chemical signatures among year-classes varied among the regions (). The only constituent that showed significant interannual variation in samples from MEX2 was Mg:Ca, but the variability of this ratio only accounted for 16% of the total variance within this region. For NWG and MEX1, almost all constituents were significantly different among year-classes; the exceptions were Mg:Ca for NWG and Mn:Ca for MEX1. In addition, Mn:Ca did not display significant interannual variation for EG or MEX2. All regions except MEX2 showed significant year-class variation in δ13C, which accounted for 8–38% of the total variance within a region. The largest proportion of total variance was attributable to significant mean δ18O (29–81%), followed by Li:Ca (9–62%) and Mg:Ca (12–58%).
FIGURE 3 Mean (±SE) region- and year-class-specific otolith constituents for age-0 red snapper sampled from six Gulf of Mexico nursery regions in 2005–2007. Nursery region codes are defined in Figure 1.
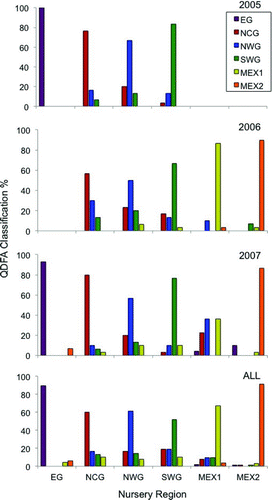
The SDA retained all element: Ca and stable isotope ratios for each year-class. Mean jackknifed classification accuracies of the QDFA models were 81.7% for 2005, 69.9% for 2006, 71.6% for 2007, and 70.3% for all year-classes combined (). Combining all year-classes resulted in a slightly higher classification success than was observed for the 2006 year-class. The lowest classification success was for MEX1 samples from the 2007 year-class (36.4%), and the highest classification success was for EG samples from the 2005 year-class (100%). With the exception of the especially low classification success for 2007 MEX1 samples, red snapper collected from NWG typically had the lowest classification success for each year-class, and the majority of misclassifications were to the NCG region. Samples from NCG had the next-lowest classification success for each year-class, with misclassifications to NWG being most prevalent. For the 2007 year-class, which was represented in samples from all nursery regions, EG and MEX2 had the highest classification success, and misclassification error was similar for EG and MEX2.
The high classification success for the 2005 year-class was apparent in its CDA, and CDA plots generally showed a trend of overlap among chemical signatures for the NCG, NWG, and SWG nursery regions for all year-classes (). The CDA plot from the model in which all year-classes were combined closely resembled the plot for the 2007 year-class (the only year-class that was sampled in all six nursery regions). Canonical structure coefficients are provided in to demonstrate the importance of each elemental and stable isotope ratio in separating otolith chemical signatures among regions (as described by Walther and Thorrold Citation2008). The most notable trend was that the first canonical variate for each year-class was primarily influenced by δ18O, generally separating EG and MEX2 from the other regions (, ). For the 2005 year-class, the Mn:Ca ratio contributed to the high loadings on the second canonical variate, most likely reflecting the separation of SWG from the other three regions. However, for the other year-classes, Mn:Ca ratios began to contribute to loadings on the first canonical variate, again reflecting the separation of EG and MEX2 from the other regions (, ). For the 2007 year-class, Li:Ca ratios contributed to separation along the second canonical variate, which appeared to correlate with the separation of EG from the other regions.
DISCUSSION
The discriminant classifications of region-specific nursery signatures for the three consecutive year-classes studied validate the utility of natural otolith tags for estimating the source of recruits to Gulf regions and for examining red snapper population connectivity. Patterson et al. (Citation2008) also demonstrated the potential for using otolith chemical signatures to discriminate among red snapper nursery regions, but those authors reported an overall higher mean classification success (80%) than was seen in the current study. However, Patterson et al. (2008) only examined three nursery regions (NCG, NWG, and SWG), whereas six regions were analyzed in the present study. The 2005 year-class had the highest classification success and the lowest number of regions sampled in the current study, whereas the 2007 year-class had the lowest classification success and the highest number of sampled regions. Thus, it would appear that the increase in regions resulted in a decrease in classification accuracy. However, misclassifications in the NCG, NWG, and SWG regions were mainly distributed among those regions, and removal of EG, MEX1, and MEX2 did not significantly alter the classification errors.
