Abstract
The recovery of several top predators in the Gulf of Mexico is likely to increase predation on and competition with other target and nontarget species, possibly causing the abundance of those species to decline. While changes are taking place at the upper trophic levels, exploitation of prey species and climate change are altering productivity at the lower levels. An Ecopath with Ecosim model was developed to simulate the ecosystem impacts of Reef Fish Fishery Management Plan Amendment 30B (which aims to rebuild Gag Mycteroperca microlepis) and Amendment 31 (which reduces effort in the longline fishery). We also evaluated the impact of a hypothetical increase in the exploitation of baitfish and future changes to phytoplankton productivity. The model predicted that rebuilding Gag will cause the biomass of Black Sea Bass Centropristis striata to be 20% lower than it is now and those of Black Grouper M. bonaci, King Mackerel Scomberomorus cavalla, and other shallow-water groupers to be 5–10% lower. Reducing effort in the longline fishery will lead to biomass declines for Black Sea Bass (13%) and Vermilion Snapper Rhomboplites aurorubens (7%). Harvesting baitfish at historically high levels caused the biomass of Red Snapper Lutjanus campechanus, Vermilion Snapper, Greater Amberjack Seriola dumerili, King Mackerel, and numerous species of dolphins and seabirds to be 5–12% lower after 20 years, while biomass increased for species whose diet consists of benthic-associated prey. This paper demonstrates that ecosystem models can be used to quantify the potential ecological impacts of management goals and that the predictions of such models should be considered alongside stock projections from single-species models that assume a constant environment. We intend for this research effort to lead to a more focused and coherent strategy for ecosystem-based fishery management in the Gulf of Mexico.
Received January 30, 2014; accepted September 9, 2014
A basic tenet of ecosystem-based fisheries management (EBFM) is that species are interconnected and that fishing, along with other human and natural perturbations, has the potential to impact entire ecosystems (Link Citation2010). Ecosystem impacts, whether induced by fishing or environmental change, typically arise through predator–prey interactions. Removing predators can cause an increase in the abundance of their prey and a decline in species two trophic levels below them, a phenomenon known as a trophic cascade (Carpenter et al. Citation1985; Frank et al. Citation2005; Steneck Citation2012). Harvesting prey, even at sustainable rates, can impact the growth and reproductive success of predators, ultimately causing their populations to decline (Walters and Martell Citation2004; Walters et al. Citation2005; Smith et al. Citation2011; Pikitch et al. Citation2012). Competition also plays a structuring role in ecosystems (Pianka Citation1974). In regards to trophic-dynamic models, competition requires that a change in the abundance of one species cause reciprocal changes in the abundance of other species that utilize the same resource (Hollowed et al. Citation2000). Simulation models have shown that competitive interactions were important in structuring demersal fish communities, especially during periods when predator abundances were high (Overholtz and Tyler Citation1986; Collie and DeLong Citation1999). Because of predation and competition, rebuilding plans for depleted predator species are likely to have consequences for other members of the community (Hartman Citation2003; Andersen and Rice Citation2010).
Reef fish such as groupers (Epinephelidae) and snappers (Lutjanidae) support some of the most valuable recreational and commercial fisheries in the southeastern United States and Gulf of Mexico. In Citation2009, the commercial fishery landed over 6,400 metric tons of reef fishes on the west coast of Florida with a dockside value of nearly US$32 million, and recreational anglers captured an estimated 3,400 metric tons of reef fish (National Marine Fisheries Service, Office of Science and Technology; http://www.st.nmfs.noaa.gov/index). Over the last 50 years, several reef fish species have been severely depleted. In Citation2009, Red Snapper Lutjanus campechanus, Greater Amberjack Seriola dumerili, and Gag Mycteroperca microlepis were all determined to be overfished and undergoing overfishing, and rebuilding plans are currently in place (NMFS Citation2011a). Alternatively, the stock of Red Grouper Epinephelus morio in the Gulf of Mexico has been increasing since the mid-1990s (SEDAR Citation2009a). The simultaneous increase in the stock sizes of a suite of top predators will increase predation on and competition with other target and nontarget species, possibly causing their abundances to decline. While these changes are taking place at the upper trophic levels, the exploitation of prey species and climate change are altering productivity at the lower levels. Despite these impending changes to the ecosystem, management goals are still based on model projections that assume no change in ecological circumstances.
In the Gulf of Mexico, reef fish are managed by the Gulf of Mexico Fishery Management Council (GMFMC) using a combination of recreational bag limits, minimum size limits, commercial trip limits, gear restrictions, annual catch limits, seasonal closures, area closures, and individual fishing quotas. Rule changes proposed in Reef Fish Fishery Management Plan Amendment 30B aim to end the overfishing of Gag and respond to the improved status of Red Grouper (GMFMC Citation2008). To limit the bycatch of the endangered loggerhead sea turtle Caretta caretta, Amendment 31 establishes an endorsement requirement, seasonal area closure, and hook limit in the bottom longline fishery that is expected to reduce overall effort in that fishery by 48–67% (GMFMC Citation2009). These two regulations are expected to increase the biomass of Gag and other reef fish captured by bottom longlines. However, the effect that this will have on other species in the system through predation and competition has not been evaluated. Moreover, the response of predator populations to variability in the abundance of their prey, whether induced by fishing or climate change, is not well known.
Ecological forecasting has become a common goal for EBFM because it can provide resource managers a comprehensive picture of how the ecosystem will respond to a diverse set of policy options (Clark et al. Citation2001; Valette-Silver and Scavia Citation2003). Ecosystem models are increasingly being utilized as ecological prediction tools because they provide the capability to simulate the entire ecosystem from primary producers to top predators and fisheries. Ecopath with Ecosim (EwE) is an ecosystem modeling package that simulates population dynamics and explicitly accounts for trophic interactions, fisheries, and environmental forcing (Christensen and Walters Citation2004). Ecosim has been used to simulate the ecosystem response to climate change (Ainsworth et al. Citation2011), fisheries (Heymans et al. Citation2009), bycatch (Walters et al. Citation2008), invasive species (Pinnegar et al. Citation2014), marine aquaculture (Forrestal et al. Citation2012), organic pollution (Libralato and Solidoro Citation2009), and bioaccumulation of toxins (Booth and Zeller Citation2005). Because Ecosim is a biomass-dynamic model with only coarse age and size representation, it is not capable of simulating tactical management measures such as bag limits and size limits. Despite their widespread use, Ecosim and other ecosystem models have played only a limited role in actual fisheries management decisions because of their large data requirements and high levels of uncertainty (Plaganyi and Butterworth Citation2004).
