Abstract
Sturgeons (Acipenseridae) are one of the most threatened taxa worldwide, including species in North Carolina and South Carolina. Populations of Atlantic Sturgeon Acipenser oxyrinchus in the Carolinas have been significantly reduced from historical levels by a combination of intense fishing and habitat loss. There is a need for estimates of current abundance, to describe status, and for estimates of historical abundance in order to provide realistic recovery goals. In this study we used N-mixture and distance models with data acquired from side-scan sonar surveys to estimate abundance of sturgeon in six major sturgeon rivers in North Carolina and South Carolina. Estimated abundances of sturgeon greater than 1 m TL in the Carolina distinct population segment (DPS) were 2,031 using the count model and 1,912 via the distance model. The Pee Dee River had the highest overall abundance of any river at 1,944 (count model) or 1,823 (distance model). These estimates do not account for sturgeon less than 1 m TL or occurring in riverine reaches not surveyed or in marine waters. Comparing the two models, the N-mixture model produced similar estimates using less data than the distance model with only a slight reduction of estimated precision.
Received May 3, 2014; accepted October 14, 2014
Sturgeon (Acipenseridae) populations worldwide have declined from historical levels as a result of a combination of factors, including overharvest and habitat alteration (Secor Citation2002; Pitkitch et al. Citation2005; Nelson et al. Citation2013). In the eastern USA, Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus was listed as “endangered” under the U.S. Endangered Species Act (ESA) in 2012. Under the listing, five separate Atlantic Sturgeon distinct population segments (DPS) were established. One DPS was categorized as threatened, but the other four were listed as endangered, including the Carolina and South Atlantic DPSs (). The Carolina DPS includes riverine populations ranging from the Roanoke River in North Carolina to the Santee–Cooper system in South Carolina. The South Atlantic DPS includes rivers from the Ashepoo–Combahee–Edisto basin in South Carolina to the St. Johns River, Florida. Given the status of sturgeons, it is important to assess and monitor the state of populations. Unfortunately, sturgeons have not been well studied in many Southeastern systems since the closure of commercial U.S. fisheries in 1998 (ASSRT Citation2007; Peterson et al. Citation2008). Reliable estimates of abundance are necessary for making management decisions and developing recovery strategies.
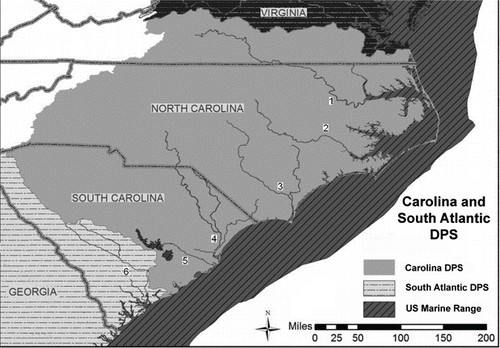
FIGURE 1. Lower portions of six river systems in the Carolina and South Atlantic distinct population segments (DPS) surveyed using side-scan sonar; from north to south they are the Roanoke (1), Neuse (2), and Cape Fear (3) rivers in North Carolina and the Pee Dee/Waccamaw (4), Santee (5), and Edisto (6) rivers in South Carolina.
A common method for estimating absolute abundance of fish species is mark–recapture; however, there are drawbacks to these studies, including cost and potential harm to the study species. The endangered status of sturgeon limits the sampling methods researchers can employ because extra care must be taken when sampling and handling specimens (Damon-Randall et al. Citation2010; Kahn and Mohead Citation2010). Hydroacoustics provides a nonintrusive method for sampling endangered species. Sturgeon and related Paddlefish Polyodon spathula have been identified as potentially suitable targets for hydroacoustic studies (Nealson and Brundage Citation2007; Bergman Citation2011; Nelson et al. Citation2013). Side-scan sonar is a relatively old hydroacoustic technology that has been used increasingly in fisheries studies in recent years (Kaeser and Litts Citation2008; Foote Citation2009). In a previous paper, we described a procedure to rapidly survey sturgeon populations using side-scan sonar and occupancy modeling approaches (Flowers and Hightower Citation2013). We reexamine those data here, with a goal of estimating Atlantic Sturgeon abundance in the Carolina DPS. Large body size, including a maximum length of over 3 m (Gross et al. Citation2002) and distinctive shape make this species an ideal hydroacoustic target. This anadromous species, spends large amounts of time feeding and migrating in marine environments, using freshwater rivers for spawning and a summer dormant period (ASSRT Citation2007). During summer, Atlantic Sturgeon may aggregate in lower portions of rivers, often in deep holes near the freshwater–saltwater interface, although locations vary with river characteristics and flow conditions (Moser and Ross Citation1995; Collins et al. Citation2000).