TABLE 2 Single-factor ANOVA results for year-class variation in otolith elemental and stable isotope ratios for age-0 red snapper collected from six Gulf of Mexico (Gulf) nursery regions (MS = mean square; %ω2 = variance component percentage of the total variance for each ANOVA; *P < 0.05; **P < 0.01; ***P < 0.0001).
FIGURE 4 Jackknifed classification percentages estimated with quadratic discriminant function analysis (QDFA) of otolith chemical signatures from age-0 red snapper that were sampled in six Gulf of Mexico nursery regions during 2005–2007 (bar colors represent QDFA-assigned regions; x-axis shows the region of sample collection). “ALL” indicates all three year-classes combined. Nursery region codes are defined in Figure 1.
The circulation patterns of the Gulf can affect the way elements, stable isotopes, and nutrients are dispersed across the continental shelf. For instance, the Mississippi–Atchafalaya River system discharge, which accounts for 90% of the freshwater input into the Gulf (Rabalais et al. Citation1996), forms a stratified coastal current that usually flows westward along the Louisiana coast and can extend as far as the south Texas coast (Justić et al. Citation1995). In close proximity is the Mobile River basin (Alabama), which is the fourth-largest source of freshwater discharge in the nation (Warner et al. Citation2005). During autumn, winter, and spring, alongshore easterly winds create an exchange of river and shelf waters between the Louisiana–Texas and Mississippi–Alabama shelves, with maximum exchange occurring during northeast wind events (Walker et al. Citation2005). Thus, it is not surprising that otolith chemical signatures were similar among the NCG, NWG, and SWG regions. Although interannual differences were present, NCG and NWG samples tended to have similar ratios of Mg:Ca, Sr:Ca, and δ18O. Red snapper that were collected from SWG differed more than fish from the other two regions, mostly owing to higher ratios for Mg:Ca and Mn:Ca. Although not all of the present results are consistent with the results of Patterson et al. (Citation2008), some similarities are evident. For instance, in both studies, the SWG samples had higher Mg:Ca than samples from the other two regions, and NWG samples had lower Mn:Ca, which was similar between NCG and SWG samples. Patterson et al. (Citation2008) concluded that red snapper otolith chemical signatures were a reflection of oceanographic and hydrological differences among regions. Environmental parameters (e.g., salinity and temperature) and physiological regulations can affect the assimilation of elements into otoliths (see Elsdon and Gillanders Citation2003 for a review). Therefore, differences between the studies would be expected due to changes in water elemental concentrations, temperature, and salinity over time (as noted by Elsdon et al. Citation2008), whereas similarities are possibly caused by persistent hydrological and oceanographic processes within the NCG, NWG, and SWG regions.
Although red snapper samples from EG, MEX1, and MEX2 were not collected for all year-classes, when samples were available these regions consistently had otolith chemical concentrations that differed from those of the NCG, NWG, and SWG regions. The exception was MEX1 samples, which tended to have concentrations similar to those of the NCG, NWG, and SWG samples, primarily for the 2007 year-class. The southern Gulf coastal waters are influenced by river runoff from the Grijalva–Usumacinta River system, which produces the second-largest volume of freshwater discharge into the Gulf (Signoret et al. Citation2006). The river plume is displaced westward towards the Tamaulipas–Veracruz (TAVE) shelf; this displacement is caused by a westward coastal current. In the spring and summer, there is an upcoast current on the TAVE shelf that reaches the southern Texas continental shelf, where it encounters a downcoast current favoring offshore transport. The TAVE shelf current reverses to a downcoast current during the fall and winter and extends to the southern Bay of Campeche, where it meets an opposing alongshelf current, generating seasonal offshore transport. The reversal of the current allows water from the Mississippi and Atchafalaya rivers to reach the TAVE shelf (Zavala-Hidalgo et al. Citation2003). Hence, the high freshwater inflow from the Grijalva and Usumacinta rivers, along with seasonal inflow from the Mississippi–Atchafalaya River system, likely contributes to the similarities between samples from MEX1 and samples from the NCG, NWG, and SWG regions.