To date, there have been few attempts at using ecosystem models to evaluate the impacts of harvest policies and environmental change on the fisheries and ecosystems in the Gulf of Mexico (Okey et al. Citation2004; Walters et al. Citation2008). Limited by data requirements, ecosystem modeling in the Gulf of Mexico has lagged behind that in regions such as Alaska and the northeastern United States, which have a long history of data collection programs and especially food web investigations (Link and Almeida Citation2000; Aydin et al. Citation2007; Link et al. Citation2010; Boldt et al. Citation2012). In this study, we developed an EwE model of the West Florida Shelf (WFS) to predict the biomass changes caused by Reef Fish Fishery Management Plan Amendments 30B and 31, a hypothetical increase in the exploitation of baitfish, and changes to primary production. Like all ecosystem models, this model is a simplified representation of a far more complex system. To make it useful to management, we attempted to strike a balance between capturing what we believe to be the major ecological processes and keeping the model flexible, functional, and interpretable. This research serves as a case study for EBFM in the Gulf of Mexico and demonstrates that ecosystem models can provide quantitative and predictive information that is useful for fisheries assessment and management in this region.
METHODS
Model description.—The EwE model that we developed centered on regulated species on the WFS, including reef fishes, coastal migratory pelagic species, and highly migratory pelagic species as defined by the GMFMC and the National Marine Fisheries Service. The area modeled is approximately 170,000 km2 and extends from the Florida Panhandle south to a boundary that excludes the Florida Keys and out to the 250-m isobaths contour. Particular emphasis was given to groupers and snappers that inhabit reefs on the WFS and support valuable commercial and recreational fisheries. Gag, Red Grouper, Black Grouper M. bonaci, and Yellowedge Grouper Hyporthodus flavolimbatus were represented in the model by three age stanzas (0–1, 1–3, and 3+ years) to capture basic ontogenetic changes in diet, habitat, and fishery selectivity. Red Snapper, Spanish Mackerel Scomberomorus maculatus, and King Mackerel S. cavalla were divided into juveniles (0–1 years) and adults (1+ years). Other reef fishes and pelagic fishes were included either as single-species biomass groups or aggregated into groups of similar species. Coastal and inshore species were included because they interact with reef fish juveniles yet to migrate offshore. Aggregate groups of nontarget fishes, invertebrates, zooplankton, and primary producers were necessary for a complete food web. The resulting model consisted of 70 biomass pools, including one each for dolphins and seabirds, 43 fish groups (of which 11 are nonadult life stages), 18 invertebrate groups, 4 primary producers, and 3 detritus groups ().
TABLE 1. Biomass, catch (including dead discards), instantaneous total mortality (Z), and instantaneous fishing mortality (F) representing historical (1950) and present-day Citation(2009) Ecopath models. Biomass and catch are in thousands of metric tons; Z and F are per year.
Biomass (B; metric tons/km2) values were taken from single-species stock assessments, estimated by dividing observed catches by assumed fishing mortality (B = C/F) or derived from survey data. The production rate (P/B) or instantaneous total mortality (Z) was calculated by adding an assumed natural mortality to the fishing mortality from stock assessments or by using empirical equations for mortality (Pauly Citation1980; Ralston Citation1987). Estimates of consumption (Q) were derived empirically using equations that incorporate data on morphometrics, ambient water temperature, and diet (Pauly Citation1989; Palomares and Pauly Citation1998). The diet compositions of fish were estimated by combining data from the Florida Fish and Wildlife Conservation Commission (FWC) fisheries-independent monitoring program's trophic database with information available in the literature using weighted averages that account for the number of nonempty stomachs, the locations at which they were collected, and the quality of the data (see Supplement A for details). Much effort was put into the derivation of parameters for invertebrates in an earlier WFS model (Okey and Mahmoudi Citation2002; Okey et al. Citation2004), and we used those values as initial input for this reef fish–centric model.
The fishery included four recreational (shore-based, private boat, charter boat, and headboat) and nine commercial (vertical line, bottom longline, pelagic longline, pelagic troll, gill/trammel net, cast net, purse seine, trawl, fish trap, and crab trap) fishing “fleets.” Commercial landings were obtained from trip tickets in the Florida Marine Resources Information System, and discards were based on bycatch reports (NMFS Citation2011b) and information from other observer programs (Pierce et al. Citation1998; Passerotti et al. Citation2010; NMFS–Southeast Fisheries Science Center, personal communication; FWC, Fish and Wildlife Research Institute, personal communication). Recreational landings and discards were made available by the Marine Recreational Fisheries Statistics Survey, and headboat landings were obtained from the Southeast Fisheries Science Center's headboat survey. After entering the required input data, the Ecopath model was “mass-balanced” by making small adjustments to diet, mortality, and biomass so that fishing and predation mortality rates did not exceed total mortality.
Model calibration.—Before being used to make predictions, the Ecosim model was calibrated to time series of observed trends in abundance and catch over the period 1950–2009. Reference time series were obtained directly from stock assessments or taken from fisheries-independent and other survey data. Fleet-specific fishing effort from the Vessel Operating Units database (Jason Rueter, National Oceanic and Atmospheric Administration, Southeast Regional Office, personal communication), and species-specific fishing mortality rates from Southeast Data Assessment and Review stock assessments (www.sefsc.noaa.gov/sedar) were used as forcing time series. Chlorophyll-a production along the WFS is dependent on a variety of factors, including the outflow from the Mississippi River (Gilbes et al. Citation1996, Citation2002; Castillo et al. Citation2001). Therefore, we used nutrient loads from the Mississippi River as a proxy for phytoplankton production on the WFS (Goolsby and Battaglin Citation2000; Aulenbach et al. Citation2007). Because the calibration simulation began in 1950, the biomass, catch, and total mortality parameters from the Citation2009 Ecopath model were first rescaled to represent a historical (1950s) condition (). This involved increasing biomass, reducing catch, and reducing total mortality to a level closer to natural mortality. In most cases, the stock assessment or time series data provided the information necessary to make such adjustments. The diet matrices were the same in the Citation2009 and 1950 models except in a few cases in which minor adjustments were required for mass balance.
The most important parameters when calibrating Ecosim models are the vulnerability exchange rates (vij) between prey i and predator j. These vulnerability parameters represent the rates at which prey move from an invulnerable state to a vulnerable state, and there is one parameter for each predator–prey interaction (Ahrens et al. Citation2012). At very high values of vij (>100) prey become vulnerable to predators at faster rates and the invulnerable pools are quickly depleted. This essentially implies a linear relationship between predator biomass and predation mortality and can lead to unstable Lotka–Volterra dynamics. At low values of vij (<2), predation mortality rates remain relatively constant at their Ecopath base values when predator abundances change. To fit the model to time series, manual adjustments were made to the foraging arena parameters, especially the vijs, to correct for any gross divergence from the data. For example, groups for which biomass declined to zero required that the vijs be reduced or that feeding time adjustment be turned on to generate stronger compensatory improvements in survival at low stock sizes. After correcting for obvious errors, we executed an automated search that adjusts the vijs to minimize the sum of squared deviations (SS) between predicted and observed biomass and catch data. This process was repeated iteratively, focusing on the group with the highest SS, until the model was able to reproduce the major patterns in biomass and catch for all groups over the entire time period.