Sturgeon density can be obtained from counts based on side-scan images and the length and width of the survey transect. These counts can be analyzed with an N-mixture model (Royle Citation2004) that uses a distribution, such as a Poisson, to simulate abundance and binomial distribution to simulate detection. Count data can also be analyzed using distance sampling methods (Buckland et al. Citation2001; Royle et al. Citation2004) based on the distance on side-scan images from sturgeon to the survey transect. We compare results from the various data types and models and provide abundance estimates for Atlantic Sturgeon recovery planning.
METHODS
Study sites.—Field work took place in six river systems in North Carolina and South Carolina (). Systems were chosen based on available information about the status of sturgeon populations or the potential for presence of sturgeon in that system. Five of the rivers were located in the Carolina DPS (Roanoke, Neuse, Cape Fear, Pee Dee, and Santee) while the sixth was located in the South Atlantic DPS (Edisto). The Tar River was the only Carolina DPS river excluded from the survey, based on smaller size (Oakley and Hightower Citation2007) and lack of recent anecdotal evidence of sturgeon. Mark–recapture studies or other types of sturgeon population studies have not been performed on these systems.
Side-scan surveys.—Surveys were conducted in coastal rivers near the freshwater–saltwater interface where sturgeon have been observed (Moser and Ross Citation1995; Collins et al. Citation2000; Peterson et al. Citation2008). Repeated side-scan sonar surveys were performed over 3 d at each of the six rivers. The three sampling days were as close as possible, often consecutive, in order to reduce the chance of movement between sites, as needed to minimize violations of the population closure assumptions of these methods. Riverine survey reaches began downstream in tidal portions of elevated salinity (>15 mg/L) and ranged upstream a distance of at least 30 km inland on the main stem or to a point where river depths were too shallow to effectively operate the side-scan sonar. The total distance surveyed in each river varied, ranging from 40 to 80 km because of braids and side channels. Full details regarding survey methods were provided in Flowers and Hightower (Citation2013).
We used an Edgetech 4125-P side-scan sonar unit operated in high-frequency (1,250 kHz) mode, with a total swath width of 50 m. The unit was deployed at a constant depth of approximately 1 m below the water's surface for all systems and surveys. Side-scan sonar data were collected and processed using Chesapeake Technology's SonarWiz.Map software. When a potential sturgeon was observed on side-scan images, the target was marked, its GPS coordinates were taken, and the target's body length and distance from the transect were measured.
Each river was an individual study area subdivided into 2-km sites, for a total of 179 sites. Sites of 1 km and 4 km were previously evaluated for their effect on occupancy, with results bracketing the 2-km sites (Flowers and Hightower Citation2013). The large amount of data collected during the surveys required extended time for analysis. Side-scan sonar file processing took 1–3 d/survey day, depending on how many targets needed to be recorded.
Abundance estimation.—Only targets clearly identified as sturgeon (Flowers and Hightower Citation2013) were used in our analyses. For the N-mixture model, side-scan observer data were compiled into a data matrix containing river, site, and the three daily counts of individual sturgeon. Sites were arranged in rows (N = 179), while river index and daily counts were listed in columns (N = 4). A separate matrix for distance sampling data incorporated corrected horizontal distance from a side-scan transect's centerline to each sturgeon identified in a given site for each survey day. The number of rows in this matrix was equal to the number of distance observations by site (N = 1,436) and had columns representing river, site, sample day, and distance (N = 4). A “slant range correction” was used to account for positioning errors because the cross-track coordinates of a side-scan image are a function of range to the sonar rather than horizontal distance on the bottom (Cobra et al. Citation1992). Although our side-scan swath was 25 m/side, based on slant-range, the maximum horizontal range was actually 24 m.
The counts and distance data sets were analyzed using procedures in package unmarked (Fiske and Chandler Citation2011) in program R (R Development Core Team 2009). For both analytical methods, we assumed that we correctly identified targets classified as sturgeon, although additional sturgeon were assumed to be present but not identifiable from a particular side-scan image. Count data were analyzed in unmarked using the pcount procedure, based on the N-mixture framework proposed by Royle (Citation2004). The general form of the N-mixture model for site abundance is(1) and for the detection process is
(2)
where λ is the abundance per site i and p is the detection probability. A discrete distribution, such as the Poisson or negative-binomial, is used for f, with support restricted to Ni ≥ 0 (Fiske and Chandler Citation2011). The θ are additional parameters of f other than the abundance rate for distributions such as the negative binomial (Fiske and Chandler Citation2011).