TABLE 3 Total canonical structure coefficients (canonical variates [var] 1 and 2) for discriminant function analyses comparing region- and year-class-specific otolith chemical signatures of age-0 red snapper for the 2005–2007 year-classes.
FIGURE 5 Canonical plot scores of otolith chemical signatures for age-0 red snapper (2005–2007 year-classes) sampled from six nursery regions in the Gulf of Mexico (canonical = canonical variate). Ellipses indicate 95% confidence levels; “ALL” indicates all three year-classes combined. Nursery region codes are defined in Figure 1.
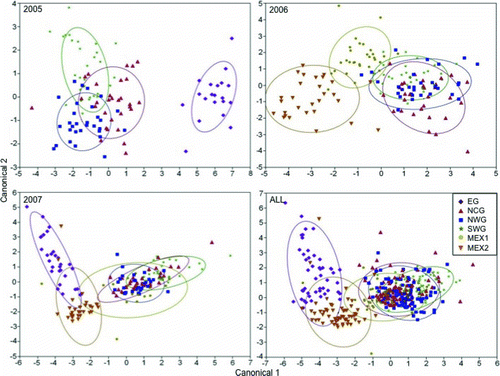
Prevailing upwelling winds cause circulation on the western Campeche Bank to flow westward along the coast throughout the entire year (Zavala-Hidalgo et al. Citation2003). These circulation patterns likely prevent mixing between MEX1 and MEX2 coastal waters, as evidenced by the differences in otolith chemical signatures between the two regions. The EG and MEX2 region samples were greatly enriched in δ18O compared with samples from the other nursery regions. Evaporation and freshwater input can alter δ18O values, resulting in heavier isotopes being associated with seawater and lighter isotopes being deposited into freshwater systems via precipitation (Lenanton et al. Citation2003). Since lighter isotopes are associated with freshwater, the present trend in δ18O most likely reflects the riverine influence on the NCG, NWG, SWG, and MEX1 regions. Furthermore, EG and MEX2 samples had lower Ba:Ca values. Barium follows a nutrient-type profile, with higher concentrations in riverine and near-coastal waters (Thorrold et al. Citation1997); thus, our Ba:Ca results further confirm the dominant riverine influence on the NCG, NWG, SWG, and MEX1 regions. Another notable difference was that EG and MEX2 had significantly lower Mn:Ca than the other regions. Hanson et al. (Citation2004) reported that the Mn concentration in otoliths of gags Mycteroperca microlepis increased with latitude, corresponding to the same trend in coastal sediment Mn concentration along the west Florida shelf. Thus, latitudinal differences, an absence of heavy freshwater input, and a lack of water mixing as a result of circulation patterns likely contributed to the distinctness of EG and MEX2 chemical signatures relative to those of the other regions.