As a further diagnostic, we evaluated how groups responded under no fishing and very high fishing mortality. We also compared the values of fishing mortality at the maximum sustainable yield (Fmsy) from Ecosim with those estimated by single-species stock assessment models. In Ecosim, Fmsy was estimated using the “MSY Search” interface that runs the model to equilibrium under a range of fishing mortality rates while holding all other groups stationary. These diagnostics were performed to correct for spurious parameter estimates obtained during the calibration process for reasons such as lack of adequate contrast in the historical biomass trend data.
To conduct forward-projecting policy simulations with the Citation2009 present-day model, we rescaled the vijs from the calibrated historical model so that the maximum possible predation mortalities were the same in both the historical and Citation2009 models. This was done by multiplying each vij from the historical model by the ratio of historical to present-day predation mortality rates for the same predator–prey interaction, i.e., where
is the rescaled vulnerability for the present-day model, M2ij is the predation mortality from the 1950s model, and
is the predation mortality from the Citation2009 model. Biomass accumulation was added to the Citation2009 model to account for the initial rate of biomass change occurring during the first year of forecasting Citation(2009); this was calculated as the change in biomass during the last year of the historical simulation. All input parameter values estimated for the historical (calibrated) and Citation2009 models are available in Supplements B and C and can be examined and changed using the EwE 6.4 user interface. Comments describing the source and derivation of input values are embedded in each cell in which data were entered.
Policy screening.—We prescribed two actual management actions (Reef Fish Fishery Management Plan Amendments 30B and 31), a hypothetical expansion in the baitfish fishery, and two alternative scenarios of future phytoplankton productivity. Although we simulated the impact of each scenario on the biomass of all of the species in the model, in the results that we present we focus on recreationally and commercially valuable species. In each case, we conducted a 20-year projection using the present-day model with vulnerability exchange rates rescaled as described above. Fishing mortality, fishing effort, and phytoplankton production were held constant at either the prescribed test values or the Citation2009 Ecopath base values throughout each simulation.
To determine the impacts on species biomass, we compared the change in biomass (ΔB = Bend/Bstart) for each scenario with that of the status quo scenario. The percent change in biomass from status quo (%ΔB) was calculated as 100 · ([ΔBscenario/ΔBstatus quo] – 1). In the status quo scenario the model was projected forward 20 years using the baseline fishing mortality rates and the fishing effort in the Citation2009 Ecopath model. Thus, the status quo scenario simulated a continued increase in biomass for species that were recovering as of Citation2009 (e.g., Greater Amberjack, Red Grouper, and Red Snapper) and declining stock sizes for species whose fishing mortality rates had not yet been reduced below the overfishing limit (Gag).
For each scenario, we conducted a deterministic run over 20 years using the base parameterization with the Citation2009 biomass, catch, and fishing mortalities. We also performed 100 Monte Carlo simulations to establish the sensitivity of the model predictions to uncertainty in Ecopath biomass values. The Monte Carlo simulations randomly selected a biomass value for each species from a uniform distribution, where the mean was the Citation2009 base value and the upper and lower limits were based on knowledge about the uncertainty in the source data and the estimates derived from them. If the random draws of biomass did not violate mass balance in Ecopath, then a Monte Carlo trial was conducted in Ecosim. Otherwise, another draw was made. For each 20-year simulation,%ΔB values were calculated along with standard errors and 95% confidence intervals from the 100 Monte Carlo simulations.
Rebuilding Gag stocks.—The Citation2009 stock assessment for Gulf of Mexico Gag determined the stock to be overfished and undergoing overfishing (SEDAR Citation2009b). Amendment 30B was adopted to address the overfished status and develop a stock rebuilding plan for Gag (GMFMC Citation2008). The rebuilding plan called for reducing fishing mortality to about a third of the Citation2009 level, to be achieved through a combination of larger size limits, smaller bag limits, and/or shorter seasons (SEDAR Citation2009b). The baseline (Ecopath) fishing mortality rate of Gag was 0.52 in Citation2009, so the fishing mortality on Gag was set at Frebuild = 0.16 for the duration of the simulation.
Longline effort reduction.—A Citation2008 report indicated that bottom longline gear took between 339 and 1,884 loggerhead sea turtles over an 18-month period, far exceeding the allowable take of 85 turtles (NMFS 2005, Citation2008). To reduce the frequency of interactions between bottom longlines and sea turtles, Amendment 31 prohibited the use of bottom longline gear in depths shallower than 64 m (i.e., the 35-fathom depth contour) from June through August; reduced the number of longline vessels to those with annual average landings of at least 18 metric tons during 1999–2007; and restricted the number of hooks per vessel to 1,000, of which only 750 may be fished at a time (GMFMC Citation2009). The overall reduction in effective effort based on these regulations is expected to be between 48% and 67%. For this scenario, we chose a middle value of 60% by which to reduce effort in the longline fishery.
Increased exploitation of baitfishes.—On the WFS, Scaled Sardine Harengula jaguana, Spanish Sardine Sardinella aurita, Atlantic Thread Herring Opisthonema oglinum, Round Scad Decapterus punctatus, and other less dominant clupeids and small carangids are commonly referred to as “baitfish.” These small pelagic planktivores make up an important forage base on the WFS and support a commercial baitfish fishery with average annual landings of 2,500 metric tons from 2006 to 2010 (Florida Marine Resources Information System; http://myfwc.com/research/saltwater/fishstats/commercial-fisheries/landings-in-florida/). While the current and historical stock sizes and fishing mortality rates of baitfish are not well known for the WFS, we assumed that a low fishing mortality rate of 0.02 in Citation2009 was reasonable given the magnitude of the biomass and the small size and scale of the fishery. During 1989, effort and catch in the baitfish fishery were both almost 20 times higher than they were from 2006 to 2010. To evaluate the impact of harvesting baitfish at historically high levels, we simulated a 20-fold increase in effort in the purse seine fishery, which generated a fishing mortality rate of about 0.40 on the baitfish complex. For comparison, the average annual landings in the Gulf Menhaden Brevoortia patronus reduction fishery from 2006 to 2010 were 436,160 metric tons, with an average fishing morality rate of 0.43 (SEDAR Citation2011).
Changes in primary production.—How complex marine ecosystems will respond to climate change is uncertain, and there are plausible hypotheses for both lower and higher overall productivity in the future. Warming sea temperatures have been shown to decrease phytoplankton productivity by reducing mixing throughout the water column and lowering nutrient supply (Behrenfeld et al. Citation2006; Doney Citation2006). Severe and prolonged droughts will reduce the delivery of nutrients from freshwater sources and lower the productivity in coastal and estuarine areas (Wiseman et al. Citation1999; Wetz et al. Citation2011). On the other hand, it has been hypothesized that increased productivity will occur in warmer, more stratified waters due to enhanced atmospheric nitrogen fixation at the surface (Karl et al. Citation1997). To investigate how the WFS might respond to broad changes in productivity driven by climate change, we considered two scenarios. In one scenario phytoplankton productivity increased 1% each year for 20 years; in the other, productivity decreased 1% each year. Linear forcing functions without seasonality or random variation were used to simplify the analysis and interpretation of results.