This method uses temporally repeated counts of individuals at a site to estimate the abundance of a closed population. The framework uses a combination of two different processes to model detection and abundance. Detection probability is modeled using a binomial distribution, while abundance is modeled using a Poisson, negative binomial, or zero-inflated Poisson distribution. Sites were assumed to be closed for the duration of the experiment, and probability of detecting an individual animal was assumed to be constant (Royle Citation2004).
Distance sampling data were analyzed using the gdistsamp function based on the method proposed by Royle et al. (Citation2004) and extended by Chandler et al. (Citation2011). This model is similar to the N-mixture model, although it adds a component to the likelihood to address variability in detection probability as a function of range from the survey transect. Here transect-level abundance is modeled the same as the N-mixture model, but the detection process is modeled as(3)
where Ni is the latent abundance at site i, as with the N-mixture model, and πij is the multinomial cell probability for transect i in distance-class j, computed by integrating a detection function such as the half-normal (with scale parameter σ) over each distance interval (Fiske and Chandler Citation2011). Abundance is modeled using a Poisson or negative-binomial distribution. Four functions (uniform, half-normal, exponential, hazard), described in further depth by Buckland et al. (Citation2001), were used to model detection, with distance data formatted in 4-m bins over the 24 m/side width of the side-scan swath. As above, sites were assumed to be closed over sampling occasions. Additional assumptions related to distance sampling were (1) sturgeon were distributed according to some stochastic process based on an underlying density, (2) sturgeon were detected at their initial locations, prior to any movement (to avoid double-counting), and (3) distances were correctly grouped by intervals (Buckland et al. Citation2001). It is generally assumed for distance surveys that survey lines are randomly placed, but here, we used a single mid-river survey line. Thus, we further assumed that sturgeon were randomly distributed so that our estimated abundances were representative of the entire surveyed sections of rivers.
For all models, scenarios were run using river as a site-level covariate to model the variability in abundance across rivers. For each method (count and distance), the most appropriate model was selected using Akaike's information criterion (AIC). We evaluated model selection using ΔAIC, i.e., the difference between the best model and each model, and AIC weight (AICwt), representing the relative likelihood of each model. Goodness of fit for the best models was evaluated using the parametric bootstrapping function in unmarked, parboot. One hundred bootstraps were run for each model, and the Freeman–Tukey test was used to assess fit. Estimates of abundance and 95% confidence intervals were calculated for each of the best models using the predict function in unmarked.
We estimated the proportion of the river scanned based on the total surface area and the area surveyed by side-scan sonar (48 m × 179 2-km sites). River area measurements were performed in Google Earth Pro (Google, Inc.). Our side-scan surveys only covered 17.2 km2, which was 0.13 of the area of the survey reach for all rivers combined. Proportions for individual rivers were 0.13 (Roanoke), 0.08 (Neuse), 0.26 (Cape Fear), 0.11 (Pee Dee), 0.29 (Santee), and 0.11 (Edisto).
RESULTS
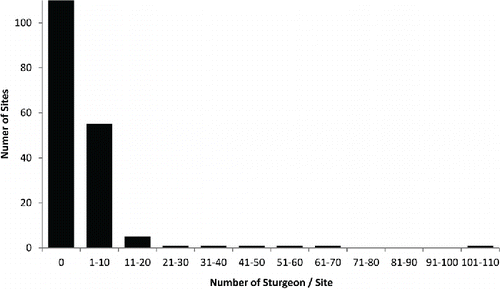
Counts of sturgeon during surveys ranged from 0 to 109 across sites (). Of the 179 sites, 113 had no sturgeon detected, 55 had between 1 and 10 sturgeon, and only 11 had more than 10 sturgeon. Clearly, sturgeon were not uniformly distributed across sites or systems. When detected, they were predominantly alone or in small numbers and not in large aggregations, except in a few instances.
FIGURE 2. The distribution of Atlantic Sturgeon counts per site across all rivers (179 sites) sampled in the Carolina and South Atlantic distinct population segments. Most sites had detections of either no sturgeon or 1–10. The maximum number of sturgeon counted at a single site was 109.
The best performing N-mixture model used a negative binomial distribution for abundance and included river as a covariate (). There was negligible support for a Poisson distribution for abundance (with or without zero inflation) or models without river-specific estimates. Estimated abundance varied substantially among sites and rivers, the highest observed and estimated counts being for the Pee Dee and Edisto rivers (; ). The Pee Dee River had the highest estimated abundance for a single river (1,944) and comprised a large fraction of estimated total abundance for the Carolina DPS (). The selected model appeared to be adequate based on a goodness-of-fit test (). The data do appear to be overdispersed, with estimated dispersion α > 0, at 0.13.