The efficacy of using otolith chemical concentrations as natural tags is partially dependent upon the temporal stability of the signature. Studies of the temporal stability of otolith chemical signatures have shown either differences between two consecutive years (Patterson et al. Citation1999; Gillanders and Kingsford Citation2000) or negligible differences over 2-year intervals, with drastic changes occurring after 4–13 years (Campana et al. Citation2000). Even though interannual differences are present, studies have shown that separation patterns among regions can still be similar and that regional differences are the cause of variability in otolith chemical signatures (Edmonds et al. Citation1992; Campana and Gagné Citation1995). Although thorough statistical testing of temporal stability was not possible in this study because of the unbalanced design, the NCG, NWG, and SWG regions demonstrated significant year-class differences, which were also reported by Patterson et al. (Citation2008). However, it is interesting to note that when otolith chemical concentrations were combined for all three year-classes, the classification success for the combined year-classes was not much lower than that observed for the 2007 year-class and was a slight improvement over that observed for the 2006 year-class. Therefore, although development of cohort-specific otolith chemical signatures would be appropriate for Gulf red snapper, the unbalanced design of this study suggests that it may be worthwhile to examine the usefulness of a signature developed from all three year-classes combined.
The results of this study indicate that element: Ca and stable isotope ratios can be used as natural tags to distinguish age-0 red snapper originating from different Gulf nursery regions. The ultimate goal of this research is the application of these natural tags to estimate the source of recruits among Gulf regions. Specifically, more work should be undertaken to (1) estimate the source of recruits to the west Florida shelf, (2) examine the connectivity between populations of the western Gulf and northeast Mexico, and (3) further explore mixing dynamics between populations east and west of the Mississippi River. The use of natural tags to study postsettlement movement and population connectivity could be beneficial to the management and recovery of red snapper stocks.
ACKNOWLEDGMENTS
This research was supported by the Marine Fisheries Initiative under Grant Number NA05NMF4331072 awarded to W. Patterson and J. Cowan. We are especially grateful to A. DeBose and K. Johnson for facilitating collection of age-0 red snapper during the NMFS Fall Groundfish Survey in the northern Gulf; J. Mareska for providing additional NCG juvenile samples; G. Martínez for sampling fish in the MEX1 and MEX2 regions; E. Scott-Denton (NMFS), J. Jamison (Gulf and South Atlantic Fisheries Foundation), and shrimp observers for their invaluable help in procuring age-0 red snapper samples from along the west Florida shelf; A. Hamilton, W. Ingram, and B. Mahmoudi for attempting to collect age-0 fish during the NMFS Small Pelagics Survey and the Fish and Wildlife Research Institute Baitfish Survey along the west Florida shelf; Z. Chen for assistance in running the SF-ICP mass spectrometry samples; D. Winter for running the isotope ratio mass spectrometry samples; and two anonymous reviewers for helpful feedback that improved the manuscript. We also thank B. Kline and A. Fischer for their assistance in the laboratory.
Subject editor: Donald Noakes, Thompson Rivers University, British Columbia, Canada
REFERENCES
- Addis , D. T. , Patterson , W. F. III and Dance , M. A. 2008 . Site fidelity and movement of reef fishes tagged at unreported artificial reef sites off northwest Florida . Proceedings of the Gulf and Caribbean Fisheries Institute , 60 : 297 – 304 .
- Campana , S. E. 1999 . Chemistry and composition of fish otoliths: pathways, mechanisms and applications . Marine Ecology Progress Series , 188 : 263 – 297 .
- Campana , S. E. , Chouinard , G. A. , Hanson , J. M. , Fréchet , A. and Brattey , J. 2000 . Otolith elemental fingerprints as biological tracers of fish stocks . Fisheries Research , 46 : 343 – 357 .
- Campana , S. E. and Gagné , J. A. 1995 . “ Cod stock discrimination using ICPMS elemental assays of otoliths ” . In Recent developments in fish otolith research: Belle W. Baruch library in marine sciences, volume 19 , Edited by: Secor , D. H. , Dean , J. M. and Campana , S. E. 671 – 691 . Columbia : University of South Carolina Press .
- Camper , J. D. , Barber , R. C. , Richardson , L. R. and Gold , J. R. 1993 . Mitochondrial DNA variation among red snapper (Lutjanus campechanus) from the Gulf of Mexico . Molecular Marine Biology and Biotechnology , 2 : 154 – 161 .