RESULTS
The model was capable of reproducing historical trends in abundance and catch for the period 1950–2010, with a total SS of 223.24 (). The nonstationary behavior in the status quo simulation (solid black lines in ) was a result of biomass accumulation rates calculated from the historical model. The biomass accumulation rates for most species were positive, leading to increasing stock sizes reflecting generally more conservative management in recent years. Gag, which was overfished and undergoing overfishing in Citation2009, was predicted to continue declining to approximately 50% of its Citation2009 stock size with a biomass of 3,773 metric tons (). The biomass of Red Grouper was predicted to be approximately 9,000 metric tons higher in year 20, a 28% increase from Citation2009. Under the status quo, Red Snapper (27% increase) continue to recover because of the reduced fishing mortality achieved by their rebuilding plans. Biomass was predicted to increase for Black Grouper (16%), Greater Amberjack (8%), Yellowedge Grouper (14%), Atlantic Goliath Grouper (40%), Vermilion Snapper (7%), and Gray Triggerfish (14%), while Tilefish were predicted to decline by 5%. The other shallow-water grouper (SWG) and deepwater grouper (DWG) species also increased over the 20-year simulation. All of the species in the coastal migratory pelagic group increased in the status quo simulation, King Mackerel by 22%, Spanish Mackerel by 18%, and Cobia by 13%. Dolphins and seabirds showed only a little change in biomass (±2%), while the sardine–herring–scad baitfish complex declined 9% as predators recovered.
FIGURE 1. Predicted biomass (solid lines) from the Ecosim model and observed trends in biomass (circles) for selected species, with the associated sums of squares in parentheses. Observed trends in abundance were obtained from stock assessments by the Southeast Data Assessment and Review, the Florida Fish and Wildlife Conservation Commission, NOAA's Southeast Fisheries Science Center, and the International Commission for the Conservation of Atlantic Tunas.
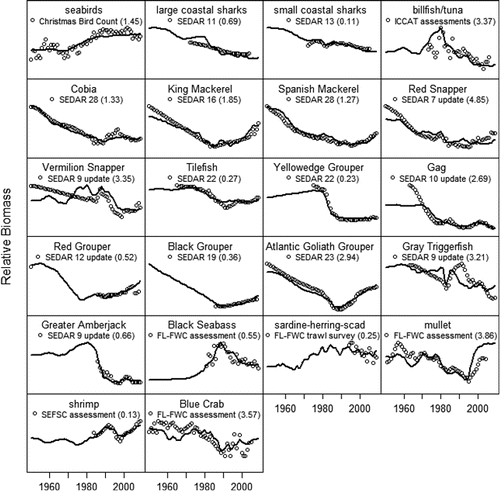
FIGURE 2. Predicted (solid lines) and observed catch (circles) for selected species from 1950 to 2009, with the associated sums of squares in parentheses. For visualization purposes, the scales of the y-axes are not shown. Observed catch was taken from stock assessments by the Southeast Data Assessment and Review and the Florida Fish and Wildlife Conservation Commission (FL-FWC) or obtained from trip tickets in the FL-FWC Marine Resources Information System.
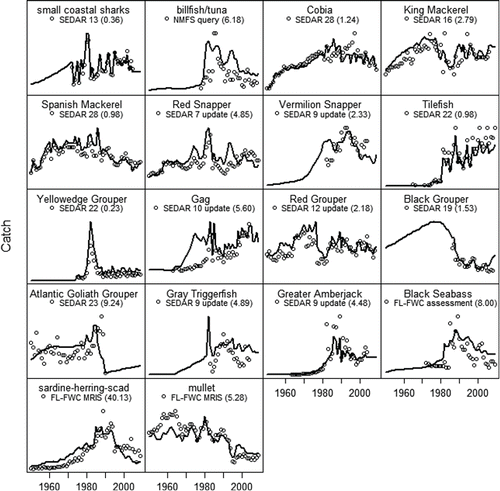
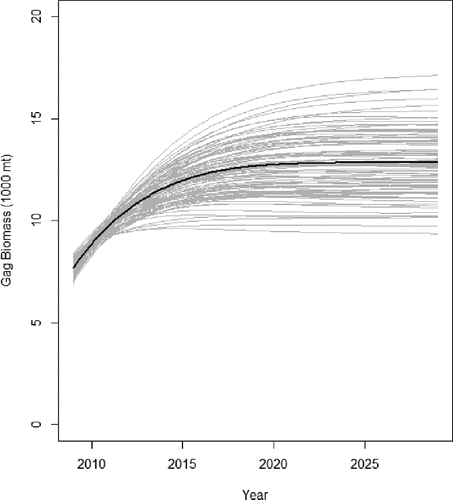
FIGURE 3. Future biomass trajectories simulated by the Ecosim model. Scenarios that caused an increase or decrease in biomass from the status quo are indicated by lines above or below the solid black lines. In some cases there was little change, and those scenarios may be obscured by the status quo line. The dotted line represents the Ecopath base 2009 biomass level.
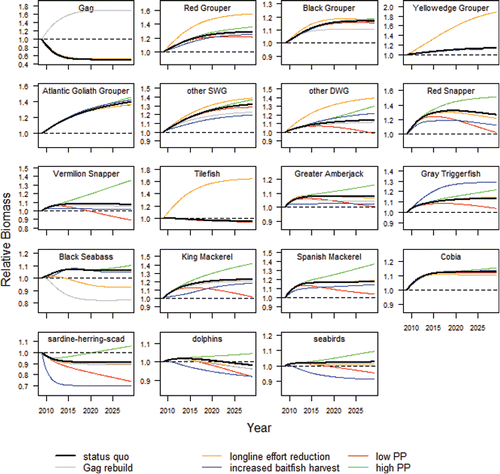
TABLE 2. Impact of three policy options and two primary productivity scenarios on select taxa. The 2009 Ecopath baseline biomass and the predicted biomass in 2029 under the status quo are expressed in thousands of metric tons; the other values are percent changes from the 2029 status quo biomass. The values in parentheses are the 95% confidence limits estimated from 100 Monte Carlo simulations.
Rebuilding Gag Stocks
Under an Frebuild of 0.16, Gag increased 70% over its Citation2009 biomass to 12,835 metric tons in year 20, which was 240% larger than the biomass predicted under the overfishing scenario in the status quo simulation (). One hundred Monte Carlo trials produced stock biomass estimates ranging from about 9,000 to 17,000 metric tons (), with 95% confidence intervals between 12,000 and 13,000 metric tons (). For reference, the single-species stock assessment biomass projections for Gag were 10,000 and 13,500 metric tons after 10 years under Frebuild values of 0.19 and 0.14, respectively (SEDAR Citation2009b). The biomass of Black Sea Bass was predicted to be 20% lower than the status quo in this scenario. The contribution of Black Sea Bass to the diet of Gags was just 1%, and the baseline predation mortality rate was 0.15/year. The predation mortality rate was more than twice as high under rebuilding (0.19/year) than under the status quo (0.09/year). The total predation mortality rates of Black Sea Bass increased from 0.55/year in Citation2009 to 0.58/year in the status quo simulation and 0.64/year in this scenario, an increase of about 10% after 20 years. Black Grouper, SWG, DWG, Vermilion Snapper, Greater Amberjack, Black Sea Bass, King Mackerel, and the sardine–herring–scad complex all had lower 95% confidence intervals that were more than 5% below the status quo ().