TABLE 1. Comparison of N-mixture models as applied to Atlantic Sturgeon in the Carolina and South Atlantic distinct population segments with and without river as a covariate for abundance and using alternative distributions for abundance. Model fit is compared using Akaike's information criterion (AIC); a lower value indicates a better performing model. The number of parameters in each model is denoted by nPars, ΔAIC is the difference between AIC value and the best model, and AICwt represents the relative likelihood of each model.
TABLE 2. Abundance estimates and 95% confidence intervals in parentheses for riverine Atlantic Sturgeon >1 m TL within six North Carolina and South Carolina rivers (see ). Results for each analytical approach are from the model with lowest Akaike information criterion. Maximum counts (per survey day) and number of survey sites for each river system are listed for comparison. The Carolina distinct population segment (DPS) estimate is a total for all rivers except the Edisto.
FIGURE 3. Site-specific estimates of Atlantic Sturgeon abundance in the Carolina and South Atlantic distinct population segments derived by the distance (solid) and N-mixture (dashed) models and overlaid onto counts of sturgeon per site for each survey day. Model estimates followed trends in site counts, and there was little difference in estimates between models.
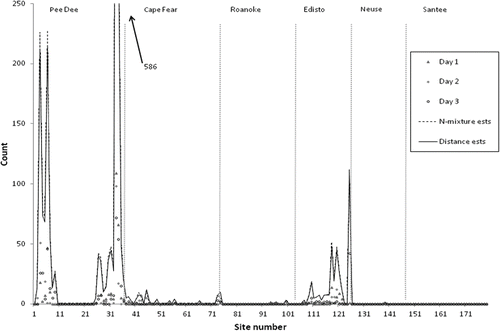
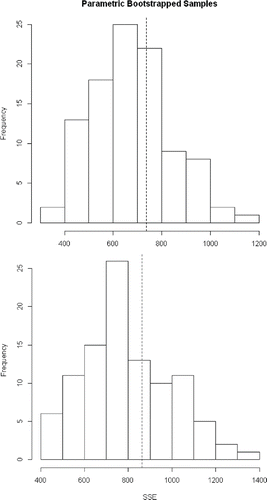
FIGURE 4. Results of parametric bootstrap goodness-of-fit testing as related to Atlantic Sturgeon in the Carolina and South Atlantic distinct population segments. The upper panel is for the N-mixture model; the lower panel is for the distance model. The dotted line is the Freeman–Tukey test statistic. Both models adequately explain the observed data.
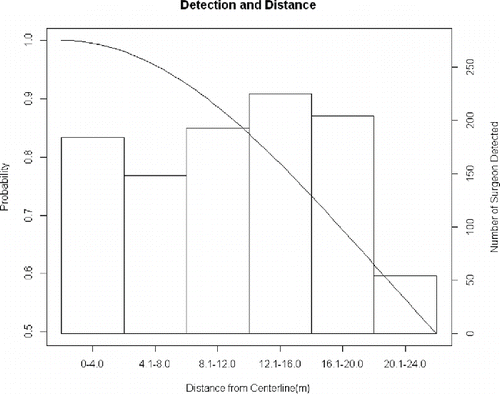
The best-performing distance model also utilized a negative binomial distribution for abundance, with river as a covariate (). As in the N-mixture models, there was negligible support for a Poisson distribution for abundance. The preferred model used a half-normal distribution for describing detection probability. The pattern of detections by range bins () was not well described by the half-normal distribution (or any other alternative), but the half-normal did account for the low detections at ranges greater than 20 m. Detections probably decreased at the far edge of the swath because acoustic shadows would have been outside the sonar image field-of-view. Detections increased gradually at distances of 8–20 m off the transect, perhaps because the more gradual angle relative to the sonar provided a clearer acoustic shadow for identification. Abundance estimates from the distance model were quite similar to those of the N-mixture model, the Carolina DPS estimate being 1,823 (; ). The preferred distance model was supported by the goodness-of-fit test (). The data do appear to be overdispersed, with estimated dispersion α > 0, at 0.274.
TABLE 3. Comparison of distance models as applied to Atlantic Sturgeon in the Carolina and South Atlantic distinct population segments with and without river as a covariate for abundance and using alternative distributions for abundance and detection. Model fit is compared using Akaike's information criterion (AIC); a lower value indicates a better performing model. For all but the top performing model, AIC weights were <0.001. The number of parameters in each model is denoted by nPars, ΔAIC is the difference in a model's AIC value and the best model, and AICwt represents the relative likelihood of each model.