- Cowen , R. K. , Lwiza , K. M. M. , Sponaugle , S. , Paris , C. B. and Olson , D. B. 2000 . Connectivity of marine populations: open or closed? . Science , 287 : 857 – 859 .
- Dorval , E. , Jones , C. M. , Hannigan , R. and van Montfrans , J. 2005 . Can otolith chemistry be used for identifying essential seagrass habitats for juvenile spotted seatrout, Cynoscion nebulosus, in Chesapeake Bay? . Marine and Freshwater Research , 56 : 645 – 653 .
- Edmonds , J. S. , Lenanton , R. C. J. , Caputi , N. and Morita , M. 1992 . Trace elements in the otoliths of yellow-eye mullet (Aldrichetta forsteri) as an aid to stock identification . Fisheries Research , 13 : 39 – 51 .
- Elsdon , T. S. and Gillanders , B. M. 2003 . Reconstructing migratory patterns of fish based on environmental influences on otolith chemistry . Reviews in Fish Biology and Fisheries , 13 : 217 – 235 .
- Elsdon , T. S. , Wells , B. K. , Campana , S. E. , Gillanders , B. M. , Jones , C. M. , Limburg , K. E. , Secor , D. H. , Thorrold , S. R. and Walther , B. D. 2008 . Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences . Oceanography and Marine Biology An Annual Review , 46 : 297 – 330 .
- Fischer , A. J. , Baker , M. S. Jr. and Wilson , C. A. 2004 . Red snapper (Lutjanus campechanus) demographic structure in the northern Gulf of Mexico based on spatial patterns in growth rates and morphometrics . U.S. National Marine Fisheries Service Fishery Bulletin , 102 : 593 – 603 .
- Gillanders , B. M. and Kingsford , M. J. 1996 . Elements in otoliths may elucidate the contribution of estuarine recruitment to sustaining coastal reef populations of a temperate reef fish . Marine Ecology Progress Series , 141 : 13 – 20 .
- Gillanders , B. M. and Kingsford , M. J. 2000 . Elemental fingerprints of otoliths of fish may distinguish estuarine ‘nursery’ habitats . Marine Ecology Progress Series , 201 : 273 – 286 .
- GMFMC (Gulf of Mexico Fishery Management Council) . 2010 . “ Final regulatory amendment to the reef fish fishery management plan to set total allowable catch for red snapper ” . Tampa , Florida : GMFMC .
- Gold , J. R. and Saillant , E. 2007 . “ Population structure of red snapper in the northern Gulf of Mexico ” . In Red snapper ecology and fisheries in the U.S. Gulf of Mexico , Edited by: Patterson , W. F. III , Cowan , J. H. Jr. , Fitzhugh , G. R. and Nieland , D. L. 201 – 216 . Bethesda , Maryland : American Fisheries Society, Symposium 60 .
- Goodyear , C. P. 1995 . “ Red snapper in U.S. waters of the Gulf of Mexico ” . Miami : Southeast Fisheries Science Center, Miami Laboratory, Stock Assessment Report MIA-95/96-05 .
- Hanson , P. J. , Koenig , C. C. and Zdanowicz , V. S. 2004 . Elemental composition of otoliths used to trace estuarine habitats of juvenile gag Mycteroperca microlepis along the west coast of Florida . Marine Ecology Progress Series , 267 : 253 – 265 .
- Jackson , M. W. , Cowan , J. H. Jr. and Nieland , D. L. 2007 . “ Demographic differences in northern Gulf of Mexico red snapper reproductive maturation: implications for the unit stock hypothesis ” . In Red snapper ecology and fisheries in the U.S. Gulf of Mexico , Edited by: Patterson , W. F. III , Cowan , J. H. Jr. , Fitzhugh , G. R. and Nieland , D. L. 217 – 227 . Bethesda , Maryland : American Fisheries Society, Symposium 60 .
- Johnson , D. R. , Perry , H. M. , Lyczkowski-Shultz , J. and Hanisko , D. 2009 . Red snapper larval transport in the northern Gulf of Mexico . Transactions of the American Fisheries Society , 138 : 458 – 470 .