Longline Effort Reduction
The impact of a 60% reduction in bottom longline effort had direct positive effects on the biomass of several reef fish species, including Gag (5%), Red Grouper (20%), Yellowedge Grouper (65%), SWG (6%), DWG (22%), and Tilefish (74%). Biomass declined for Vermilion Snapper (7%) and Black Sea Bass (13%). The biomass of the baitfish complex was 6% lower than under the status quo, and stochastic sensitivity runs (Monte Carlo trials with randomly varying Ecopath biomasses) indicated that it could be as much as 11% lower. Consequently, the impact on pelagic species was negative but within 5% for the base scenario. The 95% confidence intervals from stochastic runs were centered on zero for Spanish Mackerel and Cobia, indicating that the impact, though small, could be in either direction depending on the starting biomasses of other species.
Increased Exploitation of Baitfish
Increasing effort in the purse seine fishery 20-fold, to a historical high, reduced the biomass of baitfish by 23% from the status quo (). The biomasses of SWG, Red Snapper, Vermilion Snapper, Amberjack, King Mackerel, dolphins, and seabirds were between 5% and 12% lower after 20 years of harvesting baitfish at F = 0.40 than under status quo conditions. Monte Carlo simulations predicted the loss in biomass to be no more than 15% for a given predator. The base model predicted a greater than 5% increase in biomass for DWG and Gray Triggerfish and produced mostly positive Markov chain–Monte Carlo values for Atlantic Goliath Grouper, Tilefish, and Cobia.
Changes in Primary Production
As expected, the model predicted widespread reductions in fish biomass as productivity declined and increases in biomass when it increased. Under a low-production regime, the biomasses of Red Grouper, Red Snapper, Vermilion Snapper, Amberjack, and Gray Triggerfish were all at least 5% less than under the status quo (). More severe impacts on biomass were predicted for Red Snapper (−19%), Vermilion Snapper (−16%), King Mackerel (−12%), and Spanish Mackerel (−17%). The impacts of reduced productivity on the pelagic baitfish group were between −18% and −28%, whereas the impacts on benthic-associated prey species such as reef carnivores, small coastal carnivores, coastal omnivores, shrimp, and crabs were between 0% and −12%. Monte Carlo simulation trials for Atlantic Goliath Grouper, Tilefish, Black Sea Bass, and Cobia all showed improvements in biomass under lower primary production.
DISCUSSION
The overall conclusion from these simulations is that there are winners and losers in all policy options. Management options oriented toward a single species, such as rebuilding an overfished stock, had less widespread and more modest (±5%) impacts on biomass than policies affecting a suite of species. Simulations which involved perturbations to the middle of the food web or changes to primary production had more drastic impacts over a broader set of species. None of the harvest policies or environmental conditions that we considered was predicted to cause any species to collapse.
Differential use of resources (i.e., resource partitioning) may partly explain why competition caused the biomass of some species to decrease and that of others to increase. For example, the high utilization of anchovies by Spanish Mackerel (22% of diet) and of crabs by Cobia (32% of diet) likely provided some relief in the longline scenario from competition with groupers, whose diets are dominated by sardines, herrings, and smaller reef fishes (see Supplement A). Pelagic species such as the sardine–herring–scad complex and anchovies are more tightly coupled to changes in phytoplankton abundance than benthic prey items. In general, reef fish diets are partially composed of benthic and demersal prey items (e.g., shrimps, crabs, and grunts). The ability of reef fish to access benthic energy channels may stabilize their biomass when pelagic forage fish are removed or phytoplankton production causes changes in their abundance (Rooney et al. Citation2006).
Competitive interactions are believed to influence reef fish communities in the eastern Gulf of Mexico (Smith Citation1979). The biomasses of Vermilion Snapper and Black Sea Bass were predicted to decline in response to an increase of other predators, suggesting that these two species are at a competitive disadvantage. Black Sea Bass were observed in higher densities on experimental reefs where Gag were excluded (Lindberg et al. Citation2006), and Vermilion Snapper became more abundant after a decrease in resident piscivores (groupers) on artificial reefs (Dance et al. Citation2011). These observations at artificial and experimental reefs in the northeastern Gulf of Mexico support the predictions made by the Ecosim model. In another case, divers in the northeastern Gulf of Mexico observed a school of Greater Amberjacks driving prey downward toward the reef where Gag were waiting to feed (Stallings and Dingeldein Citation2012). This illustrates fine-scale competition for food between these two predators and that separate foraging arenas can exist for multiple predators over a single prey resource, water column, and reef. It also raises the possibility that vulnerability exchange rates can be mediated by multiple species that pursue the same prey in different microhabitats (e.g., at different depths).
Ecosim only predicts the impacts due to trophic interactions and ignores any competition for habitat. There are opposing hypotheses about the role of competition for habitat in structuring reef fish communities (Sale and Williams Citation1982), and there is conflicting evidence for habitat limitation in the Gulf of Mexico (Bohnsack Citation1989; Grossman et al. Citation1997; Shipp and Bortone Citation2009). Assuming that Vermilion Snapper and Black Sea Bass do compete with other species for habitat and that they are at a disadvantage in those interactions, we would expect the impacts to be greater than predicted from trophic interactions alone. Other species such as Gag and Red Snapper, for which the model predicted little to no impact because of a small overlap in diet or low predation mortalities, could in fact be affected by competition for space, a process not accounted for by the Ecosim model.
Fish abundance trajectories were expected to vary among species but were similar to projections made in the stock assessments for most species (SEDAR stock assessments, available at www.sefsc.noaa.gov/sedar/). The status quo biomasses were similar in magnitude and direction of change to projections made with single-species models for Gag, Red Grouper, Red Snapper, Greater Amberjack, Yellowedge Grouper, Atlantic Goliath Grouper, and Vermillion Snapper. The similarity between the status quo forecasts made by Ecosim and those of the stock assessment models should not come as a surprise because many groups were calibrated using historical data generated by the stock assessment and are therefore expected to have similar biomass dynamics. While this does not validate the model, it does facilitate direct comparison between predictions made by Ecosim and those of single-species models and allows us to characterize the environmental uncertainty not captured by the single-species models. Divergences between the predictions made by Ecosim and those of the single-species models (i.e., for Cobia and King Mackerel) could be due to incorrect parameter estimates (especially the vulnerability exchange and biomass accumulation rates) or the failure of the single-species models to capture some important environmental process.
The predicted responses of predators to forage fish depletion are consistent with—and perhaps slightly more conservative than—those made by other ecosystem models. When half of a predator's diet is composed of forage fish, ecosystem models tend to predict a 20–40% loss of predator biomass when forage fishes are reduced to between 80% and 40% of their virgin stock sizes (Pikitch et al. Citation2012). The model described here predicted predator biomass to decline by at most 15% when baitfish were harvested at the high rates of the 1980s. A diverse prey resource, such as that available in the Gulf of Mexico, would likely lessen the impact because predators can switch to prey that are more abundant and opportunistic prey species can replace niches left behind by those targeted in the fishery.