FIGURE 5. Detection probability for the distance model and distribution of side-scan sonar targets (i.e., Atlantic Sturgeon in the Carolina and South Atlantic distinct population segments) across the scan swath for all surveyed rivers (see Figure1). Target distance is measured from centerline of side-scan image to body of sturgeon target. The detection probability function shown is the half-normal for the top AIC selected distance model, σ = 20.3.
DISCUSSION
Our abundance estimates are the first of any kind on these rivers and only the fifth estimate of Atlantic Sturgeon abundance rangewide (Kocik et al. 2013; Kahn et al. 2014). Sturgeon have been observed in these rivers and sampled in various studies (Moser and Ross Citation1995; Collins and Smith Citation1997; Collins et al. Citation2000; Armstrong and Hightower Citation2002; Oakley and Hightower Citation2007; authors’ unpublished data), but no assessments have been undertaken. A recent range-wide study estimates that there may be 6,615–29,784 ocean-going Atlantic Sturgeon in the Carolina DPS (Kocik et al. Citation2013).
Our Atlantic Sturgeon population estimates are not all-inclusive. Estimates are limited to sturgeon >1 m because smaller sturgeon are difficult to identify confidently using side-scan images. Thus, all identified sturgeon were likely Atlantic Sturgeon due to their greater abundance and larger overall size. Only the largest Shortnose Sturgeon A. brevirostrum would be close to the 1 m size threshold for identification. Estimates are also limited to the reaches of rivers surveyed. This zone, the freshwater–saltwater interface, probably contained most of a river's available Atlantic Sturgeon encompassing ≥1 m, although side-scan detections of sturgeon sometimes occurred to the upper extent of survey reaches (Flowers and Hightower Citation2013, Supplement A). We did not observe sturgeon in our surveyed portion of the Santee River, resulting in a 0 population estimate, but sturgeon have been observed there in the past (Collins et al. Citation2000). A different survey design would be required to produce estimates that would apply to each entire river.
Both models require that sites be closed with respect to mortality, recruitment, and movement. Our survey passes were typically done on successive days to minimize these events, and telemetry data showed limited movement between sampling occasions (Flowers and Hightower Citation2013). Prior studies have shown that Atlantic Sturgeon occupy deep areas and show little or no movement for extended periods during summer (Moser and Ross Citation1995). Distance sampling assumes that transects are located randomly and independent of animals’ locations (Thomas et al. Citation2010). Our transects extended through the entire study reach so were therefore independent of sites containing sturgeon. However, we do recommend that future side-scan surveys of sturgeon be done with transects at varying locations cross-channel.
Based on telemetry studies (Erickson et al. Citation2011; Parauka et al. Citation2011; Oliver et al. Citation2013), sturgeon are known to move between river systems. Therefore, a sturgeon detected in a given river system may not belong to that river's reproductive population. We also do not know what proportion of sturgeon remain in river systems during summer versus move to marine habitats. Generally it is thought that juvenile Atlantic Sturgeon stay in riverine areas year-round, while adults move into rivers during spring for spawning but move out into marine environments during other times (Smith Citation1985; Bain Citation1997). Our study and others (Moser and Ross Citation1995; Collins et al. Citation2000) have observed adult and subadult Atlantic Sturgeon in riverine areas during summer. In an ongoing study in the Roanoke River–Albemarle Sound system, most sonic-tagged adult Atlantic Sturgeon were present during the summer months (two of three in 2011, five of five in 2012, and three of six in 2013), not in offshore marine waters (authors’ unpublished data). This summering behavior may be similar to observations for Gulf Sturgeon A. oxyrinchus desotoi (Wooley and Crateau Citation1985; Clugston et al. Citation1995). Additionally, presence of adult sturgeon in rivers during summer may be related to fall spawning (Smith et al. Citation1984; Balazik et al. Citation2012; Smith et al. Citation2015).
The best individual model for each method used a negative binomial distribution because the data appeared to be overdispersed, based on estimated values of the overdisperion parameter α. Precision was similar for both models, although the distance model had slightly narrower confidence intervals. The distance model used replicate counts to provide information about the probability of being available for detection (Chandler et al. Citation2011). This could represent temporary emigration or in this case fish that are present but oriented in a way that does not allow for detection. The replicate counts allow the model to relax the assumption that detection probability is 1 on the transect.