- Justić , D. , Rabalais , N. N. , Turner , R. E. and Dortch , Q. 1995 . Changes in nutrient structure of river-dominated coastal waters: stoichiometric nutrient balance and its consequences . Estuarine, Coastal and Shelf Science , 40 : 339 – 356 .
- Lenanton , R. C. J. , Valesini , F. , Bastow , T. P. , Nowara , G. B. , Edmonds , J. S. and Connard , M. N. 2003 . The use of stable isotope ratios in whitebait otolith carbonate to identify the source of prey for western Australian penguins . Journal of Experimental Marine Biology and Ecology , 291 : 17 – 27 .
- Patterson , H. M. , Thorrold , S. R. and Shenker , J. M. 1999 . Analysis of otolith chemistry in Nassau grouper (Epinephelus striatus) from the Bahamas and Belize using solution-based ICP-MS . Coral Reefs , 18 : 171 – 178 .
- Patterson , W. F. III . 2007 . “ A review of movement in Gulf of Mexico red snapper: implications for population structure ” . In Red snapper ecology and fisheries in the U.S. Gulf of Mexico , Edited by: Patterson , W. F. III , Cowan , J. H. Jr. , Fitzhugh , G. R. and Nieland , D. L. 245 – 261 . Bethesda , , Maryland : American Fisheries Society, Symposium 60 .
- Patterson , W. F. III and Cowan , J. H. Jr. 2003 . “ Site fidelity and dispersion of red snapper associated with artificial reefs in the northern Gulf of Mexico ” . In Fisheries, reefs, and offshore development , Edited by: Stanley , D. R. and Scarborough-Bull , A. 181 – 193 . Bethesda , , Maryland : American Fisheries Society, Symposium 36 .
- Patterson , W. F. III , Cowan , J. H. Jr. , Wilson , C. A. and Chen , Z. 2008 . Temporal and spatial variability in juvenile red snapper otolith elemental signatures in the northern Gulf of Mexico . Transactions of the American Fisheries Society , 137 : 521 – 532 .
- Patterson , W. F. III , Watterson , J. C. , Shipp , R. L. and Cowan , J. H. Jr. 2001 . Movement of tagged red snapper in the northern Gulf of Mexico . Transactions of the American Fisheries Society , 130 : 533 – 545 .
- Pruett , C. L. , Saillant , E. and Gold , J. R. 2005 . Historical population demography of red snapper (Lutjanus campechanus) from the northern Gulf of Mexico based on analysis of sequences of mitochondrial DNA . Marine Biology , 147 : 593 – 602 .
- Rabalais , N. N. , Turner , R. E. , Justić , D. , Dortch , Q. , Wiseman , W. J. and Sen Gupta , B. K. 1996 . Nutrient changes in the Mississippi River and system responses on the adjacent continental shelf . Estuaries , 19 : 386 – 407 .
- Rooker , J. R. , Secor , D. H. , DeMetrio , G. , Kaufman , A. J. , Rios , A. B. and Ticina , V. 2008 . Evidence of trans-Atlantic movement and natal homing of bluefin tuna from stable isotopes in otoliths . Marine Ecology Progress Series , 368 : 231 – 239 .
- Rooker , J. R. , Secor , D. H. , Zdanowicz , V. S. and Itoh , T. 2001 . Discrimination of northern bluefin tuna from nursery areas in the Pacific Ocean using otolith chemistry . Marine Ecology Progress Series , 218 : 275 – 282 .
- Saillant , E. and Gold , J. R. 2006 . Population structure and variance effective size of red snapper (Lutjanus campechanus) in the northern Gulf of Mexico . U.S. National Marine Fisheries Service Fishery Bulletin , 104 : 136 – 148 .
- SAS (Statistical Analysis System) . 2006 . “ Statistics, version 9.1 ” . Cary , North Carolina : SAS Institute .