Changes in primary production were predicted to have rather large effects on the biomass of fish on the WFS. The magnitude of the impacts predicted in the low and high primary production scenarios is consistent with those predicted by a suite of Ecosim models from Australia (Brown et al. Citation2010). These models showed that consumer biomass is proportional to the primary production rate and that a 20% change in primary production would lead to a similar change in biomass. It is likely that primary production will be highly variable in the future, making the ecosystem response far less predictable. For example, an increase in phytoplankton biomass could lead to declines in submerged aquatic vegetation (Greening and Janicki Citation2006) or cause widespread hypoxic zones (Breitburg Citation2002; Diaz and Rosenberg Citation2008; Justic et al. Citation2002) that will have negative, nonlinear effects on marine organisms. Nevertheless, the simple linear simulations explored here offer some insight into how bottom-up processes impact the entire ecosystem and provide a framework for forecasting more detailed climate change scenarios.
There are several caveats and limitations associated with the EwE approach, which are described elsewhere (Christensen and Walters Citation2004). A few of the more important caveats to consider when interpreting such projections include the lack of spatial representation, the lack of a response in fishing effort to changing biomass, and the absence of management feedback as stocks recover (or decline). For instance, in our model effort reduction in the longline fishery was achieved through a combination of regulations, including spatial closures and depth restrictions. Prohibiting longlines in depths less than 35 fathoms, as is the rule in Amendment 31, will shift effort farther offshore and therefore not benefit the deepwater species (Yellowedge Grouper, Tilefish, and other DWG) nearly as much as predicted in the nonspatial Ecosim model. Preliminary simulations conducted using Ecospace, a spatially explicit component of EwE, predict biomass to decline for deepwater species under Amendment 31.
There are two basic types of uncertainty in ecosystem models, that associated with the data used to derive the input parameters and that associated with model structure. Structural uncertainty manifests itself in the definition of biomass pools and functional relationships and the choice of environmental drivers (Pauly et al. Citation2000). This type of uncertainty was addressed early in model development through a series of internal reviews, iterative improvements, and a workshop at which the model was reviewed by a group of scientists familiar with the WFS and reef fish species.
Regarding data, the two most critical sources of uncertainty are the diet compositions for large-bodied, offshore predators and the biomass of the forage species. Quality stomach contents from deepwater reef species are difficult to obtain due to barotrauma (which can lead to stomach eversion) and the inability to sample with active, nonbaited gear. Much of the data used to establish the diet compositions of adult reef fish were outdated and obtained using baited gear, or were only available from small samples. Sampling beyond that conducted in traditional fisheries-independent surveys is needed to more adequately describe the diet compositions of these ecologically and economically important predator species.
Baitfish are one of the most ecologically important groups in this system, yet there is considerable uncertainty about their biomass. Houde Citation(1976) estimated the biomass of baitfish (Atlantic Thread Herring, Scaled Sardine, and Spanish Sardine) from egg and larval surveys in the eastern Gulf of Mexico to be nearly 1 million metric tons during the early 1970s. Recent estimates based on the FWC baitfish trawl and an acoustic survey conducted offshore of west-central Florida are 50,000 and 800,000 metric tons, respectively (Keith Fischer, FWC, personal communication). Nearly every commercially and recreationally important species utilizes the baitfish resource to some extent. Therefore, it is critical to gain a better understanding of their abundance, productivity, and contribution to the diets of predator species.
In general, the model does indicate that trophic impacts are potentially strong and can lead to ecological trade-offs that will trigger management actions, especially for species currently near a threshold or under a rebuilding plan. For instance, simultaneously rebuilding the stocks of multiple species with similar diets may require lower target catch limits or fishing mortality rates than those estimated using single-species models that do not account for the rebuilding of competing species. While ecosystem-based fisheries science and modeling has grown greatly over the last decade, agencies have had difficulty incorporating it into the management process. Even with the caveats and uncertainties, there is great utility in food web models that can quantify the changes in biomass and mortality arising from trophic interactions and environmental change. Ecosystem models such as ours are intended to complement single-species stock assessment and management. For example, they can generate vectors of time-varying natural mortality as an input to single-species models or be used to simultaneously evaluate the performance of several management options. Because large-scale, long-term ecological experiments are impractical, if not impossible, scientists will continue to rely on simulation models to predict the impacts of broad-scale management actions and environmental change on complex ecosystems. The work presented here demonstrates how the West Florida Shelf may respond to natural and anthropogenic perturbations, and we hope this effort will lead to a more focused and coherent strategy for EBFM throughout the Gulf of Mexico.
Chagaris et al Supplemental files
Download Zip (1,009.9 KB)ACKNOWLEDGMENTS
This research was supported by Florida Sea Grant and the Florida Fish and Wildlife Conservation Commission with funds from Sport Fish Restoration. We also acknowledge Sherry Larkin, Bill Pine, Bill Lindberg, and the Florida FWC Stock Assessment group for their participation and critique at various stages of model development. Sergio Alvarez, Ed Camp, Jake Tetzlaff, the FWC Fisheries Independent Monitoring Program, and the National Marine Fisheries Service assisted by providing support and necessary data. Lastly, we thank all those who participated in the science and stakeholder workshops.
REFERENCES
- Ahrens, R. N. M., C. J. Walters, and V. Christensen. 2012. Foraging arena theory. Fish and Fisheries 13:41–59.
- Ainsworth, C. H., J. F. Samhouri, D. S. Busch, W. W. L. Cheung, J. Dunne, and T. A. Okey. 2011. Potential impacts of climate change on northeast Pacific marine food webs and fisheries. ICES Journal of Marine Science 68:1217–1229.
- Andersen, K. H., and J. C. Rice. 2010. Direct and indirect community effects of rebuilding plans. ICES Journal of Marine Science 67:1980–1988.
- Aulenbach, B. T., H. T. Buxton, W. T. Battaglin, and R. H. Coupe. 2007. Streamflow and nutrient fluxes of the Mississippi–Atchafalaya River basin and subbasins for the period of record through 2005. U.S. Geological Survey, Open-File Report 2007–1080, Reston, Virginia.
- Aydin, K., S. Gaichas, I. Ortiz, D. Kinzey, and N. Friday. 2007. A comparison of the Bering Sea, Gulf of Alaska, and Aleutian Islands large marine ecosystems through food web modeling. NOAA Technical Memorandum NMFS-AFSC-178.
- Behrenfeld, M. J., R. T. O'Malley, D. A. Siegel, C. R. McClain, J. L. Sarmiento, G. C. Feldman, A. J. Milligan, P. G. Falkowski, R. M. Letelier, and E. S. Boss. 2006. Climate-driven trends in contemporary ocean productivity. Nature 444:752–755.
- Bohnsack, J. A. 1989. Are high densities of fishes at artificial reefs the result of habitat limitation or behavioral preference? Bulletin of Marine Science 44:631–645.