For both models, estimated detection probability was a product of true detection probability and availability of an animal within the site to be detected by the survey. These two components can sometimes be estimated separately, such as in occupancy modeling with multiple gears (Hines et al. 2010; Flowers and Hightower 2013). However, there is insufficient information available in this study to parse the effects of detection probability and availability using only one survey gear. While our assumption of site closure was probably not violated for the 2-km sites, it was likely that all sturgeon did not remain within our side-scan swaths during our surveys, based on the variability of sturgeon counted in individual sites over the course of our surveys. If sturgeon were moving randomly in and out of our survey swaths, our abundance estimates should reflect the numbers of sturgeon in our surveyed rivers. If sturgeon movement was more limited, abundance estimates may need to be scaled up to account for areas not surveyed by the sonar.
The distance model incorporates information about the spatial distribution of animals within the survey swath to make inference about changes in detection probability with distance, while the N-mixture model assumes an average detection probability throughout the swath. Even though the half-normal function was selected as the best to model detection across the sampling swath, it did not appear to fit the distance observations well (). We evaluated the results of other poorer fitting distributions, but abundance estimates were comparable to the half-normal. Another option was to truncate our data observations to a maximum distance of 20 m. In this case the uniform distribution fit best and estimates were more similar to those of the N-mixture model. Distance-sampling methods may not be well suited for use with side-scan sonar because the survey width is arbitrarily truncated by the width of the sonar swath, which explains why estimates were so similar for each model. Apparent changes in detection probability across the swath may be more of an artifact of interpreting side-scan images rather than of detection itself. Distance sampling may be more effective over wider swath widths, where there is greater contrast between image quality of near and far targets.
CONCLUSIONS
Side-scan sonar can be used to survey sturgeons and potentially other large fishes and various analytical approaches, such as N-mixture and distance models, are well suited for side-scan data. It is interesting that both abundance estimation models provided such similar estimates despite using different data sets and assumptions about detection. The N-mixture model requires less data (counts only, no distances off transect) than the distance-sampling model and only had slightly more uncertainty around abundance estimates. Further studies are needed to determine whether the pattern in our distance data are a result of the distribution of sturgeon within our study, characteristics of side-scan data, or both. If patterns are related to side-scan characteristics, then new detection functions and analysis techniques may be needed to properly analyze data.
Our abundance modeling provided useful information about the status of sturgeon within our sampled rivers systems. We were able to produce estimates of sturgeon populations in river systems using a fraction of the effort of traditional netting programs and without having to handle our target species. Covariates that could influence abundance can also be incorporated into all models (Kéry and Schaub Citation2012). Abundance estimates from side-scan surveys can be used in conjunction with other data sources, such as genetic abundance estimates or traditional mark–recapture tagging studies to improve population abundance estimates. Side-scan sonar can provide absolute abundance estimates in discrete areas while tagging (especially with sonic tags) could provide the information needed for expanding to systemwide estimates. Netting would also allow for information to be collected on smaller individuals and species composition. Side-scan sonar can also provide habitat information about sturgeon locations and identify potential areas where netting operations could be performed safely.
ACKNOWLEDGMENTS
Funding for this project was provided by National Oceanographic and Atmospheric Administration. We thank T. Jackson for his work in the field and in the laboratory reviewing side-scan and video data. We appreciate C. Collier and M. Loeffler (North Carolina Division of Marine Fisheries), and W. Post (South Carolina Department of Natural Resources) allowing us the use of their sonic-tagged sturgeon during the course of the study. We also thank N. Cole, J. Hughes, and A. Prince for assistance during preliminary phases of our side-scan sonar work. B. Gardner and K. Pollock provided comments on an earlier version of the manuscript. The Cooperative Fish and Wildlife Research Unit is jointly supported by North Carolina State University, North Carolina Wildlife Resources Commission, U.S. Geological Survey, U.S. Fish and Wildlife Service, and Wildlife Management Institute. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
REFERENCES
- Armstrong, J. L., and J. E. Hightower. 2002 Potential for restoration of the Roanoke River population of Atlantic Sturgeon. Journal of Applied Ichthyology 18:475–480.
- ASSRT (Atlantic Sturgeon Status Review Team). 2007. Status review of Atlantic Sturgeon (Acipenser oxyrinchus oxyrinchus). Report to National Marine Fisheries Service, Northeast Regional Office, Gloucester, Massachusetts.
- Bain, M. B. 1997. Atlantic and Shortnose sturgeons of the Hudson River: common and divergent life history attributes. Environmental Biology of Fishes 48:347–358.