- Schroepfer , R. L. and Szedlmayer , S. T. 2006 . Estimates of residence and site fidelity for red snapper Lutjanus campechanus on artificial reefs in the northeastern Gulf of Mexico . Bulletin of Marine Science , 78 : 93 – 101 .
- SEDAR (Southeast Data, Assessment, and Review) . 2005 . “ Stock assessment report of SEDAR 7: Gulf of Mexico red snapper ” . Charleston , South Carolina : SEDAR .
- SEDAR (Southeast Data, Assessment, and Review) . 2009 . “ SEDAR update assessment of red snapper in the Gulf of Mexico ” . Charleston , South Carolina : SEDAR .
- Signoret , M. , Monreal-Gómez , M. A. , Aldeco , J. and Salas-de-León , D. A. 2006 . Hydrography, oxygen saturation, suspended particulate matter, and chlorophyll-α fluorescence in an oceanic region under freshwater influence . Estuarine, Coastal and Shelf Science , 69 : 153 – 164 .
- Strelcheck , A. J. , Cowan , J. H. Jr. and Patterson , W. F. III . 2007 . “ Site fidelity, movement, and growth of red snapper: implications for artificial reef management ” . In Red snapper ecology and fisheries in the U.S. Gulf of Mexico , Edited by: Patterson , W. F. III , Cowan , J. H. Jr. , Fitzhugh , G. R. and Nieland , D. L. 147 – 162 . Bethesda , Maryland : American Fisheries Society, Symposium 60 .
- Sturgeon , R. E. , Willie , S. N. , Yang , L. , Greenberg , R. , Spatz , R. O. , Chen , Z. , Scriver , C. , Clancy , V. , Lam , J. W. and Thorrold , S. 2005 . Certification of a fish otolith reference material in support of quality assurance for trace element analysis . Journal of Analytical Atomic Spectrometry , 20 : 1067 – 1071 .
- Thorrold , S. R. , Jones , C. M. and Campana , S. E. 1997 . Response of otolith microchemistry to environmental variations experienced by larval and juvenile Atlantic croaker (Micropogonias undulatus) . Limnology and Oceanography , 42 : 102 – 111 .
- Thorrold , S. R. , Jones , C. M. , Swart , P. K. and Targett , T. E. 1998 . Accurate classification of juvenile weakfish Cynoscion regalis to estuarine nursery areas based on chemical signatures in otoliths . Marine Ecology Progress Series , 173 : 253 – 265 .
- Walker , N. D. , Wiseman , W. J. Jr. , Rouse , L. J. Jr. and Babin , A. 2005 . Effects of river discharge, wind stress, and slope eddies on circulation and the satellite-observed structure of the Mississippi River plume . Journal of Coastal Research , 21 : 1228 – 1244 .
- Walther , B. D. and Thorrold , S. R. 2008 . Continental-scale variation in otolith geochemistry of juvenile American shad (Alosa sapidissima) . Canadian Journal of Fisheries and Aquatic Sciences , 65 : 2623 – 2635 .
- Warner , K. A. , Bonzongo , J. C. J. , Roden , E. E. , Ward , G. M. , Green , A. C. , Chaubey , I. , Lyons , W. B. and Arrington , D. A. 2005 . Effect of watershed parameters on mercury distribution in different environmental compartments in the Mobile Alabama River basin, USA . Science of the Total Environment , 347 : 187 – 207 .
- Wilkinson , L. , Blank , G. and Gruber , C. 1996 . “ Desktop data analysis with SYSTAT ” . Upper Saddle River , New Jersey : Prentice-Hall .
- Zavala-Hidalgo , J. , Morey , S. L. and O’Brien , J. J. 2003 . Seasonal circulation on the western shelf of the Gulf of Mexico using a high-resolution numerical model . Journal of Geophysical Research , 108 : 3389 doi: 10.1029/2003JC001879