- Boldt, J. L., T. W. Buckley, C. N. Rooper, and K. Aydin. 2012. Factors influencing cannibalism and abundance of Walleye Pollock (Theragra chalcogramma) on the eastern Bering Sea shelf, 1982–2006. U.S. National Marine Fisheries Service Fishery Bulletin 110:293–306.
- Booth, S., and D. Zeller. 2005. Mercury, food webs, and marine mammals: implications of diet and climate change for human health. Environmental Health Perspectives 113:521–526.
- Breitburg, D. 2002. Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries 25:767–781.
- Brown, C. J., E. A. Fulton, A. J. Hobday, R. J. Matear, H. P. Possingham, C. Bulman, V. Christensen, R. E. Forrest, P. C. Gehrke, N. A. Gribble, S. P. Griffiths, H. Lozano-Montes, J. M. Martin, S. Metcalf, T. A. Okey, R. Watson, and A. J. Richardson. 2010. Effects of climate-driven primary production change on marine food webs: implications for fisheries and conservation. Global Change Biology 16:1194–1212.
- Carpenter, S. R., J. F. Kitchell, and J. R. Hodgson. 1985. Cascading trophic interactions and lake productivity. BioScience 35:634–639.
- Castillo, C. E. D., P. Coble, R. Conmy, F. Muller-Karger, L. Vanderbloemen, and G. Vargo. 2001. Multispectral in situ measurements of organic matter and chlorophyll fluorescence in seawater: documenting the intrusion of the Mississippi River plume in the West Florida Shelf. Limnology and Oceanography 46:1836–1843.
- Christensen, V., and C. Walters. 2004. Ecopath with Ecosim: methods, capabilities, and limitations. Ecological Modelling 172:109–139.
- Clark, J. S., S. R. Carpenter, M. Barber, S. Collins, A. Dobson, J. Foley, D. M. Lodge, M. Pascual, R. Pielke Jr., W. Pizer, C. Pringle, W. Reid, K. A. Rose, O. Sala, W. H. Schlesinger, D. H. Wall, and D. Wear. 2001. Ecological forecasts: an emerging imperative. Science 293:657–659.
- Collie, J. S., and A. K. DeLong. 1999. Multispecies interactions in the Georges Bank fish community: ecosystem approaches for fisheries management. Alaska Sea Grant College Program, AK-SG-99-01, Fairbanks.
- Dance, M. A., W. F. Patterson III, and D. T. Addis. 2011. Fish community and trophic structure at artificial reef sites in the northeastern Gulf of Mexico. Bulletin of Marine Science 87:301–324.
- Diaz, R. J., and R. Rosenberg. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321:926–929.
- Doney, S. C. 2006. Oceanography: plankton in a warmer world. Nature 444:695–696.
- Forrestal, F., M. Coll, D. J. Die, and V. Christensen. 2012. Ecosystem effects of Bluefin Tuna, Thunnus thynnus, aquaculture in the NW Mediterranean Sea. Marine Ecology Progress Series 456:215–231.
- Frank, K. T., B. Petrie, J. S. Choi, and W. C. Leggett. 2005. Trophic cascades in a formerly cod-dominated ecosystem. Science 308:1621–1623.
- Gilbes, F., F. E. Muller-Karger, and C. E. Del Castillo. 2002. New evidence for the West Florida Shelf Plume. Continental Shelf Research 22:2479–2496.
- Gilbes, F., C. Tomas, J. J. Walsh, and F. E. Muller-Karger. 1996. An episodic chlorophyll plume on the West Florida shelf. Continental Shelf Research 16:1201–1224.
- GMFMC (Gulf of Mexico Fishery Management Council). 2008. Final Reef Fish Amendment 30B. National Marine Fisheries Service, NA05NMF4410003, Tampa, Florida.
- GMFMC (Gulf of Mexico Fishery Management Council). 2009. Final Reef Fish Amendment 31. National Marine Fisheries Service, NA05NMF4410003, Tampa, Florida.
- Goolsby, D. A., and W. A. Battaglin. 2000. Nitrogen in the Mississippi River basin: estimating sources and predicting flux to the Gulf of Mexico. U.S. Geological Survey, Fact Sheet 135–00, Reston, Virginia.
- Greening, H., and A. Janicki. 2006. Toward reversal of eutrophic conditions in a subtropical estuary: water quality and seagrass response to nitrogen loading reductions in Tampa Bay, Florida, USA. Environmental Management 38:163–178.
- Grossman, G. D., G. P. Jones, and W. J. Seaman. 1997. Do artificial reefs increase regional fish production? A review of existing data. Fisheries 22(4):17–23.
- Hartman, K. J. 2003. Population-level consumption by Atlantic coastal Striped Bass and the influence of population recovery upon prey communities. Fisheries Management and Ecology 10:281–288.
- Heymans, J. J., U. R. Sumaila, and V. Christensen. 2009. Policy options for the northern Benguela ecosystem using a multispecies, multifleet ecosystem model. Progress in Oceanography 83:417–425.
- Hollowed, A. B., N. Bax, R. Beamish, J. Collie, M. Fogarty, P. Livingston, J. Pope, and J. C. Rice. 2000. Are multispecies models an improvement on single-species models for measuring fishing impacts on marine ecosystems? ICES Journal of Marine Science 57:707–719.
- Houde, E. D. 1976. Abundance and potential for fisheries development of some sardine-like fishes in the eastern Gulf of Mexico. Pages 73–83 in J. B. Higman, editor. Proceedings of the 28th Annual Session of the Gulf and Caribbean Fisheries Institute. Gulf and Caribbean Fisheries Institute, Miami.
- Justic, D., N. N. Rabalais, and R. E. Turner. 2002. Modeling the impacts of decadal changes in riverine nutrient fluxes on coastal eutrophication near the Mississippi River delta. Ecological Modelling 152:33–46.
- Karl, D., R. Letelier, L. Tupas, J. Dore, J. Christian, and D. Hebel. 1997. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388:533–538.
- Libralato, S., and C. Solidoro. 2009. Bridging biogeochemical and food web models for an end-to-end representation of marine ecosystem dynamics: the Venice Lagoon case study. Ecological Modelling 220:2960–2971.
- Lindberg, W. J., T. Frazer, K. Portier, F. Vose, J. Loftin, D. Murie, D. Mason, B. Nagy, and M. Hart. 2006. Density-dependent habitat selection and performance by a large mobile reef fish. Ecological Applications 16:731–746.
- Link, J. S. 2010. Ecosystem-based fisheries management: confronting trade-offs. Cambridge University Press, New York.
- Link, J. S., and F. P. Almeida. 2000. An overview and history of the food web dynamics program of the Northeast Fisheries Science Center, Woods Hole, Massachusetts. NOAA Technical Memoradum NMFS-NE-159.
- Link, J. S., E. A. Fulton, and R. J. Gamble. 2010. The northeast US application of ATLANTIS: a full system model exploring marine ecosystem dynamics in a living marine resource management context. Progress in Oceanography 87:214–234.