- Balazik, M. T., G. C. Garman, J. P. Van Eenennaam, J. Mohler, and L. C. Woods III. 2012. Empirical evidence of fall spawning by Atlantic Sturgeon in the James River, Virginia. Transactions of the American Fisheries Society 141:1465–1471.
- Bergman, P. 2011. Videography monitoring of adult sturgeon in the Feather River basin, CA. Report to Anadromous Fish Restoration Program, Cramer Fish Sciences, Gresham, Oregon.
- Buckland, S. T., D. R. Anderson, K. P. Burnham, J. L. Laake, D. L. Borchers, and L. Thomas. 2001. Introduction to distance sampling. Oxford University Press, Oxford, UK.
- Chandler, R. B., J. A. Royle, and D. I. King. 2011. Inference about density and temporary emigration in unmarked populations. Ecology 92:1429–1435.
- Clugston, J. P., A. M. Foster, and S. H. Carr. 1995. Gulf Sturgeon, Acipenser oxyrinchus desotoi, in the Suwannee River, Florida, USA. In A. D. Gershanovich and T. I. J. Smith, editors. Proceedings international symposium on sturgeons. Russian Federal Research Institute of Fisheries and Oceanography Publications, Moscow.
- Cobra, D. T., A. V. Oppenheim, and J. S. Jaffe. 1992. Geometric distortions in side-scan sonar images: a procedure for their estimation and correction. IEEE (Institute of Electrical and Electronics Engineers) Journal of Oceanic Engineering 17:252–268.
- Collins, M. R., and T. I. J. Smith. 1997. Distributions of Shortnose and Atlantic sturgeons in South Carolina. North American Journal of Fisheries Management 17:995–1000.
- Collins, M. R., T. I. J. Smith, W. C. Post, and O. Pashuk. 2000. Habitat utilization and biological characteristics of adult Atlantic Sturgeon in two South Carolina rivers. Transactions of the American Fisheries Society 129:982–988.
- Damon-Randall, K., R. Bohl, S. Bolden, D. Fox, C. Hager, B. Hickson, E. Hilton, J. Mohler, E. Robbins, T. Savoy, and A. Spells. 2010. Atlantic Sturgeon research techniques. NOAA Technical Memorandum NMFS-NE-215.
- Erickson, D. L., A. Kahnle, M. J. Millard, E. A. Mora, M. Bryja, A. Higgs, J. Mohler, M. DuFour, G. Kenney, J. Sweka, and E. Pikitch. 2011. Use of pop-up satellite archival tags to identify oceanic-migratory patterns for adult Atlantic Sturgeon, Acipenser oxyrinchus oxyrinchus Mitchell, 1815. Journal of Applied Ichthyology 27:356–365.
- Fiske, I., and R. B. Chandler. 2011. unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software 43:1–23.
- Flowers, H. J., and J. E. Hightower. 2013. A novel approach to surveying sturgeon using side-scan sonar and occupancy modeling. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science [online serial] 5:211–223.
- Foote, K. G. 2009. Acoustic methods: brief review and prospects for advancing fisheries research. Fish and Fisheries Series 31:313–343.
- Gross, M. R., J. Repka, C. T. Robertson, D. H. Secor, and W. Van Winkle. 2002. Sturgeon conservation: insights from elasticity analysis. Pages 13–30 in W. Van Winkle, P. Anders, D. Dixon, and D. Secor, editors. Biology, management and protection of North American sturgeons. American Fisheries Society, Symposium 28, Bethesda, Maryland.
- Hines, J. E., J. D. Nichols, J. A. Royle, D. I. MacKenzie, A. M. Gopalaswamy, N. Samba Kumar, and K. U. Karanth. 2010. Tigers on trails: occupancy modeling for cluster sampling. Ecological Applications 20:1456–1466.
- Kaeser, A. J., and T. L. Litts. 2008. An assessment of deadhead logs and large woody debris using side scan sonar and field surveys in streams of southwest Georgia. Fisheries 33:589–597.
- Kahn, J., C. Hager, J. C. Watterson, J. Russo, K. Moore, and K. Hartman. 2014. Atlantic Sturgeon annual spawning run estimate in the Pamunkey River, Virginia. Transactions of the American Fisheries Society 143:1508–1514.
- Kahn, J., and M. Mohead. 2010. A protocol for use of Shortnose, Atlantic, Gulf, and Green sturgeons. NOAA Technical Memorandum NMFS-OPR-45.
- Kéry, M, and M. Schaub 2012. Bayesian population analysis using WinBUGS: a hierarchical perspective. Academic Press, Waltham, Massachusetts.
- Kocik, J., C. Lipsky, T. Miller, P. Rago, and G. Shepherd. 2013. An Atlantic Sturgeon population index for ESA management analysis. Northeast Fisheries Science Center, Reference Document 13–06, Woods Hole, Massachusetts.
- Moser, M. L., and S. W. Ross. 1995. Habitat use and movements of Shortnose and Atlantic sturgeons in the lower Cape Fear River, North Carolina. Transactions of the American Fisheries Society 124:225–234.
- Nealson, P. A., and H. M. Brundage III. 2007. Feasibility assessment of split-beam hydroacoustic techniques for monitoring adult Shortnose Sturgeon in the Delaware River. Pages 405–415 in J. Munro, D. Hatin, J. E. Hightower, K. McKown, K. J. Sulak, A. W. Kahnle, and F. Caron, editors. Anadromous sturgeons: habitats, threats, and management. American Fisheries Society, Symposium 56, Bethesda, Maryland.
- Nelson, T. C., P. Doukakis, S. T. Lindley, A. D. Schreier, J. E. Hightower, L. R. Hildebrand, R. E. Whitlock, and M. A. H. Webb. 2013. Research tools to investigate movements, migrations, and life history of sturgeons (Acipenseridae), with an emphasis on marine-oriented populations. PLoS ONE [online serial] 8:e71552.
- Oakley, N. C., and J. E. Hightower. 2007. Status of Shortnose Sturgeon in the Neuse River, North Carolina. Pages 273–284 in J. Munro, D. Hatin, J. E. Hightower, K. McKown, K. J. Sulak, A. W. Kahnle, and F. Caron, editors. Anadromous sturgeons: habitats, threats, and management. American Fisheries Society, Symposium 56, Bethesda, Maryland.
- Oliver, M. J., M. W. Breece, D. A Fox, D. E. Haulsee, J. T. Kohut, J. Manderson, and T. Savoy. 2013. Shrinking the haystack: using an AUV in an integrated ocean observatory to map Atlantic Sturgeon in the coastal ocean. Fisheries 38:210–216.
- Parauka, F. M., M. S. Duncan, and P. A. Lang. 2011. Winter coastal movement of Gulf of Mexico sturgeon throughout northwest Florida and southeast Alabama. Journal of Applied Ichthyology 27:343–350.
- Peterson, D. L., P. Schueller, R. DeVries, J. Fleming, C. Grunwald, and I. Wirgin. 2008. Annual run size and genetic characteristics of Atlantic Sturgeon in the Altamaha River, Georgia. Transactions of the American Fisheries Society 137:393–401.
- Pitkitch, E. K., P. Doukakis, L. Lauck, P. Chakrabarty, and D. L. Erickson. 2005. Status, trends, and management of sturgeon and Paddlefish fisheries. Fish and Fisheries 6:233–265.
- R Development Core Team. 2009. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available: www.R-project.org. (April 2014).
- Royle, J., D. K. Dawson, and S. Bates. 2004. Modeling abundance effects in distance sampling. Ecology 85:1591–1597.
- Royle, J. A. 2004. N-mixture models for estimating population size from spatially replicated counts. Biometrics 60:108–115.
- Secor, D. H. 2002. Atlantic Sturgeon fisheries and abundances during the late nineteenth century. Pages 89–101 in W. Van Winkle, P. Anders, D. Dixon, and D. Secor, editors. Biology, management and protection of North American sturgeons. American Fisheries Society, Symposium 28, Bethesda, Maryland.
- Smith, J. A., H. J. Flowers, and J. E. Hightower. 2015. Fall spawning of Atlantic Sturgeon in the Roanoke River, North Carolina. Transactions of the American Fisheries Society 144:48–54.
- Smith, T. I. J. 1985. The fishery, biology, and management of Atlantic Sturgeon, Acipenser oxyrinchus, in North America. Environmental Biology of Fishes 14:61–72.
- Smith, T. I. J., D. E. Marchette, and G. F. Ulrich. 1984. The Atlantic Sturgeon fishery in South Carolina. North American Journal of Fisheries Management 4:164–176.
- Thomas, L., S. T. Buckland, E. A. Rexstad, J. L. Laake, S. Strindberg, S. L. Hedley, J. R. B. Bishop, T. A. Marques, and K. P. Burnham. 2010. Distance software: design and analysis of distance sampling surveys for estimating population size. Journal of Applied Ecology 47:5–14.
- Wooley, C. M., and E. J. Crateau. 1985. Movement, microhabitat, exploitation, and management of Gulf of Mexico sturgeon, Apalachicola River, Florida. North American Journal of Fisheries Management 5:590–605.