- NMFS (National Marine Fisheries Service). 2005. Endangered Species Act: Section 7 consultation on the continued authorization of reef fish fishing under the Gulf of Mexico Reef Fish Fishery Management Plan and Proposed Amendment 23. NMFS, Southeast Region Biological Opinion (February 15), St. Petersburg, Florida.
- NMFS (National Marine Fisheries Service). 2008. Estimated takes of sea turtles in the bottom longline portion of the Gulf of Mexico reef fish fishery July 2006 through 2007 based on observer data. NMFS, Southeast Fisheries Science Center Contribution PRD-07/08-15, Miami.
- NMFS (National Marine Fisheries Service). 2011a. Annual report to Congress on the status of U.S. fisheries: 2010. National Marine Fisheries Service, Silver Spring, Maryland.
- NMFS (National Marine Fisheries Service). 2011b. U.S. national bycatch report. NOAA Technical Memorandum NMFS-F/SPO-117E.
- Okey, T. A., and B. Mahmoudi. 2002. An ecosystem model of the West Florida Shelf for use in fisheries management and ecological research, volume II. Model construction. Florida Marine Research Publications 163.
- Okey, T. A., G. A. Vargo, S. Mackinson, M. Vasconcellos, B. Mahmoudi, and C. A. Meyer. 2004. Simulating community effects of sea floor shading by plankton blooms over the West Florida Shelf. Ecological Modelling 172:339–359.
- Overholtz, W. J., and A. V. Tyler. 1986. An exploratory simulation model of competition and predation in a demersal fish assemblage on Georges Bank. Transactions of the American Fisheries Society 115:805–817.
- Palomares, M. L. D., and D. Pauly. 1998. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature, and salinity. Marine and Freshwater Research 49:447–453.
- Passerotti, M. S., J. K. Carlson, and S. J. B. Gulak. 2010. Catch and bycatch in U.S. southeast gill-net fisheries, 2009. NOAA Technical Memorandum NMFS-SEFSC-600.
- Pauly, D. 1980. On the interrelationship between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES Journal of Marine Science 39:175–192.
- Pauly, D. 1989. Food consumption by tropical and temperate fish populations: some generalizations. Journal of Fish Biology 35:11–20.
- Pauly, D., V. Christensen, and C. Walters. 2000. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES Journal of Marine Science 57:1–10.
- Pianka, E. R. 1974. Evolutionary ecology. Harper and Row, New York.
- Pierce, D. J., J. E. Wallin, and B. Mahmoudi. 1998. Spatial and temporal variations in the species composition of bycatch collected during a Striped Mullet (Mugil cephalus) survey. Gulf of Mexico Science 16:15–27.
- Pikitch, E. K., P. D. Boersma, I. L. Boyd, D. O. Conover, P. Cury, T. Essington, S. S. Heppell, E. D. Houde, M. Mangel, D. Pauly, E. Plagányi, K. Sainsbury, and R. S. Steneck. 2012. Little fish, big impact: managing a crucial link in ocean food webs. Lenfest Ocean Program, Washington, D.C.
- Pinnegar, J. K., M. T. Tomczak, and J. S. Link. 2014. How to determine the likely indirect food web consequences of a newly introduced nonnative species: a worked example. Ecological Modelling 272:379–387.
- Plaganyi, E. E., and D. S. Butterworth. 2004. A critical look at the potential of Ecopath with Ecosim to assist in practical fisheries management. African Journal of Marine Science 26:261–287.
- Ralston, S. 1987. Mortality rates of snappers and groupers. Pages 375–404 in J. J. Polovina and S. Ralston, editors. Tropical snappers and groupers: biology and fisheries management. Westview Press, Boulder, Colorado.
- Rooney, N., K. McCann, G. Gellner, and J. C. Moore. 2006. Structural asymmetry and the stability of diverse food webs. Nature 442:265–269.
- Sale, P. F., and D. M. Williams. 1982. Community structure of coral reef fishes: are the patterns more than those expected by chance? American Naturalist 120:121–127.
- SEDAR (Southeast Data Assessment and Review). 2009a. SEDAR 12 update: Gulf of Mexico Red Grouper stock assessment report. SEDAR, North Charleston, South Carolina.
- SEDAR (Southeast Data Assessment and Review). 2009b. SEDAR 10 update: Gulf of Mexico Gag stock assessment report. SEDAR, North Charleston, South Carolina.
- SEDAR (Southeast Data Assessment and Review). 2011. SEDAR 27 Gulf Menhaden stock assessment report. SEDAR, North Charleston, South Carolina.
- Shipp, R. L., and S. A. Bortone. 2009. A perspective of the importance of artificial habitat on the management of Red Snapper in the Gulf of Mexico. Reviews in Fisheries Science 17:41–47.
- Smith, G. B. 1979. Relationship of eastern Gulf of Mexico reef–fish communities to the species equilibrium theory of insular biogeography. Journal of Biogeography 6:49–61.
- Smith, A. D. M., C. J. Brown, C. M. Bulman, E. A. Fulton, P. Johnson, I. C. Kaplan, H. Lozano-Montes, S. Mackinson, M. Marzloff, L. J. Shannon, Y. Shin, and J. Tam. 2011. Impacts of fishing low–trophic level species on marine ecosystems. Science 333:1147–1150.
- Stallings, C. D., and A. L. Dingeldein. 2012. Intraspecific cooperation facilitates synergistic predation. Bulletin of Marine Science 88:317–318.
- Steneck, R. S. 2012. Apex predators and trophic cascades in large marine ecosystems: learning from serendipity. Proceedings of the National Academy of Sciences of the USA 109:7953–7954.
- Valette-Silver, N. J., and D. Scavia. 2003. Ecological forecasting: new tools for coastal and ecosystem management. NOAA Technical Memorandum NOS NCCOS 1.
- Walters, C., V. Christensen, S. J. D. Martell, and J. F. Kitchell. 2005. Possible ecosystem impacts of applying MSY policies from single-species assessment. ICES Journal of Marine Science 62:558–568.
- Walters, C., S. J. D. Martell, V. Christensen, and B. Mahmoudi. 2008. An Ecosim model for exploring Gulf of Mexico ecosystem management options: implications of including multistanza life history models for policy predictions. Bulletin of Marine Science 83:251–271.
- Walters, C. J., and S. J. D. Martell. 2004. Fisheries ecology and management. Princeton University Press, Princeton, New Jersey.
- Wetz, M. S., E. A. Hutchinson, R. S. Lunetta, H. W. Paerl, and J. C. Taylor. 2011. Severe droughts reduce estuarine primary productivity with cascading effects on higher trophic levels. Limnology and Oceanography 56:627–638.
- Wiseman, W. J. Jr., N. N. Rabalais, M. J. Dagg, and T. E. Whitledge, editors. 1999. Nutrient-enhanced coastal ocean productivity in the northern Gulf of Mexico: understanding the effects of nutrients on a coastal ecosystem. National Oceanic and Atmospheric Administration, Coastal Ocean Program, Decision Analysis Series 14, Silver Spring, Maryland.